Abstract
Importance
Unruptured intracranial aneurysms (UIAs) are relatively common in the general population and are being increasingly diagnosed; a significant proportion are tiny (≤3 mm) aneurysms. There is significant heterogeneity in practice and lack of clear guidelines on the management of incidental, tiny UIAs. It is important to quantify the implications of different management strategies in terms of health benefits to patients.
Objective
To evaluate the effectiveness of routine treatment (aneurysm coiling) vs 3 strategies for imaging surveillance compared with no preventive treatment or routine follow-up of tiny UIAs.
Design, Setting, and Participants
A decision-analytic model-based comparative effectiveness analysis was conducted from May 1 to June 30, 2017, using inputs from the medical literature. PubMed searches were performed to identify relevant literature for all key model inputs, each of which was derived from the clinical study with the most robust data and greatest applicability. Analysis included 10 000 iterations simulating adult patients with incidental detections of UIAs 3 mm or smaller and no history of subarachnoid hemorrhage.
Interventions
The following 5 management strategies for tiny UIAs were evaluated: annual magnetic resonance angiography (MRA) screening, biennial MRA screening, MRA screening every 5 years, aneurysm coiling and follow-up, and no treatment or preventive follow-up.
Main Outcomes and Measures
A Markov decision model for lifetime rupture was constructed from a societal perspective per 10 000 patients with incidental, tiny UIAs. Outcomes were assessed in terms of quality-adjusted life-years. Probabilistic, 1-way, and 2-way sensitivity analyses were performed.
Results
In this analysis of 10 000 iterations simulating adult patients with a mean age of 50 years, the base-case calculation shows that the management strategy of no treatment or preventive follow-up has the highest health benefit (mean [SD] quality-adjusted life-years, 19.40 [0.31]). Among the management strategies that incorporate follow-up imaging, MRA every 5 years is the best strategy with the next highest effectiveness (mean [SD] quality-adjusted life-years, 18.05 [0.62]). The conclusion remains robust in probabilistic and 1-way sensitivity analyses. No routine follow-up remains the optimal strategy when the annual growth rate and risk of rupture of growing aneurysms are varied. When the annual risk of rupture of nongrowing UIAs is less than 1.7% (0.23% in base case scenario), no follow-up is the optimal strategy. If annual risk of rupture is more than 1.7%, coiling should be performed directly.
Conclusions and Relevance
Given the current literature, no preventive treatment or imaging follow-up is the most effective strategy in patients with aneurysms that are 3 mm or smaller, resulting in better health outcomes. More aggressive imaging surveillance for aneurysm growth or preventive treatment should be reserved for patients with a high risk of rupture. Given these findings, it is important to critically evaluate the appropriateness of current clinical practices, and potentially determine specific guidelines to reflect the most effective management strategy for patients with incidental, tiny UIAs.
This comparative effectiveness analysis evaluates the effectiveness of routine treatment (aneurysm coiling) vs 3 different strategies for imaging surveillance compared with no preventive treatment or routine follow-up of tiny unruptured intracranial aneurysms.
Key Points
Question
What is the optimal management of tiny (≤3 mm) unruptured intracranial aneurysms?
Findings
In this comparative effectiveness analysis, calculations show that routine preventive treatment (coiling) or aggressive imaging follow-up have lower health benefits from a societal perspective than no preventive treatment or imaging follow-up.
Meaning
Routine treatment or frequent imaging follow-up is not effective in the general population with tiny unruptured intracranial aneurysms, but may be more appropriate in selected patients at high risk of rupture.
Introduction
Unruptured intracranial aneurysms (UIAs) are relatively common in the general population and are being increasingly diagnosed owing to more frequent use of less invasive imaging techniques and higher resolution of images. A large number (≤87.6%) of these incidental UIAs are small, measuring less than 3 to 4 mm. Small aneurysms (≤7 mm) uncommonly cause aneurysmal symptoms and are labeled as incidental. The natural history of UIAs remains poorly understood, and only 0.25% of UIAs eventually rupture, contributing to uncertainty regarding their optimal management.
