Abstract
Respiratory syncytial virus (RSV) infection is the most common cause of lower respiratory tract infection and the leading cause of hospitalization among young children, incurring high annual costs among US children under the age of 5 years. Palivizumab has been found to be effective in reducing hospitalization and preventing serious lower respiratory tract infections in high-risk infants. This paper presents a systematic review of the cost-effectiveness studies of palivizumab and describes the main highlights of a round table discussion with clinical, payer, economic, research method, and other experts. The objectives of the discussion were to (1) review the current state of clinical, epidemiology, and economic data related to severe RSV disease; (2) review new cost-effectiveness estimates of RSV immunoprophylaxis in US preterm infants, including a review of the field’s areas of agreement and disagreement; and (3) identify needs for further research.
Keywords: expert review, immunoprophylaxis, pediatrics, cost-effectiveness, respiratory syncytial virus, value
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infection among young children and the most common cause of hospitalization among those younger than 1 year [1]. Among high-risk infants, RSV hospitalization is associated with substantial costs from prolonged intensive care and/or mechanical ventilation [2]. Studies suggest that among US children under the age of 5 years, RSV infection is responsible for anywhere from 57 500 to more than 100 000 hospitalizations and 2.1 million outpatient visits annually [3]. From a population perspective, RSV is expensive, costing more than $650 million annually in the United States, according to estimates from 2000 [4]. Additional costs such as caregiver absences from work and recurrent wheezing episodes in infants who experience severe illness also can contribute to the substantial cost burden of RSV disease.
Infants who are born prematurely (defined as <37 weeks gestational age [wGA]) and those with underlying concomitant disorders such as chronic lung disease (CLD) or congenital heart disease (CHD) are at increased risk for severe, sometimes life-threatening RSV disease [5]. Environmental risk factors for RSV infection include pollution, living in crowded conditions or at higher altitude, low parental education or socioeconomic status, and child care attendance. More severe RSV disease is correlated with preterm birth and young chronologic age [3, 6, 7].
There is currently no vaccine available to prevent RSV infection in infants. Developing a vaccine for RSV is very challenging given the young chronologic age of the target population and concerns regarding their immune responsiveness [8]. In 1998 the US Food and Drug Administration (FDA) approved immunoprophylaxis with palivizumab to prevent RSV infection for those at high risk of RSV disease. Palivizumab is a monoclonal antibody that must be injected once each month during the RSV season; the season typically lasts from November through March [9]. Palivizumab has been found to be effective in reducing hospitalizations and preventing serious lower respiratory tract infections in high-risk infants. The cost of immunoprophylaxis varies, as the dose is weight dependent. A single course of palivizumab is estimated to range from $1500 to $4300 per month, costing as much as $6000 to $20 000 per child for 4 to 5 doses in 1 RSV season [10, 11]. A significant portion of the children receiving immunoprophylaxis are covered by state Medicaid plans [12], although eligibility criteria for coverage vary from state to state. The price of immunoprophylaxis per child/year has remained relatively steady over the past 5 years.
Since the FDA approval of palivizumab, the American Academy of Pediatrics (AAP) has updated its RSV patient management guidance 4 times, focusing on subgroups of children who are at high risk of RSV infection. In 2014, the AAP issued the most recent update as a policy statement and concluded that preterm infants born between 29 and 35 wGA without CLD, hemodynamically significant CHD, or other coexisting conditions have only a small risk (<5%) of RSV hospitalization. Table 1 shows a comparison of 2012 guideline and 2014 AAP policy for RSV immunoprophylaxis. The 2014 policy recommends that the subpopulation of premature infants without other qualifying conditions who previously qualified to receive prophylaxis under the 2012 guidelines not be offered prophylaxis. In addition, infants with CHD who are older than 1 year during RSV season are no longer recommended to receive immunoprophylaxis according to the 2014 AAP policy [13].
Table 1.
Selected Elements of the 2012 and 2014 AAP RSV Immunoprophylaxis Guidance
| Population | 2012 AAP Guideline [30] | 2014 AAP Policy [13] |
|---|---|---|
| ≤28 wGA | Recommended | Recommended |
| 29–31 wGA | ≤6 mo CA at RSV season start | Not recommended unless other qualifying conditions |
| 32–34 wGA | Recommended if <90 days old and 1 risk factor is present: 1. day care attendance 2. siblings who are >5 y |
Not recommended unless other qualifying conditions |
| 35 wGA | Not recommended unless other qualifying conditions | Not recommended unless other qualifying conditions |
| Chronic lung disease | ≤24 mo CA and requiring medical therapy within 6 mo of RSV season start | • ≤12 mo CA at RSV season start • 12–24 mo CA and requiring medical therapy within 6 mo of RSV season start |
| Congenital heart disease | ≤24 mo CA at RSV season start | ≦12 mo CA at RSV season start with noncyanotic heart disease |
Abbreviation: CA = chronologic age since birth.
