Abstract
Previous studies have shown that the influence of a behaviorally irrelevant distractor on saccade reaction times (SRTs) varies depending on the temporal and spatial relationship between the distractor and the saccade target. We measured distractor influence on SRTs to a subsequently presented target, varying the spatial location and the timing between the distractor and the target. The distractor appeared at one of four equally eccentric locations, followed by a target (either 50 ms or 200 ms after) at one of 136 different locations encompassing an area of 20° square. We extensively tested two humans and two monkeys on this task to determine interspecies similarities and differences, since monkey neurophysiology is often used to interpret human behavioral findings. Results were similar across species; for the short interval (50 ms), SRTs were shortest to a target presented close to or at the distractor location and increased primarily as a function of the distance from the distractor. There was also an effect of distractor-target direction and visual field. For the long interval (200 ms) the results were inverted; SRTs were longest for short distances between the distractor and target and decreased as a function of distance from distractor. Both SRT patterns were well captured by a two-dimensional dynamic field model with short-distance excitation and long-distance inhibition, based upon known functional connectivity found in the superior colliculus that includes wide-spread excitation and inhibition. Based on these findings, we posit that the different time-dependent patterns of distractor-related SRTs can emerge from the same underlying neuronal mechanisms common to both species.
Keywords: superior colliculus, attention, facilitation, inhibition of return, remote distractor effect
Introduction
Our visual system is constantly bombarded with stimuli, some of which are relevant but most of which are irrelevant to behavior. The presence of a task-irrelevant distractor has been shown to have multiple effects on saccades to targets. For example, distractors have been shown to both increase (e.g., Maylor & Hockey, 1985; Posner & Cohen, 1984; Walker, Deubel, Schneider, & Findlay, 1997; Walker, Kentridge, & Findlay, 1995) or decrease SRT (e.g., Briand, Larrison, & Sereno, 2000; Posner & Cohen, 1984; Ro, Pratt, & Rafal, 2000), increase target selection errors (Dorris, Olivier, & Munoz, 2007; McPeek, Han, & Keller, 2003), modify saccade amplitude (Ro et al., 2000), and increase saccade trajectory curvature (Godijn & Theeuwes, 2002a, 2002b; McPeek et al., 2003; Walker, McSorley, & Haggard, 2006). Here we investigate the effects and potential underlying mechanisms of irrelevant stimuli on saccade initiation through combined monkey and human behavior and modeling work. It is important to contrast and compare human and monkey behavioral responses, given that monkey behavioral neurophysiology is frequently used to interpret human behavior.
The effect of a distractor on the timing of subsequent saccade initiation depends on its spatial and temporal relationship to the target, even when it is behaviorally irrelevant. A number of studies investigating the remote distractor effect (RDE) have demonstrated that the presence of a distractor at a distant location close in time to the saccade target increases SRT (Born & Kerzel, 2008; Dorris et al., 2007; Honda, 2005; Levy-Schoen, 1969; Ludwig, Gilchrist, & McSorley, 2005; Walker et al., 1997, 1995; White, Gegenfurtner, & Kerzel, 2005). These studies have shown that the greatest increases in reaction time occurs when the distractor appears within 20 ms (either before or after) of the target but there are still effects 80 ms before to 60 ms after the target depending on the contrast of the distractor (Bompas & Sumner, 2009; McSorley, McCloy, & Lyne, 2012; Reingold & Stampe, 2002; Ross & Ross, 1980; Walker, Fitzgibbon, & Goldberg, 1995; White et al., 2005). In addition, studies have also demonstrated that the RDE effect depends not only on the spatial distance between the target and distractor, but also on the distance of the distractor from fixation, mostly within the contralateral visual field (Griffiths, Whittle, & Buckley, 2006; Honda, 2005; McSorley et al., 2012; Walker et al., 1997).
A divergent group of studies has shown similar results, where using a classic Posner paradigm (Posner & Cohen, 1984), they demonstrated that a cue (distractor) appearing just before the target (50 ms) at a distant location results in longer SRTs compared to those in response to a target with no distractor appearing beforehand (Fecteau, Bell, & Munoz, 2004; Fecteau & Munoz, 2005; Khan, Heinen, & McPeek, 2010; Klein, 2000; Posner & Cohen, 1984; Posner, Rafal, Choate, & Vaughan, 1985). Prolonging the interval between the appearance of the distractor and that of the target to 160 ms or longer results in shorter reaction times to the target (Fecteau et al., 2004; Fecteau & Munoz, 2005; Ro et al., 2000; Ross & Ross, 1980).
Complementary findings emerge when the distractor is presented at the same location as the target. If the distractor is presented slightly before the target (33 ms to 200 ms), SRTs are decreased (Briand et al., 2000; Fecteau et al., 2004; Fecteau & Munoz, 2005; Khan et al., 2010) and sometimes labeled as attentional facilitation or capture (Jonides & Irwin, 1981; Klein, 2000; Theeuwes, Kramer, Hahn, Irwin, & Zelinsky, 1999). However other studies have shown little or no effects of distractors on SRTs when presented close to the target (e.g., McSorley & Findlay, 2003; Walker et al., 1997). In contrast, when the distractor is presented well before the target (200 ms or longer), SRTs are longer (Abrams & Dobkin, 1994a; Briand et al., 2000; Fecteau et al., 2004; Fecteau & Munoz, 2005; Hunt & Kingstone, 2003; Maylor & Hockey, 1985; Posner & Cohen, 1984; Pratt & Neggers, 2008; Rafal, Egly, & Rhodes, 1994). This phenomenon is classically referred to as inhibition of return (Posner & Cohen, 1984; Posner et al., 1985).
Taken together, these findings suggest a characteristic and dynamic pattern with regards to the temporal and spatial relationship between a distractor and a target for SRTs. Though some studies have tested multiple locations and/or multiple timings (Bompas & Sumner, 2009; Briand et al., 2000; Buonocore & McIntosh, 2012; Fecteau & Munoz, 2005; Prinzmetal, Taylor, Myers, & Nguyen-Espino, 2011; Ro et al., 2000; Walker et al., 1997), none have done so systematically and thoroughly across visual space, time, and species. Investigating these factors together could result in a number of insights. For example, it is unclear whether attentional facilitation, inhibition of return and the remote distractor effect involve similar or different mechanisms (Hoffmann et al., 1995; Klein, 2000; Tipper et al., 1997). Additionally, it remains unknown whether within a specific stimulus-onset asynchrony (SOA), SRTs vary gradually with distractor-target distance or whether this change is abrupt across hemispheres, as has been suggested by some studies (Ro et al., 2000; Sheliga, Riggio, & Rizzolatti, 1995).
It has been widely suggested that these patterns of facilitation and inhibition may be due to underlying excitatory and inhibitory connections between neurons in multiple visual and saccade processing areas in the brain (Cavanaugh, Joiner, & Wurtz, 2012; Fecteau & Munoz, 2006; Fino & Yuste, 2011; Hikosaka & Wurtz, 1985; Kätzel, Zemelman, Buetfering, Wölfel, & Miesenböck, 2011; Leigh & Zee, 2006; Mize, Jeon, Hamada, & Spencer, 1991; Moschovakis, Scudder, & Highstein, 1996; Munoz & Fecteau, 2002; Munoz & Istvan, 1998; Munoz & Wurtz, 1993; Olivier, Dorris, & Munoz, 1999). Others have proposed that the pattern is due to an interaction between fixation and saccade related neurons (Casteau & Vitu, 2012; Findlay & Walker, 1999; Walker et al., 1997), where the increased latency of the saccade from remote distractors is due to increased fixational activity, particularly when the remote distractor is presented closer to fixation than the target. Within the saccadic literature, the superior colliculus (SC) is of particular interest because of its well understood neuronal architecture and its involvement in saccade production (Goldberg & Colby, 1992; Munoz, Dorris, Paré, & Everling, 2000; Robinson & McClurkin, 1989; Sparks & Hartwich-Young, 1989; Wurtz & Optican, 1994) as well as distractor-related effects (Dorris et al., 2007; Dorris, Klein, Everling, & Munoz, 2002; Fecteau & Munoz, 2005; McPeek, 2008; Sapir, Soroker, Berger, & Henik, 1999). Indeed, a number of studies have attempted to describe how distractors influence SRTs overall using dynamic field models (Arai & Keller, 2005; Kopecz, 1995; Kopecz & Schöner, 1995; Marino, Trappenberg, Dorris, & Munoz, 2012; Satel, Wang, Trappenberg, & Klein, 2011; Trappenberg, Dorris, Munoz, & Klein, 2001; Wilimzig, Schneider, & Schöner, 2006), although other models such as fixation gating models also exist (Casteau & Vitu, 2012; Findlay & Walker, 1999). The dynamic field models endeavor to describe many effects of the distractor on the target based on spatial properties such as the excitatory and inhibitory connections between neurons representing visual space (Arai, Keller, & Edelman, 1994; Dorris et al., 2007; Godijn & Theeuwes, 2002b; Kopecz & Schöner, 1995; Marino et al., 2012; Munoz & Fecteau, 2002; Olivier et al., 1999; Satel et al., 2011; Trappenberg et al., 2001), as well as temporal properties of neuronal activity (Bell, Fecteau, & Munoz, 2004; Wilimzig et al., 2006), and short term depression or habituation (Bell, Corneil, Munoz, & Meredith, 2003; Dukewich, 2009; Fecteau & Munoz, 2005; Fischer, Gezeck, & Huber, 1995; Satel et al., 2011). Here we focus specifically on dynamic field models as they are largely based on physiological evidence of functional lateral interconnections in the SC (Meredith & Ramoa, 1998; Munoz & Fecteau, 2002; Munoz & Istvan, 1998; Olivier et al., 1999) likely mediated both by intracollicular excitation and inhibition (e.g., Isa & Hall, 2009; Phongphanphanee et al., 2014) and extracollicular excitatory and inhibitory inputs. Almost all current dynamic field models are limited in that they describe the distractor-related effects on reaction time in one dimension (i.e., with respect to the distance between the distractor and the saccade target), which does not take into account the two-dimensional spatial representation and connectivity within brain areas such as the SC (Robinson & McClurkin, 1989). Excitatory and inhibitory connections vary in 2D space (Dorris et al., 2007; Munoz & Fecteau, 2002; Olivier et al., 1999) and, thus, may not be adequately described in one dimension (e.g., as a function of distance). The only 2D model describing SRTs that exists to our knowledge focused on bottom-up versus top-down signal competition (Marino et al., 2012). We aimed to build a two-dimensional, time dependent model to determine whether previously proposed neural dynamics within the SC can adequately explain the behavioral pattern of reaction times over time and 2D space in response to the distractor and in addition, to gain insight into the underlying neuronal mechanisms that can explain the pattern of behavioral SRTs.
An additional motivation for this work was to also investigate similarities and differences between the saccadic response patterns of humans and nonhuman primates to distractors. Most studies focus on one species alone and it is unclear to what extent different findings can be attributed to interspecies differences. Separately, however, monkeys and humans show similar behavioral responses for tasks involving attentional facilitation (Fecteau & Munoz, 2005), inhibition of return (Dorris et al., 2002; Fecteau & Munoz, 2005; Klein, 2000), RDE (Walker et al., 1997; White et al., 2013), warning effects (Dick, Kathmann, Ostendorf, & Ploner, 2005; Fecteau & Munoz, 2007), covert attention (Krauzlis, Lovejoy, & Zénon, 2013; Lee & McPeek, 2013) and target selection (McPeek, 2008). Monkey neurophysiology and behavior are currently the leading models for humans and have provided invaluable knowledge into the functions of the brain. As such, it is crucial to establish similarities in behavior (within the same paradigm) to be able to confidently generalize from monkeys to humans. Moreover, behavioral similarities also provide support for similar underlying neuronal mechanisms.
To this end, we measured SRTs to targets presented at various locations across visual space with a distractor appearing randomly at one of four locations equidistant from fixation and at either 50 ms or 200 ms before the target. Two humans and two monkeys participated in the experiment.
Methods
Humans
Subjects
Two subjects (ages: 24 and 31, female), both of whom are authors (AK and NT), participated in the experiment. The experimental protocol was preapproved by the Smith-Kettlewell Institutional Review Board in compliance with NCPHS, US. Both subjects had normal vision.
Apparatus
Subjects were seated in front of a 17-inch, high-resolution Nanao color monitor (1.76 min arc/pixel) with a refresh rate of 60 Hz that was controlled by a Macintosh computer and viewed from a distance of 48 cm. Stimuli were presented using Matlab (The Math Works Inc., Natick MA) and functions from the PsychToolbox (Brainard, 1997; Pelli, 1997). Saccades kinematics were recorded using a video-based Eyelink 1000 (SR Research, Mississauga, Canada) at 1000 Hz. Prior to each block of trials, the eye tracker was calibrated by having the observer fixate a series of nine positions on the display (eight surrounding the periphery of the display and the center) while the gains and offsets were set. A chinrest maintained the viewing distance and stabilized the head for accurate eye tracking.