The American Heart Association and American Stroke Association guidelines for management of patients with UIAs were updated in 2015. However, these guidelines do not specify separate recommendations for small (3-7 mm) and tiny (≤3 mm) aneurysms, although their natural history, risk of rupture, and success of treatment might be different from those of aneurysms measuring more than 7 mm. A recent meta-analysis found that the reported rupture rate for tiny aneurysms was 0% in 5 of 7 studies and less than 0.4% in the remaining 2 studies. These small aneurysms are frequently treated because aneurysmal subarachnoid hemorrhage (SAH) is reportedly the result of rupture of aneurysms with diameters 5 mm or less. A recent meta-analysis of endovascular coiling of tiny intracranial aneurysms concluded that coiling can be performed safely and effectively, with favorable long-term angiographic and neurologic outcomes. However, coil embolization of aneurysms that are 3 mm or less is particularly challenging, and the meta-analysis found a 7% intraprocedural rupture rate and a 4% incidence of thromboembolic complications. These findings also must be interpreted in the context of a very low reported risk of rupture of small aneurysms. Patients with no history of SAH who are harboring aneurysms measuring 7 mm or less are often followed up conservatively using magnetic resonance angiography (MRA) to assess changes in size and/or morphologic characteristics. However, the utility, duration, and frequency of follow-up imaging are also not clearly established.
In a 2015 international survey of 203 neurosurgeons, most endorsed treatment of all asymptomatic aneurysms regardless of size. A more recent study showed that 11% of treating physicians always or usually recommend treatment of anterior circulation aneurysms measuring less than 5 mm without a family or personal history of SAH. Another 30% of physicians treated these small aneurysms 40% to 60% of the time. Follow-up imaging schedules were reported to be highly variable.
We performed a comparative effectiveness analysis from a societal perspective to assess the following 5 strategies in managing tiny UIAs measuring 3 mm or less: annual surveillance using MRA, biennial surveillance using MRA, surveillance using MRA every 5 years, coiling and MRA follow-up, and no treatment or preventive follow-up.
Methods
We define tiny aneurysms as those measuring 3 mm or less and small aneurysms as those measuring 3 to 7 mm. A decision tree with Markov modeling was constructed from a societal perspective with TreeAge Pro Suite 2014 (TreeAge Software Inc). By using computational simulation, decision analytic modeling can be considered as a complement to performing a large-cohort randomized clinical trial. The advantages include being able to compare several strategies and estimating the optimal strategy based on the most favorable outcomes for patients. The model covered the life span of a patient with the above-mentioned 5 strategies as potential options. With probabilistic sampling, the model simulated parallel cohorts of patients with tiny UIAs treated by different strategies and computed the respective outcomes for comparison. Outcomes were assessed in terms of quality-adjusted life-years (QALYs), which is a comprehensive utility metric accounting for both life expectancy and quality of life for patients in a specified health state. Institutional review board approval was not sought because no patient data are included in the study.
Model Structure
The model starts with a 50-year-old patient, representing the base case scenario as a patient of mean age harboring an intracranial aneurysm measuring 3 mm or smaller. A simplified flowchart of the model is presented in eFigure 1 in the Supplement. In all 5 strategies, the risk of death from other causes is considered on a yearly basis, constant across strategies but different across years. The presence of multiple aneurysms would put patients at a higher risk of SAH and the risk was compounded.
If coiling is performed after detection of an aneurysm, the patient can experience complications, die from the procedure, or have an uneventful recovery. After coiling, we assume that the patient will be followed up with MRA at 6 months and 1 year, and undergo imaging annually in subsequent years. After coiling, patients will also have the risks of regrowth or recanalization with retreatment, as well as the possibility of rebleeding.
No Preventive Option
If no follow-ups are performed, the patient would have an annual risk of rupture. If such an event does occur, the patient is assumed to be treated with coiling and may subsequently experience mild, moderate, or severe disability, or die from the SAH. After coiling, follow-up is similar to that in unruptured aneurysms.
Imaging Follow-up
If preventive screening is performed annually for an unruptured aneurysm, growth can be observed on each follow-up. As patients with growing aneurysms would be expected to be at a higher risk of rupturing, patients are assumed to undergo coiling with changes in size or morphologic findings, with subsequent similar risks after coiling and imaging follow-up. Rupture can occur in nongrowing aneurysms, which would not be preventable by imaging surveillance.
When screening is performed every 2 or 5 years, the subtree structures (eFigure 1 in the Supplement) are the same as the annual screening strategy, but the effectiveness is discounted in 2- or 5-year intervals, and clinical parameters are compounded.
No complications owing to imaging were included in the model because of the noninvasive nature and lack of radiation exposure with MRA.
Clinical Parameters
An overall discount rate of 3% for effectiveness was used in the model, as per the standard practice of comparative effectiveness analysis in the United States. Half cycle correction was performed for all strategies.
