Abstract
Vegetables are important constituents of the human diet. Heavy metals and nitrate are among the major contaminants of vegetables. Consumption of vegetables and fruits with accumulated heavy metals and nitrate has the potential to damage different body organs leading to unwanted effects. Breeding vegetables with low heavy metal and nitrate contaminants is a cost-effective approach. We investigated 38 water spinach genotypes for low Cd and nitrate co-accumulation. Four genotypes, i.e. JXDY, GZQL, XGDB, and B888, were found to have low co-accumulation of Cd (<0.71 mg/kg dry weight) and nitrate (<3100 mg/kg fresh weight) in the edible parts when grown in soils with moderate contamination of both Cd (1.10 mg/kg) and nitrate (235.2 mg/kg). These genotypes should be appropriate with minimized risk to humans who consume them. The Cd levels in the edible parts of water spinach were positively correlated with the concentration of Pb or Zn, but Cd, Pb, or Zn was negatively correlated with P concentration. These results indicate that these three heavy metals may be absorbed into the plant in similar proportions or in combination, minimizing the influx to aerial parts. Increasing P fertilizer application rates appears to prevent heavy metal and nitrate translocation to shoot tissues and the edible parts of water spinach on co-contaminated soils.
Keywords: Genotypic difference, Heavy metal, Nitrate, Soil pollution, Water spinach
1. Introduction
Cadmium (Cd) is one of the most toxic and mobile heavy metals that affect human health through the food chain. Agricultural practices, intensive industrial activity, and urban expansion have accelerated the release of Cd into soil, water, and air (Lane et al., 2015). About 27 860 000 m2 of agricultural soils in China are polluted with Cd (Liu et al., 2015) and Cd contamination has become one of the most important barriers to agricultural sustainability in China (Zhang et al., 2002). Cd in agricultural soils is taken up by plants and then enters humans and animals through the food chain (Kirkham, 2006). Phytoremediation is the best strategy to reduce the risk of Cd entry into food chain.
Fertilizer application is one of the key factors in improving crop productivity. However, excessive use of chemical fertilizers, especially nitrogen (N) fertilizers, results in severe environmental problems (Hakeem et al., 2013). China is one of the largest consumers of N fertilizers in the world, but the average N use efficiency is low (only about 35%) (Wang et al., 2014), which results in waste of resources and environmental contamination, and also poses a serious hazard to human health (Xu et al., 2012; Chen et al., 2014). Nitrate is potentially carcinogenic and may increase the incidence of gastric, bladder, and oesophageal cancers (Gulis et al., 2002). However, nitrate can decrease blood pressure, thus reducing the risk of cardiovascular disease, myocardial infarction, and stroke. Daily consumption of nitrate-rich vegetables is associated with beneficial effects for patients with gastric ulcer, renal failure, and metabolic syndrome (Habermeyer et al., 2015).
Vegetables are nutritious and are assumed to be safe to consume; people are unaware that some parts of the vegetable may be contaminated with heavy metals and are a major source of human exposure to Cd and nitrate (Tang et al., 2016; Fan et al., 2017). Water spinach (Ipomoea aquatica Forsk.), an important leafy vegetable in eastern and southern Asia, can absorb Cd and nitrate naturally into their vacuoles (Wang et al., 2009; Xin et al., 2010). Therefore, evaluation of water spinach genotypes for low Cd and nitrate accumulation when grown on contaminated soils has become a research priority in minimizing human exposure to these co-contaminants.
Accumulation of Cd by plants varies with species and genotype. New strategies have been applied to breeding and screening heavy metal low accumulator vegetable genotypes, such as Chinese cabbage (Brassica chinensis L.) (Liu et al., 2010; Wang X et al., 2015), pakchoi (Brassica rapa L. ssp. chinensis) (Chen et al., 2012), soybean (Glycine max Merr.) (Arao et al., 2003; Sugiyama et al., 2011), welsh onion (Allium fistulosum L.) (Li et al., 2012), and sweet potato (Ipomoea batatas (L.) Lam.) (Huang et al., 2015). Nitrate concentration in high-accumulation genotypes was several times greater than that in low-accumulation genotypes, such as lettuce (Lactuca sativa L.) (Escobar-Gutiérrez et al., 2002; Burns et al., 2011a), leaf mustard (Brassica juncea (L.) Czern.) (Sharma et al., 2010), and taro (Colocasia esculenta (L.) Schott) (Kristl et al., 2016). Vegetable genotypes with a lower nitrate concentration need to be identified for agricultural production and improved human health.
Soils in southern China are often polluted by multiple contaminants. Among them Cd and nitrate contaminations are attributed to irrigation practices, use of low-grade organic fertilizers, and heavy application of N fertilizers, all of which can affect growth, metal tolerance, and metal accumulation in water spinach. Screening different vegetable genotypes with low absorption of Cd and nitrate provides an opportunity to safeguard human consumption.
Previous studies were limited to pot and hydroponics where correlations between different contaminants and nutrients were not observed. This study aimed to investigate co-accumulative remediation capacity of Cd and nitrate in field conditions among 38 genotypes of water spinach.
2. Materials and methods
2.1. Soil characterization
The experiment was performed in greenhouses (Chunyi farm) located at 30°23′37″ N and 120°2′13″ E, Hangzhou, Zhejiang Province, China with an average temperature of 30 °C throughout the day. The soil had been moderately contaminated by Cd and nitrate during several decades of intensive vegetable production (Tang et al., 2016). The depth of soil sampling for analysis was 10‒15 cm, and four soil samples were collected to represent the area. The preliminary soil assessment and initial concentrations of metals in the soil (Table 1) were determined according to the previous methods (Li, 2000; Bao, 2008; Tang et al., 2016).
Table 1.
Physicochemical properties of soil in the field experiment
| pH | Organic matter (g/kg) | Total N (g/kg) | Total P (g/kg) | Total K (g/kg) | Available N (g/kg) | Available P (g/kg) |
| 5.39±0.05 | 23.07±0.69 | 1.22±0.03 | 0.60±0.07 | 1.69±0.14 | 97.35±6.74 | 77.59±10.11 |
|
| ||||||
|
| ||||||
| Available K (g/kg) | Total Cd (g/kg) | Total Pb (g/kg) | DTPA Cd (g/kg) | DTPA Pb (g/kg) | Nitrate-N (g/kg) | |
|
| ||||||
| 296.87±5.06 | 1.10±0.18 | 32.66±0.33 | 0.23±0.01 | 0.96±0.12 | 235.21±6.64 | |
DTPA: diethylene triamine pentaacetic acid. Reprinted from Tang et al. (2016), Copyright 2016, with permission from Elsevier
2.2. Sample collection and cultivation
Thirty-eight genotypes of water spinach (I. aquatica F.) were obtained from a local seed market in Hangzhou, China. Their genus, origin, and characteristics are listed in Table 2. Split plot design was used with three replicates for major plots and genotypes for the minor plots. Each minor plot was 10 m2. Approximately 900 seeds of each genotype were soaked overnight in aerated deionized water at (23±0.3) °C, disinfected with 0.7 g/L NaClO for 30 min, drained and sown in the field in June 2014. Planting density and field management were the same as in conventional farming practice.