Procedure
Subjects began each trial by fixating on a central dot (white circle on a black background, diameter = 0.19°) at center (Figure 1A). After an interval of 750 ms, a distractor (light gray circle, diameter = 0.23°) was presented at one of four oblique positions (e.g., 45° up and to the left at a distance of 7.07° in the figure) for 33.33 ms (see Bennett & Pratt, 2001). A delay period followed with only the fixation target for either 16.66 ms or 166.66 ms (corresponding to a stimulus-onset-asynchrony (SOA) of 50 and 200 ms). These values were determined through pilot experiments to reliably show shorter and longer SRTs at the distractor location compared to a second location in the opposite hemifield, and are in accordance with previous findings (Briand et al., 2000; Fecteau & Munoz, 2005; Klein, 2000). Next a target (a dark gray square with a diameter of 0.23°) was presented at one of 136 locations on the screen (Figure 1B) subtending a 10° × 10° range (horizontal and vertical target locations were separated by 1° intervals). The fixation target was extinguished when the target was illuminated. The target remained illuminated for 500 ms followed by a blank screen for 1000 ms signaling the next trial. Subjects were asked to make a saccade to the target as soon as it appeared and then return to the center fixation when it reappeared for the next trial and to ignore the distractor. Apart from the four distractor location conditions, there was an additional condition where no distractor was presented but the timing remained the same (shorter and longer time from trial onset to target onset). The distractor condition, delay and target location were pseudorandomly chosen for each trial.
Figure 1.
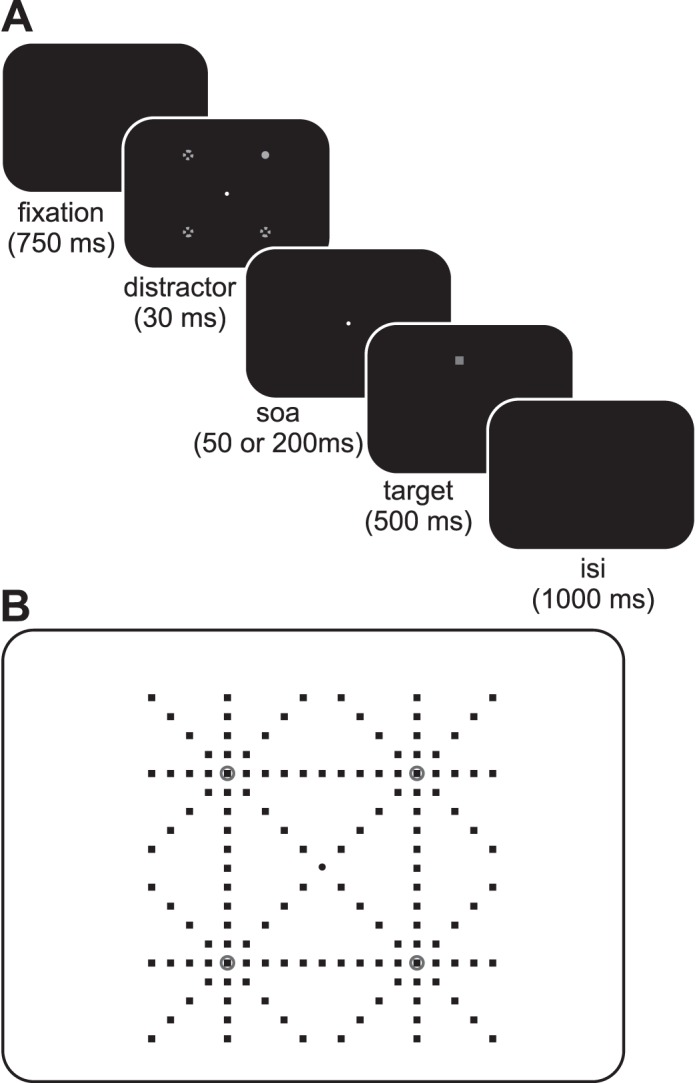
Trial sequence and stimuli locations. (A) Each trial began with the presentation of a fixation spot (white circle) at center for 750 ms. Next a distractor (light gray solid circle) appeared at one of 4 possible locations (dotted circle outlines) for 30 ms. There was also a condition where no distractor appeared. Following an SOA of 50 or 200 ms (corresponding to a delay of 20 to 170 ms), a target (dark gray square) appeared for 500 ms. The interstimulus interval (ISI) was 1000 ms. (B) Targets could appear at one of 136 different locations subtending a 10° by 10° square. The gray open circles depict the possible locations of the distractor. Fixation is at center depicted by the black circle in the center.
A block of trials, equaling one trial per condition totaled 1360 trials (5 distractor conditions × 2 delays × 136 target locations). Each block was separated into eight sessions of 170 trials each, consisting of 17 randomly chosen target locations (from the set of 136) × 2 delays × 5 distractor conditions. Subjects performed on average two or three sessions per day for a total of 12,560 trials for subject AK (approximately nine trials per condition) and 6,270 trials for subject NT (approximately five trials per condition).
Monkeys
Subjects
Two male rhesus monkeys (Macaca mulatta) weighing 7 and 11 kg participated in the experiment. The experimental protocol was preapproved by the Institutional Animal Care and Use Committee at the Smith-Kettlewell Eye Research Institute and complied with the guidelines of U.S. Public Health Service policy on Humane Care and Use of Laboratory Animals.
Apparatus and procedure
Animals HY and JA initially learned to come out of their cages and sit comfortably in a primate chair. A head-restraint system comprised of a cylindrical post attached to the chair and a mating socket attached to the subject allowed head-stabilized eye movement tracking. Experiments were conducted in a dimly illuminated room. Stimuli were presented on a 29″ color CRT (Viewsonic GA29), with a spatial resolution of 800 × 600 pixels and a noninterlaced refresh rate of 75 Hz. Stimuli were generated by a Macintosh computer using software constructed by the Video Toolbox Library (Pelli, 1997). Eye position and velocity were measured using a video-based Eyelink 1000 (SR Research, Mississauga, Canada) at 1000 Hz. At the start of each session, the eye tracker was calibrated at five locations (central fixation and 15° along the horizontal and vertical axes) to set the initial gains and offset. Monkeys were required to fixate within 2° of the fixation dot during the fixation period. Due to mechanical reasons, Monkey HY was seated 33 cm whereas Monkey JA was seated 39 cm from the screen. This resulted in slightly different variations in the distances between the targets and distractors. Specifically, the target array subtended a 10° × 10° range for Monkey JA and an 11.8° × 11.8° range for Monkey HY; since all SRTs will be interpreted relatively, we plotted HY's SRTs in the same range as the other three subjects.
The trial sequence for the monkey experiments was the same as for the human experiments except that the target could be either an equiluminant red or green square, randomly determined. The cue was presented for 26.67 ms (75 Hz display screen). A delay period followed, where only the fixation target was displayed for either 26.67 ms or 173.33 ms (corresponding to a stimulus-onset-asynchrony (SOA) of 53.33 ms, rounded to 50 ms for simplicity, and 200 ms).
Drift in fixation was monitored and corrected throughout each session. The eye movements were recorded online to ensure that the monkeys made the correct movement toward the target. The monkey received juice rewards for making an eye movement within a region outlined by an imaginary box around the target, subtending one third of the target eccentricity.
A single block for the monkeys therefore included two colors for a total of 2,720 trials per block (data was subsequently collapsed across target color). Each block was separated into 10 sessions of 280 or 260 trials each (from the set of 136 target locations × 2 delays × 5 distractor conditions × 2 colors) The animals worked until satiated and received supplemental water as necessary. The animals typically worked for five days and were allowed access to water on weekends. The weight and health of the animals were recorded and monitored regularly. Monkey HY performed a total of 30,572 trials (approximately 11 trials/conditions) and monkey JA performed 36,316 trials (approximately 13 trials/conditions).
Data analysis
All data were analyzed using Matlab (The Math Works Inc., Natick, MA). The presence of a saccade was calculated using velocity criteria (human velocity threshold = 25°/s, monkey velocity threshold = 45°/s).
Offline analysis on both the monkey and human data on saccade reaction times (SRT) for the first saccade was performed. Saccades with reaction times of less than 70 ms for monkeys (Fecteau & Munoz, 2005) or less than 100 ms for humans (Kalesnykas & Hallett, 1987; Wenban-Smith & Findlay, 1991) were considered anticipatory and were removed from the dataset. SRTs of more than 300 ms were also removed (Fecteau & Munoz, 2005; Kalesnykas & Hallett, 1987; Wenban-Smith & Findlay, 1991). This removal comprised 1.9% of trials for AK, 1.4% for NT, 3.7% for JA, and 0.01% for HY. In addition, trials in which the saccade endpoint had an amplitude error of more than 3° or a directional error of more than 10° from the target position were removed from the dataset. After these selection criteria, the total number of trials remaining was 10,413 for subject AK (82.9%), 5,810 for NT (92.7%), 30,600 for monkey JA (84.3%) and 25,037 for monkey HY (81.9%).
For all statistical analyses and figures, we used the median reaction times for each of the 136 target positions.
Model
To investigate the underlying mechanisms leading to pattern of SRTs across the visual field for short versus long SOAs, we extended a previously proposed one-dimensional dynamic field model (Trappenberg et al., 2001) to two dimensions. Dynamic field models have previously been used to describe target-distractor interactions in the superior colliculus (Satel et al., 2011; Trappenberg et al., 2001; Wilimzig et al., 2006) based on known physiological mechanisms.
Whereas an earlier two-dimensional extension of the neural field model (Marino et al., 2012) focused on bottom-up versus top-down signal competition, we focus here on SOA-dependent distractor-related effects on SRTs. This interaction is based on overall lateral functional connectivity collapsed across the SC, with no differentiation between across and within hemifields. Indeed, there is physiological evidence for wide spread excitatory and inhibitory connections (Dorris et al., 2007; Marino et al., 2012; Munoz & Fecteau, 2002; Munoz & Istvan, 1998; Olivier et al., 1999; Phongphanphanee et al., 2014) that could support a mechanism with proximal excitation and distal inhibition, such that the effective connectivity strength wij from network unit j to network unit i looked like a Mexican hat:
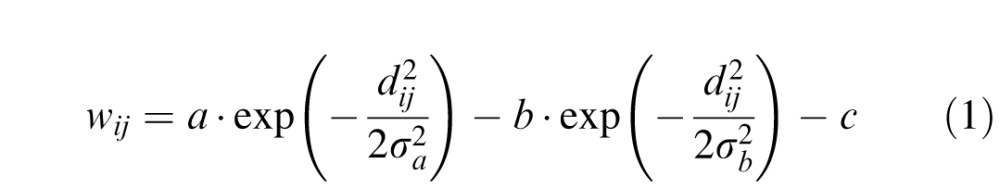 |
where 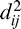 = (xi − xj)2 + (yi − yj)2 is the absolute distance between network units i and j in the two-dimensional dynamic field and a = 72, b = 24, c = 6.4, σa = 0.6, σb = 1.8. The activation of each node in the network was governed by the following dynamic neural field equation (Trappenberg et al., 2001):
= (xi − xj)2 + (yi − yj)2 is the absolute distance between network units i and j in the two-dimensional dynamic field and a = 72, b = 24, c = 6.4, σa = 0.6, σb = 1.8. The activation of each node in the network was governed by the following dynamic neural field equation (Trappenberg et al., 2001):
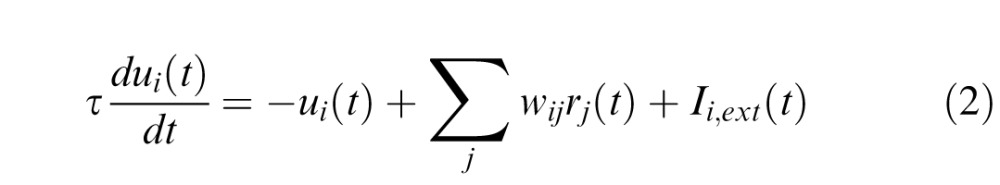 |
with the output from unit j being rj(t) = 1 / (1 + exp(−β · uj(t))) (β = 0.125) and the total external input to unit i being denoted Ii,ext(t). Similarly to previous studies (Satel et al., 2011), the external input was composed of fixation activity Ii,fix(t), cue (distractor) related activity Ii,cue(t), target-related activity Ii,tar(t), movement-related activity Ii,mov(t) and inhibitory activity from the substantia nigra pars reticulata Ii,SNr(t). The spatiotemporal profile of these external inputs was the following:
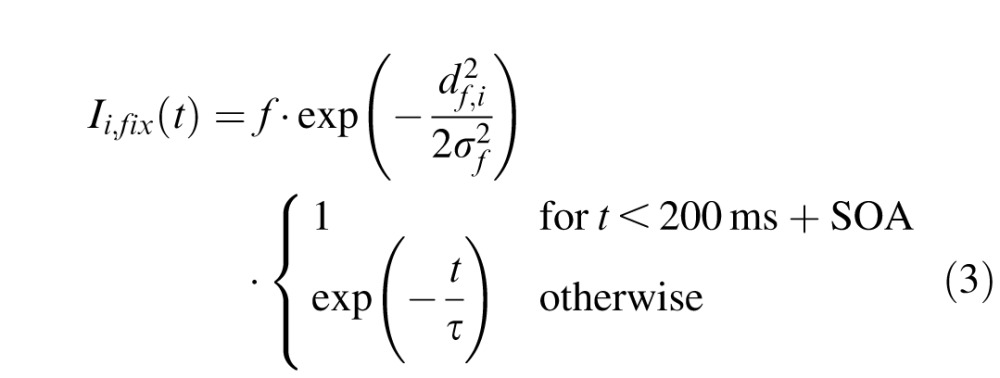 |
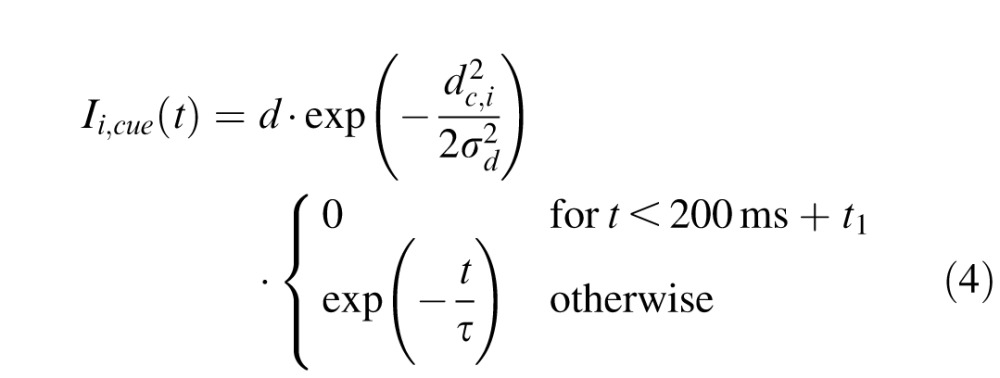 |
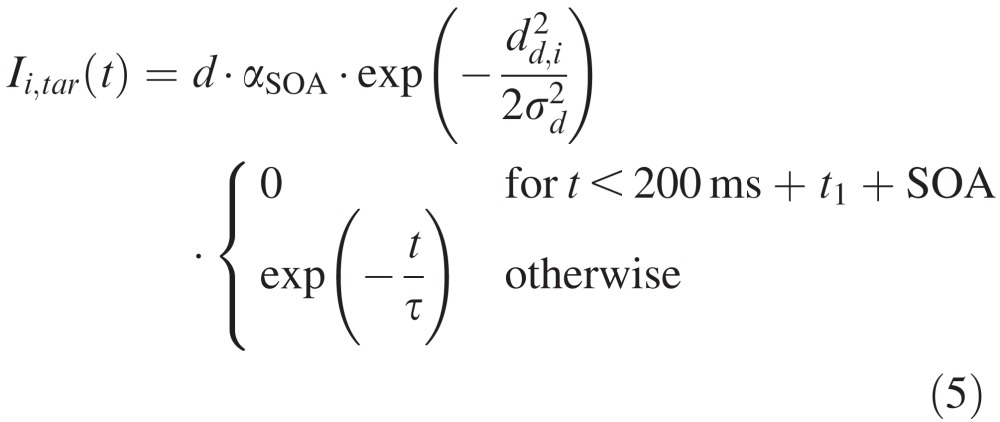 |
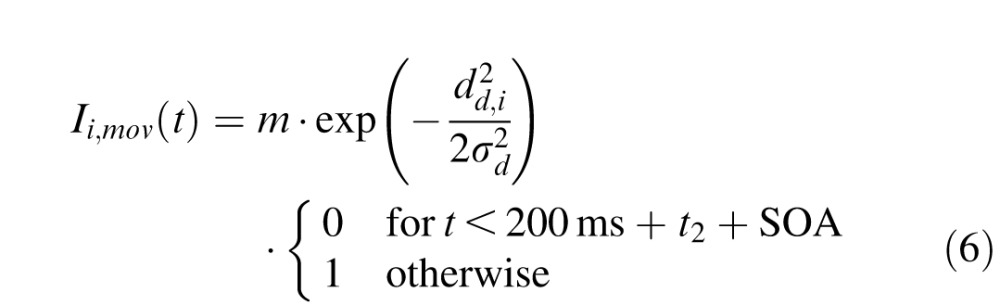 |
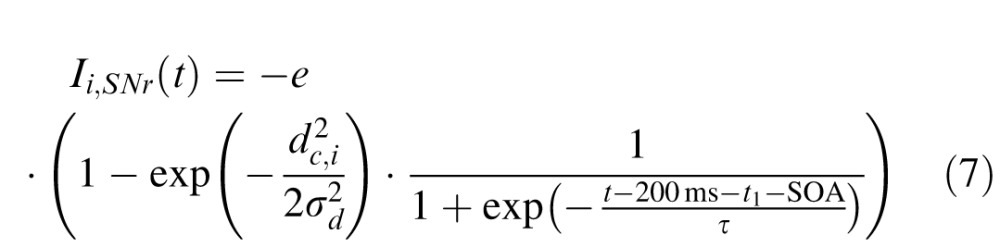 |
with the distance between node i and the fixation zone df,i, the distractor location dc,i or the target location dd,i. Parameters were f = 6, d = 60, e = 5, σd = 0.7, σf = 0.3, and delays t1 = 70 ms and t2 = 120 ms. SOA was either 50 ms or 200 ms as in the experimental data and the time constant τ = 25 ms. To replicate the earlier 1D model, we included the so-called foreperiod effect (Fecteau & Munoz, 2005), m was a linearly increasing function for SOA < 200 ms (m = 21.9 + 0.1008 · SOA) and a linearly decreasing function for SOA > 200 ms (m = 42.12 − 0.0072 × SOA). Following Satel et al. (2011), we also modeled stimulus-dependent, short term depression in Equation 5, which was captured in αSOA, such that
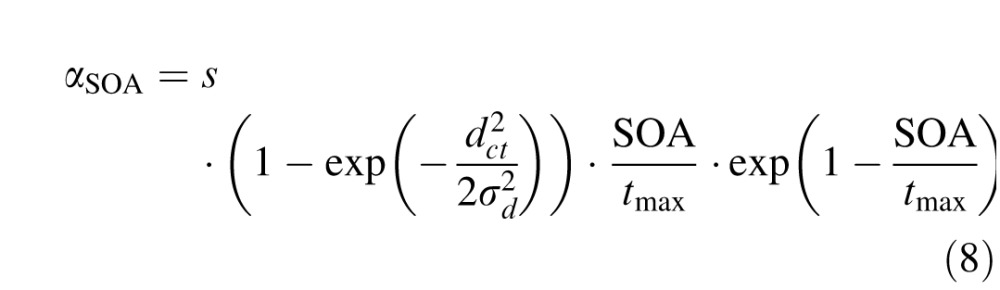 |
with dct being the distractor-target distance and parameters s = 0.45, tmax = 100 ms.
In our simulations, we used a dynamic neural field that spanned ±4 mm horizontally and ±3 mm vertically in superior colliculus coordinates and units were uniformly spaced in 0.25 mm distance. This results in 825 network units. Distractor and target locations for simulations were chosen as in the experimental data and transformed into superior colliculus coordinates. Simulation time steps were 1 ms, and we considered saccades to be triggered when the activity of any unit in the network reached a fixed threshold of 0.8.
Results
No distractor condition
We first calculated SRTs in the no-distractor condition, using the median SRT for each of the 136 target positions to account for possible nonnormal SRT distributions. Overall, monkeys had shorter reaction times than humans; monkeys made saccades with an average median SRT of 126.2 ms compared to humans at 176.7 ms, t(542) = 55, p < 0.001. Within each species, HY had slightly shorter SRTs than JA, 123.2 ms versus 129.2 ms, t(270) = 4.8, p < 0.001; and NT had slightly shorter SRTs than AK, 175.1 ms versus 178.3 ms, t(270) = 2.4, p < 0.05. Both humans and monkeys show a varied pattern of SRTs, which depended on both target eccentricity and direction as has been shown previously (Dafoe, Armstrong, & Munoz, 2007; Honda & Findlay, 1992; Kalesnykas & Hallett, 1994; Previc, 1990; Weber, Aiple, Fischer, & Latanov, 1992).
Distractor location
Nondistractor related spatial effects on SRTs, such as eccentricity and quadrant, varied for each subject. In order to measure only the effect of the distractor on SRTs, we calculated reaction times in the distractor conditions by subtracting the median SRTs for each target location in the no-distractor condition from the SRTs at the corresponding target location for each distractor condition within each SOA and subject. This subtraction serves to remove idiosyncratic effects of SRTs within the visual work space, thus helping to elucidate the effect of the distractor on SRTs to target at different locations. We then calculated the median of these difference SRTs (dSRTs) for each target location, SOA and subject.
Distractors were flashed at four different possible locations so that the location of the distractor was not predictable. In order to directly compare and collapse across distractor location, we rotated target locations so that the distractor was in the same position in all four distractor conditions (the three other distractor conditions were rotated to match the upper right distractor condition). We wished to maintain the relative positions of the targets across the horizontal meridian (i.e., relative to the distractor location) in order to be able to test any across/within left versus right hemifield effects. To do so, we flipped the positions of the targets in the upper right and lower left quadrants for the two distractor conditions orthogonal to the upper right distractor condition (i.e., the lower right and the upper left distractor conditions) before rotating the target positions. This resulted in the four distractor conditions matching in terms of the position of the target relative to the distractor and maintaining hemifield relationships. We then collapsed across the four distractor conditions.
SRTs with distractor present
Figure 2 A–H show difference median SRTs for the short and long SOAs for all four subjects as a function of target location collapsed across the four distractor locations (range of trials per target position; AK: 14–46, NT: 12–40, JA: 40–114, HY: 42–99). The distractor location is depicted by the white cross in the upper right quadrant.
Figure 2.
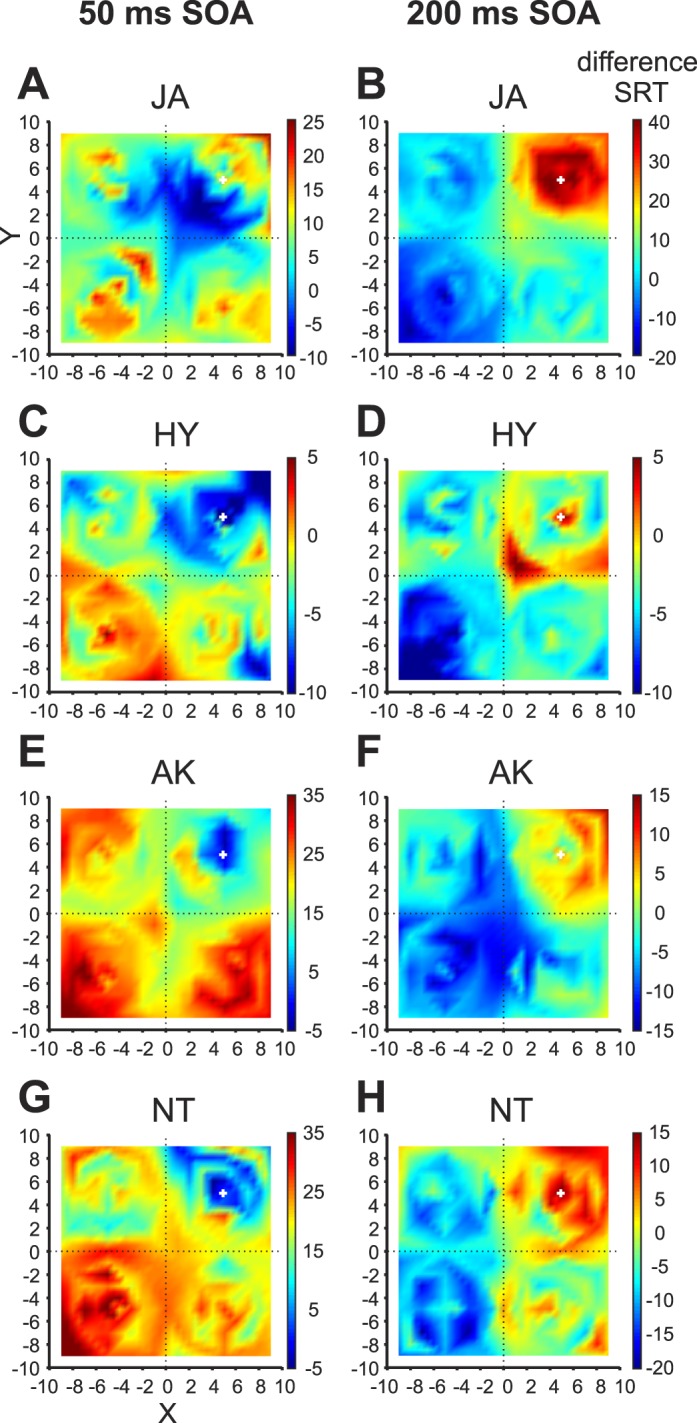
Difference SRTs (dSRTs) for short and long SOA conditions. Median dSRTs are plotted as a function of spatial location relative to the horizontal (x axis) and vertical (y axis) distance in degrees from the central fixation position (black dotted crosshairs) separately for each subject (rows) and each SOA condition (columns). The (collapsed) distractor location is depicted by the white cross in the upper right quadrant. DSRTs are color coded according to the legend for each subject (red signifies longer SRTs than the no distractor condition and blue signifies shorter SRTs compared to the no distractor condition) and are extrapolated across target positions.
While these effects of the distractor were stronger in some subjects than in others, they showed a similar pattern across all subjects. In the short SOA, the effects of the distractor were similar across all subjects. Saccades in the short SOA (A, C, E, and G) had shorter SRTs than the no distractor condition (negative difference SRTs, towards blue; see color legend) when the target was presented close to the distractor location and had longer SRTs than the no distractor condition (positive difference SRTs, towards red) as the distance from the distractor increased. For the long SOA this pattern was reversed (B, D, F, and H). Within species, monkey HY showed a much more localized distractor effect within the distractor quadrant compared to JA for both short and long SOA conditions, whereas humans showed similar effects of the distractor.
We attempted to quantify how these difference SRTs varied across the visual space tested relative to the distractor location. First, we quantified whether SRTs varied as a function of the distance between the target and the distractor. In Figure 3, we plotted median difference SRTs as a function of absolute distance from the distractor location separately for each subject and each SOA. The linear regression fits along with R2 values and significances are shown. As can be seen, SRTs significantly increased for the short SOA condition as a function of absolute distance from the target to the distractor for all subjects. Across all subjects, the fits show that a change in distance of 1° leads to a change in SRT ranging from 0.4 to 1.4 ms for the short SOA. For the long SOA condition, SRTs decreased as a function of the absolute distance, with changes of SRT ranging from 0.4 ms/° to 3 ms/°. Whereas the humans showed similar slopes, they were very different across the two monkeys. In particular, JA showed a much higher negative slope compared to HY for the short SOA condition.
