Abstract
The well-known detrimental effects of cadmium (Cd) on plants are chloroplast destruction, photosynthetic pigment inhibition, imbalance of essential plant nutrients, and membrane damage. Jasmonic acid (JA) is an alleviator against different stresses such as salinity and drought. However, the functional attributes of JA in plants such as the interactive effects of JA application and Cd on rapeseed in response to heavy metal stress remain unclear. JA at 50 μmol/L was observed in literature to have senescence effects in plants. In the present study, 25 μmol/L JA is observed to be a “stress ameliorating molecule” by improving the tolerance of rapeseed plants to Cd toxicity. JA reduces the Cd uptake in the leaves, thereby reducing membrane damage and malondialdehyde content and increasing the essential nutrient uptake. Furthermore, JA shields the chloroplast against the damaging effects of Cd, thereby increasing gas exchange and photosynthetic pigments. Moreover, JA modulates the antioxidant enzyme activity to strengthen the internal defense system. Our results demonstrate the function of JA in alleviating Cd toxicity and its underlying mechanism. Moreover, JA attenuates the damage of Cd to plants. This study enriches our knowledge regarding the use of and protection provided by JA in Cd stress.
Keywords: Rapeseed, Cadmium, Jasmonic acid, Antioxidant enzyme, Malondialdehyde, Ultrastructure
1. Introduction
The last few decades have been significant in terms of the studies conducted on the metal pollutant accumulation in soil. Metal pollutants reduce crop productivity and quality and are extremely dangerous to the food chain. Moreover, they disturb the ecosystem balance and threaten human health (Chen et al., 1999). Anthropogenic activities, including chemical fertilizer application, waste water irrigation, and animal waste, are dominant sources of heavy metal (e.g. cadmium (Cd), plumbum (Pb), zinc (Zn), and copper (Cu)) deposition into the soil (Wu and Zhang, 2010; Jiao et al., 2012).
Cd, which is often applied with phosphorous fertilizers, is among the most hazardous metal pollutants to plants and humans (Pinto et al., 2004). Various strategies, such as split nitrogen application in form of ammonium or urea fertilizer, are being adopted for vegetable cultivation in Cd-contaminated soil (Fan et al., 2017), but still Cd has the highest pollution index in China (Niu et al., 2013) and is known for its carcinogenic, mutagenic, and teratogenic effects on human health. Despite being a non-essential element for plant metabolism, Cd uptake by the roots and transportation to the shoot can easily be accomplished in plants.
Cd is mobile in nature and it can bring physiological, biochemical, and genetic changes in plants. The well-recognized detrimental effects of Cd on plants are: (1) chloroplast structure destruction that leads to the inhibition of photosynthetic pigments and photosynthesis that ultimately decreases growth and yield (Shamsi et al., 2010); (2) irregular homeostasis of essential plant nutrients (e.g. calcium (Ca), magnesium (Mg), iron (Fe), and Zn), thereby leading to nutrient deficiency and eventual plant death (López-Millán et al., 2009); and (3) membrane degradation due to the production of reactive oxygen species (ROS) (Shamsi et al., 2008).
Flowering plants usually have defense responses that protect them from a continuously changing environment. Initially, the cell wall could resist the entry of Cd or other harmful metals into the cells. However, after Cd has crossed the cell wall, plant cells launch other detoxifying mechanisms such as phytochelation and stress protein upregulation against Cd. In addition, plant cells contain superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX), catalase (CAT), glutathione peroxidase (GPX), and glutathione reductase, a group of antioxidant enzymes that actively participate in stress conditions (Larson, 1988). SOD produces hydrogen peroxide (H2O2) from ROS generated during oxidative stress. H2O2 is reduced to H2O and O2 by CAT and GPX (in the cytoplasm and other cellular compartments) or APX (in the ascorbate-glutathione cycle). This enzymatic defense system is augmented by stress signaling molecules that further improve defense system regulation in response to Cd stress. These signaling molecules, including jasmonic acid (JA), nitric oxide, salicylic acid, and ethylene, can directly or indirectly attenuate Cd phytotoxicity. In China, especially in the Zhejiang Province, rapeseed and rice are grown in rotation. Irrigation with contaminated water greatly pollutes the fields with heavy metals, especially Cd, that affect crops and pose serious threats to human health (Meng et al., 2009). Cd is one of the most toxic elements worldwide and has the highest pollution index in China (Niu et al., 2013).
JA is a natural plant hormone involved in many biological processes (Creelman and Mullet, 1997). The intensive research in stress physiology revealed its role as a signaling or stress-modulating molecule against various environmental stresses. Currently, JA is receiving much attention due to its multifunctional defense properties in plants against several abiotic stresses, including salinity (Tsonev et al., 1998), drought (Creelman and Mullet, 1997), and herbicides (Kaya and Doganlar, 2016). However, studies of its role in plants under heavy metal stress are few (Chen et al., 2014), especially regarding the interactive effects of Cd and JA application on rapeseed oil. Thus, the aim of present study was to know: (1) the effects of exogenous JA application in rapeseed genotypes under Cd stress; (2) whether JA enhances gas exchange by protecting the chloroplast against oxidative stress and thereby keeping the ionic balance by reducing Cd uptake to the plant shoots; and (3) whether JA application modulates the antioxidant enzymes that can reduce and/or negate the detrimental effects of Cd on rapeseed oil.
2. Materials and methods
2.1. Soil properties
The soil collected from the experimental field of Huajiachi Campus, Zhejiang University, Hangzhou, China, was air-dried at room temperature with a water content of around 8%‒10% with regular mixing. Approximately 8 kg of ground and sieved soil was loaded into a plastic pot (10 L, 20 cm height) to the brim. The physicochemical properties were the same as in our previous experiment (Ali et al., 2015).
2.2. Plant materials and experimental treatments
The pot experiment was done in the experimental wire house in Zhejiang University under natural light conditions. This study was conducted on three rapeseed genotypes (Zheshuang-72 (ZS72), Zhejiang-619 (ZJ619), and Zheshuang-758 (ZS758)) that were characterized and certified in different decades (Guan et al., 2012; Hussain et al., 2013, 2014).
Seeds were initially grown in trays containing a growing medium of 1/2 compost, 1/4 vermiculite, and 1/4 sand (v/v/v). Seedlings were kept at low temperature in the growth chamber for a month to provide vernalization treatment. Morphologically homogenous seedlings were selected and transplanted into pots for further investigation. The experiment used a completely randomized design with three replicates. Five seedlings per pot per treatment per replication were grown initially and only two healthy seedlings per pot were kept until the end of the experiment. After two weeks of acclimatization, treatments were applied as follows: (1) control (untreated), (2) 75 mg/kg Cd, (3) 150 mg/kg Cd, (4) 300 mg/kg Cd, (5) 25 μmol/L JA, (6) 75 mg/kg Cd+25 μmol/L JA foliar spray, (7) 150 mg/kg Cd+25 μmol/L JA foliar spray, and (8) 300 mg/kg Cd+25 μmol/L JA foliar spray. CdCl2 was used as a source of Cd and applied only once to the soil. Exogenous applications of JA (25 μmol/L) and distilled water containing acetone (control) were made four times with an interval of 3 d per application using a foliar spray. For the foliar spray, JA solution was prepared by dissolving JA in 100 μl of absolute ethanol and a concentration of 25 μmol/L was achieved with the appropriate volume of distilled water. The control solution contained acetone dissolved in water (Thaler et al., 1999). Data on physiological parameters were collected after a week of the last treatment. Leaf samples were taken per treatment and immediately stored at −80 °C for further analysis.
2.3. Measurement of leaf gas exchange
Net photosynthetic rate (P n), stomatal conductance (G s), intercellular CO2 concentration (C i), and transpiration rate (T r) were measured in the leaves of rapeseed genotypes using an infrared gas analyzer and portable photosynthesis system (LI-COR 6400, Lincoln, NE, USA) on a clear sunny morning, with an air temperature of 25 to 30 °C, CO2 concentration of 400 μmol/mol, relative air humidity of 80%–90%, and photosynthetic photon flux density (PPFD) of 1000 μmol/(m2∙s).
2.4. Analysis of photosynthetic pigments in leaves
Chlorophyll (chl a and chl b) content and carotenoid content were analyzed following the method of Wang et al. (2009). Briefly, 0.1 g of fresh leaf sample was sliced with scissors to provide maximum contact with the extract (acetone, ethanol, and distilled water; 4.5:4.5:1, v/v/v). The samples were placed in a glass tube containing the extract and stored at 4 °C for 48 h. After that, the solution absorbance was recorded at 645 and 663 nm spectrophotometrically.
2.5. Analyses of malondialdehyde content and antioxidant enzyme activity
The leaf sample (0.5 g) was ground and homogenized in 8 ml ice-cold 50 mmol/L phosphate buffer solution (PBS, pH 7.8) containing Na2HPO4∙12H2O (16.385 g/L)+NaH2PO4∙2H2O (0.663 g/L). The homogenized solution was centrifuged at 4 °C for 15 min at 12 000 r/min. The supernatant of each sample was collected and stored at 4 °C before being used to determine the malondialdehyde (MDA) content and antioxidant enzyme activity.