Table 2.
Tested genotypes of water spinach
| Serial No. | Accession name | Origin* | Horticultural characteristics |
|
| Petioles | Blades | |||
| 1 | JXDY | Jiangxi | Green | Extra-big |
| 2 | JADY | Jiangxi | Green | Big |
| 3 | GDLY | Jiangxi | White | Small |
| 4 | GZTB | Guangdong | White | Extra-big |
| 5 | G501 | Guangdong | Light yellow | Small |
| 6 | GZQL | Hong Kong | Green | Small |
| 7 | XGDB | Fujian | White | Big |
| 8 | G268 | Fujian | Green | Small |
| 9 | TWBD | Jiangxi | White | Big |
| 10 | TWLY | Thailand | Green | Small |
| 11 | TWBL | Guangdong | White | Small |
| 12 | TWYX | Taiwan | Green | Small |
| 13 | TWZY | Indonesia | Green | Small |
| 14 | T311 | Taiwan | White | Small |
| 15 | TAIG | Thailand | Green | Small |
| 16 | TGLY | Thailand | Green | Small |
| 17 | T221 | Guangxi | Green | Small |
| 18 | TGJY | Thailand | Green | Small |
| 19 | TGLL | Jiangsu | Green | Small |
| 20 | YXBL | Guangdong | White | Small |
| 21 | X606 | Guangdong | Light green | Small |
| 22 | Y601 | Beijing | Green | Small |
| 23 | CHFE | Hebei | Green | Extra-big |
| 24 | QCUI | Tianjin | Green | Extra-big |
| 25 | XIAO | Jiangsu | Green | Small |
| 26 | LZHU | Thailand | Green | Small |
| 27 | GDZY | Thailand | Green | Small |
| 28 | GLCQ | Jiangxi | Green | Small |
| 29 | DAYE | Jiangxi | Green | Extra-big |
| 30 | BQBA | Fujian | Green | Small |
| 31 | CBDY | Jiangxi | White | Extra-big |
| 32 | LIUY | Jiangxi | Green | Small |
| 33 | BGDY | Hubei | White | Big |
| 34 | LYQG | Fujian | Green | Small |
| 35 | HSZY | Shanghai | Green | Small |
| 36 | B888 | Jiangxi | White | Small |
| 37 | DQGU | Jiangxi | Light green | Small |
| 38 | QGZY | Hebei | Green | Small |
Region (province or city of China) or country (Thailand, Indonesia)
2.3. Fresh weight and biomass determination
The experiment was performed according to the previous method (Tang et al., 2017). All genotypes were grown in green house for four weeks without application of fertilizer. Plants excised at 0.5 cm above the base were used for fresh weight (FW) and biomass determination. Mature and immature leaves (leaf blades and petioles) were separated and FWs were estimated. After rinsing with tap water, ten representative plants selected from each genotype were combined together to make a composite sample. Subsamples were dried in an oven at 105 °C for 30 min, and then at 65 °C until a constant weight was attained and the biomass of shoots was then recorded.
2.4. Heavy metal analysis and nitrate determination of plant samples
The concentrations of Cd and other metals (K, Ca, Mg, Fe, Zn, Cu, Pb, Mn, and Se) of plant samples, which were digested with HNO3 and HClO4 (5:1, v/v), were determined using inductively coupled plasma mass spectrometer (ICP-MS, 7500a, Aligent, USA), according to the previous method (Bao, 2008). Fresh samples were placed in 10 ml deionized water and heated in a boiling water bath for 30 min. Then the nitrate concentration in the water extract was determined using an ultraviolet spectrophotometer (Lambda 350V-vis, Perkin Elmer, Singapore), according to salicylic acid colorimetric method described by Li (2000). Analyses of other mineral elements (N and P) and nutritional indices (chlorophyll, protein, vitamin C, and cellulose) were performed according to the pervious methods (Tang et al., 2016).
2.5. Statistical analysis
The statistical evaluation was represented as the mean±standard error (SE) of four replicates. The differences between 38 genotypes of water spinach were estimated by the least significant difference (LSD) method and the correlation was analyzed by Bivariate analysis (SPSS 20.0) and graphical representation was generated by Origin Pro 8.0.
3. Results
3.1. Fresh weight and biomass yields
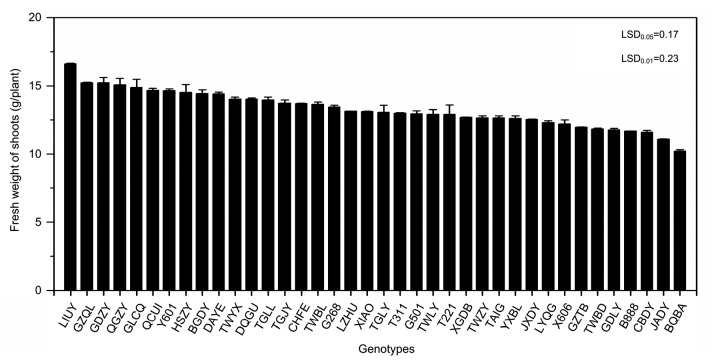
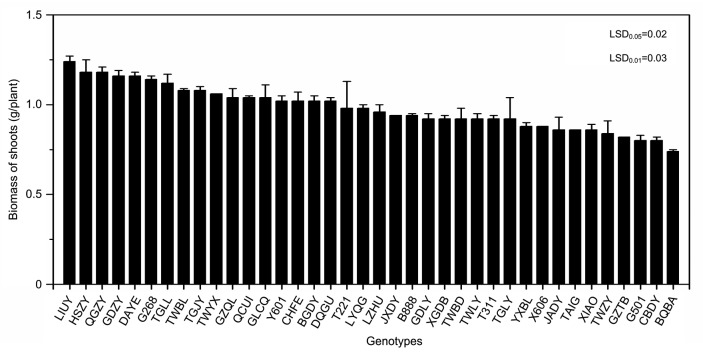
FWs and biomass of 38 water spinach genotypes were determined (Figs. 1 and 2) after growth for four weeks. FW ranged from 16.62 g/plant (LIUY) to 10.22 g/plant (BQBA), approximately a 1.6-fold difference between the highest and the lowest levels with a mean value of 13.30 g/plant (Fig. 1). Biomass values ranged from 1.24 g/plant (LIUY) to 0.74 g/plant (BQBA), approximately a 1.7-fold difference with a mean value of 0.98 g/plant (Fig. 2).
Fig. 1.