Figure 3.
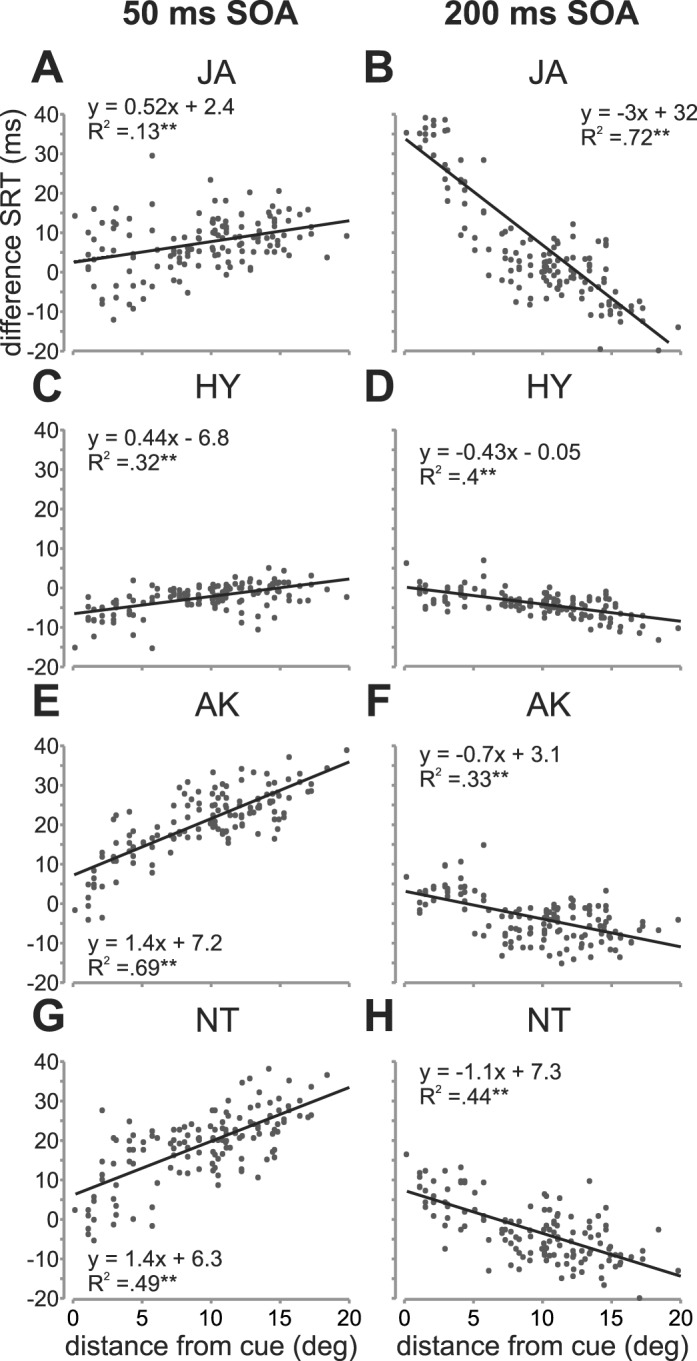
DSRTs as a function of target-distractor distance. Median dSRTs are plotted as a function of the absolute distance between the target and the distractor separately for each subject (rows) and each SOA condition (columns). Linear fits to the dSRTs are shown along with the corresponding equations and R2 values. ** depict significance at p < 0.01.
Additionally, the intercept values provide some insight with regards to dSRTs when the target appeared at the distractor location. As can be seen in the figures, during the short SOA condition, the distractor appearing at the target location had a small effect on SRTs, decreasing them by less than 8 ms at most compared to the no distractor condition. For subject NT, there was actually an increase in SRTs by approximately 6 ms. Indeed, for three subjects (AK, JA, and NT), the greatest effect of the distractor was a large increase in SRTs when targets appeared at far distances from the distractor rather than a decrease at close distances. HY, however, shows no change from the no-distractor condition at far distances. For the long SOA condition, both humans show a similar pattern, where SRTs were not greatly increased at close distances (3 to 7 ms), but rather were decreased at far distances (−15 to −20 ms). This pattern, though attenuated, was also true of HY (−12 ms). In contrast, JA showed highly increased SRTs (30 ms) at close distances as well as highly decreased SRTs at long distances (−25 ms).
In summary, dSRTs vary significantly as a function of distance from the distractor and the target for both the long and the short SOA condition. In addition, with an exception, the greatest influence of the distractor appears to be when the target appeared at far distances rather than at close distances.
SRTs vary as a function of direction from distractor
In addition to distance, we investigated whether dSRTs also vary as a function of direction. We compared whether dSRTs varied consistently as a function of distance (0° to 15°) across three main directions from the distractor location, i.e., 180° (targets within directions of 160° to 200°), 225° (205° to 245°) and 270° (250° to 290°; see inset in Figure 4D). We selected these three direction bins because of the large range of distances within each direction. If there was only an effect of distance, we would expect no differences in SRTs across the different directions. We plotted median dSRTs as a function of distance from the distractor (from 0° to 15°) separately for the three directions as well as their corresponding linear fits for each subject and condition (Figure 4). These slopes varied across the three directions to different degrees for the different subjects and conditions. This was confirmed through ANCOVAs with direction as a factor, controlling for distance as a covariate. All subjects showed significant effects for direction across both SOA conditions: AK, 50 ms, F(1, 99) = 5.5, p < 0.01; AK, 200 ms, F(1, 99) = 188, p < 0.01; JA, 50 ms, F(1, 99) = 3.3, p < 0.05; HY, 50 ms, F(1, 99) = 4.1, p < 0.05; HY, 200 ms, F(1, 99) = 6.7, p < 0.01; NT, 50 ms, F(1, 99) = 16.2, p < 0.01; NT, 200 ms, F(1, 99) = 12.6, p < 0.01; except for JA in the long SOA condition (p > 0.05).
Figure 4.
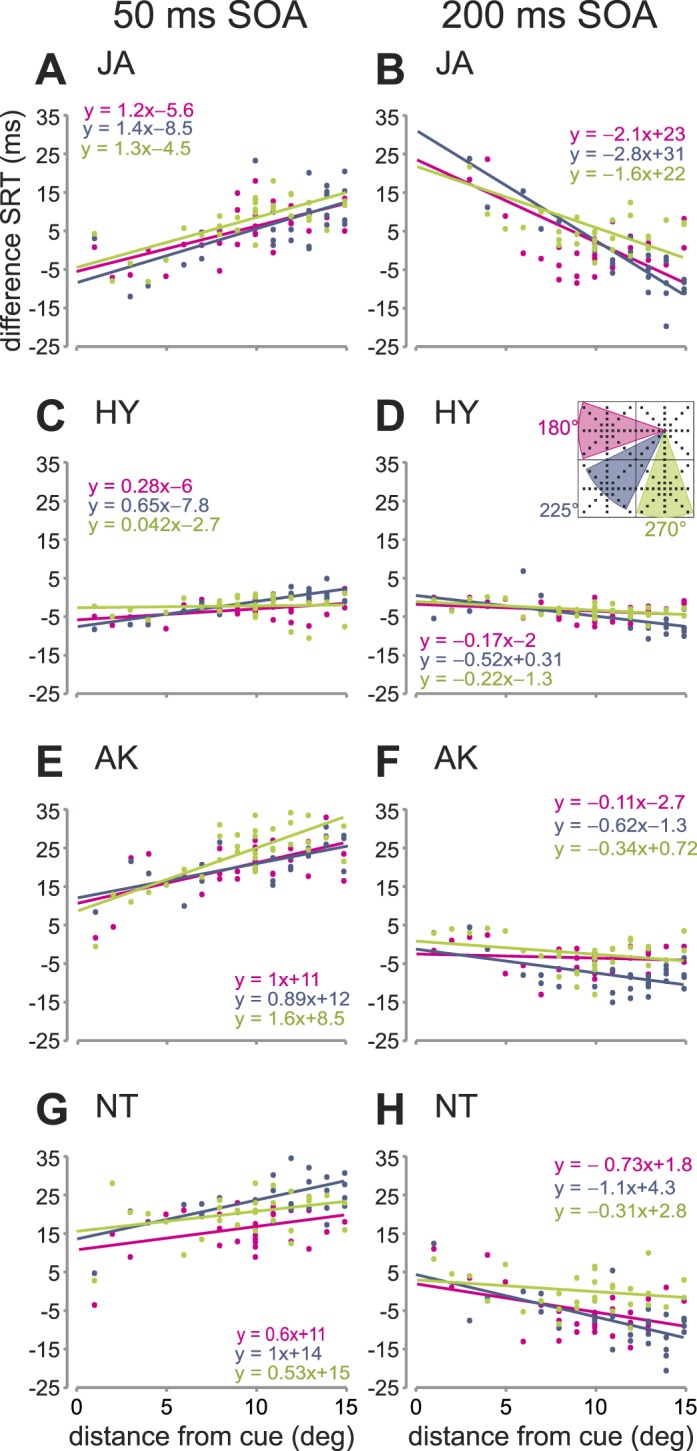
DSRTs as a function of direction. Median dSRTs are plotted as a function of target-distractor distance separately for three directions, 180° (red; see inset in D), 225° (blue), and 270°(green) for different subjects (rows) and different SOA conditions (columns). Corresponding linear fits are also shown.
In addition to distance, SRTs also varied across different directions for both short and longer distances from the distractor position. Thus, SRTs depend not only on the distance between the distractor and the target but also on the direction.
SRTs vary as a function of quadrant
We also tested whether there was an effect of quadrant as suggested by previous studies (Ro et al., 2000; Sheliga, Craighero, Riggio, & Rizzolatti, 1997). While it is expected that there will be differences across the quadrants due to both the distance and the direction effect, we were particularly interested in determining whether there were any overall quadrant effects in the two orthogonal conditions (where distances but not directions from the distractor are equivalent) across all targets within that quadrant, i.e., orthogonal same—same hemifield orthogonal to the distractor quadrant vs. orthogonal opposite—opposite hemifield orthogonal to the distractor quadrant. Figure 5 depicts median SRTs plotted as a function of quadrant separately for SOA condition and species. In agreement with the findings shown in Figure 3, SRTs increased (A and C) or decreased (B and D) as a function of the quadrants closest to furthest away from the distractor quadrant. Moreover all subjects show a small but consistent difference in the two orthogonal conditions. In the short SOA condition, there were significantly shorter SRTs for humans in the orthogonal opposite compared to the orthogonal same quadrant; repeated-measures t tests: AK mean difference = −4.6 ms, t(53) = 5.23, p < 0.01; and NT mean difference = −3.7 ms, t(53) = 2.01, p < 0.01; as well as for JA, −2.54 ms, t(53) = 3.42, p < 0.01; but not for HY, −0.73 ms, p > 0.05. In the long SOA condition, JA and NT showed significantly shorter SRTs for the orthogonal opposite compared to the orthogonal same quadrant: JA mean difference = −4.97 ms, t(53) = 6.9, p < 0.01; and NT mean difference = −6.12 ms, −(53) = 4.44, p < 0.01; whereas HY and AK did not (HY mean difference = −0.2 ms; and AK mean difference = −0.67 ms, p > 0.05.)
Figure 5.
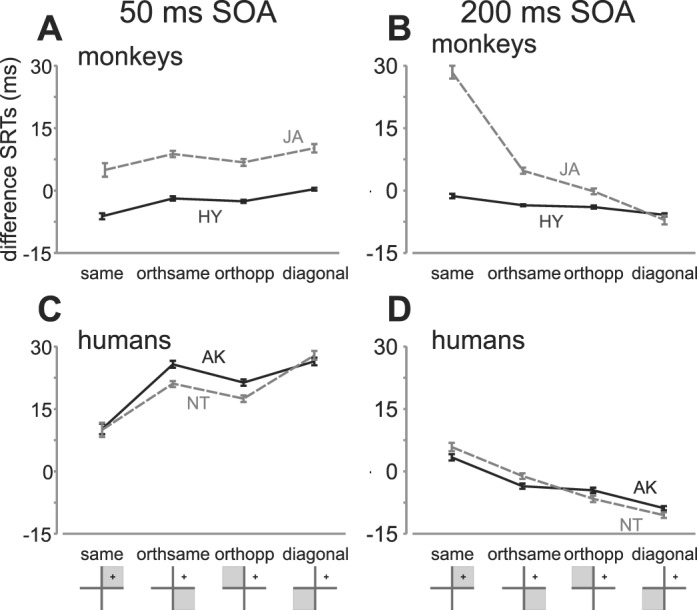
DSRTs as a function of quadrant relative to distractor. Median dSRTs for each quadrant relative to the distractor are shown for monkeys (HY—solid black lines; JA—dashed gray lines; top row) and humans (AK and NT; bottom row) for the short (left column) and long (right column) SOA conditions. The four quadrant locations are depicted in the x-axis label as gray regions. Error bars are standard errors of the medians.
In sum, the two SOA conditions show different patterns of SRTs across quadrants. In the short SOA condition, the SRTs in the orthogonal opposite quadrant were slightly shorter than those in the orthogonal same quadrant. This was also the case for the long SOA condition, and not opposite as would be expected. This significant effect of quadrant (for most subjects and conditions) cannot be due to the distance effect, as distances across all the targets within the two quadrants were equivalent. However, this effect could also be due to the direction effect shown in Figure 4. As the effect of quadrant and direction are not independent, it is difficult to tease them apart.