Identifying which children should be targeted for RSV immunoprophylaxis remains a challenge for parents and health care providers of premature infants. Conflicting evidence about the cost-effectiveness of palivizumab has led to changes in clinical recommendations on the use of RSV immunoprophylaxis and created confusion. Demand to better understand RSV disease and the cost-effectiveness of immunoprophylaxis is growing from providers, payers, and parents [14].
In this paper, we present a systematic review of the cost-effectiveness studies of palivizumab. In addition, we describe the main highlights of a round table discussion with clinical, payer, research methods, and other experts about the available cost-effectiveness evidence for RSV immunoprophylaxis and the next steps for future research. The objectives of the round table were to (1) review the current state of clinical, epidemiology, and economic data related to severe RSV disease in US infants born between 29 and 35 wGA; (2) review new cost-effectiveness estimates of the value of RSV immunoprophylaxis in US preterm infants, including a review of assumptions and scenarios to understand the field’s areas of agreement and disagreement; and (3) identify areas of common ground and areas of evidence uncertainty as well as needs for further research.
METHODS
Literature Review
We conducted a systematic search for published cost-effectiveness analyses using the Tufts Cost-Effectiveness Analysis (CEA) Registry, as well as PubMED, Cochrane collaboration library of systematic reviews, and health technology assessment body reports for the National Institute for Clinical Excellence (NICE) in the United Kingdom and the Canadian Agency for Drugs and Technologies in Health (CADTH) to inform the round table discussion. The CEA Registry is a database containing detailed information from a large systematic review of cost-utility analyses in the peer-reviewed literature, including more than 14 570 standardized cost-effectiveness ratios, as well as more than 21 900 utility weights. The methodology underlying the CEA Registry has been described previously [15]. For this study, we limited our search to articles that reported an original cost-effectiveness analysis study where the intervention was RSV prophylaxis with palivizumab; that studied either a US, Canadian, or UK population; and were published between January 1998 and December 2016. The search was conducted with the following terms: “RSV,” “respiratory syncytial virus,” “palivizumab” or “prophylaxis,” and “cost effectiveness” or “cost-effectiveness.” We referred to health technology assessment reports in order to capture all the original studies that might not have otherwise come up on our searches. While meta-analyses did not fit our inclusion criteria, we checked the references and literature reviews performed as part of the meta-analysis in order to enrich our own literature search.
Further, we assessed the risk of bias for each study, using modified criteria for assessing study design and validity in clinical research [16]. The criteria included quality of evidence that supported health-economic model assumptions, as well as the level of validation of model internal consistency with techniques such as 1-way deterministic and multivariate probabilistic sensitivity analyses. Two reviewers independently assessed each study, rated its risk of bias (low, medium, or high) and met to reach consensus on their findings.
We evaluated the methods and assumptions used in these published cost-effectiveness analyses to determine what was primarily driving differences in the results of the published analyses and how they compared with the 2014 AAP policy. Specifically, we reviewed the patient populations included in the analysis, the epidemiological and cost assumptions, the time horizons and perspectives of the analyses, and the specific outcome measures. The perspective of a cost analysis can have a large impact on results. For example, a study might be narrowly focused on a particular part of the health care sector or might take into account broader impacts of disease and treatment, such as impact on caregivers, their costs, health, and productivity.
Round Table Discussion
The round table meeting was held on November 11, 2016, in Boston, Massachusetts, and included discussants from various health care perspectives, including clinicians, payers, policy experts, and health economists. Participants were invited based on having one of the roles above, scientific interest in the area of RSV, and availability to attend an in-person meeting. Additionally, we invited members of the American Academy of Pediatrics Committee on Infectious Diseases; however, they declined the invitation. Researchers from University of Washington and Tufts Medical Center reviewed and presented the available evidence, retaining independence to publish and disseminate the conclusions of the research and the round table discussion. AstraZeneca, the manufacturer of palivizumab, sponsored the meeting. In order to describe, from a variety of different stakeholder viewpoints, the issues and gaps around the evidence on RSV immunoprophylaxis and to generate a discussion between the various stakeholders, we used focus group methodology: a moderated semistructured discussion among the panel participants, allowing each participant a chance to speak and respond to a set of topic questions and comments raised by other participants. The discussions focused on 2 key topics: (1) epidemiological and clinical challenges and (2) value assessment challenges.