Shoot fresh weights of 38 water spinach genotypes grown in co-contaminated soils
Bars represent standard error of the mean with four replicates. Data analysis was performed using LSD method
Fig. 2.
Shoot biomass of 38 water spinach genotypes grown in co-contaminated soils
Bars represent standard error of the mean with four replicates. Data analysis was performed using LSD method
3.2. Cd and nitrate uptake
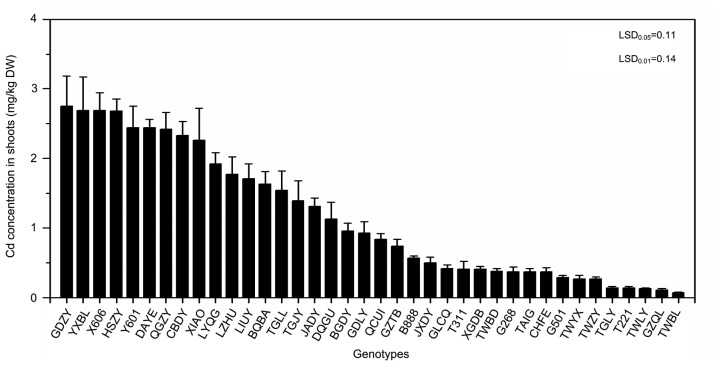
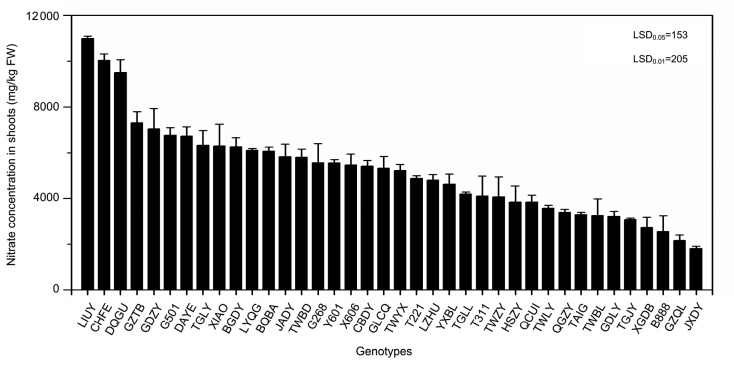
Cd uptake ranged from 2.75 mg/kg dry weight (DW) (GDZY) to 0.07 mg/kg DW (TWBL), a difference of more than 39-fold with a mean value of 1.15 mg/kg DW (Fig. 3). Nitrate concentrations among all genotypes ranged from 10 983 mg/kg FW (LIUY) to 1809 mg/kg FW (JXDY), a 6-fold difference with a mean value of 5177 mg/kg FW (Fig. 4).
Fig. 3.
Cd concentration of 38 water spinach genotypes grown in co-contaminated soils
Bars represent standard error of the mean with four replicates. Data analysis was performed using LSD method
Fig. 4.
Nitrate concentration in 38 water spinach genotypes grown in co-contaminated soils
Bars represent standard error of the mean with four replicates. Data analysis was performed using LSD method
3.3. Correlations between Cd, nitrate, and other elements
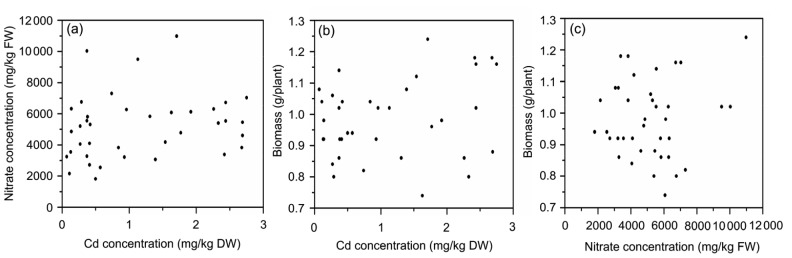
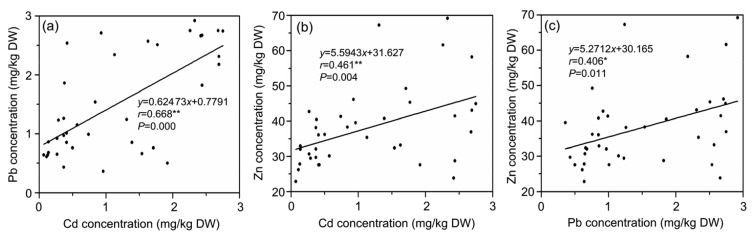
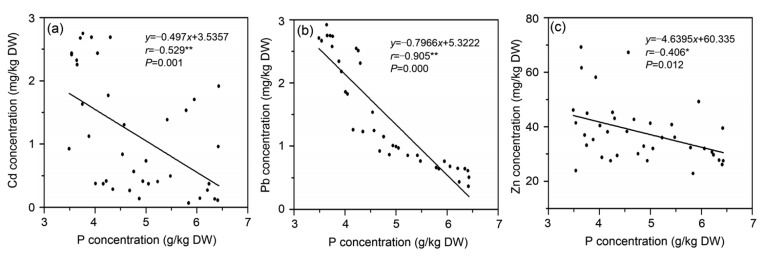
No correlation was observed between Cd and nitrate concentrations (Fig. 5a). However, there was a significant positive correlation between Cd, Pb, and Zn concentrations (Fig. 6) and a significant negative correlation between Cd or Pb and P (Figs. 7a and 7b), and between Zn and P (Fig. 7c). There was a positive correlation between nitrate and chlorophyll concentrations (Fig. 8b), and a significant positive correlation between nitrate and vitamin C (Fig. 8a). However, no significant correlation between Cd, nitrate and biomass yield in water spinach (Figs. 5b and 5c) was observed.
Fig. 5.
Correlation coefficients between Cd, nitrate concentrations, and plant biomass of water spinach genotypes in Cd and nitrate contaminated soils
(a) Nitrate vs. Cd; (b) Plant biomass vs. Cd; (c) Plant biomass vs. nitrate
Fig. 6.
Correlation coefficients between Cd, Pb, and Zn concentrations in water spinach genotypes in Cd and nitrate contaminated soils
(a) Pb vs. Cd; (b) Zn vs. Cd; (c) Zn vs. Pb. * Significance at P<0.05; ** Significance at P<0.01
Fig. 7.
Correlation coefficients between P concentrations and Cd, Pb, and Zn accumulation levels in water spinach genotypes in Cd and nitrate contaminated soil
(a) Cd vs. P; (b) Pb vs. P; (c) Zn vs. P. * Significance at P<0.05; ** Significance at P<0.01
Fig. 8.
