Abstract
Early studies indicated that testicular nuclear receptor 4 (TR4) could function as a suppressor in the transcriptional regulation of the HBV core gene expression, which might then influence the development of hepatocellular carcinoma (HCC). The direct linkage between TR4 and HCC progression, however, remained unclear. Here, via a human clinical sample survey, we found that 13 of the 18 HCC patients studied had lower TR4 expression in metastatic lesions than in matched primary HCC lesions, suggesting that TR4 may play a negative role in HCC metastasis. Results from in vitro cell migration/invasion studied confirmed that TR4 could suppress HCC cell migration/invasion. Mechanism dissection revealed that TR4 might function through downregulating ephrin type-A receptor 2 (EphA2) expression at the transcriptional level via direct binding to the TR4REs located on the 5′ promoter of EphA2 to suppress HCC cell migration/invasion. Targeting the EphA2 via EphA2-siRNA partially reversed the enhanced HCC cell migration/invasion with confirmed TR4 knockdown. Notably, results from preclinical studies using in vivo mouse model with orthotopic xenograft of HCC LM3 cells also confirmed the in vitro findings. Taking these findings together, preclinical studies using multiple in vitro HCC cell lines and an in vivo mouse model all led to the conclusion that TR4 may function as a suppressor of HCC metastasis and that targeting this newly identified TR4-EphA2 signaling may improve our ability to suppress HCC metastasis.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common and lethal malignant tumors, accounting for 70–90% of primary liver cancers [1–3]. It has been reported that liver cancer is the second leading cause of cancer death worldwide, with an estimated 782,500 new cases and 745,500 deaths occurring during 2012, in which China alone accounted for about 50% of the total numbers of cases and deaths [3].
The common risk factors for HCC are chronic hepatitis B virus (HBV) infection, hepatitis C virus infection, consumption of food contaminated with aflatoxin, obesity, type 2 diabetes, non-alcoholic fatty liver disease, cirrhosis related to heavy alcohol consumption, and smoking [3]. The high HCC rates in sub-Saharan Africa and parts of Asia, such as China, largely reflect the elevated prevalence of chronic HBV infection [4].
The standard treatments for HCC include surgical resection, liver transplantation, local ablation therapy, transhepatic arterial chemotherapy and embolization, and systemic treatment. Among these, surgical resection, liver transplantation, and local ablation therapy are considered as curative treatments [5, 6], which are suitable for early-stage HCC patients, accounting for about 30% of all cases [7–9]. However, almost all of these patients eventually relapse with recurrence and metastasis, which is the main lethal factor after treatment. Thus it is necessary to investigate the mechanism of HCC metastasis to achieve better treatment.
Testicular nuclear receptor 4 (TR4), one of the key transcriptional regulators belonging to the nuclear receptor superfamily, can bind to direct repeat AGGTCA sequences in gene promoters to regulate gene expression [10]. It has been demonstrated that TR4 plays significant roles in normal spermatogenesis [11], normal ovarian function [12], cerebellum development [13], glucose and lipid metabolism [14, 15], oxidative stress [16], DNA damage/repair [17], as well as HCC progression via binding to DR1 on the HBV core promoter to suppress its transcriptional regulation [18, 19].
Here we investigated the role of TR4 in HCC metastasis using immunohistochemistry (IHC) staining of TR4 from clinical tumor tissues, in vitro migration/invasion assays, and an in vivo metastasis mouse model. The results demonstrated that TR4 could suppress HCC cell migration and invasion by downregulating EphA2 expression.
Results
Lower TR4 expression in metastatic lesions of HCC patients
We first examined TR4 expression in primary HCC and matched metastatic lesions from 18 HCC patients using IHC staining (Table 1, Fig. 1a–d). There were 15 men and 3 women, all of these patients were infected with HBV, combined with liver cirrhosis in 9 patients. And the correlation analysis revealed there was no obvious correlation with TR4 expression and cirrhosis (R = 0.46, P = 0.055). German Immunoreactive Score (IRS) was calculated to measure the protein levels, and the results revealed that 13 patients had lower TR4 expression in metastatic lesions than in their matched primary HCC lesions, while such levels in the other 5 patients were equal, with significant difference (P = 0.014). We also analyzed the TR4 expression in the clinical samples of these 18 patients by reverse transcriptase quantitative PCR (RT-qPCR). As metastatic tissues are difficult to extract without the contamination of normal tissues or primary HCC tissues, we compared TR4 expression between normal liver tissues and primary HCC tissues, and the results showed lower expression of TR4 in primary HCC tissues (P = 0.043; Fig. 1e). The results above suggest that TR4 may play an inhibitory role during HCC metastasis.
Table 1.