All clinical parameters were derived from recently published large cohort studies or meta-analyses specific to patients with small aneurysms. The annual growth rate (1.22%) and risk of rupture (0.23%) were extracted from a study by Sonobe et al with a large cohort of patients harboring tiny aneurysms. The study by Sonobe et al was the only one that included both rupture and growth rates for tiny aneurysms. The outcome of coiling, including mortality, morbidity, and retreatment rates, was derived from a recent systematic review and meta-analysis by Yamaki et al. The incidence of de novo aneurysm formation was reported to be 0.97% per person-year by Zali et al, with more than 7 years of follow-up. We assumed that the risks of rupture of de novo aneurysms were the same as for existing aneurysms, and that their risks of rupture were independent of one another. For the model, all growths were assumed to be detected and growth seen on results of imaging was assumed to be true positive, as angiography would be performed subsequently to confirm the findings. Most clinical parameters, when possible, were assigned β distributions, which are flexible and bounded by 0 and 1, making it useful for varying probability inputs in probabilistic sensitivity analysis.
We assigned differential annual mortality rates from nonaneurysmal causes, as the model was of a lifetime horizon. The differential mortality rates were computed from the 2010 United States Life Tables. Patients with moderate to severe disability would have a 17% excess rate of mortality.
Outcomes
The health state parameters were based on a previous cost-effectiveness analysis by Greving et al, and included a disutility for patient awareness of having an unruptured aneurysm. We assigned a temporary 5% disutility for discomfort and anxiety from the coiling procedure. We assumed that if a patient experienced more than 1 episode of SAH, he or she would develop moderate to severe disability or die. A full list of parameters is presented in Table 1.
Table 1. All Parameters.
| Variable | Mean Value With Reference(s), % | Distribution | Source |
|---|---|---|---|
| Clinical Parameters | |||
| Annual growth rate of UIA <3 mm | 1.22 | β (α = 11, β = 890), SD = 0.37% | Sonobe et al, 2010 |
| Annual rupture rate of growing UIA | 0 | Normal, SD = 9.25%, lower bound at 0% | Sonobe et al, 2010 |
| Annual rupture rate of nongrowing UIA | 0.23 | β (α = 2, β = 861), SD = 0.16% | Sonobe et al, 2010 |
| Annual incidence of de novo aneurysm formation | 0.97 | β (α = 9, β = 919), SD = 0.32% | Zali et al, 2014 |
| Proportion of patients with SAH with good long-term neurologic outcomes | 52.5 | β, SD = 5% | Yamaki et al, 2016 |
| Proportion of patients with SAH developing moderate to severe disability | 17.5 | Calculated by 1 − proportion of patients with SAH with good long-term neurologic outcome − SAH mortality | Sonobe et al, 2010 |
| SAH mortality | 30 | β, SD = 3% | Yamaki et al, 2016 |
| Proportion of good long-term neurologic outcome after coiling | 89 | β, SD = 5% | Yamaki et al, 2016 |
| Perioperative mortality associated with endovascular coiling | 3 | β, SD = 1% | Yamaki et al, 2016 |
| Risk of moderate disability from coiling | 8 | Calculated by 1 − proportion of good long-term neurologic outcome − mortality after coiling | Yamaki et al, 2016 |
| Rate of retreatment after coiling in unruptured aneurysms | 7 | β, SD = 1% | Yamaki et al, 2016 |
| Rate of retreatment after coiling in ruptured aneurysms | 7 | β, SD = 3% | Yamaki et al, 2016 |
| Rate of rebleeding after coiling | 0.16 | β (13, 8338) | Molyneux et al, 2015 |
| Effectiveness, QALYs | |||
| Well | 1 | NA | NA |
| Awareness of the UIA (range) | 0.92 (0.87-1.0) | Triangular | Greving et al, 2009 |
| Mild disability (range) | 0.72 (0.65-0.80) | Triangular | Greving et al, 2009 |
| Moderate to severe disability (range) | 0.41 (0.25-0.65) | Triangular | Greving et al, 2009 |
| Coiling | 5% disutility | NA | NA |
| SAH (range) | 0.64 (0.52-0.71) | Triangular | Bor et al, 2010 |
Abbreviations: NA, not applicable; QALYs, quality-adjusted life-years; SAH, subarachnoid hemorrhage; UIA, unruptured intracranial aneurysm.
Statistical Analysis
Base case calculation was carried out using the mean value for each parameter. Probabilistic sensitivity analysis simulation was performed with 10 000 iterations, modeling 10 000 patients. In addition, key variables, including annual growth rate, overall rupture rate, and utility of SAH, are varied across a wide range in 1-way, 2-way, and probabilistic sensitivity analyses.