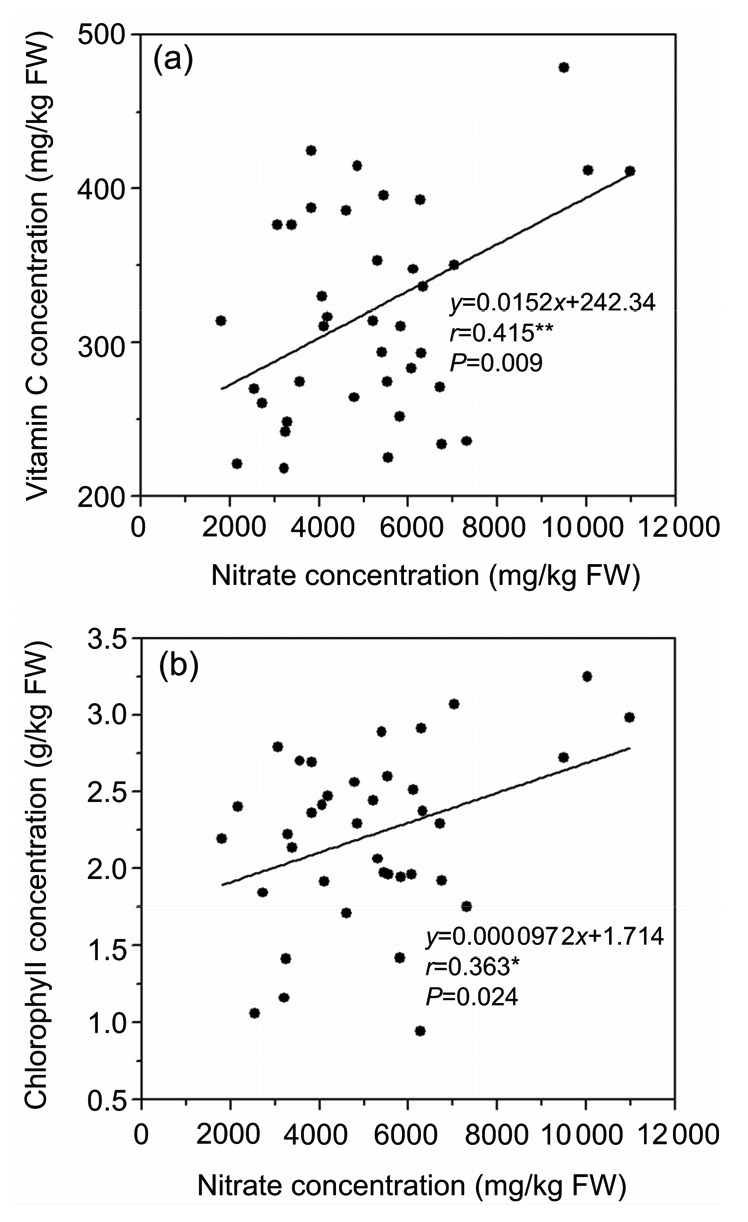
Correlation coefficients between nitrate concentrations and vitamin C, chlorophyll accumulation levels in water spinach genotypes in Cd and nitrate contaminated soil
(a) Vitamin C vs. nitrate; (b) Chlorophyll vs. nitrate. * Significance at P<0.05; ** Significance at P<0.01
4. Discussion
Water spinach is a common leafy vegetable which provides rich nutrients to the diet. All the selected water spinach showed successive growth in Cd and nitrate co-contaminated soil. The FW or biomass yield is close to the mean value. Water spinach tolerates high Cd and nitrate stress relatively well. The results were consistent with previous studies (Wang et al., 2007). Since there is no visible symptom of toxicity when water spinach is grown in Cd and nitrate co-contaminated soils, the potential health risk may be high from inadvertently consuming contaminated water spinach. The National Food Safety Standard of China GB 2762-2012 (Ministry of Health of the People’s Republic of China, 2012) sets the safe limit for Cd contamination of fresh leafy vegetables at 0.05 mg/kg FW. Since the water content of water spinach is 93%, it can be calculated that the safe consumption level of Cd in water spinach shoot is 0.71 mg/kg DW. Given this standard, 17 water spinach genotypes tested in our study can be safe for consumption. The maximum permissible concentration (MPC) of nitrate is 3100 mg/kg FW (Zhou et al., 2000), which means that only five water spinach genotypes can be safe for consumption. Using the combined standards for Cd and nitrate, four genotypes were recognized as safe, i.e. JXDY, GZQL, XGDB, and B888 (Table 3), which are low Cd and nitrate co-accumulators and suitable to grow in slightly or moderately contaminated soils without any risk to human health.
Table 3.
Cd and nitrate concentrations in the shoots of four safe water spinach genotypes
| Genotypes | Cd (mg/kg DW) | Nitrate (mg/kg FW) |
| JXDY | 0.50±0.08 | 1809.6±96.4 |
| GZQL | 0.11±0.02 | 2154.9±236.2 |
| XGDB | 0.41±0.04 | 2723.7±443.6 |
| B888 | 0.57±0.03 | 2549.3±668.0 |
Data are expressed as mean±standard error with four replicates
Previous studies showed that low doses of heavy metals may promote plant hormone secretion and regulate plant growth and development (Liu et al., 2010). However, the distinct tolerance mechanisms of water spinach genotypes to Cd are not fully understood. Variations in Cd accumulation among genotypes may be related to uptake, transfer, and bioaccumulation of Cd in shoots (Uraguchi et al., 2009) and roots (Lux et al., 2011) and are influenced by several environmental factors such as light, cold, and humidity (Cheng et al., 2006; Li et al., 2015), including properties of the rhizosphere (Arao et al., 2009; Zheng and Zhang, 2011).
The accumulation of Cd in plants varies greatly not only among plant species but also among genotypes or cultivars within the same species (Yu et al., 2006; Liu et al., 2007; Wang et al., 2007; Martin et al., 2012). The results of the present study showed that different genotypes of water spinach had different Cd retention abilities, resulting in different Cd accumulations. Genotype-dependent Cd accumulation of water spinach largely depends on bioprocesses occurring in shoots and roots (Xin et al., 2013a). Some evidence indicates that Cd subcellular distribution may be associated with Cd tolerance and detoxification in plants. Distribution of Cd in plants is influenced by cross-membrane transport systems, the existence of intracellular binding sites, vacuole sequestration, xylem and phloem transport, and root retention (Grant et al., 2008). In most cases heavy metals are retained in plant root cell wall, restricting their translocation to shoot and minimizing plant damage (Wang Y et al., 2015). Similar results were reported by Xin et al. (2013a, 2013b) and Huang et al. (2016). Retention of Cd in the cell wall is the effective and most important mechanism for Cd detoxification in all water spinach tissues, especially in young leaves (Xin et al., 2013b), though this may vary between genotypes (Huang et al., 2016). The presence of thicker phloem and outer cortex cell walls in the low Cd cultivars may explain why low Cd cultivar roots were able to retain more Cd, thus reducing Cd translocation to shoots (Xin et al., 2013b).