Clinical data and TR4 expression of primary HCC and its matched metastatic lesions from 18 patients
| Patient | Age | Gender | HBV infection | HCV infection | Alcoholic hepatitis | Cirrhosis | Metastatic lesion | TR4 expression in primary lesion | TR4 expression in metastatic lesion |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | M | + | − | − | − | Lung metastasis | Strong (12) | Moderate (6) |
| 2 | 43 | M | + | − | − | + | Bile duct tumor thrombus | Strong (12) | Weak (4) |
| 3 | 33 | M | + | − | − | + | Portal vein tumor thrombus | Strong (9) | Weak (4) |
| 4 | 60 | M | + | − | − | + | Bile duct tumor thrombus | Strong (9) | Weak (4) |
| 5 | 57 | M | + | − | − | + | Lymph node metastasis | Moderate (8) | Weak (4) |
| 6 | 41 | M | + | − | − | − | Bile duct tumor thrombus | Moderate (8) | Weak (4) |
| 7 | 61 | M | + | − | − | + | Portal vein tumor thrombus | Moderate (8) | Weak (2) |
| 8 | 69 | M | + | − | − | − | Bile duct tumor thrombus | Moderate (6) | Weak (2) |
| 9 | 66 | M | + | − | − | + | Bile duct tumor thrombus | Moderate (8) | Weak (2) |
| 10 | 58 | M | + | − | − | − | Portal vein tumor thrombus | Moderate (6) | Weak (4) |
| 11 | 40 | M | + | − | − | − | Portal vein tumor thrombus | Weak (4) | Negative (1) |
| 12 | 49 | M | + | − | − | − | Bile duct tumor thrombus | Weak (4) | Negative (1) |
| 13 | 49 | F | + | − | − | − | Portal vein tumor thrombus | Weak (2) | Negative (1) |
| 14 | 55 | F | + | − | − | + | Portal vein tumor thrombus | Moderate (8) | Moderate (6) |
| 15 | 62 | M | + | − | − | + | Inferior vena cava tumor thrombus | Moderate (6) | Moderate (6) |
| 16 | 63 | F | + | − | − | − | Bile duct tumor thrombus | Weak (4) | Weak (2) |
| 17 | 61 | M | + | − | − | − | Portal vein tumor thrombus | Weak (2) | Weak (2) |
| 18 | 52 | M | + | − | − | + | Portal vein tumor thrombus | Weak (2) | Weak (2) |
German Immunoreactive Score (IRS) was calculated to measure TR4 expression
Fig. 1. IHC staining results investigating TR4 level in primary HCC and their matched metastatic lesions.
Eighteen pairs of clinical specimens of primary HCC and their matched metastatic lesions were obtained from Sir Run Run Shaw Hospital, Zhejiang University, School of Medicine, Hangzhou, China. IHC staining was performed using TR4 antibody (1:100). a, b TR4 expression in one matched primary and metastatic clinical sample: TR4 expression level is strong in primary HCC lesion (a) and weak in its bile duct tumor thrombus (b). c, d TR4 expression in another matched primary and metastatic clinical sample: TR4 expression level is moderate in primary HCC lesion (c) and weak in its lymph node metastasis (d). e RT-qPCR results showed lower expression of TR4 (P = 0.043) and higher EphA2 expression (P = 0.020) in primary HCC tissues compared with their matched normal liver tissues. P-values presented in figures, *P < 0.05
TR4 suppresses HCC cell migration and invasion
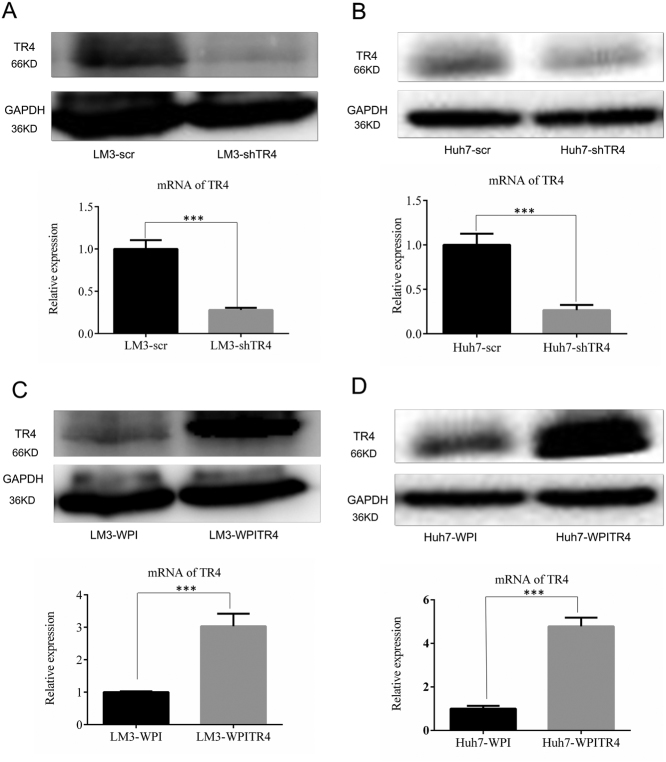
To confirm the above preliminary clinical data, we then examined the role of TR4 in HCC progression in the in vitro cell lines. We first manipulated TR4 expression in two HCC cells (LM3 and Huh7) by either knocking down TR4 with TR4-shRNA (Fig. 2a, b) or adding functional TR4-cDNA via a lentiviral system (Fig. 2c, d).
Fig. 2. Successful manipulation of TR4 expression in LM3 and Huh7 cells.
a, b Knocking down efficiency of TR4 in LM3 and Huh7 cells. Upper and lower panels show TR4 expression at protein and mRNA levels, respectively. c, d Overexpression efficiency of TR4 in LM3 and Huh7 cells. Upper and lower panels show TR4 expression at protein and mRNA levels, respectively. P-values presented in figures, ***P < 0.001
We then applied the MTS proliferation assay [20] to examine the impact on the growth of HCC cells of altering their TR4 expression. The results revealed that little change occurred after altering the TR4 expression in both LM3 and Huh7 cell lines (Fig. 3a–d).