Results
Base Case Calculation
In the base case calculation, all imaging strategies were dominated by the strategy of no scheduled follow-up: all imaging strategies showed lower effectiveness than no follow-up, which had an expected health benefit of a mean (SD) 19.40 (0.31) QALYs. Among the imaging strategies, imaging every 5 years is the best strategy with the next highest effectiveness (mean [SD] QALYs, 18.05 [0.62]). Coiling is the least favorable option because of high risks of complications and the least favorable outcome (mean [SD] QALYs, 17.53 [0.30]). The detailed results are presented in Table 2.
Table 2. Base Case Calculation Results.
| Management Strategy | Expected Health Benefit, Mean (SD), QALYs |
|---|---|
| No follow-up | 19.40 (0.31) |
| Follow-up every 5 y | 18.05 (0.62) |
| Annual follow-up | 17.93 (0.56) |
| Biennial follow-up | 17.65 (0.58) |
| Coiling | 17.53 (0.30) |
Abbreviation: QALYs, quality-adjusted life-years.
Probabilistic Sensitivity Analysis
We performed probabilistic sensitivity analyses to simulate a cohort of 10 000 patients as iterations. The 2 strategies for comparison were follow-up every 5 years vs no follow-up, since they were the 2 strategies with the highest QALYs from the base case calculations. In the simulation, follow-up every 5 years is better only 0.050% (95% CI, 0.037%-0.066%) of the time. No scheduled follow-up is the optimal strategy in the remaining iterations.
Sensitivity Analyses
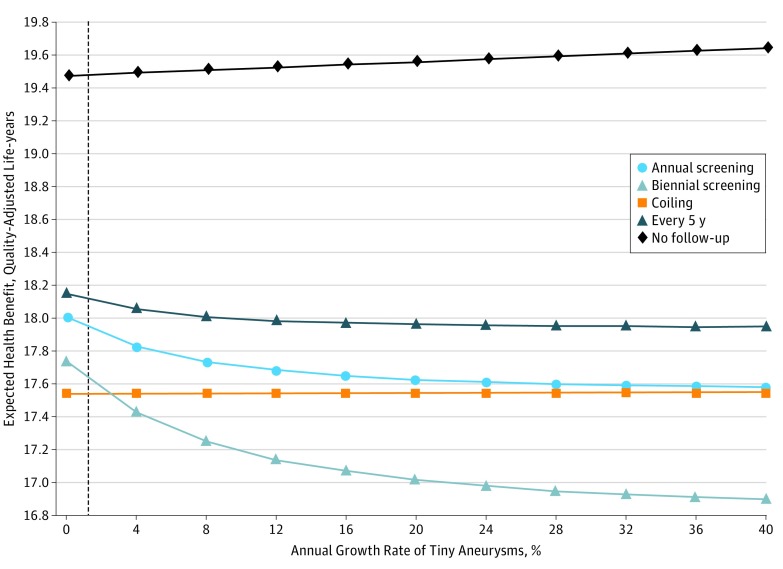
The growth rate of tiny UIAs was varied across a wide range while keeping other variables fixed. The result shows that no follow-up is better throughout the entire range. If imaging is to be performed, the most effective imaging strategy is every 5 years, irrespective of the growth rate (Figure 1).
Figure 1. One-Way Sensitivity Analysis Varying the Annual Growth Rate of Tiny Aneurysms.
A higher health benefit is more favorable. No follow-up remains the optimal strategy throughout the range of 0% to 40% annual growth rate of unruptured intracranial aneurysms. The dotted vertical line indicates base case value.