Nitrate is taken up by plants from fertilizer, but this differs depending on the genotype and environment (Burns et al., 2011b). Nitrate concentration in plants depends mainly on environmental, genetic, and nutritional factors (Anjana et al., 2009). When plants are provided with excess nitrate, only a small portion taken up by roots may be immediately translocated to and assimilated by shoots, while the majority of the absorbed nitrate is stored in vacuoles in both roots and shoots (Luo et al., 2006). Nitrate accumulation in plant tissues acts not only as a temporary store, but also as a replacement osmoticum for other plant solutes for maintaining turgor and driving leaf expansion (Burns et al., 2010). Excessive N accumulation in plant tissues is attributed to the imbalance between uptake and assimilation (Cárdenas-Navarro et al., 1999). As a result, the uptake of nitrate may reduce osmatic pressure that limits the storage of organic solutes in vacuole (Wojciechowska and Kołton, 2014).
Although there is strong evidence for the genotypical effects on Cd or nitrate accumulation in vegetable plants (Arao et al., 2003; Burns et al., 2011a; Sugiyama et al., 2011; Wang X et al., 2015; Kristl et al., 2016), less is known about the higher specificity. The present study indicated that no correlation occurred between plant Cd, nitrate, and biomass, suggesting that yield, Cd and nitrate levels may be independent of each other. This suggests that these three traits could be improved separately or in combination for high yield with low concentrations of Cd and nitrate.
Heavy metals, such as Cd, Pb, and Zn, are phytotoxic when present in excessive amounts and can interfere with photosynthetic and respiratory activities, mineral nutrition, enzymatic activity, membrane functions, and hormone balance (Clijsters and van Assche, 1985). The significant positive correlation between Cd or Pb and Zn in water spinach genotypes may indicate that they have similar uptake mechanisms. Similar results were reported in previous studies (Wu and Zhang, 2002; Liu et al., 2003; Dong et al., 2006; Li et al., 2015). These heavy metals are probably transported by similar transporters in the form of compounds or chelate complexes, and the mobilizing function of root exudates is effective not only for Cd but also for Pb and Zn (Kabata-Pendias, 2011). Cohen et al. (1998) reported that IRT1 may facilitate the transport of heavy metals in the form of divalent cations such as Cd2+, Pb2+, and Zn2+. Almost all Cd hyper-accumulation plants could accumulate high concentrations of Pb and Zn (He et al., 2002). All of these suggest that these three metals may have similar transport mechanisms, although the interaction of Cd with some nutrients in soil is not fully understood.
P is a macronutrient and has an important role in plant physiology including metabolism. When P is deficient, plant growth and crop yield are reduced, as P is an essential element for the synthesis of nucleic acids, phospholipids, and adenosine triphosphate (ATP) (Yin et al., 2016). Our results indicated that there was a significant negative correlation between Cd, Pb, or Zn and P concentration in water spinach plants (Fig. 7). Similar results have also been reported (Keller and Römer, 2001; Zhang et al., 2002; Dheri et al., 2007). Application of P-containing materials influences the bioavailability of heavy metals such as Cd, Pb, and Zn in soil (Qiu et al., 2011). Jiang et al. (2007) reported that increased P in soil resulted in substantial precipitation of heavy metal-P complexes in the cell wall and vacuoles in maize (Zea mays L.), and a similar effect was reported in strawberry (Fragaia ananassa D.) (Nuzahath et al., 2013). P-heavy metal interactions reduce the availability of heavy metals in soil and limit their mobility in plants (Clemens, 2006). The supply of adequate and balanced mineral nutrients to crops has the potential to improve plant tolerance, growth, development, and productivity under stress environments (Mitchell et al., 2000; Astolfi et al., 2004). However, the proportion of macronutrient fertilizers in agriculture is often out of balance in China. Our present study points out that increasing the P supply to heavy metal (Cd, Pb, or Zn)-contaminated soils reduces Cd, Pb, or Zn concentration in water spinach.
Chlorophyll concentration is influenced by environmental factors and N fertilization (Barickman and Kopsell, 2016) and the form and ratio of N in plants (Borowski and Michalek, 2008). Previous research demonstrated that higher ratios of NO3 −-N to NH4 +-N positively influenced chlorophyll concentrations in the leaf tissue of kale (Brassica oleracea L. var. acephala) (Kopsell et al., 2007) and leaf lettuce (L. sativa L.) (Stagnari et al., 2015). Chlorophyll concentration in low nitrate accumulation genotypes oilseed rape (Brassica napus L.) was reported to be significantly lower than that in high nitrate accumulation genotypes (Han et al., 2016). In the present study, there was a positive correlation between nitrate and chlorophyll concentration (Fig. 8b), which was consistent with previous studies. There was a significant positive correlation between nitrate and vitamin C in water spinach genotypes (Fig. 8a). Similar results in spinach (Spinacia oleracea L.) were previously reported by Conesa et al. (2009) and Koh et al. (2012). The levels of vitamin C and nitrate in leafy vegetables are two key indices for evaluating their nutritional quality (Konstantopoulou et al., 2010), and vitamin C content in leafy vegetables is mainly controlled by the form and ratio of N (Sørensen et al., 1994). The significant positive correlation between nitrate and vitamin C concentration in water spinach genotypes suggests that it would be difficult to reduce nitrate and increase vitamin C concentration simultaneously, so it is necessary to improve these two traits separately.
5. Conclusions
We have identified four genotypes of water spinach, JXDY, GZQL, XGDB, and B888, as low co-accumulators for Cd and nitrate, indicating that they should be the preferred genotypes for growing in slightly or moderately contaminated soils, minimizing risk to human health. It should be possible to combine high yields with low concentrations of Cd and nitrate as these variables are independent of each other. Increasing P fertilizer rates appears to increase tolerance in water spinach to Cd, Pb, and Zn toxicity and nitrate concentrations. Our experiment has shown that screening vegetable genotypes for those which do not exceed allowable levels of a contaminant is a cost-effective strategy for minimizing the risk of contaminants to human health via the food chain.