Fig. 3. TR4 suppresses HCC cell migration and invasion.
a, b MTS proliferation assay demonstrates that knocking down TR4 has little influence on LM3 and Huh7 cells proliferation. c, d MTS proliferation assay demonstrates that overexpression of TR4 has little influence on LM3 and Huh7 cells proliferation. e, i Knocking down TR4 promotes LM3 cells migration and invasion. f, j Knocking down TR4 promotes Huh7 cells migration and invasion. g, k Overexpression of TR4 suppresses LM3 cells migration and invasion. h, l Overexpression of TR4 suppresses Huh7 cells migration and invasion. The migrated or invaded cells were stained with crystal violet (0.1%) and positively stained cell numbers in six randomly picked areas were averaged. Experiments are repeated three times and mean ± SD values are shown in quantification. P-values presented in figures, ***P < 0.001
However, the results from migration assay revealed that HCC cell migration was significantly enhanced after knocking down TR4 in both LM3 (Fig. 3e) and Huh7 cells (Fig. 3f). Furthermore, when we replaced the migration assay with the invasion assay, we found similar results showing that knocking down TR4 significantly enhanced HCC cell invasion in LM3 (Fig. 3i) and Huh7 cells (Fig. 3j).
We also used the opposite approach with the overexpression of TR4 to examine the impact of TR4 on HCC cell migration and invasion. The results revealed that cell migration and invasion abilities were significantly suppressed in HCC LN3 cells after adding TR4-cDNA (Fig. 3g, h, k, l). Together, the results from Figs. 2 and 3 using two different approaches of knocking down or adding TR4 in two different HCC cell lines all demonstrated that TR4 suppressed HCC cell migration/invasion.
Mechanism dissection of how TR4 suppresses HCC cell migration and invasion: via suppressing the EphA2 expression
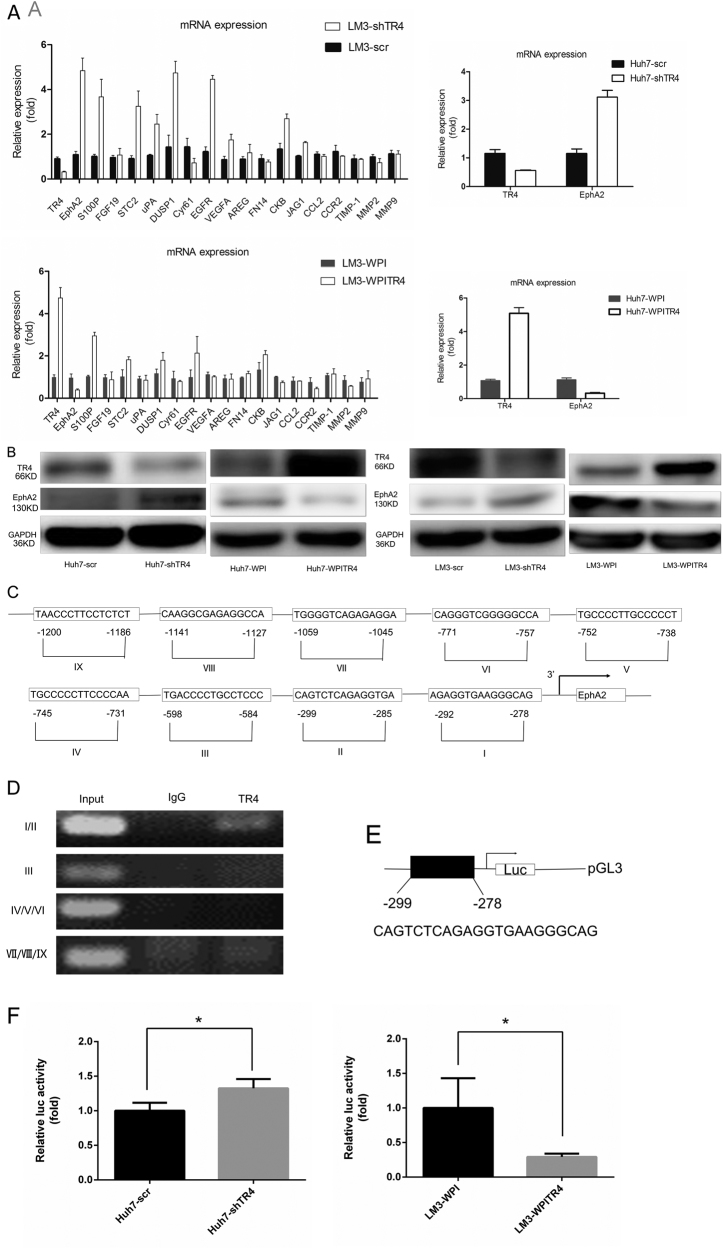
To dissect the molecular mechanism by which TR4 suppresses HCC cell migration/invasion, we screened the different expression of HCC metastasis-related genes between TR4-knocked-down Huh7 cells (Huh7-shTR4) and their scramble cells (Huh7-scr) by transcriptome sequencing. We found that targeting TR4 altered the expression of some metastasis-associated genes in Huh7-shTR4 cells compared with those in their scramble cells (supplementary Table 1). We selected some of these genes and applied qPCR assay to further verify the results.
Among these metastasis-associated genes, we noted that the expression of EphA2 mRNA was significantly increased when we knocked down TR4 in the LM3 cell line, while the opposite result was obtained when we added TR4-cDNA (Fig. 4a). We also tested EphA2 mRNA expression after modulating TR4 expression in Huh7 cell line, and similar results were observed (Fig. 4a). We further examined its expression at the protein level using western blotting and found that knocking down TR4 resulted in increased EphA2 protein expression and adding TR4-cDNA resulted in decreased EphA2 protein expression in both Huh7 and LM3 cells (Fig. 4b).