In summary, the influence of the distractor on SRTs was not localized to the distractor location but rather spread to the entire visual space measured. SRTs varied as a function of distance from the distractor location, with opposite effects for the short and long SOA conditions, i.e., an increase of SRT with increasing distance in the short SOA condition and a decrease with increasing distance in the long SOA condition. In addition, SRTs also varied as a function of direction when distance was accounted for. Finally, SRTs varied at the level of the quadrant relative to the distractor, with slight differences between the two SOA conditions. These SRT patterns may be a behavioral reflection of underlying lateral excitatory and inhibitory neuronal connections in areas involved in saccade generation (e.g., superior colliculus). We will investigate this possibility in the next section.
Individual differences among subjects
We compared the difference SRTs across the two monkeys and humans. For the short SOA condition, there was a significant difference between the two monkeys: HY: M = −2.6 ms, SD = 3.6 ms; and JA: M = 7.4 ms, SD = 6.8 ms; t(270) = 15.1, p < 0.001. This was also the case for the long SOA condition: HY: M = −4.1 ms, SD = 3.1 ms; and JA: M = 9.2 ms, SD = 14.6 ms; t(270) = 10.3, p < 0.001. As can be seen, monkey JA had a much broader range of SRTs (Figure 2A and B) compared to HY (Figure 2C and D). This may be due to differences in the amount of experience with saccade tasks between the two monkeys; monkey HY was highly trained whereas monkey JA was trained to a lesser degree. Humans showed no significant differences for either the short [AK: M = 20.7 ms, SD = 8.4 ms; and NT: M = 19.2 ms, SD = 9 ms; t(270) = 1.5, p > 0.05; or the long SOA condition [AK: M = −3.5 ms, SD = 5.6 ms; and NT: M = −3 ms, SD = 7.6 ms; t(270) = 0.7, p > 0.05]. As can be seen in the figures, both humans showed similar patterns of SRTs.
Model simulations
In this section we set out to evaluate whether behavioral results are consistent with current knowledge about SC functional connectivity. It has been previously suggested that the effect of distractor-target interactions on SRTs can be well captured by considering SC network dynamics (Arai et al., 1994; Arai & Keller, 2005; Fischer et al., 1995; Kopecz, 1995; Marino et al., 2012; Satel et al., 2011; Trappenberg et al., 2001). Specifically, it has been shown that there are short distance excitatory connections and long distance inhibitory connections within the SC (Meredith & Ramoa, 1998; Munoz & Fecteau, 2002; Munoz & Istvan, 1998; Olivier et al., 1999), and recent evidence from mouse colliculus indicates that this pattern of connectivity is seen in the superficial layers, while the intermediate layers have both long-range excitatory and inhibitory connections (Isa & Hall, 2009; Phongphanphanee et al., 2014). The superficial SC layers, which respond to visual stimuli, in turn project to the intermediate layers, which govern saccade generation (Helms, Ozen, & Hall, 2004; Isa, Endo, & Saito, 1998; Lee, Helms, Augustine, & Hall, 1997; Phongphanphanee, Kaneda, & Isa, 2008). In addition, temporal processes within the SC such as summation of activity (Bell et al., 2003; Bell et al., 2004; Dukewich, 2009) and short term depression (Bell et al., 2003; Bell et al., 2004; Fecteau & Munoz, 2005) combined with the lateral interactions may explain the mirror patterns of SRTs seen for the short and long SOA conditions as previously posited (Satel et al., 2011).
Here we extended a previous 1D dynamic SC model (Satel et al., 2011; Trappenberg et al., 2001) to 2D (see Methods section). This new model incorporates the short-distance excitatory and long-distance inhibitory connections in two dimensions. To gain insight into the potential neurophysiological mechanisms underlying distractor-target interactions across space and time, we investigated whether this model could accurately capture our dataset.
Figure 6A and B depict simulated difference SRTs projected onto superior colliculus space for the short (A) and long (B) SOA conditions. The distractor location in SC space is shown by the white dot. Using (a) a combination of short-distance excitatory and long-distance inhibitory connections in 2D, (b) a foreperiod effect (Fecteau & Munoz, 2005) and (c) a stimulus-dependent short term depression; the model simulated shorter SRTs when targets were placed closer the distractor location (Figure 6A). The SRTs increased when the distance from the distractor increased. Within the opposite SC (corresponding to the opposite visual field) the SRTs were generally uniformly longer. The opposite is true for the long SOA condition (Figure 6B), where SRTs were longest at the distractor location and decreased as the distance between the target and distractor increased. For comparison, we transformed the spatial target locations into SC coordinates, and plotted the SRTs onto SC positions for the two monkeys (JA, C and D, HY, E and F) as well as the two humans (AK, G and H; NT, I and J). Note that the SC coordinates used are based on neurophysiological findings from monkeys (Marino et al., 2012; Ottes, Van Gisbergen, & Eggermont, 1986; Van Gisbergen, Van Opstal, & Tax, 1987). As can be seen, the model SRTs resembled the patterns shown by both monkeys and humans, namely shortest median SRTs near the distractor location, an increase in SRTs with longer distances and almost uniformly longer SRTs in the opposite half of the SC for the short SOA condition and the opposite pattern for the long SOA condition. Thus, the observed SRT patterns of distractor-target interactions could naturally arise from the known inherent network properties of the SC without the need of additional mechanisms.
Figure 6.
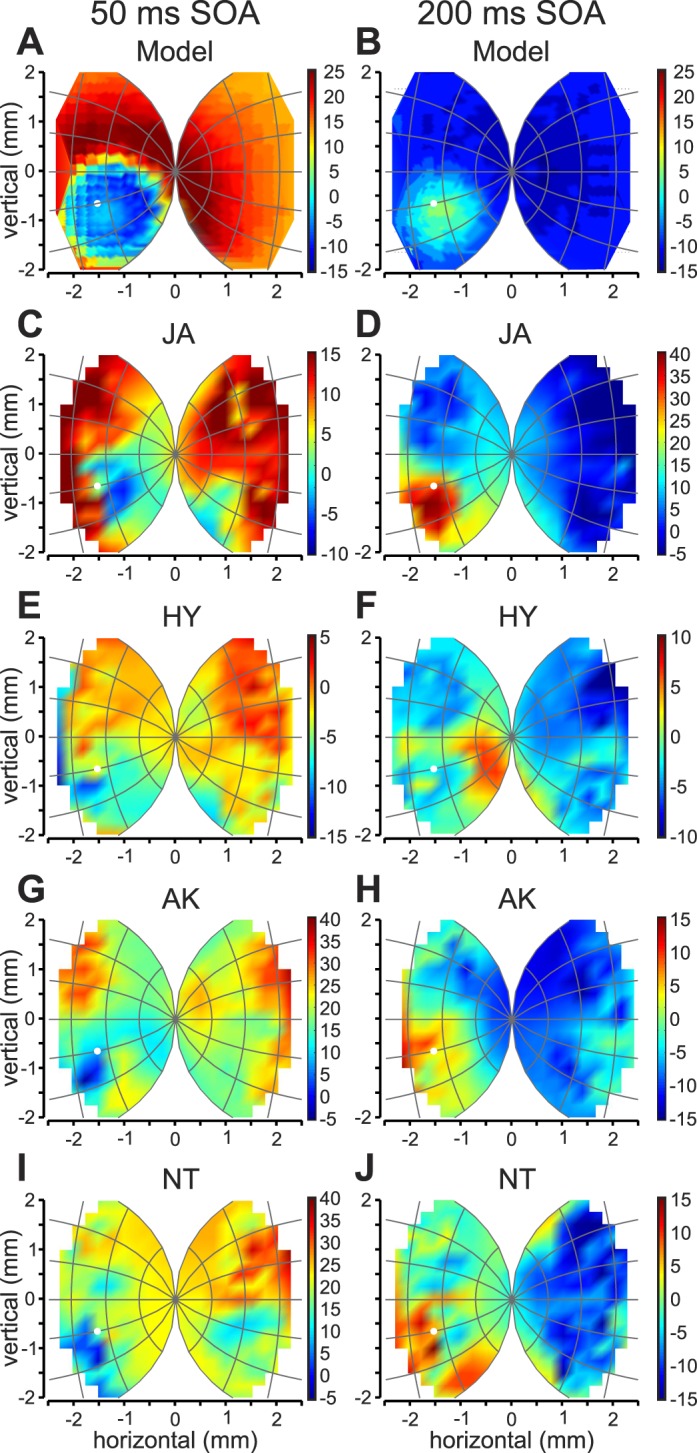
Model simulations and behavioral data in SC coordinates. (A and B) dSRTs simulated by the model are plotted in SC coordinates for the short (A) and long (B) SOA conditions. The color scale is shown for each color plot, where red depicts SRTs that are longer than in the no distractor condition and blue depicts SRTs that are shorter than the no distractor condition. Behavioral median dSRTs for monkey JA (C and D), HY (E and F), AK (G and H) and NT (I and J) are also plotted in the same SC coordinates based on monkey physiology. Note that the color scales are different for each plot.
Discussion
We measured the influence of the distractor on SRTs to a subsequently presented target systematically and in detail across different timings, different species, and across a relatively broad range of visual space. The behavioral patterns of SRTs for both the short and the long SOA conditions for both monkeys and humans showed a gradual change in SRTs that increased or decreased respectively as a function of distractor-target distance with a small effect of direction as well as a hemifield effect, where SRTs were slightly shorter in the opposite hemifield compared to the same hemifield for both short and long SOAs. A 2D dynamic field model based on known SC properties performed well at simulating the patterns of SRTs, supporting the involvement of SC in distractor-target interactions in saccade planning.
Relevance to previous behavioral studies
The findings from this study are consistent with and encompass together many previous studies on facilitation and IOR that have investigated only one or two aspects of this relationship (Abrams & Dobkin, 1994b; Bennett & Pratt, 2001; Blangero et al., 2010; Briand et al., 2000; Dorris, Taylor, Klein, & Munoz, 1999; Khan et al., 2010; Maylor & Hockey, 1985; Munoz et al., 2000; Posner et al., 1985; Pratt & Hirshhorn, 2003; Ro et al., 2000; Sheliga et al., 1997; Trappenberg et al., 2001). In particular, we show that SRTs vary systematically as a function of distance from the distractor, even within the opposite visual field, rather than uniform SRTs as have been suggested by some (Maylor & Hockey, 1985; Ro et al., 2000; Tassinari & Berlucchi, 1995). Moreover, the direction of the distractor relative to the target is also relevant (Maylor & Hockey, 1985; Sheliga et al., 1997). We also found a small effect of visual field consistent with previous studies (Maylor & Hockey, 1985; Ro et al., 2000; Sheliga et al., 1997) though this result may be confounded by distance and/or direction. Recently Albares et al. (2011) suggested that the presence of a distractor may result in a global inhibitory effect, increasing SRTs overall compared to trials where no distractor is presented at all within a block and therefore effects of facilitation and inhibition shown in the above studies including this one may be simply distractor-relative effects. Since we did not have entire blocks of trials with no distractor present, we cannot determine whether the SRTs are indeed overall longer. Nevertheless, the interaction between the distractor and target as a function of distance, direction and quadrant are highly robust and consistent and likely reflect underlying neuronal mechanisms which play a role in determining SRTs, in addition to a possible overall inhibitory mechanism resulting from the presence of distractors within a block of trials (Albares et al., 2011; Ballanger, 2009; Criaud, Wardak, Ben Hamed, Ballanger, & Boulinguez, 2012). Thus, our results are in accordance with previous studies which have suggested that IOR and attentional capture or facilitation may be implemented by the same mechanisms (Patel, Peng, & Sereno, 2010) particularly within the saccadic system (Fecteau & Munoz, 2005). However, it remains unclear whether, outside the saccadic system, all instances of IOR are implemented by the same underlying mechanisms (Bennett & Pratt, 2001; Hunt & Kingstone, 2003; Klein, 2000; Reuter-Lorenz & Rosenquist, 1996; Ro & Rafal, 1999; Tipper et al., 1997).
The pattern of SRTs including the spread of inhibition and excitation and the quadrant effect are also consistent with numerous studies showing the remote distractor effect (Buonocore & McIntosh, 2012; Gandhi & Keller, 1999; Honda, 2005; Levy-Schoen, 1969; Ludwig et al., 2005; McSorley et al., 2012; Walker et al., 1997; Walker, Kentridge, & Findlay, 1995; White et al., 2005). It has been demonstrated that the RDE is strongest when the distractor is presented within ±20 ms of the target (Bompas & Sumner, 2009). Hence, it is not certain whether the current findings with the target presented 50 ms after the distractor are also part of the same mechanism or not (Bompas & Sumner, 2009; Walker, Kentridge, & Findlay, 1995). It has also been suggested that the RDE may be related to the eccentricity of the distractor relative to the target rather than the absolute distance between the target and distractor (Casteau & Vitu, 2012; Walker, Kentridge, & Findlay, 1995; Walker et al., 1997). However, as we did not vary the eccentricity of the distractor, we cannot determine if our findings are consistent with this.