RESULTS
Literature Review
General Methods and Model Inputs
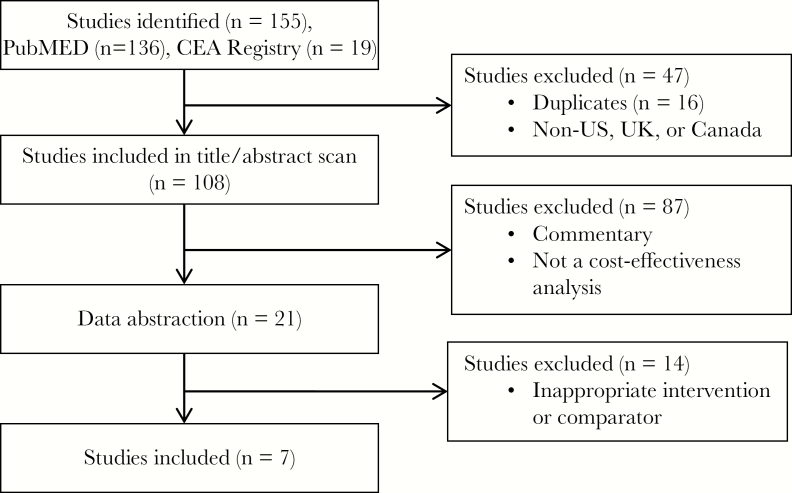
We identified 7 studies that met our inclusion criteria (Figure 1 and Table 2). Most of the studies used similar methods for cost-effectiveness analyses and employed consistent data. Most of the eligible studies examined a 5-month RSV season corresponding to 5 doses of palivizumab. Six out of 7 studies conducted the analysis from a health care payer perspective, incorporating direct medical costs of the immunoprophylaxis and hospitalizations due to RSV over a lifetime time horizon, and examined the short-term benefits of reducing the number of hospitalizations and of reducing morbidity and mortality. Only 4 studies attempted to include a broader societal perspective in their analysis, and only 3 included both the health care sector and the societal perspective, as recommended by the Second Panel on Cost-Effectiveness in Health and Medicine [17]. All 7 studies we reviewed used quality-adjusted life-years (QALYs) as the outcome measure. Five of the 7 studies were sponsored by pharmaceutical companies, and the remaining 2 did not disclose the funding source. In our review of risk of bias, 3 studies were found to be at medium risk [18–20] and 4 were considered at high risk of bias [21–24].
Figure 1.
Literature search and selection flow diagram.
Table 2.
Summary of Economic Analyses of Palivizumab Prophylaxis
| Study, Country | Population (Perspective) | Time Horizon | Discount Rates | Influential Input Parameters | Outcome Measure | Methodology | Results | Threshold | Sponsor |
|---|---|---|---|---|---|---|---|---|---|
| Bentley et al. 2013, UK [18] | Infants ≤35 wGA and ≤6 mo at the start of the RSV season, ≤24 mo with CLD, and ≤24 mo with CHD (payer) | Lifetime | 3.5% | Direct medical costs (hospitalization, administration, medicine), risk/cost of asthma, mortality, life expectancy, LOS, rate/risk reduction of hospitalization, health utility values | Cost per QALY | Decision tree following clinical path for high-risk infants | ICER was $41 361 for infants with CHD; $23 868 for infants with CLD; $4788 for <29 wGA; $37 612 for 29–32 wGA; and $123 347 for 33–35 wGAa | $37 000/ QALYa | AbbVie |
| Mahadevia et al. 2012, US [22] | Premature infants <35 wGA per AAP 2006 and 2009 guidelines from both public and private sectors (provider) | Lifetime | 3% | Direct costs included medical costs (administration, medicine), and indirect costs included transportation and time lost due to illness | Cost per QALY | Decision tree focused on hospitalization using clinical trials and published data | ICER was $44 774/ QALY for 32–34 wGA with 2009 AAP RFs; $79 477/ QALY for 32–35 wGA with 2006 AAP RFs; $464 476/ QALY for 32–35 wGA with ≤1 RF | $25 000/QALY $50 000/QALY $75 000/QALY $300 000/ QALY |
MedImmune |
| Weiner et al. 2012, US [23] | Premature infants <35 wGA per AAP 2006 and 2009 guidelines within Medicaid (provider) | Lifetime | 3% | Direct costs included medical costs (administration, medicine), and indirect costs included transportation and time lost due to illness | Cost per QALY | Decision tree focused on hospitalization using clinical trials and published data | ICER was $16 037/ QALY for 32–34 wGA with 2009 AAP RFs; $32 244/ QALY for 32–35 wGA for 2006 AAP RF; $281 892/ QALY for 32–35 wGA with 1 RF | $25 000/QALY $50 000/QALY $75 000/QALY $300 000/ QALY |
MedImmune |
| Lanctôt et al. 2008, Canada [19] | Infants 32–35 wGA with RSV or symptoms and 0–5 RFs (payer/ societal) | Lifetime | 5% | Direct costs included medical costs (administration, medicine), asthma costs, and indirect costs of work loss and productivity, rate of hospitalization, LOS and mortality | Cost per QALY | Decision tree following path for infants in different RF subgroups between 32–35 wGA | ICER was $801 297/ QALY for infants with 0 RFs; $143 267/ QALY with 1 RF; $81 331/ QALY with 2 RFs; $26 667/ QALY for 3 RFs; $808/ QALY for 4 RFs | $50 000/QALY | Abbott |