Fig. 4. TR4 regulates EphA2 expression at the transcriptional level.
a Knocking down TR4 in LM3 and Huh7 cells results in increased mRNA expression of EphA2, and overexpression of TR4 in LM3 and Huh7 cells results in decreased mRNA expression of EphA2. b EphA2 western blotting test results. Knocking down TR4 in Huh7 and LM3 cells results in increased EphA2 expression. Overexpression of TR4 in Huh7 and LM3 cells results in decreased EphA2 expression. (c) Nine putative TR4-response-elemenst (TR4REs) in the 2-Kb region of EphA2 promoter are predicted by ALGGEN-PROMO program. d ChIP assay reveals that TR4RE 1/2 but not the rest of other TR4REs are the potential binding sites. e Construction of pGL3-EphA2 promoter containing TR4-binding element sequence. f Luciferase assay results. Knocking down TR4 results in increased luciferase activity in Huh7 cells (left panel) and overexpression of TR4 results in decreased luciferase activity in LM3 cells (right panel). (*P < 0.05, **P < 0.01)
Taken together, the results from supplementary Table 1 and Fig. 4a, b suggest that TR4 suppresses EphA2 expression.
Mechanism dissection of how TR4 suppresses EphA2 expression: via transcriptional regulation
TR4 is one of the transcriptional regulators, and knocking down or overexpressing it in LM3 cells can result in significantly increased or decreased expression of EphA2 at the mRNA level, respectively. We further investigated the molecular mechanisms at the transcriptional level and applied the ALGGEN-PROMO (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB) program to analyze the 2-Kb region of the EphA2 promoter and found nine putative TR4-response elements (TR4REs) (Fig. 4c). We then applied a chromatin immunoprecipitation (ChIP) binding assay and found that TR4 could bind to TR4RE1/2, but not the other TR4REs (Fig. 4d). We then constructed an EphA2-luciferase reporter by inserting the 2000-bp 5′ promoter region of EphA2 containing TR4REs into the PGL3 luciferase plasmid (Fig. 4e) and tested whether the expression of this promoter-mediated luciferase activity could be changed after altering TR4 expression in HCC LM3-WPITR4 cells and Huh7-shTR4 cells. The results revealed that knocking down TR4 could increase the luciferase activity in Huh7 cells (Fig. 4f, left panel) and adding TR4 could decrease such activity in LM3 cells (Fig. 4f, right panel).
Taken together, the results from Fig. 4c–f suggest that TR4 can suppress EphA2 expression at the transcriptional regulation via direct binding to the TR4REs located on the 5′ promoter of EphA2.
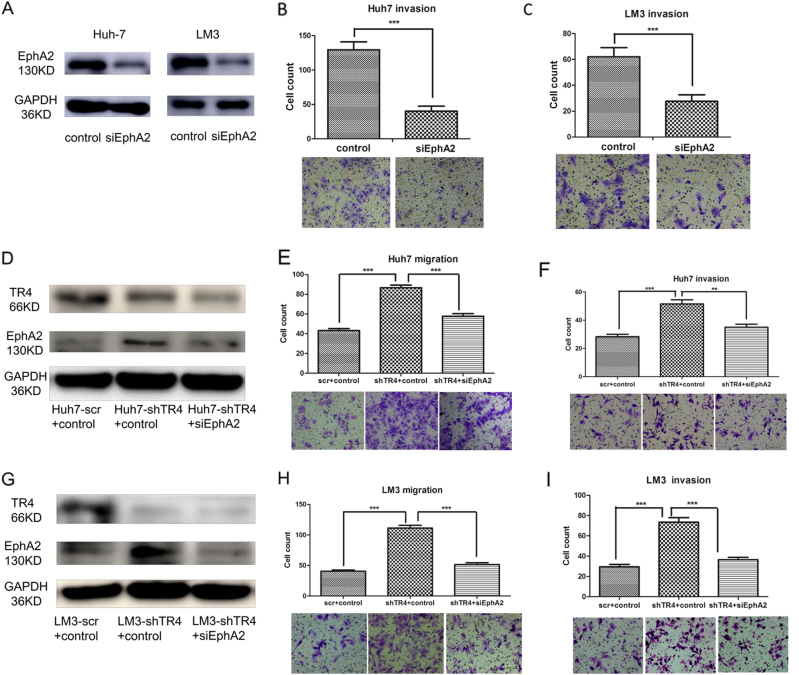
EphA2 plays critical roles in mediating TR4-suppressed HCC cell migration and invasion
For further investigation of whether EphA2 plays critical roles in mediating TR4-suppressed HCC cell migration and invasion, we first manipulated EphA2 expression in HCC cell to verify whether it plays a critical role in the suppression of HCC cell invasion. We knocked down EphA2 in both Huh7 and LM3 cells by EphA2-siRNA (Fig. 5a). Chamber cell co-culture invasion assay revealed that HCC cell invasion was significantly suppressed after knocking down EphA2 in both Huh7 (Fig. 5b) and LM3 cells (Fig. 5c). We then performed neutralization/interruption experiments by transfecting EphA2-siRNA into Huh7-shTR4 cells (Fig. 5d). The results revealed that the disruption of EphA2 by EphA2-siRNA can partially reverse the increasing migration (Fig. 5e) and invasion abilities (Fig. 5f) of Huh7 cells with TR4 knockdown. Similar results were also obtained when we replaced Huh7 cells with LM3 cells (Fig. 5g–i). We also compared EphA2 expression between normal liver tissues and primary HCC tissues in 18 HCC patients, and the results showed higher expression of EphA2 in primary HCC tissues (P = 0.020; Fig. 1e).