2D dynamic field model
Using a 2D dynamic field model, we show that both patterns of SRTs can be explained using the same underlying neuronal mechanisms within the same area of the brain, e.g., SC, through short-distance excitatory and long-distance inhibitory functional connections as has been previously suggested (Satel et al., 2011). The appearance of the distractor increased activity for locations at or close to the distractor through excitatory connections and decreased activity further away from the distractor (including the opposite visual field) through inhibitory connections. When the target appeared shortly after, the activity related to it was influenced by the previous ongoing activity related to the distractor (Bell et al., 2004; Dukewich, 2009; Fecteau & Munoz, 2006). Based on a fixed threshold trigger mechanism for saccade initiation (at least within a task; see Jantz, Watanabe, Everling, & Munoz, 2013), it would take longer for a saccade to be initiated if building on previously inhibited activity, compared to previously enhanced activity. Moreover, because of the gradients of inhibitory connections, inhibition increased when the distractor was positioned further away from the target. Therefore, for targets appearing further away, activity will have to build on more inhibited activity, taking longer to reach thresholds, resulting in longer SRTs. In the model, IOR was also achieved through the same short-distance excitatory and long-distance inhibitory connections. In this case, the appearance of the distractor resulted in the same increase of activity at and close to the distractor location as well as decreasing activity at further distances. However over time, the activity related to subsequent stimuli at the same location is suppressed. This suppression of activity is considered to be a purely sensory mechanism implemented at the level of the superficial superior colliculus (Fecteau et al., 2004; Fecteau & Munoz, 2005) and called short term depression (Satel et al., 2011) or sometimes habituation (Boehnke et al., 2011; Dukewich, 2009; Fecteau & Munoz, 2005; Huber, 2008). Because of the underlying neural connections, this decreased activity at the distractor location also decreases activity at locations close to the distractor and increases activity at locations further away from the distractor. Ensuing target related activity builds on this previous activity in the same manner as explained above, but now resulting in the opposite pattern of SRTs; shorter SRTs at further locations where activity is already enhanced, requiring less time to reach saccade trigger thresholds, and longer SRTs at closer locations.
Our model is based on the effective functional SC connectivity rather than capturing its detailed anatomy or physiology. Many physiological studies have revealed short-distance excitatory and long-distance inhibitory interactions throughout the SC (Dorris et al., 2007; Marino et al., 2012; Munoz & Fecteau, 2002; Munoz & Istvan, 1998; Olivier et al., 1999; Phongphanphanee et al., 2014). More recent evidence from mouse slice preparation (Phongphanphanee et al., 2014) suggests that the intermediate layers might show stronger long-range excitatory connections than previously suggested. Importantly, this was an in vitro study testing for lateral connections within the superficial and intermediate layers of the SC independent of the entire network involved in producing saccades. This study was unique in employing horizontal slices of SC, instead of coronal or sagittal slices which sever the lateral connections (Lee & Hall, 2006).Therefore, it is possible that the intermediate layers of the SC can utilize a mechanism of short-range excitation and long-range inhibition and it is possible that the long-range excitatory connections are for a different function. Thus, these physiological findings do not tell us how the functional network works as a whole. Indeed, many previous stimulation and recording studies in vivo within the monkey SC show evidence for short-distance excitatory and long-distance inhibitory effects, clearly demonstrating that the functional interactions are not necessarily explained by or negated by physiological findings on a small part of the network. Regardless, the connectivity exists for short-range excitatory and long-range inhibitory effects in both superficial and intermediate SC layers. In our view, the complex connectivity within the SC as well as with other structures including the basal ganglia, thalamus, and the cortex appears to produce effective overall functional dynamics in support of the short-range excitatory and long-range inhibitory connectivity profiles, as shown previously (Dorris et al., 2007; Isa & Hall, 2009).
The dynamic field model is based on competition related to visual and motor signals based on effective short-distance excitatory and long-distance inhibitory neuronal connectivity within the SC resulting in a certain pattern of SRTs depending on the relationship between the distractor and the saccade target. An alternate theory relying on the competition between a specialized fixation system and a saccade system has been put forth (Casteau & Vitu, 2012; Walker et al., 1997), which posits that distractors exert their behavioral effects by activating fixation neurons which in turn inhibit saccade-related neurons. It has recently been shown that fixation neurons in the SC might be part of a continuum towards very small (micro) saccades (Hafed, Goffart, & Krauzlis, 2009; Hafed & Krauzlis, 2012). Alternatively, it has been proposed that the omnipause neurons may function as the fixation system (Casteau & Vitu, 2012) and they receive visual responses across a broad region of the visual field (Everling, Pare, Dorris, & Munoz, 1998). In this case, SRTs depend on the eccentricity of the distractor relative to the fovea, with greater effects when the distractor is close to the fovea.
We observed a consistent pattern of change in SRTs related to the distractor-target relationship utilizing a single distractor eccentricity. Given the spatial configuration of the stimuli, our findings are consistent with an interpretation of competitive interaction between the target and distractor locations in terms of lateral connectivity rather than interactions between fixation and move processes, as has also been shown by others (Dorris et al., 2007; Olivier et al., 1999; White et al., 2005; but see also McSorley et al., 2012). However we did not vary the eccentricity of the distractor relative to the fixation; in order to distinguish between the fixation-move and the lateral competition hypotheses, both target and distractor locations should be systematically varied within and across hemifields.
Underlying neuronal mechanisms
The behavioral SRT patterns were well modeled by a dynamic neuronal field model that simulated known properties of the SC (see Methods). As such, the SC, known to be highly involved in both oculomotor (Sparks & Hartwich-Young, 1989; Sparks, 1986) and attentional processes (Krauzlis et al., 2013) is a strong contender. Most studies agree that attentional facilitation is implemented at least in part by the SC (Fecteau et al., 2004; Fecteau & Munoz, 2005; Ikeda, Yoshida, & Isa, 2011; Yoshida et al., 2012), consistent with findings from neurophysiological studies which suggest that attentional capture may be regulated by the SC through a simple summation of distractor and target-related activity. Additionally, neuronal recordings, lesion studies and behavioral findings support the involvement of the SC in IOR (Abrams & Dobkin, 1994b; Dorris et al., 2002; Fecteau & Munoz, 2005; Posner et al., 1985; Rafal, Posner, Friedman, Inhoff, & Bernstein, 1988; Sapir et al., 1999; Sereno, Briand, Amador, & Szapiel, 2006).
Nevertheless, there are other areas in the brain that could also play a major role in the distractor related modulation of SRTs, including the frontal eye fields (Goldberg & Bruce, 1990; Goldberg & Segraves, 1989; Hanes & Schall, 1996; Rafal, 2006; Schall & Bichot, 1998), the parietal cortex (Bisley & Goldberg, 2010; Colby & Goldberg, 1999; Falkner, Krishna, & Goldberg, 2010; Suzuki & Gottlieb, 2013) and the basal ganglia (Deijen, Stoffers, Berendse, Wolters, & Theeuwes, 2006; Hikosaka, Takikawa, & Kawagoe, 2000). For example, Dorris et al. (2002) showed that SRTs correlated with reduced activity in the intermediate layers of the SC during an IOR task, but that this suppression of activity may not originate from the SC, but rather upstream. It remains unclear whether this inhibition was a result of overall inhibition related to top-down modulation by the cortex (Albares et al., 2011; Basso & Wurtz, 1998; Constantinidis & Steinmetz, 2005; Dorris & Munoz, 1998; Glimcher & Sparks, 1992; Marino & Munoz, 2009) or specifically related to the spatial and temporal relationship between the distractor and the saccade target, but it suggests that signals entering the SC from other areas are involved.
Monkeys versus humans
The pattern of SRTs as a function of distractor-target distance, direction, relative quadrant, and timing were remarkably similar across monkeys and humans and validate monkeys as a good model for human saccadic behavior for distractor related effects as well as attentional effects (e.g., Lee & McPeek, 2013). The similarities between the monkey and human SRTs also provide support for the idea that similar underlying mechanisms in both species produce these distractor-target interactions. Indeed, many cortical areas including the parietal and frontal cortices show similarities between humans and monkeys (Astafiev et al., 2003; Koyama et al., 2004; Leoné, Toni, & Medendorp, 2014; Orban, Van Essen, & Vanduffel, 2004) as does the SC (Krebs et al., 2010; Schneider & Kastner, 2005). The main systematic difference between humans and monkeys was the shorter baseline SRTs for the monkeys (129 ms) compared to humans (177 ms), reflective of previous studies (e.g., Fecteau et al., 2004). This could be attributed to overtraining in the monkeys. Additionally, it is thought that shorter SRTs for monkeys are due to shorter connections and transmission pathways across visual and motor areas in the monkey or to overtraining on saccade tasks.
Conclusions
We explored the influence of a behaviorally irrelevant distractor on subsequent SRTs to targets presented at various locations across visual space and at two different times after the distractor for both humans and monkeys. We found that both species showed similar patterns of SRTs which depended on the timing, distance, direction, and possibly visual field of the saccade target relative to the distractor. In addition, we demonstrated that a 2D dynamic field model of the superior colliculus which included a short term depression/habituation process replicated the pattern of SRTs for both short and long SOAs well, supporting the role of the SC in distractor-target interactions.
Acknowledgments
AZK was funded by a Canadian Institutes of Health Research Fellowship. GB is funded by the National Science and Engineering Research Council (Canada), the Ontario Research Fund (Canada), the Canadian Foundation for Innovation (Canada), and the Botterell Foundation (Queens University, Kingston, ON, Canada). RMM is funded by National Eye Institute grant R01-EY014885.
Commercial relationships: none.
Corresponding author: Aarlenne Zein Khan.
Email: aarlennek@gmail.com.
Address: School of Optometry, University of Montreal, Montreal, Quebec, Canada.
Contributor Information
Aarlenne Z. Khan, aarlennek@gmail.com, http://www.opto.umontreal.ca/visattac/.
Douglas P. Munoz, doug.munoz@queensu.ca, http://brain.phgy.queensu.ca/doug/www/.
Naomi Takahashi, Email: naomi2kahashi@gmail.com.
Gunnar Blohm, gunnar.blohm@queensu.ca, http://www.compneurosci.com/.
Robert M. McPeek, rmcpeek@sunyopt.edu, http://poseidon.sunyopt.edu/mcpeek/McPeek_Lab/Home.html.
References
- Abrams, R. A., Dobkin R. S.. (1994a). Inhibition of return: Effects of attentional cuing on eye movement latencies. Journal of Experimental Psychology: Human Perception and Performance, 20 3, 467– 477. [DOI] [PubMed] [Google Scholar]
- Abrams R. A., Dobkin R. S.. (1994b). The gap effect and inhibition of return: Interactive effects on eye movement latencies. Experimental Brain Research, 98 3, 483– 487. [DOI] [PubMed] [Google Scholar]
- Albares M., Criaud M., Wardak C., Nguyen S. C. T., Ben Hamed S., Boulinguez P.. (2011). Attention to baseline: Does orienting visuospatial attention really facilitate target detection? Journal of Neurophysiology, 106 2, 809– 816. [DOI] [PubMed] [Google Scholar]
- Arai K., Keller E. L.. (2005). A model of the saccade-generating system that accounts for trajectory variations produced by competing visual stimuli. Biological Cybernetics, 92 1, 21– 37. [DOI] [PubMed] [Google Scholar]
- Arai K., Keller E. L., Edelman J. A.. (1994). Two-dimensional neural network model of the primate saccadic system. Neural Networks, 7 6-7, 1115– 1135. [Google Scholar]
- Astafiev S. V., Shulman G. L., Stanley C. M., Snyder A. Z., Van Essen D. C., Corbetta M.. (2003). Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. The Journal of Neuroscience, 23 11, 4689– 4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanger B. (2009). Top-down control of saccades as part of a generalized model of proactive inhibitory control. Journal of Neurophysiology, 102 5, 2578– 2580. [DOI] [PubMed] [Google Scholar]
- Basso M. A., Wurtz R. H.. (1998). Modulation of neuronal activity in superior colliculus by changes in target probability. The Journal of Neuroscience, 18 18, 7519– 7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. H., Corneil B. D., Munoz D. P., Meredith M. A.. (2003). Engagement of visual fixation suppresses sensory responsiveness and multisensory integration in the primate superior colliculus. The European Journal of Neuroscience, 18 10, 2867– 2873. [DOI] [PubMed] [Google Scholar]