MDA was analyzed according to the method described by Hodges et al. (1999). In brief, a solution containing 5% (v/v) trichloracetic acid with 2.5 g of thiobarbituric acid and enzyme extract was heated in a hot water bath at 95 °C for 15 min and cooled immediately on ice. To collect the supernatant, the samples were centrifuged at 4800 r/min for 10 min and measured spectrophotometrically at 532 nm (E 532=0.155 L/(mol∙cm)). Non-specific turbidity was corrected by subtracting the absorbance index obtained at 600 nm.
SOD (EC 1.15.1.1) analysis was done according to Giannopolitis and Ries (1977) as follows: one unit of SOD activity is the amount of enzyme required to inhibit 50% of p-nitro blue tetrazolium chloride photoreduction at 560 nm. POD (EC 1.11.1.7) activity and CAT (EC 1.11.1.6) activity were measured according to the methods of Cakmak and Marschner (1992). POD activity was determined using the increasing absorbance value of a solution, containing 50 mmol/L PBS (pH 7.8), 1.5 mmol/L guaiacol, 300 mmol/L H2O2, and enzyme extract, at 470 nm from guaiacol oxidation (E 470=26.6 L/(mol∙cm)). CAT activity was measured by using the reduction in the absorbance index of a reaction mixture (50 mmol/L PBS (pH 7.8), 300 mmol/L H2O2, and the enzyme extract) caused by the decomposition of H2O2 (E 240=39.4 L/(mol∙cm)) at 240 nm. APX (EC 1.11.1.11) activity was measured by analyzing a reaction solution containing 50 mmol/L PBS (pH 7.8), 7.5 mmol/L ascorbate, 300 mmol/L H2O2, and enzyme extract, following the method of Nakano and Asada (1981). This measurement is technically based on the principle of monitoring ascorbic acid oxidation by H2O2 at 290 nm (E 290=2.8 L/(mol∙cm)).
2.6. Analysis of nutrient elements
Oven-dried (65 to 70 °C for 72 h) leaf samples were ground, weighed, and placed in a muffle furnace to be incinerated for 12 h at 500 °C. The ash was digested with 5 ml of 30% HNO3, and diluted using deionized H2O. A flame atomic absorption spectrometer (Shimadzu, AA-6300, Kyoto, Japan) was used to analyze the concentrations of Cd and other mineral elements including Ca, Mg, Fe, and Zn.
2.7. Subcellular analysis using transmission electron microscopy
For subcellular analysis, transmission electron microscopy (TEM) studies were conducted on the fresh leaf sections (about 1 mm2) from the middle of the topmost leaf in all three rapeseed genotypes. The assay was done as follows: (1) fixation of the leaf sample in 4% (v/v) glutaraldehyde in 0.2 mol/L PBS (pH 7.2) overnight; (2) postfixation in 1% (0.01 g/ml) osmium tetroxide (OsO4) for 1 h; (3) washing in 0.2 mol/L PBS (pH 7.2) for 1–2 h; (4) dehydration in a graded ethanol series (50%, 60%, 70%, 80%, 90%, 95%, and 100%) followed by acetone (100%); (5) filtration of the samples and embedding in Spurr’s resin; and finally, (6) preparation of ultra-thin sections (80 nm) and mounting of the samples on copper grids for visualization under TEM (JEOL TEM-1230 EX) at an accelerating voltage of 60.0 kV.
2.8. Statistical analysis
MSTAT-C software for DOS (MSTATC version 2.10, 1989) was used for statistical analyses. The data were mainly presented as mean±standard error (SE) and analyzed using the one-way analysis of variance (ANOVA) technique. The significance levels considered were P≤0.01 or P≤0.05. Summaries of the analyses are given in Tables 1 and 2. To conduct multiple comparisons of the significant means, the least significant difference (LSD) test was applied (Steel and Torrie, 1980). OriginPro v7.5 (OriginLab, Northampton, MA) was used to design the graphs and allot the SE bars.
Table 1.
Analysis of variance for gas exchange, pigment composition, and MDA content
| Treatment | DF | P n | G s | C i | T r | Chl a | Chl b | Chl a+b | Car | MDA |
| G | 2 | ** | ** | ns | ** | ** | ** | ** | ** | ** |
| Cd | 3 | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| JA | 1 | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| G×Cd | 6 | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| G×JA | 2 | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Cd×JA | 3 | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| G×Cd×JA | 6 | ns | ns | ns | ns | ns | ns | ns | ns | ns |
G: genotypes; Cd: cadmium; JA: jasmonic acid; DF: degree of freedom; P n: photosynthetic rate; G s: stomatal conductance; C i: intercellular CO2 concentration; T r: transpiration rate; Chl a: chlorophyll a; Chl b: chlorophyll b; Chl a+b: chlorophyll a+chlorophyll b; Car: carotenoid; MDA: malondialdehyde; ns: not significant;
: significant at P≤0.01
Table 2.
Analysis of variance of antioxidant enzyme activity, Cd accumulation, and essential elements
| Treatment | DF | CAT | APX | POD | SOD | Cd | Ca | Mg | Zn | Fe |
| G | 2 | ** | ** | ns | ** | ** | ** | ** | ns | ** |
| Cd | 3 | ns | ** | ** | ** | ** | ** | ** | ** | ** |
| JA | 1 | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| G×Cd | 6 | ns | ns | ns | ** | ** | ns | ns | ns | ** |
| G×JA | 2 | ** | ns | ns | ns | * | ns | ** | ns | ** |
| Cd×JA | 3 | ** | * | ** | ** | ** | ** | ns | ns | ** |
| G×Cd×JA | 6 | ns | ns | ns | ns | ns | ns | ns | ns | ** |
G: genotypes; Cd: cadmium; JA: jasmonic acid; DF: degree of freedom; CAT: catalase; APX: ascorbate peroxidase; POD: peroxidase; SOD: superoxide dismutase; Cd: cadmium; Ca: calcium; Mg: magnesium; Zn: zinc; Fe: iron; ns: not significant;
: significant at P≤0.05;
: significant at P≤0.01
3. Results
3.1. Leaf gas exchange under Cd and JA application
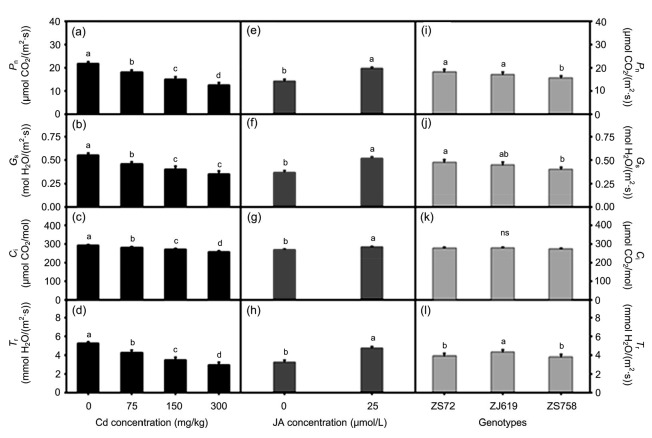
To assess the sole and interactive effects of Cd, JA, and genotypes on photosynthesis, the leaf gas exchange parameters were measured (Figs. 1 and 2). Increased Cd levels resulted in decreased gas exchange in leaves. For instance 42.56%, 35.99%, 11.87%, and 43.43% decreases are shown by P n, G s, C i, and T r, respectively, at 300 mg/kg Cd (Figs. 1a‒1d). Significant differences were observed in all the genotypes for gas exchange except for C i (Figs. 1i‒1l). In contrast to Cd, a sole application of JA positively regulated all gas exchange parameters. Increases of 38.39% (P n), 40.60% (G s), 5.75% (C i), and 45.36% (T r) took place after JA application compared with those in the untreated plants (Figs. 1e‒1h).
Fig. 1.
Sole effects of genotypes, cadmium, and jasmonic acid on leaf gas exchange in rapeseed
(a‒d) Cadmium; (e‒h) Jasmonic acid; (i‒l) Genotypes. (a, e, i) P n: photosynthetic rate; (b, f, j) G s: stomatal conductance; (c, g, k) C i: intercellular CO2 concentration; (d, h, l) T r: transpiration rate. Cd: cadmium; JA: jasmonic acid; ZS72: Zheshuang-72; ZJ619: Zhejiang-619; ZS758: Zheshuang-758. Differently lettering indicates statistical difference (P≤0.01) among the treatments for each parameter; ns: not significant. Data represent the mean±SE of three measurements
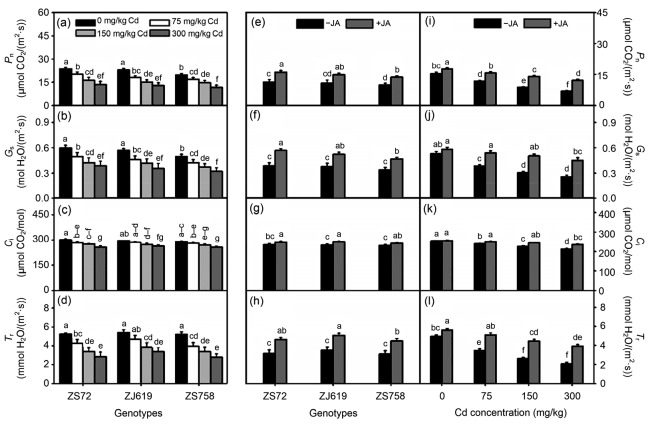
Fig. 2.