Fig. 5. Interrupting EphA2 by siEphA2 reversed the increased migration and invasion ability in TR4 knocking down HCC cells.
a WB results show the knocking down efficiency of EphA2 in Huh7 and LM3 cells. b Knocking down EphA2 suppresses Huh7 cells invasion. c Knocking down EphA2 suppresses LM3 cells invasion. d WB results show the efficiency of the disruption of EphA2 expression by transfecting with siEphA2 in Huh7-shTR4 cells. e Interrupting EphA2 can partially reverse the increasing migration in Huh7-shTR4 cells. f Interrupting EphA2 can partially reverse the increasing invasion in Huh7-shTR4 cells. g WB results show the efficiency of the disruption of EphA2 expression by transfecting with siEphA2 in LM3-shTR4 cells. h Interrupting EphA2 can partially reverse the increasing migration in LM3-shTR4 cells. i Interrupting EphA2 can partially reverse the increasing invasion in LM3-shTR4 cells. (**P < 0.01, ***P < 0.001)
Taken together, the results from Figs. 1e and 5a–i suggest that EphA2 may play critical roles in mediating TR4-suppressed HCC cell migration and invasion.
Preclinical study using in vivo mouse model showing that TR4 knocking down promotes HCC cell invasion
To confirm the validity of the above in vitro data in an in vivo mouse model, we then established an orthotopically xenografted mouse model using luciferase-expressing TR4-knock down LM3 and corresponding scramble cells. LM3 cells were transfected with pGL4.17 vector carrying luciferase and the stable clone cells expressing luciferase were selected, expanded, and infected with lentivirus carrying pLKO.1-shTR4 or pLKO.1 scramble, followed by the selection of stable cells (luc-LM3-shTR4 and luc-LM3-scr).
We divided the mice into two groups. Group 1 mice (n = 5) were injected with luc-LM3-scr cells and group 2 mice (n = 5) were injected with luc-LM3-shTR4 cells. The non-invasive In Vivo Imaging Systems (IVIS) was applied weekly to monitor tumor growth and metastasis. Six weeks later, we analyzed tumor growth and metastasis in both groups. As shown in Fig. 6a, we observed intrahepatic tumor formation in each mouse of the two groups. However, we detected a clear difference in metastasis between these two groups: no metastasis was detected in any of the five mice in group 1, while it was observed in three of the five mice in group 2. These IVIS imaging results were further confirmed after the mice were sacrificed; there was one mouse with intrahepatic metastases, one with intrahepatic and diaphragm metastases, and another with intrahepatic, diaphragm, and omentum metastases in group 2. We applied hematoxylin & eosin (H&E) staining to confirm the presence of the tumor and its metastatic lesions (Fig. 6b).
Fig. 6. In vivo mice studies using the LM3 xenograft model.
The luc-LM3-scr and luc-LM3-shTR4 cells (2 × 106) were orthotopically injected into the left lateral lobe of the liver of athymic nude mice. a IVIS imaging was used to determine the tumor size and metastasis, and the results showed that no metastasis was detected in any of the five mice that were injected with luc-LM3-scr cells. In three of the five mice that were injected with luc-LM3-shTR4 cells, tumor metastasis was observed on Day 42. b HE staining results of HCC and the metastatic tumor tissues. c IHC staining was used for detecting TR4 and EphA2 expression levels in tumor tissues obtained from the two groups of mice. d RT-qPCR was used for detecting TR4 and EphA2 expression levels in tumor tissues obtained from the two groups of mice.
We also applied IHC staining and RT-qPCR for the key molecules involved in TR4-suppressed HCC cell invasion. The results revealed that tumor tissues in group 2 mice (luc-LM3-shTR4 cells derived) had higher expression of EphA2 than tumor tissues in group 1 mice (Fig. 6c, d). Moreover, the metastatic lesions had higher EphA2 than their primary lesions (Fig. 6c, d).Taken together, the results from Fig. 6a–c suggest that TR4 can suppress HCC cell invasiveness and the mechanism behind this may involve the downregulation of EphA2 expression.
Discussion
HCC is one of the deadliest human cancers because of its high incidence of metastasis. Although many attempts have been made to improve its survival, metastasis remains the major cause of death from HCC [21, 22]. Unfortunately, the detailed mechanisms behind the metastasis in HCC have remained unclear.
Here we demonstrated that TR4 might function in suppressing HCC metastasis. This is interesting since it contradicts an early study showing that TR4 could promote prostate cancer metastasis through the tissue inhibitor of metalloproteinase 1 (TIMP-1)/matrix metalloproteinase 2 (MMP2)/MMP9 [23] and C-C chemokine motif ligand 2 (CCL2)/C-C chemokine motif receptor 2 (CCR2) signaling pathways [24]. The detailed mechanisms explaining these opposite roles remain unclear and require further elucidation. To dissect the mechanisms involved, we speculated that TR4 might need to go through different signaling pathways to regulate different tumor metastases, and a tissue-specific factor or different TR4 expression levels in different tissues might contribute to regulating different signaling cascades.
HBV is known as a major risk factor for HCC progression [25] and a previous study also demonstrated that TR4 could suppress the transcriptional regulation of HBV core gene expression [18]. In this study, all of the included cell lines and clinical HCC tissues had an HBV infection background, so the conclusion of this study may depend on the background of HBV infection. In this study, we have tested the mRNA expression levels of TIMP-1, MMP2, MMP9, CCL2, and CCR2, and no significant differences were observed neither by knocking down nor by overexpressing TR4 in LM3 cells (Fig. 4a).
Moreover, these contrasting roles of TR4 to either enhance or suppress tumor metastasis are not unique, since other nuclear receptors such as androgen receptor also play opposite roles in various cancers, namely, functioning as a suppressor in HCC [26, 27] and prostate cancer [28, 29] metastasis but as a stimulator promoting bladder cancer [30, 31] and renal cancer [32] metastasis.