| Nuijten et al. 2007, UK [20] | Preterm infants ≤35 wk gestation, children with BPD and CHD (payer/ societal) | Lifetime | 3.5% | Prophylaxis costs, hospitalization costs, clinical complications (asthma) | Cost per QALY | Cost-utility/ cost-benefit with decision tree based on published literature, clinical trials, and UK price lists and population statistics | ICER was $20 800/ QALY with discountinga | $31 000/ QALYa | Abbott GmbH & Co. |
| Elhassan et al. 2006, US [21] | Premature infants <32 wGA (societal) | 8 y | 3% | Length of stay, asthma | Cost per QALY | Decision tree assessing hypothetical cohorts based on published data | ICER ranged from $675 780/ QALY (29–30 wGA) to $1 855 000/QALY (32 wGA); gestational age and ICER did not exhibit a strong relationship | $200 000/ QALY | none |
| Yount et al. 2004, US [24] | Children with CHD per AAP guidelines (provider and societal) | Lifetime | 3% | Direct costs included medical costs and medication costs, and indirect costs included missed work (parent) and mortality value | Cost per QALY | Decision tree and cost utility for a hypothetical cohort of 10 000 pediatric CHD patients | 203.33 life-years were saved with a cost per QALY of $US 114 337 | $100 000/ QALY | none |
Abbreviations: LOS, length of stay; RF, risk factor.
aICERs converted to $US and same year (2016).
Patient Populations Studied
The patient populations included in the study samples are shown in Table 3. Six of the 7 studies included a premature infant population, and 1 study focused only on an infant population with CHD. Two of the studies examined all main high-risk categories (prematurity, CLD, CHD), whereas 3 other studies examined other risk factors associated with RSV disease.
Table 3.
Patient Populations Examined in CEAs on the Use of Palivizumab for RSV Prophylaxis
| Included Subgroups | ||||||
|---|---|---|---|---|---|---|
| Article Author (Year), Country | Premature | CLD | CHD | CA | Other | Population Description From Article |
| Yount (2004), US [24] | X | Infants/children with CHD | ||||
| Elhassan (2006), US [21] | X | Premature infants ≤32 wGA | ||||
| Nuijten (2007), UK [20] | X | X | X | Premature infants <35 wGA with CLD or with CHD | ||
| Lanctôt (2008), Canada [19] | X | X | Premature infants 32–35 wGA | |||
| Mahadevia (2012), US (public/private) [22] |
X | X | X | 1.<32 wGA, ≤6 mo CA 2.32–34 wGA, ≤3 mo CA, 2009 AAP risk factors* 3.32–35 wGA, ≤6 mo CA, 2006 AAP risk factors* 4.32–35 wGA, ≤6 mo CA, ≤1 risk factors* *Risk factors do not include infants with CLD or CHD |
||
| Weiner (2012), US (Medicaid) [23] |
X | X | X | |||
| Bentley (2013), UK [18] | X | X | X | X | Infants with either CHD, CLD, or premature (<29–35 wGA) | |
Abbreviation: CA, chronologic age.
Variation in Reported ICERs
Table 4 shows the ranges of the reported incremental cost-effectiveness ratios (ICERs) for various groups. For the studies that examined the population of infants with CHD, ICERS ranged from $15 000/QALY to $140 000/QALY gained. This group falls within the recommended coverage criteria put forth by the AAP if the infant is younger than 12 months of age. For the studies that examined a population of infants with CLD, ICERs ranged from $31 000/QALY to $38 000/QALY gained. This group also falls within the recommended coverage criteria in the AAP if the infant is younger than 24 months of age. Cost-effectiveness ratios for a population of premature infants ranged from $800/QALY to $800 000/QALY gained. The magnitude of the ICER was different in specific subgroups, particularly as defined by wGA at birth, as well as the number of risk factors. The differences were driven by a combination of factors varying by subgroup, including the risk of adverse outcomes associated with RSV, drug effect sizes, and costs. Immunoprophylaxis is less cost-effective and less likely to be recommended by the AAP if the infant is of older gestational age (ie, 32–35 wGA) and older chronologic age, and if they have fewer risk factors.