- Bell A. H., Fecteau J. H., Munoz D. P.. (2004). Using auditory and visual stimuli to investigate the behavioral and neuronal consequences of reflexive covert orienting. Journal of Neurophysiology, 91 5, 2172– 2184. [DOI] [PubMed] [Google Scholar]
- Bennett P. J., Pratt J.. (2001). The spatial distribution of inhibition of return. Psychological Science, 12 1, 76– 80. [DOI] [PubMed] [Google Scholar]
- Bisley J. W., Goldberg M. E.. (2010). Attention, intention, and priority in the parietal lobe. Annual Review of Neuroscience, 33, 1– 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangero A., Khan A. Z., Salemme R., Deubel H., Schneider W. X., Rode G., Pisella L.. (2010). Pre-saccadic perceptual facilitation can occur without covert orienting of attention. Cortex, 46 9, 1132– 1137. [DOI] [PubMed] [Google Scholar]
- Boehnke S. E., Berg D. J., Marino R. A., Baldi P. F., Itti L., Munoz D. P.. (2011). Visual adaptation and novelty responses in the superior colliculus. The European Journal of Neuroscience, 34 5, 766– 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bompas A., Sumner P.. (2009). Temporal dynamics of saccadic distraction. Journal of Vision, 9(9): 17, 1– 14, doi:10.1167/9.9.17 [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Born S., Kerzel D.. (2008). Influence of target and distractor contrast on the remote distractor effect. Vision Research, 48 28, 2805– 2816. [DOI] [PubMed] [Google Scholar]
- Brainard D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10 4, 433– 436. [PubMed] [Google Scholar]
- Briand K. A., Larrison A. L., Sereno A. B.. (2000). Inhibition of return in manual and saccadic response systems. Perception & Psychophysics, 62 8, 1512– 1524. [DOI] [PubMed] [Google Scholar]
- Buonocore A., McIntosh R. D.. (2012). Modulation of saccadic inhibition by distractor size and location. Vision Research, 69, 32– 41. [DOI] [PubMed] [Google Scholar]
- Casteau S., Vitu F.. (2012). On the effect of remote and proximal distractors on saccadic behavior: A challenge to neural-field models. Journal of Vision, 12(12): 14, 1– 33, doi:10.1167/12.12.14 [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Cavanaugh J., Joiner W. M., Wurtz R. H.. (2012). Suppressive surrounds of receptive fields in monkey frontal eye field. The Journal of Neuroscience, 32 35, 12284– 12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby C. L., Goldberg M. E.. (1999). Space and attention in parietal cortex. Annual Review of Neuroscience, 22, 319– 349. [DOI] [PubMed] [Google Scholar]
- Constantinidis C., Steinmetz M. A.. (2005). Posterior parietal cortex automatically encodes the location of salient stimuli. Journal of Neuroscience, 25 1, 233– 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criaud M., Wardak C., Ben Hamed S., Ballanger B., Boulinguez P.. (2012). Proactive inhibitory control of response as the default state of executive control. Frontiers in Psychology, 3, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafoe J. M., Armstrong I. T., Munoz D. P.. (2007). The influence of stimulus direction and eccentricity on pro- and anti-saccades in humans. Experimental Brain Research, 179 4, 563– 570. [DOI] [PubMed] [Google Scholar]
- Deijen J. B., Stoffers D., Berendse H. W., Wolters E. C., Theeuwes J.. (2006). Abnormal susceptibility to distracters hinders perception in early stage Parkinson's disease: A controlled study. BMC Neurology, 6, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick S., Kathmann N., Ostendorf F., Ploner C. J.. (2005). Differential effects of target probability on saccade latencies in gap and warning tasks. Experimental Brain Research, 164 4, 458– 463. [DOI] [PubMed] [Google Scholar]
- Dorris M. C., Klein R. M., Everling S., Munoz D. P.. (2002). Contribution of the primate superior colliculus to inhibition of return. Journal of Cognitive Neuroscience, 14 8, 1256– 1263. [DOI] [PubMed] [Google Scholar]
- Dorris M. C., Munoz D. P.. (1998). Saccadic probability influences motor preparation signals and time to saccadic initiation. The Journal of Neuroscience, 18 17, 7015– 7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris M. C., Olivier E., Munoz D. P.. (2007). Competitive integration of visual and preparatory signals in the superior colliculus during saccadic programming. The Journal of Neuroscience, 27 19, 5053– 5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris M. C., Taylor T. L., Klein R. M., Munoz D. P.. (1999). Influence of previous visual stimulus or saccade on saccadic reaction times in monkey. Journal of Neurophysiology, 81 5, 2429– 2436. [DOI] [PubMed] [Google Scholar]
- Dukewich K. R. (2009). Reconceptualizing inhibition of return as habituation of the orienting response. Psychonomic Bulletin & Review, 16 2, 238– 251. [DOI] [PubMed] [Google Scholar]
- Everling S., Pare M., Dorris M. C., Munoz D. P.. (1998). Comparison of the discharge characteristics of brain stem omnipause neurons and superior colliculus fixation neurons in monkey: Implications for control of fixation and saccade behavior. Journal of Neurophysiology, 79 2, 511– 528. [DOI] [PubMed] [Google Scholar]
- Falkner A. L., Krishna B. S., Goldberg M. E.. (2010). Surround suppression sharpens the priority map in the lateral intraparietal area. The Journal of Neuroscience, 30 38, 12787– 12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau J. H., Bell A. H., Munoz D. P.. (2004). Neural correlates of the automatic and goal-driven biases in orienting spatial attention. Journal of Neurophysiology, 92 3, 1728– 1737. [DOI] [PubMed] [Google Scholar]
- Fecteau J. H., Munoz D. P.. (2005). Correlates of capture of attention and inhibition of return across stages of visual processing. Journal of Cognitive Neuroscience, 17 11, 1714– 1727. [DOI] [PubMed] [Google Scholar]
- Fecteau J. H., Munoz D. P.. (2006). Salience, relevance, and firing: A priority map for target selection. Trends in Cognitive Sciences, 10 8, 382– 390. [DOI] [PubMed] [Google Scholar]
- Fecteau J. H., Munoz D. P.. (2007). Warning signals influence motor processing. Journal of Neurophysiology, 97 2, 1600– 1609. [DOI] [PubMed] [Google Scholar]
- Findlay J. M., Walker R.. (1999). A model of saccade generation based on parallel processing and competitive inhibition. The Behavioral and Brain Sciences, 22 4, 661– 674. [DOI] [PubMed] [Google Scholar]
- Fino E., Yuste R.. (2011). Dense inhibitory connectivity in neocortex. Neuron, 69 6, 1188– 1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B., Gezeck S., Huber W.. (1995). The three-loop model: A neural network for the generation of saccadic reaction times. Biological Cybernetics, 72 3, 185– 196. [DOI] [PubMed] [Google Scholar]
- Gandhi N. J., Keller E. L.. (1999). Activity of the brain stem omnipause neurons during saccades perturbed by stimulation of the primate superior colliculus. Journal of Neurophysiology, 82 6, 3254– 3267. [DOI] [PubMed] [Google Scholar]
- Glimcher P. W., Sparks D. L.. (1992). Movement selection in advance of action in the superior colliculus. Nature, 355 6360, 542– 545. [DOI] [PubMed] [Google Scholar]
- Godijn R., Theeuwes J.. (2002a). Oculomotor capture and Inhibition of Return: Evidence for an oculomotor suppression account of IOR. Psychological Research, 66 4, 234– 246. [DOI] [PubMed] [Google Scholar]
- Godijn R., Theeuwes J.. (2002b). Programming of endogenous and exogenous saccades: Evidence for a competitive integration model. Journal of Experimental Psychology: Human Perception and Performance, 28 5, 1039– 1054. [DOI] [PubMed] [Google Scholar]
- Goldberg M. E., Bruce C. J.. (1990). Primate frontal eye fields. III. Maintenance of a spatially accurate saccade signal. Journal of Neurophysiology, 64 2, 489– 508. [DOI] [PubMed] [Google Scholar]
- Goldberg M. E., Colby C. L.. (1992). Oculomotor control and spatial processing. Current Opinion in Neurobiology, 2 2, 198– 202. [DOI] [PubMed] [Google Scholar]
- Goldberg M. E., Segraves M. A.. (1989). The visual and frontal cortices. Reviews of Oculomotor Research, 3, 283– 313. [PubMed] [Google Scholar]
- Griffiths H., Whittle J., Buckley D.. (2006). The effect of binocular and monocular distractors on saccades in participants with normal binocular vision. Vision Research, 46 1–2, 72– 81. [DOI] [PubMed] [Google Scholar]
- Hafed Z. M., Goffart L., Krauzlis R. J.. (2009). A neural mechanism for microsaccade generation in the primate superior colliculus. Science, 323 5916, 940– 943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed Z. M., Krauzlis R. J.. (2012). Similarity of superior colliculus involvement in microsaccade and saccade generation. Journal of Neurophysiology, 107 7, 1904– 1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes D. P., Schall J. D.. (1996). Neural control of voluntary movement initiation. Science, 274 5286, 427– 430. [DOI] [PubMed] [Google Scholar]
- Helms M. C., Ozen G., Hall W. C.. (2004). Organization of the intermediate gray layer of the superior colliculus. I. Intrinsic vertical connections. Journal of Neurophysiology, 91 4, 1706– 1715. [DOI] [PubMed] [Google Scholar]
- Hikosaka O., Takikawa Y., Kawagoe R.. (2000). Role of the basal ganglia in the control of purposive saccadic eye movements. Physiological Reviews, 80 3, 953– 978. [DOI] [PubMed] [Google Scholar]
- Hikosaka O., Wurtz R. H.. (1985). Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. Journal of Neurophysiology, 53 1, 266– 291. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P., Distler C., Mark R. F., Marotte L. R., Henry G. H., Ibbotson M. R.. (1995). Neural and behavioral effects of early eye rotation on the optokinetic system in the wallaby, Macropus eugenii. Journal of Neurophysiology, 73 2, 727– 735. [DOI] [PubMed] [Google Scholar]
- Honda H. (2005). The remote distractor effect of saccade latencies in fixation-offset and overlap conditions. Vision Research, 45 21, 2773– 2779. [DOI] [PubMed] [Google Scholar]
- Honda H., Findlay J. M.. (1992). Saccades to targets in three-dimensional space: Dependence of saccadic latency on target location. Perception & Psychophysics, 52 2, 167– 174. [DOI] [PubMed] [Google Scholar]
- Huber D. (2008). Immediate priming and cognitive aftereffects. Journal of Experimental Psychology. General, 137 2, 324– 347. [DOI] [PubMed] [Google Scholar]
- Hunt A. R., Kingstone A.. (2003). Inhibition of return: Dissociating attentional and oculomotor components. Journal of Experimental Psychology. Human Perception and Performance, 29 5, 1068– 1074. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Yoshida M., Isa T.. (2011). Lesion of primary visual cortex in monkey impairs the inhibitory but not the facilitatory cueing effect on saccade. Journal of Cognitive Neuroscience, 23 5, 1160– 1169. [DOI] [PubMed] [Google Scholar]
- Isa T., Endo T., Saito Y.. (1998). The visuo-motor pathway in the local circuit of the rat superior colliculus. The Journal of Neuroscience, 18 20, 8496– 8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T., Hall W. C.. (2009). Exploring the Superior Colliculus In Vitro. Journal of Neurophysiology, 102 5, 2581– 2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantz J. J., Watanabe M., Everling S., Munoz D. P.. (2013). Threshold mechanism for saccade initiation in frontal eye field and superior colliculus. Journal of Neurophysiology, 109 11, 2767– 2780. [DOI] [PubMed] [Google Scholar]
- Jonides J., Irwin D. E.. (1981). Capturing attention. Cognition, 10 1–3, 145– 150. [DOI] [PubMed] [Google Scholar]
- Kalesnykas R. P., Hallett P. E.. (1987). The differentiation of visually guided and anticipatory saccades in gap and overlap paradigms. Experimental Brain Research, 68 1, 115– 121. [DOI] [PubMed] [Google Scholar]
- Kalesnykas R. P., Hallett P. E.. (1994). Retinal eccentricity and the latency of eye saccades. Vision Research, 34 4, 517– 531. [DOI] [PubMed] [Google Scholar]
- Kätzel D., Zemelman B. V., Buetfering C., Wölfel M., Miesenböck G.. (2011). The columnar and laminar organization of inhibitory connections to neocortical excitatory cells. Nature Neuroscience, 14 1, 100– 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. Z., Heinen S. J., McPeek R. M.. (2010). Attentional cueing at the saccade goal, not at the target location, facilitates saccades. Journal of Neuroscience, 30 16, 5481– 5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. M. (2000). Inhibition of return. Trends in Cognitive Sciences, 4 4, 138– 147. [DOI] [PubMed] [Google Scholar]
- Kopecz K. (1995). Saccadic reaction times in gap/overlap paradigms: A model based on integration of intentional and visual information on neural, dynamic fields. Vision Research, 35 20, 2911– 2925. [DOI] [PubMed] [Google Scholar]
- Kopecz K., Schöner G.. (1995). Saccadic motor planning by integrating visual information and pre-information on neural dynamic fields. Biological Cybernetics, 73 1, 49– 60. [DOI] [PubMed] [Google Scholar]
- Koyama M., Hasegawa I., Osada T., Adachi Y., Nakahara K., Miyashita Y.. (2004). Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: Comparison of cortical eye fields with humans. Neuron, 41 5, 795– 807. [DOI] [PubMed] [Google Scholar]
- Krauzlis R. J., Lovejoy L. P., Zénon A.. (2013). Superior colliculus and visual spatial attention. Annual Review of Neuroscience, 36, 165– 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs R. M., Woldorff M. G., Tempelmann C., Bodammer N., Noesselt T., Boehler C. N., Schoenfeld M. A.. (2010). High-field FMRI reveals brain activation patterns underlying saccade execution in the human superior colliculus. PloS One, 5 1, e8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.-T., McPeek R. M.. (2013). The effects of distractors and spatial precues on covert visual search in macaque. Vision Research, 76, 43– 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P., Hall W. C.. (2006). An in vitro study of horizontal connections in the intermediate layer of the superior colliculus. The Journal of Neuroscience, 26 18, 4763– 4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. H., Helms M. C., Augustine G. J., Hall W. C.. (1997). Role of intrinsic synaptic circuitry in collicular sensorimotor integration. Proceedings of the National Academy of Sciences, USA, 94 24, 13299– 13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh R. J., Zee D. S.. (2006). The Neurology of Eye Movements. New York: Oxford University Press. [Google Scholar]