In this study, we found that EphA2 expression was increased upon knocking down TR4 in HCC cells. EphA2 is a representative member of a 16-member superfamily of receptor tyrosine kinases and functions as a key mediator of tumor progression [33, 34]. It has been reported that overexpression of EphA2 relate to tumor progression, metastasis, and prognosis in HCC [35]. Cui XD et al. found that EphA2 expression was prominent in highly invasive hepatoma cells, and its overexpression was significantly correlated with decreased differentiation and poor survival for HCC patients [36]. Another study also indicated that microRNA-miR-26b could inhibit HCC cell migration and invasion via the downregulation of EphA2 [37]. In this study, HCC cell invasion was significantly suppressed after knocking down EphA2 in both Huh7 and LM3 cells.
Further studies demonstrated that TR4 suppressed EphA2 expression at the transcriptional level. Neutralization/interruption experiments and in vivo mouse studies indicated that EphA2 may play critical roles in mediating TR4-suppressed HCC cell migration and invasion. The finding that TR4 functions by altering EphA2 expression further supports the importance of EphA2 in HCC metastasis and may provide us with a new target to suppress HCC metastasis.
In summary, preclinical studies using multiple in vitro cell lines and an in vivo mouse model all demonstrated that TR4 has a protective role in suppressing HCC metastasis via downregulating EphA2 expression. Targeting this newly identified TR4–EphA2 signal may help us to develop new therapies to improve the suppression of HCC metastasis.
Materials and methods
Cell culture
The human HCC cell lines Huh7 and LM3 were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Huh7 and LM3 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) medium containing 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37 °C in 5% CO2.
Antibodies
Anti-TR4 (PP-0107B-00) was purchased from R&D systems (Minneapolis, MN), Anti-EphA2, and Anti-GAPDH (6c5) antibodies was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Plasmids
The siTR4 sequence (5′-cgggagaaaccaagcaattg-3′) was cloned into the Age I and EcoR I sites of pLKO.1 vector to construct the pLKO.1-shTR4 plasmid. Full-length TR4 cDNA was ligated into the Pme I site of the pWPI vector to construct the pWPI-TR4 plasmid.
Lentiviral infection
For the infection of lentivirus, 293T cells were transfected with a mixture of DNAs consisting of target plasmids (pLKO.1 scramble, pLKO.1-shTR4, pWPI, and pWPI-TR4), psPAX2 packaging plasmid, and pMD2G envelope plasmid at a ratio of 4:3:2 using Lipofectamine 2000 (Invitrogen). Lentiviral supernate were then collected to infect HCC cells. After viral infection, the media was replaced with normal culture media. The stable cells were selected and established about 2 weeks later by puromycin (1 μg/ml in medium) and confirmed by quantitative real-time PCR (qPCR) and western blotting and then named as HCC-scr/HCC-shTR4 or HCC-WPI/HCC-WPITR4.
Quantitative RT-PCR
The qPCR was carried out using the SYBP Green PCR Amplification Kit (Applied Biosystems). The primers of TR4 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were designed by PrimerPremier 5.0 and synthetized by Biosune Biological Technology. The qPCR reaction condition: Step1: 95 °C, 2 min; Step2: 95 °C, 30 s; 60 °C, 30 s; 68 °C, 1 min; 40 cycles; Step3: 72 °C, 10 min. The results were analyzed by delta–delta Ct method. The sequences of primers are shown in supplementary Table 2.
Western blotting
Cells were harvested and washed twice with cold phosphate-buffered saline (PBS), then resuspended and lysed in RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 10 ng/ml phenylmethanesulfonylfluoride, 0.03% aprotinin, 1 μM sodium orthovanadate) at 4 °C for 30 min. Lysates were centrifuged for 15 min at 12,000 × g and supernatants were stored at −80 °C as whole-cell extracts. Total protein concentrations were determined by Bradford assay. Proteins were separated on 12% SDS-polyacrylamide gel electrophoresis gels and transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% bovine serum albumin and incubated with the indicated primary antibodies. Corresponding horseradish peroxidase-conjugated secondary antibodies were used against each primary antibody. Proteins were detected using the chemiluminescent detection reagents.
IHC staining
We collected 18 pairs of primary HCC and then metastatic lesions from HCC patients at Sir Run Run Shaw Hospital. IHC was then performed to evaluate TR4 expression in these samples. IHC was also performed in tumors of orthotopically xenografted mouse model to evaluate TR4 and EphA2 expression. Tissues were fixed in 10% (v/v) formaldehyde in PBS, embedded in paraffin, and cut into 5-μm sections for H&E and IHC staining. IHC staining was performed using TR4 antibody (1:100) and EphA2 antibody (1:100). German IRS was calculated to measure the protein levels. Briefly, the IRS assigns subscores for the percentage of immunoreactive cells (0–4) and immunoreactive intensity (0–3), then multiplies them to yield the IRS score, which ranged from 0 to 12. The percentage of positivity was scored as “0” (<1%), “1” (1–10%), “2” (11–50%), “3” (51–80%), and “4” (>80%). The staining intensity was scored as “0” (negative), “1” (weak), “2” (moderate), and “3” (strong). Scores were considered negative (0–1), weakly positive (2–4), moderately positive (6–8), and strongly positive (9–12).
Cell proliferation assay
For cell proliferation assay, 1000 cells were seeded into 96-well plates (per well) and incubated for different times (0, 24, 48, and 72 h). After that, 20 μl of MTS (Cell Titer 96 Aqueous One Solution Reagent; Promega) was added to the wells and then incubated at 37 °C for 4 h. The absorbance was detected at 490 nm with a microplate reader.