Interactive effects of genotypes, cadmium, and jasmonic acid on leaf gas exchange in rapeseed
(a‒d) Genotype×cadmium; (e‒h) Genotype×jasmonic acid; (i‒l) jasmonic acid×cadmium. (a, e, i) P n: photosynthetic rate; (b, f, j) G s: stomatal conductance; (c, g, k) C i: intercellular CO2 concentration; (d, h, l) T r: transpiration rate. Cd: cadmium; JA: jasmonic acid; ZS72: Zheshuang-72; ZJ619: Zhejiang-619; ZS758: Zheshuang-758. Differently lettering indicates statistical difference (P≤0.01) among the treatments for each parameter. Data represent the mean±SE of three measurements
Interactive effects of genotypes with Cd and JA application were significantly different among genotypes regarding gas exchange parameters in different Cd levels (Figs. 2a‒2d) and JA application (Figs. 2e‒2h). The largest decrease was shown by rapeseed genotypes at the highest Cd level (300 mg/kg). For instance, at 300 mg/kg Cd, ZS72, ZJ619, and ZS758 decreased by 42.88%, 43.94%, and 40.57% in P n, respectively (Fig. 2a); 35.09%, 37.42%, and 35.53% in G s, respectively (Fig. 2b); 14.00%, 10.45%, and 11.11% in C i, respectively (Fig. 2c); and 46.28%, 37.82%, and 46.55% in T r, respectively (Fig. 2d). Moreover, JA application increased the levels of all the gas exchange parameters. For ZS72, after JA application, compared with the control, P n, G s, C i, and T r increased by 42.00%, 45.94%, 5.45%, and 46.85%, respectively. Similarly, JA improved gas exchange in terms of JA and Cd interaction (Figs. 2i‒2l). Compared with their respective controls, JA application on leaves under 300 mg/kg Cd improved P n, G s, C i, and T r by 75.21%, 75.83%, 10.97%, and 89.37%, respectively (Figs. 2i‒2l).
3.2. Role of JA on leaf pigment composition under Cd stress
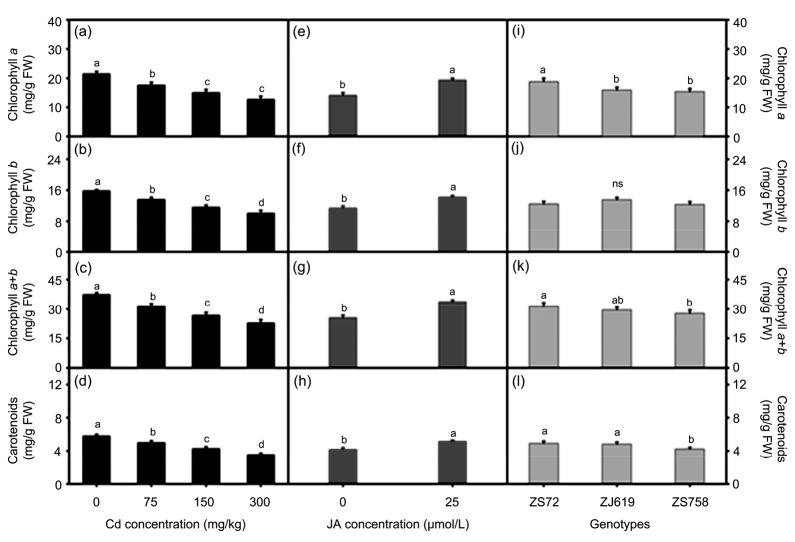
Sole effects of Cd, JA, and genotypes on photosynthetic pigment composition are shown in Fig. 3. Cd negatively regulated the photosynthetic pigments in all concentrations, but an obvious decrease was observed with 300 mg/kg Cd. The average decreases in chl a (30.04% and 40.90%), chl b (26.23% and 35.91%), chl a+chl b (28.43% and 38.78%), and carotenoids (26.27% and 39.24%) were recorded at 75 and 300 mg/kg Cd (Figs. 3a‒3d); however, JA application relatively increased chl a by 37.35%, chl b by 25.06%, chl a+chl b by 31.86%, and carotenoids by 23.50% compared with their respective controls (Figs. 3e‒3h). Genotypic differences were found in rapeseed genotypes based on photosynthetic pigment composition (Figs. 3i‒3l). Except for chl b (Fig. 3j), all genotypes were significantly different in their photosynthetic pigments.
Fig. 3.
Sole effects of genotypes, cadmium, and jasmonic acid on leaf photosynthetic pigments in rapeseed
(a‒d) Cadmium; (e‒h) Jasmonic acid; (i‒l) Genotypes. (a, e, i) Chlorophyll a; (b, f, j) Chlorophyll b; (c, g, k) Chlorophyll a+b; (d, h, l) Carotenoids. Cd: cadmium; JA: jasmonic acid; ZS72: Zheshuang-72; ZJ619: Zhejiang-619; ZS758: Zheshuang-758. Differently lettering indicates statistical difference (P≤0.01) among the treatments for each parameter; ns: not significant. Data represent the mean±SE of three measurements
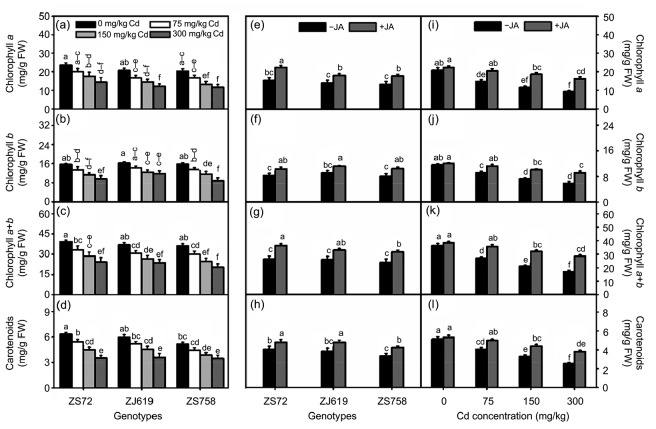
Interaction among genotypes, Cd, and JA is shown in Fig. 4. As Cd concentration increased, a significant decrease was noticed in all genotype pigments (Figs. 4a‒4d). However, JA application in genotypes and JA interaction showed a positive and significant effect. The average increases of 46.95%, 25.13%, 37.74%, and 18.80% in ZS72, 30.13%, 21.94%, 26.27%, and 25.40% in ZJ619, and 33.99%, 28.54%, 31.47%, and 27.00% in ZS758 were recorded for chl a, chl b, chl a+b, and carotenoids, respectively, after JA application compared with their respective controls (Figs. 4e‒4h). Moreover, JA rectified the deleterious effects of Cd on pigments in co-applications of Cd and JA. For instance, under 300 mg/kg Cd application, average increases of 75.40% (chl a), 54.51% (chl b), 65.67% (chl a+b), and 51.06% (carotenoids) were noticed after foliar JA application (Figs. 4i‒4l).
Fig. 4.
Interactive effects of genotypes, cadmium, and jasmonic acid on leaf photosynthetic pigments in rapeseed
(a‒d) Genotype×cadmium; (e‒h) Genotype×jasmonic acid; (i‒l) Jasmonic acid×cadmium. (a, e, i) Chlorophyll a; (b, f, j) Chlorophyll b; (c, g, k) Chlorophyll a+b; (d, h, l) Carotenoids. Cd: cadmium; JA: jasmonic acid; ZS72: Zheshuang-72; ZJ619: Zhejiang-619; ZS758: Zheshuang-758. Differently lettering indicates statistical difference (P≤0.01) among the treatments for each parameter. Data represent the mean±SE of three measurements
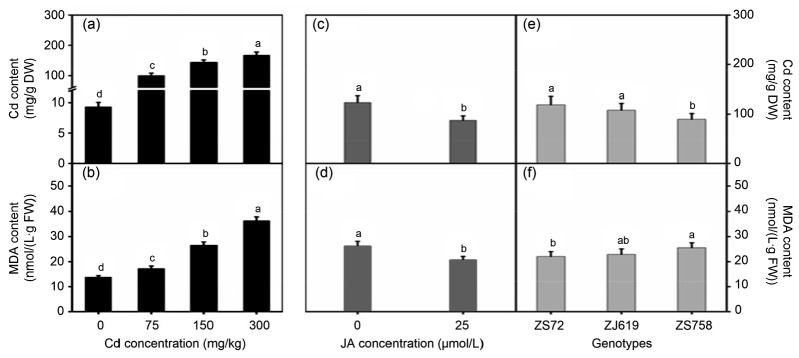
3.3. Decrease in Cd accumulation and MDA content by JA treatment under Cd stress
Fig. 5 shows the sole effects of Cd concentration, genotype, and JA levels on the Cd accumulation and MDA content in rapeseed leaves. As Cd concentration increased, Cd accumulation and MDA content in leaves were increased. For instance, average increases of 100.67, 144.73, and 167.59 mg/g DW in Cd accumulation and 24.93%, 92.46%, and 162.80% in MDA content were observed at 75, 150, and 300 mg/kg Cd, respectively (Figs. 5a and 5b). In contrast to Cd treatment, JA application decreased both Cd accumulation and MDA content in rapeseed leaves at an average of 29.12% (Cd) and 20.92% (MDA) compared with their respective controls (Figs. 5c and 5d). Moreover, genotypes showed significant differences in Cd accumulation and MDA content (Figs. 5e and 5f).