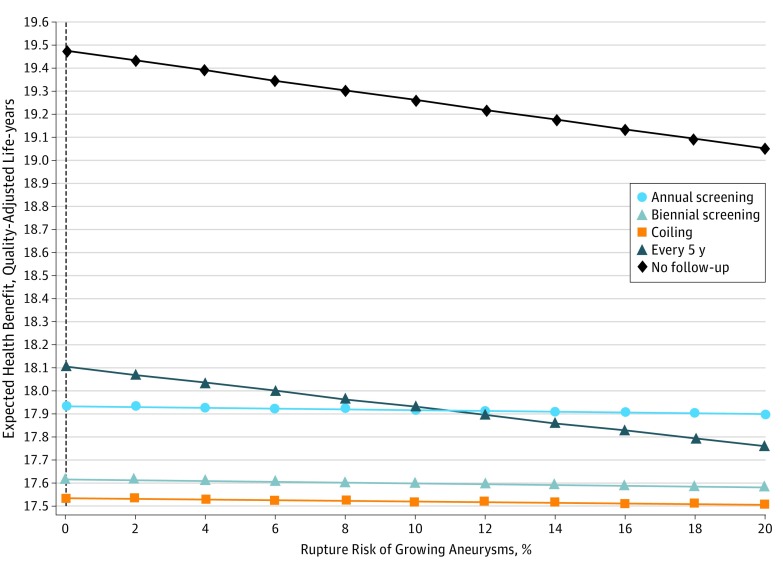
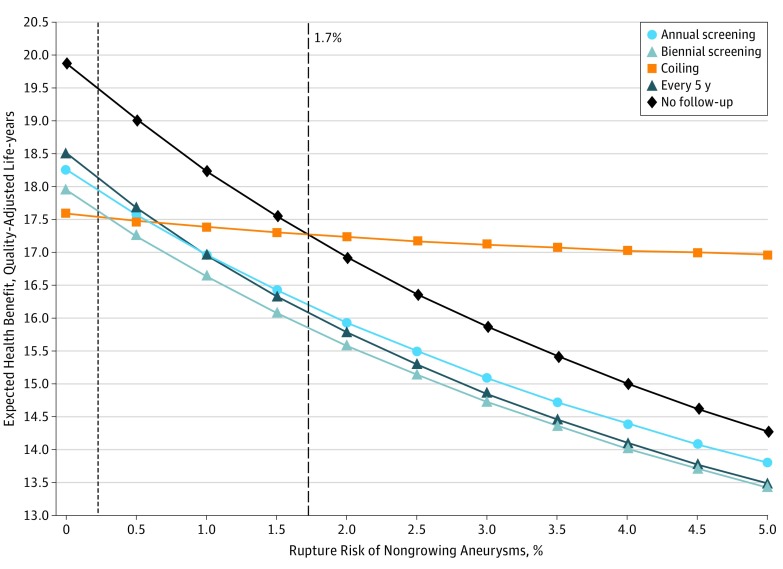
We similarly varied the risks of rupture of growing and nongrowing aneurysms. The model shows that no follow-up is best regardless of the risk of rupture (Figure 2). If imaging has to be considered, the best imaging strategy is 5-year follow-up if the risk of rupture of growing aneurysms is lower than 10.8%. Annual follow-up should be performed if the risk is higher than 10.8% (eFigure 2 in the Supplement). On the other hand, when the risk of rupture of small, nongrowing aneurysms is smaller than 1.7% per year, no follow-up is the optimal strategy. When the risk is higher, coiling should be performed directly (Figure 3).
Figure 2. One-Way Sensitivity Analysis Varying the Rupture Risk of Growing Aneurysms.
A higher health benefit is more favorable. No follow-up remains the optimal strategy throughout the range of 0% to 20% rupture risk of growing intracranial aneurysms. The dotted vertical line indicates base case value.
Figure 3. One-Way Sensitivity Analysis Varying the Rupture Risk of Nongrowing Aneurysms.
A higher health benefit is more favorable. No follow-up is the optimal strategy when the rupture risk of nongrowing intracranial aneurysms is less than 1.7%. When the risk is higher, coiling becomes the preferred strategy. The dotted vertical line indicates base case value.
A 2-way sensitivity analysis was performed, varying both the risk of rupture of growing aneurysms (0%-40%) and the proportion of growing aneurysms (0%-40%) among all tiny aneurysms (eFigure 3 in the Supplement). The result shows that when either the proportion or risk of rupture of growing aneurysms is lower than 4%, no follow-up is the optimal strategy regardless of the value of the other variable. When the rupture risk is between 4% and 8%, follow-up should be performed every 5 years if the annual growth rate of tiny aneurysms is high. When the rupture risk is greater than 8%, annual follow-up should be performed if growth rate is intermediate and coiling should be performed if the growth rate is high.
Results of sensitivity analysis further show that the conclusion does not change while varying the health states of patients with SAH after rupture of tiny aneurysms or the mortality from SAH caused by aneurysmal rupture (eFigures 4 and 5 in the Supplement).