Table 4.
Selected Cost-effectiveness Ratios for RSV Immunoprophylaxis by Population
| Reported ICER | Recommended by AAP 2014 Policy? | |
|---|---|---|
| Congenital heart disease | ||
| Children with CHD [20] | $15 000 | Yes |
| ≤24 mo CA [18] | $53 000 | Partial noa |
| Children with CHD [24] | $140 000 | Partial noa |
| Chronic lung disease | ||
| Children with bronchopulmonary dysplasia [20] | $38 000 | Yes |
| <24 mo CA [18] | $31 000 | Yesb |
| Prematurity (<6 mo CA) | ||
| 2+ risk factors/<29 wGA [18] | $6000 | Yes |
| 2+ risk factorse/29–32 wGA [18, 22, 23] | Cost-saving – $48 000 | Yesc |
| 2+ risk factors/32–35 wGA [19, 22, 23] | $800–$85 000 | Noc,d |
| <1 risk factors/32–35 wGA [19, 22] | $150 000–$800 000 | Noc,d |
| 2+ risk factors/33–35 wGA [18] | $160–000 | Noc,d |
| Premature (<6 mo CA) with increased risk of asthma after RSV infection included in analysis | ||
| 32–35 wGA [19] | $22 000 | Noc,d |
| 26–32 wGA with increased risk of asthma [21] | $1 000 000 | Noc,d |
Abbreviation: CA, chronologic age.
aAAP does not recommend for children >12 mo CA.
bAAP recommends for children 12–24 mo CA only if they continue to need medical support.
cAAP does not recommend for premature infants of >29 wGA unless they meet certain qualifying conditions.
dAAP does not recommend for 35 wGA.
eRisk factors may include comorbid conditions, such as congenital heart disease or chronic lung disease.
Societal Perspective and Spillover Effects
Only 1 of the 7 studies [24] we reviewed included infant future productivity loss estimated over the expected lifetime of the recipient. The 6 other studies did not incorporate any productivity loss impacts or spillover effects pertaining to the RSV infection. In the studies that included both perspectives, RSV immunoprophylaxis appeared more cost-effective when broader cost impacts were included.
DISCUSSION
Critical Challenges and Limitations for RSV Economic Modeling
As in all situations, decisions about appropriate policy for RSV must be made in the face of uncertainty around cost-effectiveness. In this paper, our objective was to evaluate the current state of cost-effectiveness analysis for RSV and areas for improvement in the evidence base. As outlined in the review of the literature, there were some areas of methodological agreement in published health economic studies, such as assumptions about the duration that immunoprophylaxis was offered, the types of costs considered, and the time horizon. However, several assumptions and definitions about the specific populations treated limit the ease of interpretation, comparison, and use of these analyses by clinicians and policy makers. In general, the studies found that the more targeted the risk group, the more likely that immunoprophylaxis was cost-effective; however, the studies did not systematically identify those groups in which immunoprophylaxis offered the best value. Additionally, the patient group definitions used in cost-effectiveness studies and clinical recommendations, such as the 2014 AAP policy, did not always line up, making the health economic evidence difficult to apply. Participants at the round table focused primarily on limitations in the existing evidence base that make interpretation of cost-effectiveness challenging, and ways forward to fill the evidence gaps.
Epidemiological and Clinical Data Challenges
Participants at the round table agreed that an understanding of foundational epidemiology and outcomes associated with RSV disease is essential for better understanding the potential value of immunoprophylaxis and other prevention strategies across different risk groups. The general consensus was that the foundational epidemiological data on RSV disease should be improved. Discussants further identified the following gaps in the field’s knowledge.
Incidence and Hospitalization Rates
Disease incidence rates and associated hospitalization rates for specific risk groups, such as younger gestational and/or chronologic age or underlying conditions, or a combination of risk factors, are often unclear or ill-defined in the existing literature. Anatomical and physiological immaturity of infants may increase susceptibility to a complicated respiratory infection, such as RSV. While it is clear that prematurity and younger chronologic age confer an increased risk of severe RSV disease, each study presents slightly different cut-points of gestational age at birth and/or chronologic age since birth to define and report rates in subgroups. It is also not well laid out how the presence of multiple risk factors, such as degrees of prematurity combined with underlying conditions of CLD, among others, affects the incidence rates and disease severity leading to hospitalization. There is a need for a clear summary of refined and systematically organized incidence rates of RSV and related hospitalizations, and for morbidity or mortality outcomes, by different risk factors or combinations of risk factors. Assessment of RSV risk and severity in the United States in the absence of immunoprophylaxis use has not been available since before 1998 [7], and the epidemiology of RSV in high-risk infants indicated for palivizumab is influenced by the use of immunoprophylaxis. Analysis comparing prophylaxis use to nonuse in these populations needs to account for this lack of information.