Fig. 5.
Sole effects of genotypes, cadmium, and jasmonic acid on Cd accumulation and MDA content in rapeseed
(a, b) Cadmium; (c, d) Jasmonic acid; (e, f) Genotypes. (a, c, e) Cd: cadmium content; (b, d, f) MDA: malondialdehyde content. Cd: cadmium; JA: jasmonic acid; ZS72: Zheshuang-72; ZJ619: Zhejiang-619; ZS758: Zheshuang-758. Differently lettering indicates statistical difference (P≤0.01) among the treatments for each parameter. Data represent the mean±SE of three measurements
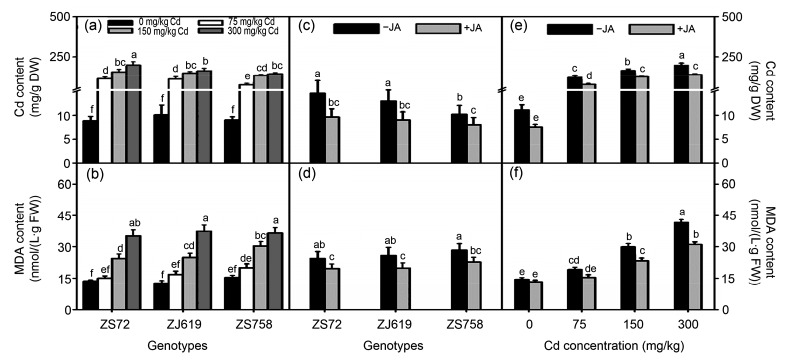
Interactions among Cd concentration, JA levels, and genotypes are shown in Fig. 6. As Cd concentration increased, a substantial increase was observed in the leaf Cd accumulation and MDA content in three rapeseed genotypes. At 300 mg/kg Cd treatment, average increases of 197.78, 162.63, and 142.38 mg/g DW in Cd accumulation were observed in ZS72, ZJ619, and ZS758, respectively (Fig. 6a), whereas 159.05%, 198.98%, and 136.77% increases were recorded for MDA content in ZS72, ZJ619, and ZS758, respectively, compared with the control (Fig. 6b). JA showed its effects by decreasing the Cd accumulation and MDA content in all genotypes. Average decreases of 33.79%, 30.01%, and 21.32% in Cd accumulation, and 19.66%, 23.38%, and 19.77% in MDA content were noticed in ZS72, ZJ619, and ZS758, respectively, after JA application (Figs. 6c and 6d). Interaction between Cd and JA application showed the alleviatory effects of JA over Cd treatments. JA application reduced Cd accumulation by 31.70%, 36.18%, 22.79%, and 29.48% and MDA by 8.18%, 20.06%, 21.83%, and 25.09% at 0, 75, 150, and 300 mg/kg Cd treatments, respectively (Figs. 6e and 6f).
Fig. 6.
Interactive effects of genotypes, cadmium, and jasmonic acid on Cd accumulation and MDA content in rapeseed
(a, b) Genotype×cadmium; (c, d) Genotype×jasmonic acid; (e, f) Jasmonic acid×cadmium. (a, c, e) Cd: cadmium content; (b, d, f) MDA: malondialdehyde content. Cd: cadmium; JA: jasmonic acid; ZS72: Zheshuang-72; ZJ619: Zhejiang-619; ZS758: Zheshuang-758. Differently lettering indicates statistical difference (P≤0.01) among the treatments for each parameter. Data represent the mean±SE of three measurements
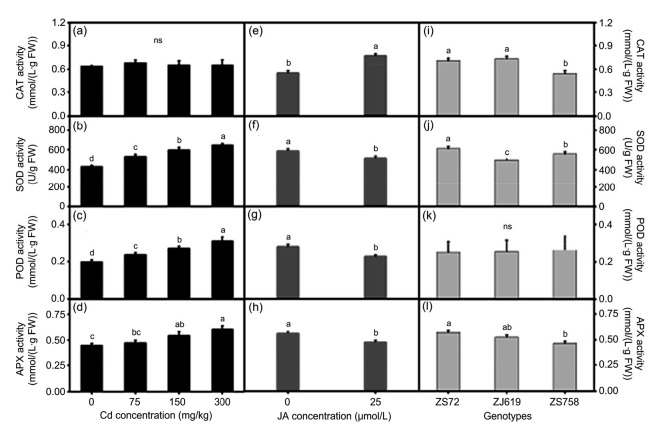
3.4. Effects of JA application on antioxidant enzyme activity under Cd stress
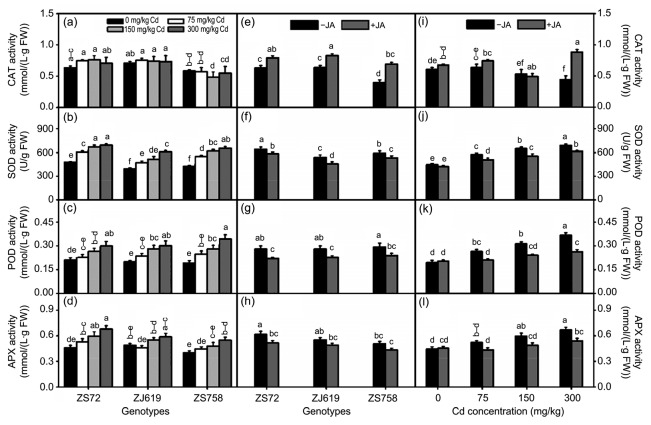
Antioxidant enzymes mainly protect plants from stress. To keep the ionic balance in stressful environments, these enzymes exhibit different increasing and decreasing patterns. Sole and interactive effects of Cd, JA, and genotypes are shown in Figs. 7 and 8. Cd application significantly enhanced antioxidant enzyme activity in a dose-dependent manner, except for CAT (Figs. 7a‒7d). JA application not only increased CAT activity but also decreased SOD, POD, and APX activity (Figs. 7e‒7h). Moreover, different responses of genotypes to JA application were observed in all antioxidant enzyme activity, except in POD (Figs. 7i‒7l). The interaction between genotypes and Cd showed that CAT activity remained non-significant in genotypes ZS72, ZJ619, and ZS758, compared with the control. Other enzymes (e.g. SOD, POD, and APX) showed an increasing trend in response to increased Cd concentration. Average increases of 44.61%, 53.54%, and 53.25% in SOD, 42.85%, 52.14%, and 79.08% in POD, and 47.65%, 20.47%, and 37.07% in APX activity were noted in ZS72, ZJ619, and ZS758, respectively, at 300 mg/kg Cd (Figs. 8a‒8d). In genotype and JA interaction, JA treatment decreased all enzyme activity except for CAT, whose activity is increased after JA application in rapeseed genotypes (Figs. 8e‒8h). JA decreased SOD, POD, and APX activity when co-applied with Cd and increased CAT activity in all applied Cd levels except 150 mg/kg. Average decreases of 10.74% (SOD), 28.19% (POD), and 18.97% (APX) were noted at 300 mg/kg after JA application (Figs. 8i‒8l).
Fig. 7.
Sole effects of genotypes, cadmium, and jasmonic acid on leaf antioxidant enzyme activity in rapeseed
(a–d) Cadmium; (e–h) Jasmonic acid; (i–l) Genotypes. (a, e, i) CAT: catalase; (b, f, j) SOD: superoxide dismutase; (c, g, k) POD: peroxidase; (d, h, l) APX: ascorbate peroxidase. Cd: cadmium; JA: jasmonic acid; ZS72: Zheshuang-72; ZJ619: Zhejiang-619; ZS758: Zheshuang-758. Differently lettering indicates statistical difference (P≤0.01) among the treatments for each parameter; ns: not significant. Data represent the mean±SE of three measurements
Fig. 8.