Footnotes
Project supported by the Key Projects from Ministry of Science and Technology of China (No. 2016YFD0800805), the Zhejiang Provincial Science and Technology Bureau (Nos. 2015C02011-3 and 2015C03020-2), and the Fundamental Research Funds for the Central University, China
Compliance with ethics guidelines: Lin TANG, Wei-jun LUO, Zhen-li HE, Hanumanth Kumar GURAJALA, Yasir HAMID, Kiran Yasmin KHAN, and Xiao-e YANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Anjana , Umar S, Iqbal M. In: Factors responsible for nitrate accumulation: a review. Lichtfouse E, Navarreter M, Debaeke P, editors. Sustainable Agriculture. Springer, Dordrecht; 2009. pp. 533–549. [DOI] [Google Scholar]
- 2.Arao T, Ae N, Sugiyama M, et al. Genotypic differences in cadmium uptake and distribution in soybeans. Plant Soil. 2003;251(2):247–253. doi: 10.1023/A:1023079819086. [DOI] [Google Scholar]
- 3.Arao T, Kawasaki A, Baba K, et al. Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice. Environ Sci Technol. 2009;43(24):9361–9367. doi: 10.1021/es9022738. [DOI] [PubMed] [Google Scholar]
- 4.Astolfi S, Zuchi S, Passera C. Role of sulphur availability on cadmium-induced changes of nitrogen and sulphur metabolism in maize (Zea mays L.) leaves. J Plant Physiol. 2004;161(7):795–802. doi: 10.1016/j.jplph.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Bao SD. Soil agricultural Chemistry Analysis Method, 3rd Ed. China Agriculture Press, Beijing, China; 2008. (in Chinese) [Google Scholar]
- 6.Barickman TC, Kopsell DA. Nitrogen form and ratio impact Swiss chard (Beta vulgaris subsp. cicla) shoot tissue carotenoid and chlorophyll concentrations. Sci Hortic. 2016;204:99–105. doi: 10.1016/j.scienta.2016.04.007. [DOI] [Google Scholar]
- 7.Borowski E, Michalek S. The effect of nitrogen form and air temperature during foliar fertilization on gas exchange, the yield and nutritive value of spinach (Spinacia oleracea L.) Folia Hortic. 2008;20(2):17–27. doi: 10.2478/fhort-2013-0110. [DOI] [Google Scholar]
- 8.Burns IG, Zhang KF, Turner MK, et al. Iso-osmotic regulation of nitrate accumulation in lettuce. J Plant Nutr. 2010;34(2):283–313. doi: 10.1080/01904167.2011.533328. [DOI] [Google Scholar]
- 9.Burns IG, Zhang KF, Turner MK, et al. Screening for genotype and environment effects on nitrate accumulation in 24 species of young lettuce. J Sci Food Agric. 2011;91(3):553–562. doi: 10.1002/jsfa.4220. [DOI] [PubMed] [Google Scholar]
- 10.Burns IG, Zhang KF, Turner MK, et al. Genotype and environment effects on nitrate accumulation in a diversity set of lettuce accessions at commercial maturity: the influence of nitrate uptake and assimilation, osmotic interactions and shoot weight and development. J Sci Food Agric. 2011;91(12):2217–2233. doi: 10.1002/jsfa.4442. [DOI] [PubMed] [Google Scholar]
- 11.Cárdenas-Navarro R, Adamowicz S, Robin P. Nitrate accumulation in plants: a role for water. J Exp Bot. 1999;50(334):613–624. doi: 10.1093/jexbot/50.334.613. [DOI] [Google Scholar]
- 12.Chen XP, Cui ZL, Fan MS, et al. Producing more grain with lower environmental costs. Nature. 2014;514(7523):486–489. doi: 10.1038/nature13609. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Li TQ, Han X, et al. Cadmium accumulation in different pakchoi cultivars and screening for pollution-safe cultivars. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2012;13(6):494–502. doi: 10.1631/jzus.B1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng WD, Zhang GP, Yao HG, et al. Genotypic and environmental variation in cadmium, chromium, arsenic, nickel, and lead concentrations in rice grains. J Zhejiang Univ-Sci B. 2006;7(7):565–571. doi: 10.1631/jzus.2006.B0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clemens S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie. 2006;88(11):1707–1719. doi: 10.1016/j.biochi.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Clijsters H, van Assche F. Inhibition of photosynthesis by heavy metal. Phytosyn Res. 1985;7(1):31–40. doi: 10.1007/BF00032920. [DOI] [PubMed] [Google Scholar]
- 17.Cohen CK, Fox TC, Garvin DF, et al. The role of iron-deficiency stress responses in stimulating heavy-metal transport in plants. Plant Physiol. 1998;116(3):1063–1072. doi: 10.1104/pp.116.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conesa E, Ninirola D, Vicente MJ, et al. The influence of nitrate/ammonium ratio on yield quality and nitrate, oxalate and vitamin C content of baby leaf spinach and bladder campion plants grown in a floating system. Acta Hortic. 2009;843:269–273. [Google Scholar]
- 19.Dheri GS, Brar MS, Malhi SS. Influence of phosphorus application on growth and cadmium uptake of spinach in two cadmium-contaminated soils. J Plant Nutr Soil Sci. 2007;170(4):495–499. doi: 10.1002/jpln.200625051. [DOI] [Google Scholar]
- 20.Dong J, Wu FB, Zhang GP. Influence of cadmium on antioxidant capacity and four microelement concentrations in tomato seedlings (Lycopersicon esculentum) Chemosphere. 2006;64(10):1659–1666. doi: 10.1016/j.chemosphere.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 21.Escobar-Gutiérrez AJ, Burns IG, Lee A, et al. Screening lettuce cultivars for low nitrate content during summer and winter production. J Hort Sci Biotechnol. 2002;77(2):232–237. doi: 10.1080/14620316.2002.11511485. [DOI] [Google Scholar]
- 22.Fan SK, Zhu J, Tian WH, et al. Effects of split applications of nitrogen fertilizers on the Cd level and nutritional quality of Chinese cabbage. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2017;18(10):897–905. doi: 10.1631/jzus.B1600272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant CA, Clarke JM, Duguid S, et al. Selection and breeding of plant cultivars to minimize cadmium accumulation. Sci Total Environ. 2008;390(2-3):301–310. doi: 10.1016/j.scitotenv.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 24.Gulis G, Czompolyova M, Cerhan JR. An ecologic study of nitrate in municipal drinking water and cancer incidence in Trnava District, Slovakia. Environ Res. 2002;88(3):182–187. doi: 10.1006/enrs.2002.4331. [DOI] [PubMed] [Google Scholar]