Cell migration and invasion assays
Briefly, 1 × 105 cells were seeded in top chambers of the transwell plates (BD Biosciences, San Jose, CA) in 1% FBS media with membrane inserts coated either with or without matrigel (8%) for invasion and migration tests, respectively. Bottom chambers were filled with DMEM medium with 10% FBS. After 16–24 h (for migration) or 36–48 h (for invasion) incubation, cells that migrated/invaded to the lower surface of the membrane were fixed and stained, and the cell numbers in six random fields were counted under the light microscope.
Neutralization/interruption experiment
TR4 knocked down LM3 and Huh7 cells were transfected with EphA2 small interfering RNA (siEphA2) and its vector as control using Lipofectamine 2000 (Invitrogen). Forty eight hours after transfection, cells were harvested for western blotting, migration, and invasion assays as mentioned above.
In vivo mice studies
The animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals and comply with the ARRIVE guidelines. Ten mice (athymic nude) were equally divided into two groups. Group 1 mice were injected with luc-LM3-scr cells and Group 2 mice were injected with luc-LM3-shTR4 cells. Cells were suspended in DMEM media and injected into the left lateral lobe of the liver (2 × 106 cells, each mouse) of these athymic nude mice by surgery. Every week, tumor growth and metastasis were monitored by in vivo imaging system. All mice of these two groups were sacrificed 6 weeks after surgery.
Plasmid construction and luciferase reporter assay
A 2000-bp promoter of EphA2 was obtained from genomic DNA of 293T cells by Phusion® High-Fidelity DNA Polymerase (NEB, Beverly, NY) and cloned into pGL3-basic vector (Promega, Madison, WI) by Gibson assembly method. For the luciferase reporter assay, cell transfection was performed using Lipofectamine 2000 (Invitrogen). Cells were co-transfected with EphA2-pGL3 and pRL-TK vector as an internal control. Forty eight hours after transfection, cells were harvested with Passive Lysis Buffer (Promega, Madison, WI), and luciferase activities were analyzed using the Dual Luciferase Reporter Assay system (Promega, Madison, WI).
ChIP assay
ChIP assay was done using the ChIP Assay Kit (Cell signaling, Irvine, CA) following the manufacturer’s protocol. The following primer pairs were used for the amplification of the TR4RE site in EphA2 promoter sequence. PCR products were analyzed by agarose gel electrophoresis.
| Forward | Reverse | |
|---|---|---|
| TR4RE 1/2 | GGAGGCAACTGCTTATTGGA | AGGCCTTCCAAAGTTTGAGC |
| TR4RE 3 | AAGCAGAGACCACCAGGAT | TTCCTCTGGGAATGGATCAG |
| TR4RE 4/5/6 | TATCAAGGGGCAGGTGGTAG | AGGCTCCAAGAGCAGAAACA |
| TR4RE 7/8/9 | ACAGGCTCTCAGAGGACCAA | CCCTTTGCCTACCTCTTCCT |
Statistical analysis
All results are expressed as mean ± standard deviation (SD). Statistical analysis of the difference between treated and control groups was performed with Student’s t-test. McNemer chi-square test was applied for pair test and Spearman rank correlation is used for correlation analysis. Each experiment/statistical test was performed three times. Values of P < 0.05 were considered as significant differences.
Electronic supplementary material
Acknowledgements
This work was supported by an NIH grant CA156700 and George Whipple Professorship Endowment and Taiwan Department of Health Clinical Trial and Research Center of Excellence grant DOH99-TD-B-111-004. Excellence grant DOH99-TD-B-111-004, Natural Science Foundation of China General Project grant 81772546, and The Fundamental Research Funds for the Central Universities Zhejiang, Provincial Natural Science Foundation of China grant LZ14H160002. We thank Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac) for editing the English text of a draft of this manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
These authors contributed equally: R Jin, H Lin, G Li.
Edited by Y Shi
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41419-018-0287-5).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chawnshang Chang, Phone: +001-585-2734500, Email: chang@urmc.rochester.edu.
Xiujun Cai, Phone: +0086-571-86006605, Email: srrsh_cxj@zju.edu.cn.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, et al. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, et al. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Franceschi S, Raza SA. Epidemiology and prevention of hepatocellular carcinoma. Cancer Lett. 2009;286:5–8. doi: 10.1016/j.canlet.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J. Hepatol. 2012;56(Suppl 1):S75–S87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 8.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin. Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 10.Rastinejad F, Perlmann T, Evans RM, Sigler PB. Structural determinants of nuclear receptor assembly on DNA direct repeats. Nature. 1995;375:203–211. doi: 10.1038/375203a0. [DOI] [PubMed] [Google Scholar]
- 11.Mu X, et al. Targeted inactivation of testicular nuclear orphan receptor 4 delays and disrupts late meiotic prophase and subsequent meiotic divisions of spermatogenesis. Mol. Cell. Biol. 2004;24:5887–5899. doi: 10.1128/MCB.24.13.5887-5899.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen LM, et al. Subfertility with defective folliculogenesis in female mice lacking testicular orphan nuclear receptor 4. Mol. Endocrinol. 2008;22:858–867. doi: 10.1210/me.2007-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YT, Collins LL, Uno H, Chang C. Deficits in motor coordination with aberrant cerebellar development in mice lacking testicular orphan nuclear receptor 4. Mol. Cell. Biol. 2005;25:2722–2732. doi: 10.1128/MCB.25.7.2722-2732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu NC, et al. Loss of TR4 orphan nuclear receptor reduces phosphoenolpyruvate carboxykinase-mediated gluconeogenesis. Diabetes. 2007;56:2901–2909. doi: 10.2337/db07-0359. [DOI] [PubMed] [Google Scholar]