Interactive effects of genotypes, cadmium, and jasmonic acid on leaf antioxidant enzyme activity in rapeseed
(a–d) Genotype×cadmium; (e–h) Genotype×jasmonic acid; (i–l) Jasmonic acid×cadmium. (a, e, i) CAT: catalase; (b, f, j) SOD: superoxide dismutase; (c, g, k) POD: peroxidase; (d, h, l) APX: ascorbate peroxidase. Cd: cadmium; JA: jasmonic acid; ZS72: Zheshuang-72; ZJ619: Zhejiang-619; ZS758: Zheshuang-758. Differently lettering indicates statistical difference (P≤0.01) among the treatments for each parameter. Data represent the mean±SE of three measurements
3.5. Mineral elements under JA and Cd treatments
The sole effects of Cd, genotypes, and JA on leaf nutrient elements, except Zn, in rapeseed were found to be significant (Table 3). A significant and negative effect on elements was observed after Cd application.
Table 3.
Sole effects of genotypes, cadmium, and jasmonic acid application on leaf nutrient elements in Brassica napus
| Factor | Ca content (mg/g DW) | Mg content (mg/g DW) | Fe content (mg/g DW) | Zn content (mg/g DW) |
| Genotype | ||||
| ZS72 | 45.10±2.48a | 8.72±0.82a | 0.25±0.01a | 0.21±0.01a |
| ZJ619 | 43.73±2.29a | 6.28±0.46b | 0.24±0.02a | 0.22±0.01a |
| ZS758 | 38.12±2.61b | 4.90±0.26b | 0.18±0.01b | 0.20±0.01a |
| Level of significance | ** | ** | ** | ns |
| Cd concentration (mg/kg) | ||||
| 0 | 53.32±1.53a | 8.73±0.73a | 0.32±0.02a | 0.30±0.01a |
| 75 | 45.66±2.17b | 7.16±0.69b | 0.25±0.02b | 0.23±0.01b |
| 150 | 39.03±2.70c | 5.79±0.64bc | 0.19±0.02c | 0.17±0.01c |
| 300 | 31.26±2.22d | 4.96±0.61c | 0.14±0.02d | 0.13±0.01c |
| Level of significance | ** | ** | ** | ** |
| JA concentration (μmol/L) | ||||
| 0 | 36.09±2.02b | 5.17±0.35b | 0.17±0.02b | 0.17±0.01b |
| 25 | 48.54±1.47a | 8.15±0.55a | 0.27±0.01a | 0.24±0.01a |
| Level of significance | ** | ** | ** | ** |
Ca: calcium; Mg: magnesium; Fe: iron; Zn: zinc; Cd: cadmium; JA: jasmonic acid; ZS72: Zheshuang-72; ZJ619: Zhejiang-619; ZS758: Zheshuang-758. Data represent the mean±SE of three measurements. Differently lettering indicates statistical difference (P≤0.01) in the same column.
Significant at P≤0.01; ns: not significant
Cd treatment decreased all the elements in a dose-dependent manner and the highest decrease was found with 300 mg/kg Cd. In contrast with Cd, JA application significantly increased nutrient elements in rapeseed leaves (Table 3).
The interactive effects of genotypes and Cd levels negatively regulated the mineral elements in leaves. Cd application decreased all the nutrient elements in a dose-dependent manner in all genotypes. The average decreases of 41.24%, 42.58%, 53.13%, and 56.66% in ZS72, 34.78%, 48.19%, 61.09%, and 52.72% in ZJ619, and 48.01%, 37.78%, 52.55%, and 54.55% in ZS758 were noted for Ca, Mg, Fe, and Zn, respectively, at 300 mg/kg Cd (Table 4).
Table 4.
Interactive effects of genotypes and cadmium application on leaf nutrient elements in Brassica napus
| Genotype | Cd (mg/kg) | Ca (mg/g DW) | Mg (mg/g DW) | Fe (mg/g DW) | Zn (mg/g DW) |
| ZS72 | 0 | 57.00±2.00a | 11.60±1.40a | 0.34±0.02ab | 0.30±0.02a |
| 75 | 47.54±3.30bc (−16.62%) | 9.11±1.70ab (−21.93%) | 0.28±0.03bc (−18.84%) | 0.23±0.02abc (−23.33%) | |
| 150 | 42.34±4.90cd (−25.74%) | 7.41±1.50bcd (−36.50%) | 0.22±0.02cde (−33.82%) | 0.18±0.02b–e (−40.00%) | |
| 300 | 33.50±3.30de (−41.24%) | 6.70±1.40b–e (−42.58%) | 0.16±0.02efg (−53.13%) | 0.13±0.02de (−56.66%) | |
| ZJ619 | 0 | 52.90±2.70ab | 8.32±0.50bc | 0.36±0.02a | 0.30±0.02a |
| 75 | 46.73±3.70bc (−11.66%) | 6.92±0.60b–e (−16.82%) | 0.27±0.03bc (−24.87%) | 0.25±0.02ab (−17.41%) | |
| 150 | 41.07±4.50cd (−22.36%) | 5.59±0.80d–f (−32.81%) | 0.18±0.03efg (−49.76%) | 0.19±0.02b–e (−38.05%) | |
| 300 | 34.20±4.00de (−34.78%) | 4.31±0.80ef (−48.19%) | 0.14±0.03fg (−61.09%) | 0.14±0.02cde (−52.72%) | |
| ZS758 | 0 | 50.00±2.80abc | 6.22±0.30c–f | 0.26±0.01cd | 0.29±0.02a |
| 75 | 42.72±4.00cd (−14.64%) | 5.45±0.30d–f (−12.37%) | 0.19±0.03def (−24.68%) | 0.21±0.02bcd (−28.40%) | |
| 150 | 33.68±4.50de (−32.70%) | 4.37±0.40ef (−23.95%) | 0.16±0.02efg (−37.97%) | 0.16±0.02cde (−43.74%) | |
| 300 | 26.00±3.70e (−48.01%) | 3.87±0.50f (−37.78%) | 0.12±0.02g (−52.55%) | 0.13±0.02e (−54.55%) | |
|
| |||||
| Level of significance | ** | ** | ** | ** | |
Cd: cadmium; Ca: calcium; Mg: magnesium; Fe: iron; Zn: zinc; ZS72: Zheshuang-72; ZJ619: Zhejiamg-619; ZS758: Zheshuang-758. Data represent the mean±SE of three measurements (percent changes compared to 0 mg/kg Cd in the same genotype). Differently lettering indicates statistical difference (P≤0.01) in the same column.
Significant at P≤0.01
However, applying JA with Cd mitigated the negative effects of Cd by increasing the nutrient element absorption. After JA application, Ca increased by 35.66%, 56.35%, and 62.02%, and Mg increased by 57.55%, 84.52%, 106.81%, in leaves under 75, 150, and 300 mg/kg Cd, respectively. In addition, Fe and Zn increased by 58.89% and 40.02% at 75 mg/kg Cd, 83.55% and 60.93% at 150 mg/kg Cd, and 139.57% and 89.43% at 300 mg/kg Cd treatment, respectively (Table 5). Moreover, JA increased the mineral elements in rapeseed genotypes. In ZS72, ZJ619, and ZS758, the average increases after JA application were respectively 35.53%, 28.47%, and 40.47% in Ca; 89.70%, 44.07%, and 29.26% in Mg; 52.73%, 59.57%, and 63.15% in Fe; and 36.55%, 34.53%, and 45.43% in Zn (Table 6).
Table 5.
Interactive effects of jasmonic acid and cadmium application on leaf nutrient elements in Brassica napus
| Cd (mg/kg) | JA (μmol/L) | Ca (mg/g DW) | Mg (mg/g DW) | Fe (mg/g DW) | Zn (mg/g DW) |
| 0 | 0 | 51.32±2.20ab | 7.81±0.70ab | 0.29±0.01bc | 0.28±0.02a |
| 25 | 55.32±2.00a (7.80%) | 9.66±1.20a (23.68%) | 0.35±0.02a (21.99%) | 0.31±0.02a (9.27%) | |
| 75 | 0 | 38.75±2.20c | 5.56±0.20cd | 0.19±0.02d | 0.19±0.01cd |
| 25 | 52.57±1.70ab (35.66%) | 8.76±1.10ab (57.55%) | 0.30±0.02ab (58.89%) | 0.27±0.05ab (40.02%) | |
| 150 | 0 | 30.45±2.10d | 4.07±0.20de | 0.13±0.01e | 0.13±0.01de |
| 25 | 47.61±2.00b (56.35%) | 7.51±0.90abc (84.52%) | 0.24±0.01cd (83.55%) | 0.22±0.01bc (60.93%) | |
| 300 | 0 | 23.86±2.00d | 3.23±0.20e | 0.08±0.01e | 0.09±0.01e |
| 25 | 38.66±1.70c (62.02%) | 6.68±0.80bc (106.81%) | 0.20±0.01d (139.57%) | 0.18±0.01cd (89.43%) | |
|
| |||||
| Level of significance | * | ** | ** | ** | |
Cd: cadmium; JA: jasmonic acid; Ca: calcium; Mg: magnesium; Fe: iron; Zn: zinc. Data represent the mean±SE of three measurements (percent changes compared to 0 μmol/L JA at the same Cd concentration). Differently lettering indicates statistical difference (P≤0.01) in the same column.
Significant at P≤0.05;
Significant at P≤0.01
Table 6.