- 25.Habermeyer M, Roth A, Guth S, et al. Nitrate and nitrite in the diet: how to assess their benefit and risk for human health. Mol Nutr Food Res. 2015;59(1):106–128. doi: 10.1002/mnfr.201400286. [DOI] [PubMed] [Google Scholar]
- 26.Hakeem KR, Mir BA, Qureshi MI, et al. Physiological studies and proteomic analysis for differentially expressed proteins and their possible role in the root of N-efficient rice (Oryza sativa L.) Mol Breeding. 2013;32(4):785–798. doi: 10.1007/s11032-013-9906-0. [DOI] [Google Scholar]
- 27.Han YL, Song HX, Liao Q, et al. Nitrogen use efficiency is mediated by vacuolar nitrate sequestration capacity in roots of Brassica napus . Plant Physiol. 2016;170(3):1684–1698. doi: 10.1104/pp.15.01377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He B, Yang XE, Ni WZ, et al. Sedum alfredii: a new lead-accumulating ecotype. Acta Bot Sin. 2002;44(11):1365–1370. (in Chinese) [Google Scholar]
- 29.Huang BF, Xin JL, Dai HW, et al. Identification of low-Cd cultivars of sweet potato (Ipomoea batatas (L.) Lam.) after growing on Cd-contaminated soil: uptake and partitioning to the edible roots. Environ Sci Pollut Res. 2015;22(15):11813–11821. doi: 10.1007/s11356-015-4449-z. [DOI] [PubMed] [Google Scholar]
- 30.Huang YY, Shen C, Chen JX, et al. Comparative transcriptome analysis of two Ipomoea aquatica Forsk. cultivars targeted to explore possible mechanism of genotype-dependent accumulation of cadmium. J Agric Food Chem. 2016;64(25):5241–5250. doi: 10.1021/acs.jafc.6b01267. [DOI] [PubMed] [Google Scholar]
- 31.Jiang HM, Yang JC, Zhang JF. Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environ Pollut. 2007;147(3):750–756. doi: 10.1016/j.envpol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Kabata-Pendias A. Trace Elements in Soils and Plants, 4th Ed. CRC Press Inc., Boca Raton, USA; 2011. [Google Scholar]
- 33.Keller H, Römer W. Cu, Zn, and Cd acquisition by two spinach cultivars depending on P nutrition and root exudation. J Plant Nutr Soil Sci. 2001;164(3):335–342. doi: 10.1002/1522-2624(200106)164:3<335::aid-jpln335>3.0.co;2-c. (in German) [DOI] [Google Scholar]
- 34.Kirkham MB. Cadmium in plants on polluted soils: effects of soil factors, hyperaccumulation, and amendments. Geoderma. 2006;137(1-2):19–32. doi: 10.1016/j.geoderma.2006.08.024. [DOI] [Google Scholar]
- 35.Koh E, Charoenprasert S, Mitchell AE. Effect of organic and conventional cropping systems on ascorbic acid, vitamin C, flavonoids, nitrate, and oxalate in 27 varieties of spinach (Spinacia oleracea L.) J Agric Food Chem. 2012;60(12):3144–3150. doi: 10.1021/jf300051f. [DOI] [PubMed] [Google Scholar]
- 36.Konstantopoulou E, Kapotis G, Salachas G, et al. Nutritional quality of greenhouse lettuce at harvest and after storage in relation to N application and cultivation season. Sci Hortic. 2010;125(2):93.e1–93e5. doi: 10.1016/j.scienta.2010.03.003. [DOI] [Google Scholar]
- 37.Kopsell DA, Kopsell DE, Curran-Celentano J, et al. Carotenoid pigments in kale are influenced by nitrogen concentration and form. J Sci Food Agric. 2007;87(5):900–907. doi: 10.1002/jsfa.2807. [DOI] [Google Scholar]
- 38.Kristl J, Ivancic A, Mergedus A, et al. Variation of nitrate content among randomly selected taro (Colocasia esculenta (L.) Schott) genotypes and the distribution of nitrate within a corm. J Food Compos Anal. 2016;47:76–81. doi: 10.1016/j.jfca.2016.01.007. [DOI] [Google Scholar]
- 39.Lane EA, Canty MJ, More SJ. Cadmium exposure and consequence for the health and productivity of farmed ruminants. Res Vet Sci. 2015;101:132–139. doi: 10.1016/j.rvsc.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Li HS. Principle and Technology of Plant Physiological and Biochemical Experiment. Higher Education Press, Beijing, China; 2000. (in Chinese) [Google Scholar]
- 41.Li N, Kang Y, Pan WJ, et al. Concentration and transportation of heavy metals in vegetables and risk assessment of human exposure to bioaccessible heavy metals in soil near a waste-incinerator site, South China. Sci Total Environ. 2015;521-522:144–151. doi: 10.1016/j.scitotenv.2015.03.081. [DOI] [PubMed] [Google Scholar]
- 42.Li XH, Zhou QX, Wei SH, et al. Identification of cadmium excluding welsh onion (Allium fistulosum L.) cultivars and their mechanisms of low cadmium accumulation. Environ Sci Pollut Res. 2012;19(5):1773–1780. doi: 10.1007/s11356-011-0692-0. [DOI] [PubMed] [Google Scholar]
- 43.Liu F, Liu XN, Ding C, et al. The dynamic simulation of rice growth parameters under cadmium stress with the assimilation of multi-period spectral indices and crop model. Field Crops Res. 2015;183:225–234. doi: 10.1016/j.fcr.2015.08.004. [DOI] [Google Scholar]
- 44.Liu JG, Li KQ, Xu JK, et al. Interaction of Cd and five mineral nutrients for uptake and accumulation in different rice cultivars and genotypes. Field Crop Res. 2003;83(3):271–281. doi: 10.1016/S0378-4290(03)00077-7. [DOI] [Google Scholar]
- 45.Liu JG, Qian M, Cai GL, et al. Uptake and translocation of Cd in different rice cultivars and the relation with Cd accumulation in rice grain. J Hazard Mater. 2007;143(1-2):443–447. doi: 10.1016/j.jhazmat.2006.09.057. [DOI] [PubMed] [Google Scholar]
- 46.Liu WT, Zhou QX, An J, et al. Variations in cadmium accumulation among Chinese cabbage cultivars and screening for Cd-safe cultivars. J Hazard Mater. 2010;173(1-3):737–743. doi: 10.1016/j.jhazmat.2009.08.147. [DOI] [PubMed] [Google Scholar]
- 47.Luo JK, Sun SB, Jia LJ, et al. The mechanism of nitrate accumulation in pakchoi [Brassica campestris L.ssp. Chinensis (L.)] Plant Soil. 2006;282(1-2):291–300. doi: 10.1007/s11104-005-6094-7. [DOI] [Google Scholar]
- 48.Lux A, Martinka M, Vaculík M, et al. Root responses to cadmium in the rhizosphere: a review. J Exp Bot. 2011;62(1):21–37. doi: 10.1093/jxb/erq281. [DOI] [PubMed] [Google Scholar]
- 49.Martin SR, Llugany M, Barceló J, et al. Cadmium exclusion a key factor in differential Cd-resistance in Thlaspi arvense ecotypes. Biol Plant. 2012;56(4):729–734. doi: 10.1007/s10535-012-0056-8. [DOI] [Google Scholar]
- 50.Ministry of Health of the People’s Republic of China. Maximum Levels of Contaminats in Foods. 2012. GB 2762-2012: National Food Standard. [Google Scholar]
- 51.Mitchell LG, Grant CA, Racz GJ. Effect of nitrogen application on concentration of cadmium and nutrient ions in soil solution and in durum wheat. Can J Soil Sci. 2000;80(1):107–115. doi: 10.4141/S98-085. [DOI] [Google Scholar]