Potential for Bias
Further, caution must be used when interpreting the reported rates of hospitalization by gestational age/chronologic age, as there could be prevalence bias in these estimates. For instance, the 28–29 wGA infants that are in the community at <3 months old are not like most infants in this group, who stay at the hospital and may have prolonged stays due to RSV, but not new hospitalizations. Incidence rates of RSV in the community may be very different from those in the academic or tertiary health care setting.
Another factor introducing potential bias into RSV incidence and hospitalization rates is inadequate testing and reporting. Currently, patients triaged based on symptoms at emergency departments and hospitals are not being tested for RSV routinely. In order to prevent underestimation, RSV would have to be tested for and reported adequately and systematically, especially in the at-risk groups who can benefit from palivizumab prophylaxis. Although routine testing in all patients is not recommended, and likely not helpful in practice, the lack of systematic incidence information remains a challenge.
Types of Resource Use
Interpreting hospitalizations and other resource use is challenging given the lack of standardization for admission, acuity, and triage. It is often not clear, for example, what specific types of resource use are considered in cost-effectiveness analyses. The possibilities span outpatient monitoring and visits, emergency department visits, varying numbers of days for inpatient stays and intensive care unit (ICU) stays. Unfortunately, data on RSV disease severity are often not assessed or presented in resource use analyses.
Seasonality
Another aspect that introduces complexity is the seasonality of RSV disease incidence, which varies annually. The disease incidence is highest during specific times of year, which vary by region, with the special exceptions of Alaska, Hawaii, parts of Florida, and Texas. Depending on the location and time of year combined with other factors, the best strategy may vary. Geographic and climate variability of RSV risk remains largely unspecified.
Longterm Consequences
Going forward, research should be undertaken to better understand the potential long-term consequences of RSV disease. There is ongoing debate over whether there is a causal relationship between RSV infection in infancy and subsequent wheezing and asthma later in life. One view is that having RSV during a certain window of time may predispose the individual to asthma later in life. Others believe that the same individuals who are likely to develop wheezing and asthma are also more susceptible to RSV infection in infancy, and the infections themselves do not modify asthma risk [25–27].
Economic Analysis Challenges and Limitations
The limitations of epidemiology data on RSV disease hamper the ability to conduct a complete economic analysis because of gaps in the underlying clinical data. The FDA indication for palivizumab supports a much larger treatment cohort. Based on epidemiology data alone, it is not clear which patients should have the highest priority and have the best chance of benefitting from immunoprophylaxis. Published studies generally do not make a clear distinction between outpatient treatment and inpatient hospitalizations, which incur different resource use and costs. Importantly, hospitalizations for RSV infection among very young premature infants may typically include costly time in the neonatal intensive care unit (NICU) or pediatric intensive care unit (PICU) [7].
Furthermore, there are natural patterns of variability in the cost estimates involved in RSV and its prophylaxis that complicate cost-effectiveness calculations. First, RSV hospitalization costs vary by patient gestational and chronologic age, with younger and more premature infants more likely to have more expensive longer stays and higher use of ICUs [11]. Second, the cost of palivizumab prophylaxis is based on infant weight, which is determined partly by the gestational age at birth, as well as the chronologic age. Smaller infants are treated with smaller doses of palivizumab, which lowers the costs of prophylaxis in younger or more premature infants [28]. These cost trends move in opposite directions, and there are potentially certain high-risk groups in which the costliest hospitalizations could be prevented with lowest-cost prophylaxis, creating a possible “sweet spot” for value. Various strategies, including batching immunoprophylaxis for high-risk preterm infants prior to NICU discharge, may produce additional efficiencies in dosing/cost ratios.