Interactive effects of genotypes and jasmonic acid application on leaf nutrient elements in Brassica napus
| Genotype | JA (μmol/L) | Ca (mg/g DW) | Mg (mg/g DW) | Fe (mg/g DW) | Zn (mg/g DW) |
| ZS72 | 0 | 38.30±3.32cd | 6.00±0.70bc | 0.20±0.02b | 0.18±0.02c |
| 25 | 51.90±2.61a (35.53%) | 11.40±0.90a (89.70%) | 0.30±0.02a (52.73%) | 0.24±0.01a (36.55%) | |
| ZJ619 | 0 | 38.20±3.63cd | 5.10±0.60c | 0.18±0.03bc | 0.19±0.02bc |
| 25 | 49.10±2.11ab (28.47%) | 7.40±0.40b (44.07%) | 0.20±0.02a (59.57%) | 0.25±0.02a (34.53%) | |
| ZS758 | 0 | 31.70±3.73d | 4.30±0.40c | 0.14±0.02c | 0.16±0.02c |
| 25 | 44.50±2.66bc (40.47%) | 5.60±0.20bc (29.26%) | 0.20±0.01b (63.15%) | 0.23±0.02ab (45.43%) | |
|
| |||||
| Level of significance | ** | ** | ** | ** | |
JA: jasmonic acid; Ca: calcium; Mg: magnesium; Fe: iron; Zn: zinc; ZS72: Zheshuang-72; ZJ619: Zhejiang-619; ZS758: Zheshuang-758. Data represent the mean±SE of three measurements (percent changes compared to 0 μmol/L JA in the same genotype). Differently lettering indicates statistical difference (P≤0.01) in the same column.
Significant at P≤0.01
3.6. Subcellular study of rapeseed leaves under JA and Cd treatments
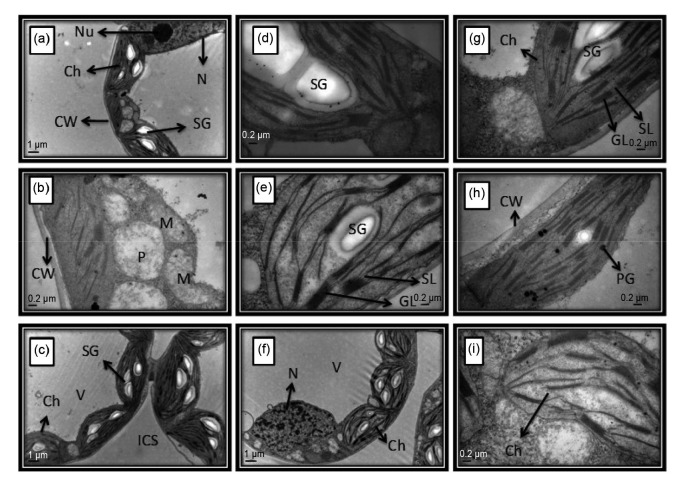
The physiological and biochemical changes observed in the leaves of rapeseed genotypes after Cd and JA treatments are observed at a subcellular level (Fig. 9). In untreated (control) plants, cells were metabolically active in terms of normal chloroplasts with starch granules in the stroma, intact cell walls, and membrane systems, active mitochondria, normal peroxisomes, and typical nuclei with nucleoli. Granal and stromal lamella maintained their architecture (Figs. 9a–9c). However, in Cd-treated leaves, the lamella were fragmented and disorganized, thylakoid membranes were disorganized with disrupted granal and stromal lamella, starch grains were present, and the nuclei had disintegrated nucleoli (Figs. 9d–9f). JA treatment alleviated the damage caused by Cd on the chloroplast. Exogenous JA application on Cd-treated plants retained the normal structures of the chloroplast and thylakoid membrane (Figs. 9g–9i).
Fig. 9.
Transmission electron micrograph of the leaf mesophyll cells
(a, d, g) Zheshuang-72; (b, e, h) Zhejiang-619; (c, f, i) Zheshuang-758. (a, b, c) Control; (d, e, f) 150 mg/kg Cd; (g, h, i) 150 mg/kg Cd+25 μmol/L JA. Labels: Ch, chloroplast; SG, starch grain; PG, plastoglobule; M, mitochondrion; GL, granal lamella; SL, stromal lamella; N, nucleus; Nu, nucleolus; P, peroxysome; V, vacoule; ICS, intercellular spaces; CW, cell wall
4. Discussion
Cd stress evokes a series of complex responses in higher plants in terms of disturbing the regular physiological and morphological processes. Thus, the mechanism(s) involved in such responses can be regulated as a cause and/or an effect of metabolic changes regarding Cd stress management. The current experiment was conducted to unravel the underlying mechanisms and effects of JA in enhancing the tolerance of rapeseed genotypes to Cd toxicity.
Significant reduction of gas exchange in plants was observed under Cd stress; however, exogenous JA application repaired the damage caused by Cd (Figs. 1 and 2). This reduction can be attributed to possible reduction in G s due to the toxic effects of Cd on guard cells, which is a major consequence of Cd stress in plants (Satler and Thimann, 1981). As a result, limited diffusion of CO2 to the site of carboxylation would take place and this can reduce P n, C i, and T r (Perfus-Barbeoch et al., 2002). JA induced improvement in G s when applied alone (Fig. 1f) and/or in a genotypic-dependent manner in response to Cd treatment (Figs. 2f and 2j). These results are contrary to the findings that jasmonates cause stomatal closure (Satler and Thimann, 1981). However, numerous studies reported that the response of G s to jasmonates depends on the concentration and time of exposure (Metodiev et al., 1996). In general, 50 μmol/L JA induces senescence-related responses (Creelman and Mullet, 1995); however, we used a concentration of 25 μmol/L JA in this study. JA-induced improvement in gas exchange can be attributed to the reduced Cd accumulation in upper parts of plant (Fig. 6e) that can protect against stomatal closure and pigment degradation. Hence, this improvement increases the photosynthetic rate (Figs. 2e and 2i) and related parameters.
The disruption of photosynthetic pigments can explain the degree of damage to the photosynthetic system from environmental stressors (Maxwell and Johnson, 2000). Drastic reductions of chlorophyll and carotenoids were observed in response to Cd stress application, whereas JA application increased photosynthetic pigments (Figs. 3 and 4), showing the negative effects of Cd in pigment regulation and the positive role of JA in protecting photosynthetic pigments and apparatuses. Little or no accumulation of photosynthetic pigments under heavy metal stress might be a consequence of chloroplast membrane peroxidation through enhanced rates of H2O2 production (Piotrowska-Niczyporuk et al., 2012). JA usually protects chlorophyll under toxic metal stress (Chen et al., 2014), whether applied alone (Figs. 3e–3h) or in combination with metals (Figs. 4i–4l), as seen in this study. These results suggested that JA elicits protective effects during photosynthesis under Cd stress. Other studies demonstrated similar findings in JA-and Pb-treated Wolffia arrhiza (Piotrowska et al., 2009) and soybean plants (Keramat et al., 2009). JA-induced protection of photosynthetic pigments can be attributed to the production of multiple secondary metabolite classes, alkaloids and phenolics, and anthocyanins in various plant species (Memelink et al., 2001). Moreover, anthocynin accumulation in epidermal cells and increased carotenoid content under JA and metal stress can protect the chloroplast (Czerpak et al., 2006).
Cd accumulation in the aboveground plant parts increased with Cd application in a dose-dependent manner. However, JA application significantly reduced Cd accumulation in rapeseed genotypes (Figs. 5a and 6e). These results agree with the findings of Piotrowska et al. (2009) which state that JA application significantly inhibits Pb accumulation in W. arrhiza. In addition, other stress hormones, such as salicylic acid (SA), abscisic acid (ABA), and brassinolide (2,4-epibrassinolide), can reduce the uptake of toxic metals (Cd or nickel (Ni)) in hydroponic and soil-grown plants (Kanwar et al., 2012; Ali et al., 2015). Reduction in heavy metal uptake in response to exogenous application of stress hormones can be attributed to the reduced T r and symplastic loading of Cd into the xylem (Lux et al., 2011). However, a relatively higher T r and reduced Cd translocation into the leaves in JA-treated plants were observed and correspond with our previous findings (Ali et al., 2015), where low Cd accumulation and high T r were observed in rapeseed after SA application. Besides transpiration, metal uptake in the plant can also be influenced by other factors, such as accumulation/exudation of organic compounds such as phenolic compounds. Kováčik et al. (2011) reported that accumulation of phenolic compounds in the aboveground parts of plants significantly repressed Ni and Cd uptakes in shoots. Moreover, significantly increased phenolic compound accumulation in plants in response to exogenous JA application was reported (Kim et al., 2007). JA treatments might increase the phenolic compounds in rapeseed leaves, thereby repressing Cd uptake in leaves.