- 15.Kim E, et al. Disruption of TR4 orphan nuclear receptor reduces the expression of liver apolipoprotein E/C-I/C-II gene cluster. J. Biol. Chem. 2003;278:46919–46926. doi: 10.1074/jbc.M304088200. [DOI] [PubMed] [Google Scholar]
- 16.Li G, et al. Oxidative stress stimulates testicular orphan receptor 4 through forkhead transcription factor forkhead box O3a. Endocrinology. 2008;149:3490–3499. doi: 10.1210/en.2008-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S, et al. Testicular nuclear receptor 4 (TR4) regulates UV light-induced responses via Cockayne syndrome B protein-mediated transcription-coupled DNA repair. J. Biol. Chem. 2011;286:38103–38108. doi: 10.1074/jbc.M111.259523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin WJ, et al. Suppression of hepatitis B virus core promoter by the nuclear orphan receptor TR4. J. Biol. Chem. 2003;278:9353–9360. doi: 10.1074/jbc.M205944200. [DOI] [PubMed] [Google Scholar]
- 19.de Martel C, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 20.Ma X, et al. MicroRNA-185 inhibits cell proliferation and induces cell apoptosis by targeting VEGFA directly in von Hippel-Lindau-inactivated clear cell renal cell carcinoma. Urol. Oncol. 2015;33:169.e1–169. doi: 10.1016/j.urolonc.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat. Dis. Int. 2008;7:237–257. [PubMed] [Google Scholar]
- 22.Wang W, Wu F, Fang F, Tao Y, Yang L. Inhibition of invasion and metastasis of hepatocellular carcinoma cells via targeting RhoC in vitro and in vivo. Clin. Cancer Res. 2008;14:6804–6812. doi: 10.1158/1078-0432.CCR-07-4820. [DOI] [PubMed] [Google Scholar]
- 23.Ding X, et al. Targeting TR4 nuclear receptor suppresses prostate cancer invasion via reduction of infiltrating macrophages with alteration of the TIMP-1/MMP2/MMP9signals. Mol. Cancer. 2015;14:16. doi: 10.1186/s12943-014-0281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding X, et al. TR4 nuclear receptor promotes prostate cancer metastasis via upregulation of CCL2/CCR2 signaling. Int. J. Cancer. 2015;136:955–964. doi: 10.1002/ijc.29049. [DOI] [PubMed] [Google Scholar]
- 25.Di Bisceglie AM. Hepatitis B and hepatocellular carcinoma. Hepatology. 2009;49(5 Suppl):S56–S60. doi: 10.1002/hep.22962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma WL, Jeng LB, Lai HC, Liao PY, Chang C. Androgen receptor enhances cell adhesion and decreases cell migration via modulating β1-integrin-AKT signaling in hepatocellular carcinoma cells. Cancer Lett. 2014;351:64–71. doi: 10.1016/j.canlet.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Ma WL, et al. Hepatic androgen receptor suppresses hepatocellular carcinoma metastasis through modulation of cell migration and anoikis. Hepatology. 2012;56:176–185. doi: 10.1002/hep.25644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie H, et al. Infiltrated pre-adipocytes increase prostate cancer metastasis via modulation of the miR-301a/androgen receptor (AR)/TGF-β1/Smad/MMP9 signals. Oncotarget. 2015;6:12326–12339. doi: 10.18632/oncotarget.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, et al. Infiltrating mast cells enhance prostate cancer invasion via altering LncRNA-HOTAIR/PRC2-androgen receptor (AR)-MMP9 signals and increased stem/progenitor cell population. Oncotarget. 2015;6:14179–14190. doi: 10.18632/oncotarget.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ou Z, et al. Tumor microenvironment B cells increase bladder cancer metastasis via modulation of the IL-8/androgen receptor (AR)/MMPs signals. Oncotarget. 2015;6:26065–26078. doi: 10.18632/oncotarget.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shang Z, et al. Antiandrogen therapy with hydroxyflutamide or androgen receptor degradation enhancer ASC-J9 enhances BCG efficacy to better suppress bladder cancer progression. Mol. Cancer Ther. 2015;14:2586–2594. doi: 10.1158/1535-7163.MCT-14-1055-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He D, et al. ASC-J9 suppresses renal cell carcinoma progression by targeting an androgen receptor-dependent HIF2α/VEGF signaling pathway. Cancer Res. 2014;74:4420–4430. doi: 10.1158/0008-5472.CAN-13-2681. [DOI] [PubMed] [Google Scholar]
- 33.Brantley-Sieders D, Schmidt S, Parker M, Chen J. Eph receptor tyrosine kinases in tumor and tumor microenvironment. Curr. Pharm. Des. 2004;10:3431–3442. doi: 10.2174/1381612043383160. [DOI] [PubMed] [Google Scholar]
- 34.Tandon M, Vemula SV, Mittal SK. Emerging strategies for EphA2 receptor targeting for cancer therapeutics. Expert Opin. Ther. Targets. 2011;15:31–51. doi: 10.1517/14728222.2011.538682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang P, et al. Overexpression of EphA2, MMP-9, and MVD-CD34 in hepatocellular carcinoma: Implications for tumor progression and prognosis. Hepatol. Res. 2009;39:1169–1177. doi: 10.1111/j.1872-034X.2009.00563.x. [DOI] [PubMed] [Google Scholar]
- 36.Cui XD, et al. EFNA1 ligand and its receptor EphA2: potential biomarkers for hepatocellular carcinoma. Int. J. Cancer. 2010;126:940–949. doi: 10.1002/ijc.24798. [DOI] [PubMed] [Google Scholar]
- 37.Li H, et al. MiR-26b inhibits hepatocellular carcinoma cell proliferation, migration, and invasion by targeting EphA2. Int J. Clin. Exp. Pathol. 2015;8:4782–4790. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.