The impact of RSV disease has both short-term and long-term consequences, as well as broader societal implications that are often not included in cost-effectiveness analyses. Although immunoprophylaxis does not prevent RSV infection, the severity of the symptoms is significantly reduced in those who are treated. In the short term, caregivers of a sick infant may need to miss work to take care of the infant and travel for appointments and hospital stays, which can result in a heavy financial toll in the form of lost workdays and travel costs. Additionally, families and caregivers may suffer adverse health consequences based on increased illness severity or duration in the unprophylaxed infant. In order to account for these aspects of RSV immunoprophylaxis value, cost-effectiveness analyses need to incorporate spillover effects on patients’ caregivers, families, and society at large, as recommended by the recent review by the Panel on Cost-Effectiveness in Health and Medicine [17].
In the long-term, the impact RSV disease has on health may range from increased susceptibility to respiratory complications, mid- to long-term lung function decline, recurrent wheezing, asthma, and otitis and rhino-conjunctivitis, as well as mortality. Length of stay data may inadequately reflect disease severity in situations where early mortality from disease is high. Costs associated with these effects go beyond the acute phase of the disease and may need to be incorporated, or at least considered as part of sensitivity analyses in cost-effectiveness analysis.
Strengths and Limitations
This review and round table discussion provides important insights into different stakeholders’ views on the evidence landscape. While the discussion was not meant to comprehensively capture all views, it identified several areas of consensus and ideas to further the field of evaluation of RSV prevention strategies. Furthermore, the cost per QALY literature is not the only possible measure of value; however, this type of CEA is considered a standard approach to quantifying value in health and medicine [17, 29].
ROUND TABLE DISCUSSION: CONCLUSIONS AND RECOMMENDATIONS
Following the discussion and identification of critical gaps in data and approaches to modeling the value of RSV immunoprophylaxis, the round table discussion outlined recommendations for next steps in advancing knowledge in this area. An understanding of foundational epidemiology and outcomes associated with RSV disease is essential for understanding the value of immunoprophylaxis and other prevention strategies across different risk groups, as described in Table 3. We highlight the following recommendations to tackle the challenges of economic modeling of RSV disease:
i. Conduct studies strengthening epidemiological data. There should be valid data on incidence rates for disease and hospitalizations, which allow for risk stratification. Even as clear risk factors have been identified, further data are needed to map out the risks of mortality and severe disease by those risk factors and their combinations in a systematic fashion. Good data should also account for the potential biases from differential testing, reporting, and treatment in certain groups that could distort the estimates.
ii. Study the long-term consequences of RSV. Further study is needed to clarify the possible associations between childhood RSV disease and asthma or other respiratory conditions developed later in life.
iii. Standardize terminology related to RSV disease. There should be clarification across the epidemiological and economic literature for defined age cutoff ranges, outcome definitions, and hospitalization rate definitions.
iv. Incorporate spillover effects into cost-effectiveness analyses to help analyze the societal cost burden of RSV infection. The economic impact of RSV extends beyond the hospital stay to affect families and the broader society.
v. Consider certain socioeconomic or other high-risk patient subgroups explicitly. A number of factors that place children at risk for severe RSV, such as poor housing conditions and environmental factors, and are associated with medical resource use that may differentially affect subgroups by socioeconomic status. It is important to analyze the effectiveness and value of RSV immunoprophylaxis by different socioeconomic groups, for example, in commercially insured vs Medicaid-insured populations.
Acknowledgments
Financial support. Researchers from University of Washington and Tufts Medical Center reviewed and presented the available evidence, retaining independence to publish and disseminate the conclusions of the research and the round table discussion. AstraZeneca, the manufacturer of palivizumab, sponsored the meeting.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J 2002; 21:629–32. [DOI] [PubMed] [Google Scholar]
- 2. Doucette A, Jiang X, Fryzek J et al. Trends in respiratory syncytial virus and bronchiolitis hospitalization rates in high-risk infants in a United States Nationally Representative Database, 1997-2012. PLoS One 2016; 11:e0152208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall CB, Weinberg GA, Iwane MK et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paramore LC, Ciuryla V, Ciesla G, Liu L. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics 2004; 22:275–84. [DOI] [PubMed] [Google Scholar]