Cd stress indirectly induces the overproduction of various ROS, such as H2O2, O2 −, and OH−, to cause membrane lipid peroxidation and MDA accumulation. MDA is a product of lipid peroxidation and indicates oxidative membrane damage. Significant increase in MDA content indicates oxidative impairment in the leaves of rapeseed genotypes under Cd stress in this study (Figs. 5b and 6b). A similar trend was observed in the pea (Popova et al., 2009) and the results can link Cd toxicity to the production of free radicals, which can hinder membrane stability and increase membrane permeability to the outside environment. However, JA protects the cell membrane lipid by alleviating lipid peroxidation during Cd stress. The benefit of exogenous JA application to plants was observed in terms of reduced oxidative stress, evidenced by the decreased MDA level.
Plant cells are equipped with enzymatic machinery that helps eliminate or reduce oxidative damage. Plant cells have defensive enzymes, including SOD, POD, APX, and CAT, against oxidative damage (Larson, 1988). Among these enzymes, SOD defends by catching superoxide radicals and converts them to H2O2, which is then moved to either APX (in the ascorbate–glutathione cycle) or CAT (in cytoplasm and other cellular compartments) and split into water and oxygen. Previously, Cd was reported to alter the antioxidative system of plants (Ahmad et al., 2011). Significant increases in the enzymatic activity of SOD, POD, and APX under Cd stress were observed, except that of CAT, which remained non-significant in all Cd levels (Figs. 7a–7d). A similar trend was reported in previous studies on different crops, such as pea (Popova et al., 2009) and Brassica juncea (Mobin and Khan, 2007), where the enzymatic activity increased as Cd concentration increased. However, CAT activity is balanced by the increase in APX activity (Fig. 7d). In contrast, a sole application of JA decreases the activity of all antioxidant enzymes, except CAT (Sorial et al., 2010). Moreover, JA also decreases SOD, POD, and APX activity when combined with Cd but increases CAT activity in all Cd levels except 150 mg/kg Cd (Figs. 8i–8l). Decreased SOD, POD, and APX activity can be attributed to the decreased Cd uptake (Fig. 6e), reduction in lipid peroxidation and ROS production by JA, and this can be confirmed from the decline in MDA content (Fig. 6f). CAT is a heme-containing enzyme; hence, increased Fe content after JA application (Table 2) can cause the increased CAT activity (Garg and Manchanda, 2009).
Cd stress can disrupt the homeostasis of macro-and micro-nutrients and related processes in plants (Ramos et al., 2002). For instance, reduced Ca, Mg, Fe, and Zn concentrations were reported in plants under Cd stress (Azevedo et al., 2005). Cd can enter the cells through an uptake system similar to one used by cations, such as Fe, Ca, and Zn. Cd could reduce the uptake and accumulation of these cations by binding with the related transporters (Clemens, 2006). However, JA treatment improves ion homeostasis either solely or in combination with Cd (Tables 2 and 4) by maintaining normal T r and stomatal openings. The JA-improved ion homeostasis can be attributed to the inhibition of Cd uptake in aboveground plant parts (Chen et al., 2014), thereby providing a clear uptake channel for the ions.
Ultra structural studies under environmental stresses are important for studying the probable mechanisms of plant defenses at the subcellular level. Previous studies on Cd effects at the subcellular level in plants revealed the chloroplast to be the most sensitive organelle to Cd toxicity (Ali et al., 2015). However, studies on the combined effects of Cd and JA in rapeseed are scarce. Severe chloroplast damage was noticed in plants under Cd stress compared with their respective controls. The damage can be explained in terms of enlarged but disrupted chloroplasts with entirely deformed thylakoid membranes and disintegrated nucleoli (Vijaranakul et al., 2001; Borges et al., 2004). Despite reduced chlorophyll and low photosynthetic activity in Cd-treated leaves, the presence of starch granules was surprising in this study (Fig. 9d). Disrupted chloroplasts with a low amount of chlorophyll are unlikely to have an energy surplus. This suggests the inability of chloroplasts to metabolize stored starch deposits (Uzunova and Popova, 2000). Nutrient deficiencies may be responsible for starch grain accumulation in Cd-treated leaves (Sandalio et al., 2001).
The recovery of chloroplast through JA application after Cd stress (Figs. 9g–9i) can be attributed to the reduced Cd accumulation and antioxidant enzyme modulation against ROS, and this could be verified by both the increase in photosynthetic pigments and gas exchange parameters in this study. Moreover, JA increases the uptake and accumulation of essential nutrient elements required for chlorophyll and other metabolic activities.
5. Conclusions
JA is helpful to plants in attenuating damage by Cd. The relief provided by JA to plants under Cd stress might be the result of the inhibition of Cd accumulation in leaves, thereby decreasing the damage intensity to the membrane system by ROS produced during oxidative stress
ses. Moreover, JA increases the uptake of essential nutrients that are required for normal growth, and some of these nutrients act as cofactors for key enzymes involved in photosynthesis; therefore JA enhances photosynthesis-related activity. Similarly, JA modulates the activity of stress enzymes to ensure maximum protection to plants. Furthermore, normal chloroplast, and increased gas exchange and photosynthetic pigments suggested that JA protects chloroplasts against ROS produced from Cd stress. This study may clarify the possible mechanisms involved in JA-induced tolerance in rapeseed against Cd toxicity. However, molecular studies should be conducted to further understand the underlying mechanisms of JA-induced tolerance in plants under Cd stress.
Acknowledgments
We thank Miss Mei LI of Zhejiang Key Laboratory of Crop Gene Resources, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, China for her technical assistance and Miss Jun-ying LI of Electron Microscopy Center, Zhejiang University for processing TEM samples.
Footnotes
Project supported by the National Basic Research Program (973) of China (No. 2015CB150205) and the National Natural Science Foundation of China (No. 31671597)
Compliance with ethics guidelines: Essa ALI, Nazim HUSSAIN, Imran Haider SHAMSI, Zahra JABEEN, Muzammil Hussain SIDDIQUI, and Li-xi JIANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Ahmad P, Nabi G, Ashraf M. Cadmium-induced oxidative damage in mustard [Brassica juncea (L.) Czern. & Coss.] plants can be alleviated by salicylic acid. South Afr J Bot. 2011;77(1):36–44. doi: 10.1016/j.sajb.2010.05.003. [DOI] [Google Scholar]
- 2.Ali E, Maodzeka A, Hussain N, et al. The alleviation of cadmium toxicity in oilseed rape (Brassica napus) by the application of salicylic acid. Plant Growth Regul. 2015;75(3):641–655. doi: 10.1007/s10725-014-9966-0. [DOI] [Google Scholar]
- 3.Azevedo BM, Bastos FGC, Viana TVA, et al. Feitos de niveis de irrigacao na cultura da melancia. Rev Cienc Agron. 2005;36:9–15. (in Portuguese) [Google Scholar]
- 4.Borges R, Miguel EC, Dias JMR, et al. Ultrastructural, physiological and biochemical analyses of chlorate toxicity on rice seedlings. Plant Sci. 2004;166(4):1057–1062. doi: 10.1016/j.plantsci.2003.12.023. [DOI] [Google Scholar]
- 5.Cakmak I, Marschner H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992;98(4):1222–1227. doi: 10.1104/pp.98.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Zheng C, Tu C, et al. Heavy metal pollution in soils in China: status and countermeasures. Ambio. 1999;28(2):130–134. [Google Scholar]
- 7.Chen J, Yan ZZ, Li XZ. Effect of methyl jasmonate on cadmium uptake and antioxidative capacity in Kandelia obovata seedlings under cadmium stress. Ecotoxicol Environ Safety. 2014;104:349–356. doi: 10.1016/j.ecoenv.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Clemens S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie. 2006;88(11):1707–1719. doi: 10.1016/j.biochi.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Creelman RA, Mullet JE. Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA. 1995;92(10):4114–4119. doi: 10.1073/pnas.92.10.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Ann Rev Plant Physiol Plant Mol Biol. 1997;48(1):355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- 11.Czerpak R, Piotrowska A, Szulecka K. Jasmonic acid affects changes in the growth and some components content in alga Chlorella vulgaris . Acta Physiol Plant. 2006;28(3):195–203. doi: 10.1007/BF02706531. [DOI] [Google Scholar]