- Leoné F. T. M., Toni I., Medendorp W. P.. (2014). Two-dimensional spatial tuning for saccades in human parieto-frontal cortex. NeuroImage, 87, 476– 489. [DOI] [PubMed] [Google Scholar]
- Levy-Schoen A. (1969). Determination et latence de la reponse oculomotrice a deux stimulus simultanes our successifs selon leur excentricite relative [Translation: Determination and latency of oculo-motor response to simultaneous and successive stimuli according to their relative eccentricity]. L'annee Psychologie, 69, 373– 392. [Google Scholar]
- Ludwig C. J. H., Gilchrist I. D., McSorley E.. (2005). The remote distractor effect in saccade programming: Channel interactions and lateral inhibition. Vision Research, 45 9, 1177– 1190. [DOI] [PubMed] [Google Scholar]
- Marino R. A., Munoz D. P.. (2009). The effects of bottom-up target luminance and top-down spatial target predictability on saccadic reaction times. Experimental Brain Research, 197 4, 321– 335. [DOI] [PubMed] [Google Scholar]
- Marino R. A., Trappenberg T. P., Dorris M. C., Munoz D. P.. (2012). Spatial interactions in the superior colliculus predict saccade behavior in a neural field model. Journal of Cognitive Neuroscience, 24 2, 315– 336. [DOI] [PubMed] [Google Scholar]
- Maylor E., Hockey R.. (1985). Inhibitory component of externally controlled covert orienting in visual space. Journal of Experimental Psychology. Human Perception and Performance, 11 6, 777– 787. [DOI] [PubMed] [Google Scholar]
- McPeek R. M. (2008). Reversal of a distractor effect on saccade target selection after superior colliculus inactivation. Journal of Neurophysiology, 99 5, 2694– 2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPeek R. M., Han J. H., Keller E. L.. (2003). Competition between saccade goals in the superior colliculus produces saccade curvature. Journal of Neurophysiology, 89 5, 2577– 2590. [DOI] [PubMed] [Google Scholar]
- McSorley E., Findlay J. M.. (2003). Saccade target selection in visual search: Accuracy improves when more distractors are present. Journal of Vision, 3(11): 20, 877– 892, doi:10.1167/3.11.20 [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- McSorley E., McCloy R., Lyne C.. (2012). The spatial impact of visual distractors on saccade latency. Vision Research, 60, 61– 72. [DOI] [PubMed] [Google Scholar]
- Meredith M. A., Ramoa A. S.. (1998). Intrinsic circuitry of the superior colliculus: Pharmacophysiological identification of horizontally oriented inhibitory interneurons. Journal of Neurophysiology, 79 3, 1597– 1602. [DOI] [PubMed] [Google Scholar]
- Mize R. R., Jeon C. J., Hamada O. L., Spencer R. F.. (1991). Organization of neurons labeled by antibodies to gamma-aminobutyric acid (GABA) in the superior colliculus of the Rhesus monkey. Visual Neuroscience, 6 1, 75– 92. [DOI] [PubMed] [Google Scholar]
- Moschovakis A. K., Scudder C. A., Highstein S. M.. (1996). The microscopic anatomy and physiology of the mammalian saccadic system. Progress in Neurobiology, 50 2–3, 133– 254. [DOI] [PubMed] [Google Scholar]
- Munoz D. P., Dorris M. C., Paré M., Everling S.. (2000). On your mark, get set: Brainstem circuitry underlying saccadic initiation. Canadian Journal of Physiology and Pharmacology, 78 11, 934– 944. [PubMed] [Google Scholar]
- Munoz D. P., Fecteau J. H.. (2002). Vying for dominance: Dynamic interactions control visual fixation and saccadic initiation in the superior colliculus. Progress in Brain Research, 140, 3– 19. [DOI] [PubMed] [Google Scholar]
- Munoz D. P., Istvan P. J.. (1998). Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. Journal of Neurophysiology, 79 3, 1193– 1209. [DOI] [PubMed] [Google Scholar]
- Munoz D. P., Wurtz R. H.. (1993). Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. Journal of Neurophysiology, 70 2, 576– 589. [DOI] [PubMed] [Google Scholar]
- Olivier E., Dorris M. C., Munoz D. P.. (1999). Lateral interactions in the superior colliculus, not an extended fixation zone, can account for the remote distractor effect. Behavioral and Brain Sciences, 22, 694– 695. [Google Scholar]
- Orban G. A., Van Essen D., Vanduffel W.. (2004). Comparative mapping of higher visual areas in monkeys and humans. Trends in Cognitive Sciences, 8 7, 315– 324. [DOI] [PubMed] [Google Scholar]
- Ottes F. P., Van Gisbergen J. A., Eggermont J. J.. (1986). Visuomotor fields of the superior colliculus: A quantitative model. Vision Research, 26 6, 857– 873. [DOI] [PubMed] [Google Scholar]
- Patel S. S., Peng X., Sereno A. B.. (2010). Shape effects on reflexive spatial selective attention and a plausible neurophysiological model. Vision Research, 50 13, 1235– 1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli D. G. (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10 4, 437– 442. [PubMed] [Google Scholar]
- Phongphanphanee P., Kaneda K., Isa T.. (2008). Spatiotemporal profiles of field potentials in mouse superior colliculus analyzed by multichannel recording. The Journal of Neuroscience, 28 37, 9309– 9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phongphanphanee P., Marino R. A., Kaneda K., Yanagawa Y., Munoz D. P., Isa T.. (2014). Distinct local circuit properties of the superficial and intermediate layers of the rodent superior colliculus. The European Journal of Neuroscience, 40 2, 2329– 2343. [DOI] [PubMed] [Google Scholar]
- Posner M. I., Cohen Y.. (1984). Components of visual orienting. Bouma H., Bouwhuis D. G. (Eds.) Attention and Performance X (pp 531– 556). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Posner, M. I., Rafal R. D., Choate L. S., Vaughan J.. (1985). Inhibition of return: Neural basis and function. Cognitive Neuropsychology, 2 3, 211– 228. [Google Scholar]
- Pratt J., Hirshhorn M.. (2003). Examining the time course of facilitation and inhibition with simultaneous onset and offset cues. Psychological Research, 67 4, 261– 265. [DOI] [PubMed] [Google Scholar]
- Pratt J., Neggers B.. (2008). Inhibition of return in single and dual tasks: Examining saccadic, keypress, and pointing responses. Perception & Psychophysics, 70 2, 257– 265. [DOI] [PubMed] [Google Scholar]
- Previc F. (1990). Functional specialization in the lower and upper visual fields in humans: Its ecological origins and implications. Behavioral and Brain Sciences, 13 3, 519– 575. [Google Scholar]
- Prinzmetal W., Taylor J. A., Myers L. B., Nguyen-Espino J.. (2011). Contingent capture and inhibition of return: A comparison of mechanisms. Experimental Brain Research, 214 1, 47– 60. [DOI] [PubMed] [Google Scholar]
- Rafal R. D. (2006). Oculomotor functions of the parietal lobe: Effects of chronic lesions in humans. Cortex, 42 5, 730– 739. [DOI] [PubMed] [Google Scholar]
- Rafal R. D., Egly R., Rhodes D.. (1994). Effects of inhibition of return on voluntary and visually guided saccades. Canadian Journal of Experimental Psychology, 48 2, 284– 300. [DOI] [PubMed] [Google Scholar]
- Rafal R. D., Posner M. I., Friedman J. H., Inhoff A. W., Bernstein E.. (1988). Orienting of visual attention in progressive supranuclear palsy. Brain, 111, 267– 280. [DOI] [PubMed] [Google Scholar]
- Reingold E. M., Stampe D. M.. (2002). Saccadic inhibition in voluntary and reflexive saccades. Journal of Cognitive Neuroscience, 14 3, 371– 388. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P. A., Rosenquist J. N.. (1996). Auditory cues and inhibition of return: The importance of oculomotor activation. Experimental Brain Research, 112, 119– 126. [DOI] [PubMed] [Google Scholar]
- Ro T., Pratt J., Rafal R. D.. (2000). Inhibition of return in saccadic eye movements. Experimental Brain Research, 130 2, 264– 268. [DOI] [PubMed] [Google Scholar]
- Ro T., Rafal R. D.. (1999). Components of reflexive visual orienting to moving objects. Perception & Psychophysics, 61 5, 826– 836. [DOI] [PubMed] [Google Scholar]
- Robinson D. L., McClurkin J. W.. (1989). The visual superior colliculus and pulvinar. Reviews of Oculomotor Research, 3, 337– 360. [PubMed] [Google Scholar]
- Ross L. E., Ross S. M.. (1980). Saccade latency and warning signals: Stimulus onset, offset, and change as warning events. Perception & Psychophysics, 27 3, 251– 257. [DOI] [PubMed] [Google Scholar]
- Sapir A., Soroker N., Berger A., Henik A.. (1999). Inhibition of return in spatial attention: Direct evidence for collicular generation. Nature Neuroscience, 2 12, 1053– 1054. [DOI] [PubMed] [Google Scholar]
- Satel J., Wang Z., Trappenberg T. P., Klein R. M.. (2011). Modeling inhibition of return as short-term depression of early sensory input to the superior colliculus. Vision Research, 51 9, 987– 996. [DOI] [PubMed] [Google Scholar]
- Schall J. D., Bichot N. P.. (1998). Neural correlates of visual and motor decision processes. Current Opinion in Neurobiology, 8 2, 211– 217. [DOI] [PubMed] [Google Scholar]
- Schneider K., Kastner S.. (2005). Visual responses of the human superior colliculus: A high-resolution functional magnetic resonance imaging study. Journal of Neurophysiology, 94 4, 2491– 2503. [DOI] [PubMed] [Google Scholar]
- Sereno A. B., Briand K. A., Amador S. C., Szapiel S. V.. (2006). Disruption of reflexive attention and eye movements in an individual with a collicular lesion. Journal of Clinical and Experimental Neuropsychology, 28 1, 145– 166. [DOI] [PubMed] [Google Scholar]
- Sheliga B. M., Craighero L., Riggio L., Rizzolatti G.. (1997). Effects of spatial attention on directional manual and ocular responses. Experimental Brain Research, 114 2, 339– 351. [DOI] [PubMed] [Google Scholar]
- Sheliga B. M., Riggio L., Rizzolatti G.. (1995). Spatial attention and eye movements. Experimental Brain Research, 105 2, 261– 275. [DOI] [PubMed] [Google Scholar]
- Sparks D. L. (1986). Translation of sensory signals into commands for control of saccadic eye movements: Role of primate superior colliculus. Physiological Reviews, 66 1, 118– 171. [DOI] [PubMed] [Google Scholar]
- Sparks D. L., Hartwich-Young R.. (1989). The deep layers of the superior colliculus. Reviews of Oculomotor Research, 3, 213– 255. [PubMed] [Google Scholar]
- Suzuki M., Gottlieb J.. (2013). Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nature Neuroscience, 16 1, 98– 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassinari G., Berlucchi G.. (1995). Covert orienting to non-informative cues: Reaction time studies. Behavioral Brain Research, 71 1-2, 101– 112. [DOI] [PubMed] [Google Scholar]
- Theeuwes J., Kramer A. F., Hahn S., Irwin D. E., Zelinsky G. J.. (1999). Influence of attentional capture on oculomotor control. Journal of Experimental Psychology: Human Perception and Performance, 25 6, 1595– 1608. [DOI] [PubMed] [Google Scholar]
- Tipper S. P., Rafal R., Reuter-Lorenz P. A., Starrveldt Y., Ro T., Egly R., Weaver B.. (1997). Object-based facilitation and inhibition from visual orienting in the human split-brain. Journal of Experimental Psychology, 23 5, 1522– 1532. [DOI] [PubMed] [Google Scholar]
- Trappenberg T. P., Dorris M. C., Munoz D. P., Klein R. M.. (2001). A model of saccade initiation based on the competitive integration of exogenous and endogenous signals in the superior colliculus. Journal of Cognitive Neuroscience, 13 2, 256– 271. [DOI] [PubMed] [Google Scholar]
- Van Gisbergen J. A., Van Opstal A. J., Tax A. A.. (1987). Collicular ensemble coding of saccades based on vector summation. Neuroscience, 21 2, 541– 555. [DOI] [PubMed] [Google Scholar]
- Walker M. F., Fitzgibbon E. J., Goldberg M. E.. (1995). Neurons in the monkey superior colliculus predict the visual result of impending saccadic eye movements. Journal of Neurophysiology, 73 5, 1988– 2003. [DOI] [PubMed] [Google Scholar]
- Walker R., Deubel H., Schneider W. X., Findlay J. M.. (1997). Effect of remote distractors on saccade programming: Evidence for an extended fixation zone. Journal of Neurophysiology, 78 2, 1108– 1119. [DOI] [PubMed] [Google Scholar]
- Walker R., Kentridge R. W., Findlay J. M.. (1995). Independent contributions of the orienting of attention, fixation offset and bilateral stimulation on human saccadic latencies. Experimental Brain Research, 103 2, 294– 310. [DOI] [PubMed] [Google Scholar]
- Walker R., McSorley E., Haggard P.. (2006). The control of saccade trajectories: Direction of curvature depends on prior knowledge of target location and saccade latency. Perception & Psychophysics, 68 1, 129– 138. [DOI] [PubMed] [Google Scholar]
- Weber H., Aiple F., Fischer B., Latanov A.. (1992). Dead zone for express saccades. Experimental Brain Research, 89 1, 214– 222. [DOI] [PubMed] [Google Scholar]
- Wenban-Smith M. G., Findlay J. M.. (1991). Express saccades: Is there a separate population in humans? Experimental Brain Research, 87 1, 218– 222. [DOI] [PubMed] [Google Scholar]
- White B. J., Gegenfurtner K. R., Kerzel D.. (2005). Effects of structured nontarget stimuli on saccadic latency. Journal of Neurophysiology, 93 6, 3214– 3223. [DOI] [PubMed] [Google Scholar]
- White B. J., Marino R. A., Boehnke S. E., Itti L., Theeuwes J., Munoz D. P.. (2013). Competitive integration of visual and goal-related signals on neuronal accumulation rate: A correlate of oculomotor capture in the superior colliculus. Journal of Cognitive Neuroscience, 25 10, 1754– 1768. [DOI] [PubMed] [Google Scholar]
- Wilimzig C., Schneider S., Schöner G.. (2006). The time course of saccadic decision making: Dynamic field theory. Neural Networks, 19 8, 1059– 1074. [DOI] [PubMed] [Google Scholar]
- Wurtz R. H., Optican L. M.. (1994). Superior colliculus cell types and models of saccade generation. Current Opinion in Neurobiology, 4 6, 857– 861. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Itti L., Berg D. J., Ikeda T., Kato R., Takaura K., Isa T.. (2012). Residual attention guidance in blindsight monkeys watching complex natural scenes. Current Biology, 22 15, 1429– 34. [DOI] [PubMed] [Google Scholar]