- 5. Haynes AK, Prill MM, Iwane MK, Gerber SI; Centers for Disease Control and Prevention (CDC) Respiratory syncytial virus–United States, July 2012-June 2014. MMWR Morb Mortal Wkly Rep 2014; 63:1133–6. [PMC free article] [PubMed] [Google Scholar]
- 6. Mauskopf J, Margulis AV, Samuel M, Lohr KN. Respiratory syncytial virus hospitalizations in healthy preterm infants: systematic review. Pediatr Infect Dis J 2016; 35:e229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson EJ, Krilov LR, DeVincenzo JP et al. SENTINEL1: an observational study of respiratory syncytial virus hospitalizations among U.S. infants born at 29 to 35 weeks’ gestational age not receiving immunoprophylaxis. Am J Perinatol 2017; 34:51–61. [DOI] [PubMed] [Google Scholar]
- 8. Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med 2001; 344:1917–28. [DOI] [PubMed] [Google Scholar]
- 9. Neuzil KM. Progress toward a respiratory syncytial virus vaccine. Clin Vaccine Immunol 2016; 23:186–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haynes LM. Progress and challenges in RSV prophylaxis and vaccine development. J Infect Dis 2013; 208 (Suppl 3):S177–83. [DOI] [PubMed] [Google Scholar]
- 11. McLaurin KK, Farr AM, Wade SW et al. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol 2016; 36:990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Markus AK, Krohe S, Garro N et al. Examining the association between Medicaid coverage and preterm births using 2010–2013 National Vital Statistics Birth Data. J Child Poverty 2017; 23. [Google Scholar]
- 13. American Academy of Pediatrics Committee on Infectious Diseases and Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014; 134:415–20. [DOI] [PubMed] [Google Scholar]
- 14. Hussman JM, Li A, Paes B, Lanctôt KL. A review of cost-effectiveness of palivizumab for respiratory syncytial virus. Expert Rev Pharmacoecon Outcomes Res 2012; 12:553–67. [DOI] [PubMed] [Google Scholar]
- 15. Thorat T, Cangelosi M, Neumann PJ. Skills of the trade: the tufts cost-effectiveness analysis registry. J Benefit Cost Anal 2012; 3:1–9. [Google Scholar]
- 16. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- 17. Cost-effectiveness in Health and Medicine. 2nd ed Oxford: Oxford University Press; 2017. [Google Scholar]
- 18. Bentley A, Filipovic I, Gooch K, Büsch K. A cost-effectiveness analysis of respiratory syncytial virus (RSV) prophylaxis in infants in the United Kingdom. Health Econ Rev 2013; 3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lanctôt KL, Masoud ST, Paes BA et al. The cost-effectiveness of palivizumab for respiratory syncytial virus prophylaxis in premature infants with a gestational age of 32-35 weeks: a Canadian-based analysis. Curr Med Res Opin 2008; 24:3223–37. [DOI] [PubMed] [Google Scholar]
- 20. Nuijten MJ, Wittenberg W, Lebmeier M. Cost effectiveness of palivizumab for respiratory syncytial virus prophylaxis in high-risk children: a UK analysis. Pharmacoeconomics 2007; 25:55–71. [DOI] [PubMed] [Google Scholar]
- 21. Elhassan NO, Sorbero ME, Hall CB et al. Cost-effectiveness analysis of palivizumab in premature infants without chronic lung disease. Arch Pediatr Adolesc Med 2006; 160:1070–6. [DOI] [PubMed] [Google Scholar]
- 22. Mahadevia PJ, Masaquel AS, Polak MJ, Weiner LB. Cost utility of palivizumab prophylaxis among pre-term infants in the United States: a national policy perspective. J Med Econ 2012; 15:987–96. [DOI] [PubMed] [Google Scholar]
- 23. Weiner LB, Masaquel AS, Polak MJ, Mahadevia PJ. Cost-effectiveness analysis of palivizumab among pre-term infant populations covered by Medicaid in the United States. J Med Econ 2012; 15:997–1018. [DOI] [PubMed] [Google Scholar]
- 24. Yount LE, Mahle WT. Economic analysis of palivizumab in infants with congenital heart disease. Pediatrics 2004; 114:1606–11. [DOI] [PubMed] [Google Scholar]
- 25. Martinez FD. Respiratory syncytial virus bronchiolitis and the pathogenesis of childhood asthma. Pediatr Infect Dis J 2003; 22:S76–82. [DOI] [PubMed] [Google Scholar]
- 26. Blanken MO, Rovers MM, Molenaar JM et al. ; Dutch RSV Neonatal Network Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368:1791–9. [DOI] [PubMed] [Google Scholar]
- 27. Piedimonte G. Respiratory syncytial virus and asthma: speed-dating or long-term relationship?Curr Opin Pediatr 2013; 25:344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shahabi A, Peneva D, Incerti D et al. Assessing variation in the cost of palivizumab for respiratory syncytial virus prevention in preterm infants. Pharmacoecon Open 2017; 10.1007/s41669-017-0042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanders GD, Neumann PJ, Basu A et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016; 316:1093–103. [DOI] [PubMed] [Google Scholar]
- 30. Pickering LK, Baker CJ, Kimberlin DW, Long SS, eds. Red Book: 2012 Report of the Committee on Infectious Diseases. Elk Grove Village, IL: American Academy of Pediatrics; 2012. [Google Scholar]