- 12.Fan SK, Zhu J, Tian WH, et al. Effects of split applications of nitrogen fertilizers on the Cd level and nutritional quality of Chinese cabbage. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2017;18(10):897–905. doi: 10.1631/jzus.B1600272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg N, Manchanda G. ROS generation in plants: boon or bane? Plant Biosyst. 2009;143(1):81–96. doi: 10.1080/11263500802633626. [DOI] [Google Scholar]
- 14.Giannopolitis CN, Ries SK. Superoxide dismutases. I. Occurrence in higher plants. Plant Physiol. 1977;59(2):309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan YZ, Zhao J, Chen SB. Study on direct sowing cultivation techniques of high-quality, high-yielding and high-resistant rapeseed variety Zheda 619. Acta Agric Jiangxi. 2012;24:26–27. [Google Scholar]
- 16.Hodges DM, DeLong JM, Forney CF, et al. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207(4):604–611. doi: 10.1007/s004250050524. [DOI] [PubMed] [Google Scholar]
- 17.Hussain N, Jabeen Z, Li YL, et al. Detection of tocopherol in oilseed rape (Brassica napus L.) using gas chromatography with flame ionization detector. J Integr Agric. 2013;12(5):803–814. doi: 10.1016/S2095-3119(13)60301-9. [DOI] [Google Scholar]
- 18.Hussain N, Li H, Jiang YX, et al. Response of seed tocopherols in oilseed rape to nitrogen fertilizer sources and application rates. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2014;15(2):181–193. doi: 10.1631/jzus.B1300036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiao WT, Chen WP, Chang AC, et al. Environmental risks of trace elements associated with long-term phosphate fertilizers applications: a review. Environ Pollut. 2012;168:44–53. doi: 10.1016/j.envpol.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 20.Kanwar MK, Bhardwaj R, Arora P, et al. Plant steroid hormones produced under Ni stress are involved in the regulation of metal uptake and oxidative stress in Brassica juncea L. Chemosphere. 2012;86(1):41–49. doi: 10.1016/j.chemosphere.2011.08.048. [DOI] [PubMed] [Google Scholar]
- 21.Kaya A, Doganlar ZB. Exogenous jasmonic acid induces stress tolerance in tobacco (Nicotiana tabacum) exposed to imazapic. Ecotoxicol Environ Safety. 2016;124:470–479. doi: 10.1016/j.ecoenv.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Keramat B, Kalantari KM, Arvin MJ. Effects of methyl jasmonate in regulating cadmium induced oxidative stress in soybean plant (Glycine max L.) Afr J Microbiol Res. 2009;31(5):240–244. [Google Scholar]
- 23.Kim HJ, Fonseca JM, Choi JH, et al. Effect of methyl jasmonate on phenolic compounds and carotenoids of romaine lettuce (Lactuca sativa L.) J Agric Food Chem. 2007;55(25):10366–10372. doi: 10.1021/jf071927m. [DOI] [PubMed] [Google Scholar]
- 24.Kováčik J, Klejdus B, Hedbavny J, et al. Significance of phenols in cadmium and nickel uptake. J Plant Physiol. 2011;168(6):576–584. doi: 10.1016/j.jplph.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Larson RA. The antioxidants of higher plants. Phytochemistry. 1988;27(4):969–978. doi: 10.1016/0031-9422(88)80254-1. [DOI] [Google Scholar]
- 26.López-Millán AF, Sagardoy R, Solanas M, et al. Cadmium toxicity in tomato (Lycopersicon esculentum) plants grown in hydroponics. Environ Exp Bot. 2009;65(2-3):376–385. doi: 10.1016/j.envexpbot.2008.11.010. [DOI] [Google Scholar]
- 27.Lux A, Martinka M, Vaculík M, et al. Root responses to cadmium in the rhizosphere: a review. J Exp Bot. 2011;62(1):21–37. doi: 10.1093/jxb/erq281. [DOI] [PubMed] [Google Scholar]
- 28.Maxwell K, Johnson GN. Chlorophyll fluorescence–a practical guide. J Exp Bot. 2000;51(345):659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- 29.Memelink J, Verpoorte R, Kijne JW. ORCAnization of jasmonate-responsive gene expression in alkaloid metabolism. Trends Plant Sci. 2001;6(5):212–219. doi: 10.1016/S1360-1385(01)01924-0. [DOI] [PubMed] [Google Scholar]
- 30.Meng H, Hua S, Shamsi IH, et al. Cadmium-induced stress on the seed germination and seedling growth of Brassica napus L., and its alleviation through exogenous plant growth regulators. Plant Growth Regul. 2009;58(1):47–59. doi: 10.1007/s10725-008-9351-y. [DOI] [Google Scholar]
- 31.Metodiev MV, Tsonev TD, Popova LP. Effect of jasmonic acid on the stomatal and nonstomatal limitation of leaf photosynthesis in barley leaves. J Plant Growth Regul. 1996;15(2):75–80. doi: 10.1007/BF00192935. [DOI] [Google Scholar]
- 32.Mobin M, Khan NA. Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol. 2007;164(5):601–610. doi: 10.1016/j.jplph.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in Spinach chloroplasts. Plant Cell Physiol. 1981;22(5):867–880. doi: 10.1093/oxfordjournals.pcp.a076232. [DOI] [Google Scholar]
- 34.Niu L, Yang F, Xu C, et al. Status of metal accumulation in farmland soils across China: from distribution to risk assessment. Environ Pollut. 2013;176:55–62. doi: 10.1016/j.envpol.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 35.Perfus-Barbeoch L, Leonhardt N, Vavasseur A, et al. Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002;32(4):539–548. doi: 10.1046/j.1365-313X.2002.01442.x. [DOI] [PubMed] [Google Scholar]
- 36.Pinto AP, Mota AM, de Varennes A, et al. Influence of organic matter on the uptake of cadmium, zinc, copper and iron by sorghum plants. Sci Total Environ. 2004;326(1-3):239–247. doi: 10.1016/j.scitotenv.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Piotrowska A, Bajguz A, Godlewska-Żyłkiewicz B, et al. Jasmonic acid as modulator of lead toxicity in aquatic plant Wolffia arrhiza (Lemnaceae) Environ Exp Bot. 2009;66(3):507–513. doi: 10.1016/j.envexpbot.2009.03.019. [DOI] [Google Scholar]
- 38.Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka E, et al. Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae) Plant Physiol Biochem. 2012;52:52–65. doi: 10.1016/j.plaphy.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Popova LP, Maslenkova LT, Yordanova RY, et al. Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings. Plant Physiol Biochem. 2009;47(3):224–231. doi: 10.1016/j.plaphy.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Ramos I, Esteban E, Lucena JJ, et al. Cadmium uptake and subcellular distribution in plants of Lactuca sp. Cd–Mn interaction. Plant Sci. 2002;162(5):761–767. doi: 10.1016/S0168-9452(02)00017-1. [DOI] [Google Scholar]
- 41.Sandalio LM, Dalurzo HC, Gómez M, et al. Cadmium-induced changes in the growth and oxidative metabolism of pea plant. J Exp Bot. 2001;52(364):2115–2126. doi: 10.1093/jexbot/52.364.2115. [DOI] [PubMed] [Google Scholar]
- 42.Satler SO, Thimann KV. Le jasmonate de methyle: nou-veau et puissant promoteur de la senescence des feuilles. Compt Rend Acad Sci Paris Ser III. 1981;293:735–740. (in French) [Google Scholar]
- 43.Shamsi IH, Wei K, Zhang GP, et al. Interactive effects of cadmium and aluminum on growth and antioxidative enzymes in soybean. Biol Plant. 2008;52(1):165–169. doi: 10.1007/s10535-008-0036-1. [DOI] [Google Scholar]
- 44.Shamsi IH, Jiang LX, Wei K, et al. Alleviation of cadmium toxicity in soybean by potassium supplementation. J Plant Nut. 2010;33(13):1926–1938. doi: 10.1080/01904167.2010.512052. [DOI] [Google Scholar]
- 45.Sorial ME, El Gamal SM, Gendy AA. Response of sweet basil to jasmonic acid application in relation to different water supplies. Biosci Res. 2010;7(1):39–47. [Google Scholar]
- 46.Steel RGD, Torrie JH. Principles and Procedures of Statistics: a Biometrical Approach, 2nd Ed. McGraw-Hill, New York; 1980. [Google Scholar]
- 47.Thaler JS, Fidantsef AL, Duffey SS, et al. Trade-offs in plant defense against pathogens and herbivores: a field demonstration of chemical elicitors of induced resistance. J Chem Ecol. 1999;25(7):1597–1609. doi: 10.1023/A:1020840900595. [DOI] [Google Scholar]
- 48.Tsonev TD, Lazova GN, Stoinova ZG, et al. A possible role for jasmonic acid in adaptation of barley seedlings to salinity stress. J Plant Growth Regul. 1998;17(3):153–159. doi: 10.1007/PL00007029. [DOI] [Google Scholar]
- 49.Uzunova AN, Popova LP. Effect of salicylic acid on leaf anatomy and chloroplast ultrastructure of barley plants. Photosynthetica. 2000;38(2):243–250. doi: 10.1023/A:1007226116925. [DOI] [Google Scholar]
- 50.Vijaranakul U, Jayaswal RK, Nadakavukaren MJ. Alteration in chloroplast ultrastructure of suspension cultured Nicotiana tabaccum cells by cadmium. Sci Asia. 2001;27:227–231. [Google Scholar]
- 51.Wang LS, Wang L, Wang L, et al. Effect of 1-butyl-3-methylimidazolium tetrafluoroborate on the wheat (Triticum aestivum L.) seedlings. Environ Toxicol. 2009;24(3):296–303. doi: 10.1002/tox.20435. [DOI] [PubMed] [Google Scholar]
- 52.Wu CF, Zhang LM. Heavy metal concentrations and their possible sources in paddy soils of a modern agricultural zone, southeastern China. Environ Earth Sci. 2010;60(1):45–56. doi: 10.1007/s12665-009-0168-4. [DOI] [Google Scholar]