- 52.Nuzahath A, Abdukadir A, Dilnur M. Effect of phosphorus on chemical forms and physiological properties of cadmium in Fragaia ananassa D. J Soil Sci. 2013;44(6):1460–1464. (in Chinese) [Google Scholar]
- 53.Qiu Q, Wang Y, Yang Z, et al. Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food Chem Toxicol. 2011;49(9):2260–2267. doi: 10.1016/j.fct.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 54.Sharma A, Sainger M, Dwivedi S, et al. Genotypic variation in Brassica juncea (L.) Czern. cultivars in growth, nitrate assimilation, antioxidant responses and phytoremediation potential during cadmium stress. J Environ Biol. 2010;31(5):773–780. [Google Scholar]
- 55.Sørensen JN, Johansen AS, Poulsen N. Influence of growth conditions on the value of crisphead lettuce. 1. Marketable and nutritional quality as affected by nitrogen supply, cultivar and plant age. Plant Foods Hum Nutr. 1994;46(1):1–11. doi: 10.1007/BF01088455. [DOI] [PubMed] [Google Scholar]
- 56.Stagnari F, Galieni A, Pisante M. Shading and nitrogen management affect quality, safety and yield of greenhouse-grown leaf lettuce. Sci Hortic. 2015;192:70–79. doi: 10.1016/j.scienta.2015.05.003. [DOI] [Google Scholar]
- 57.Sugiyama M, Ae N, Hajika M. Developing of a simple method for screening soybean seedling cadmium accumulation to select soybean genotypes with low seed cadmium. Plant Soil. 2011;341(1-2):413–422. doi: 10.1007/s11104-010-0654-1. [DOI] [Google Scholar]
- 58.Tang L, Luo WJ, Tian SK, et al. Genotypic differences in cadmium and nitrate co-accumulation among the Chinese cabbage genotypes under field conditions. Sci Hortic. 2016;201:92–100. doi: 10.1016/j.scienta.2016.01.040. [DOI] [Google Scholar]
- 59.Tang L, Luo WJ, Chen WK, et al. Field crops (Ipomoea aquatica Forsk. and Brassica chinensis L.) for phytoremediation of cadmium and nitrate co-contaminated soils via rotation with Sedum alfredii Hance. Environ Sci Pollut Res. 2017;24(23):19293–19305. doi: 10.1007/s11356-017-9146-7. [DOI] [PubMed] [Google Scholar]
- 60.Uraguchi S, Mori S, Kuramata M, et al. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot. 2009;60(9):2677–2688. doi: 10.1093/jxb/erp119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang GL, Ding GD, Li L, et al. Identification and characterization of improved nitrogen efficiency in interspecific hybridized new-type Brassica napus . Ann Bot. 2014;114(3):549–559. doi: 10.1093/aob/mcu135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang JL, Fang W, Yang ZY, et al. Inter-and intraspecific variations of cadmium accumulation of 13 leafy vegetable species in a greenhouse experiment. J Agric Food Chem. 2007;55(22):9118–9123. doi: 10.1021/jf0716432. [DOI] [PubMed] [Google Scholar]
- 63.Wang JL, Yuan JG, Yang ZY, et al. Variation in cadmium accumulation among 30 cultivars and cadmium subcellular distribution in 2 selected cultivars of water spinach (Ipomoea aquatica Forsk.) J Agric Food Chem. 2009;57(19):8942–8949. doi: 10.1021/jf900812s. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Shi Y, Chen X, et al. Screening of Cd-safe genotypes of Chinese cabbage in field condition and Cd accumulation in relation to organic acids in two typical genotypes under long-term Cd stress. Environ Sci Pollut Res. 2015;22(21):16590–16599. doi: 10.1007/s11356-015-4838-3. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Shen H, Xu L, et al. Transport, ultrastructural localization and distribution of chemical forms of lead in radish (Raphanus sativus L.) Front Plant Sci, 6:293. 2015 doi: 10.3389/fpls.2015.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wojciechowska R, Kołton A. Comparison of the ability of fifteen onion (Allium cepa L.) cultivars to accumulate nitrates. Acta Agrobot. 2014;67(1):27–32. doi: 10.5586/aa.2014.006. [DOI] [Google Scholar]
- 67.Wu FB, Zhang G. Genotypic differences in effect of Cd on growth and mineral concentrations in barley seedlings. Bull Environ Contam Toxicol. 2002;69(2):219–227. doi: 10.1007/s00128-002-0050-5. [DOI] [PubMed] [Google Scholar]
- 68.Xin JL, Huang BF, Yang ZY, et al. Responses of different water spinach cultivars and their hybrid to Cd, Pb and Cd-Pb exposures. J Hazard Mater. 2010;175(1-3):468–476. doi: 10.1016/j.jhazmat.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 69.Xin JL, Huang BF, Yang JZ, et al. Role of roots in cadmium accumulation of two water spinach cultivars: reciprocal grafting and histochemical experiments. Plant Soil. 2013;366(1-2):425–432. doi: 10.1007/s11104-012-1439-5. [DOI] [Google Scholar]
- 70.Xin JL, Huang BF, Yang ZY, et al. Comparison of cadmium subcellular distribution in different organs of two water spinach (Ipomoea aquatica Forsk.) cultivars. Plant Soil. 2013;372(1-2):431–444. doi: 10.1007/s11104-013-1729-6. [DOI] [Google Scholar]
- 71.Xu GH, Fan XR, Miller AJ. Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol. 2012;63:153–182. doi: 10.1146/annurev-arplant-042811-105532. [DOI] [PubMed] [Google Scholar]
- 72.Yin AG, Yang ZY, Ebbs S, et al. Effects of phosphorus on chemical forms of Cd in plants of four spinach (Spinacia oleracea L.) cultivars differing in Cd accumulation. Environ Sci Pollut Res. 2016;23(6):5753–5762. doi: 10.1007/s11356-015-5813-8. [DOI] [PubMed] [Google Scholar]
- 73.Yu H, Wang JL, Fang W, et al. Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci Total Environ. 2006;370(2-3):302–309. doi: 10.1016/j.scitotenv.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 74.Zhang GP, Fukami M, Sekimoto H. Influence of cadmium on mineral concentrations and yield components in wheat genotypes differing in Cd tolerance at seedling stage. Field Crop Res. 2002;77(2-3):93–98. doi: 10.1016/S0378-4290(02)00061-8. [DOI] [Google Scholar]
- 75.Zheng SN, Zhang MK. Effect of moisture regime on the redistribution of heavy metals in paddy soil. J Environ Sci. 2011;23(3):434–443. doi: 10.1016/S1001-0742(10)60428-7. [DOI] [PubMed] [Google Scholar]
- 76.Zhou ZY, Wang MJ, Wang JS. Nitrate and nitrite contamination in vegetables in China. Food Rev Int. 2000;16(1):61–76. doi: 10.1081/fri-100100282. [DOI] [Google Scholar]