Discussion
The natural history of UIAs remains poorly understood, which is especially true for small (3-7 mm) and tiny (≤3 mm) aneurysms. The 2015 American Heart Association and American Stroke Association guidelines on the management of patients with UIAs recommend intermittent imaging studies at regular intervals to follow up UIAs that are managed conservatively (class I; level of evidence B). This recommendation is based on the understanding that aneurysmal growth may increase the risk of rupture. A first follow-up study at 6 to 12 months after initial discovery is recommended, followed by subsequent follow-up yearly or every other year (class IIb; level of evidence C).
Patients with documented enlargement during follow-up should be offered treatment in the absence of prohibitive comorbidities (class I; level of evidence B). Long-term follow-up imaging may be considered after treatment, given the combined risk of aneurysm recurrence and formation of de novo aneurysm (class IIb; level of evidence B). The timing and duration of follow-up is, however, not defined for treated aneurysms as well as untreated aneurysms, and additional investigation has been deemed necessary.
No specific guidelines exist regarding the management of tiny, incidentally detected UIAs measuring 3 mm or less. The incidence of rupture in tiny UIAs in the published literature is low. The International Study of Unruptured Intracranial Aneurysms (ISUIA) had previously found the risk of rupture of anterior circulation aneurysms measuring 7 mm or less to be 0% in absence of a history of SAH. Subsequent studies have shown a good percentage of small and tiny aneurysms among all ruptured aneurysms. Kassell and Torner found that 13% of ruptured aneurysms out of 1092 cases were 5 mm or less in diameter, a significant discrepancy from the ISUIA data.
A positive correlation between aneurysm growth and rupture is critical to justify imaging surveillance to assess aneurysmal growth. The 3.1% rate of rupture for growing aneurysms compared with the 0.1% rate for stable aneurysms has been reported for all aneurysms. However, the growth and rate of rupture for aneurysms measuring 3 mm or smaller and their correlation may not be the same as for larger aneurysms. Although Villablanca et al found a positive correlation between growth and rupture in aneurysms measuring 7 mm or less, a systematic review in 2010 found this association to be variable and unpredictable.
Despite the low risk of rupture, these small aneurysms are being treated, increasingly by coiling. The risks associated with surgical clipping have not been well categorized, and coiling is being performed more frequently. Therefore, we focused on endovascular coiling for treatment of aneurysms in this study.
Our model parameters are based on the SUAVe study, which is the only study, to our knowledge, that reported on growth as well as rates of rupture in tiny aneurysms. The study found an annual risk of rupture of 0.34% for single aneurysms measuring 5 mm or less. Seven cases (1.9% of all patients) experienced ruptures during follow-up. Only 2 of these 7 cases were aneurysms measuring less than 4 mm. None of the 7 ruptured aneurysms had change in size on follow-up.
The results of our study show that routine imaging follow-ups may not be effective in following up these tiny aneurysms, based on the current literature. If imaging must be performed, follow-up every 5 years is more effective than more frequent follow-up.
Furthermore, no preventive follow-up would be the optimal strategy, irrespective of the incidence of rupture in growing aneurysms. Among the imaging strategies, imaging every 5 years is the most effective strategy. The sensitivity analysis varying the risk of rupture in nongrowing aneurysms shows that coiling becomes optimal only when the risk of rupture in nongrowing aneurysms becomes greater than 1.7%. The risk of rupture reported in the literature is much smaller. The Unruptured Cerebral Aneurysm study reported a rupture rate of 0.36% in UIAs measuring 3 to 4 mm.
In a previous cost-effectiveness analysis, Greving et al concluded that treatment was cost-effective for UIAs in 50-year-old patients with rates of rupture between 0.3% and 3.5% per year. However, the procedure-associated mortality and morbidity used in that analysis was much lower compared with the values for tiny aneurysms. Furthermore, Greving et al found that their results were highly sensitive to the utility of awareness of an untreated aneurysm, with only a slight decrease in quality of life from awareness of an aneurysm leading to substantial increase in incremental cost-effectiveness of treatment. However, the assigned utility of 0.92 is from a previous study on the outcome of finding a small aneurysm in patients who had previously undergone an operation for ruptured aneurysm. That study clearly stated that the results could not be extrapolated to screening of patients without prior SAH. Our study results are similar to those of Johnston et al from 1999, although our study is based on more recently available data, and their study did not assess the role of follow-up imaging.
More aggressive management strategies may be more appropriate in patients at higher risk of rupture. Hypertension, age less than 50 years, multiple aneurysms, posterior aneurysm location, and a larger size ratio have all been postulated to be high-risk features in UIAs. Although these risk factors might tilt decision making toward aneurysm ablation, to our knowledge, there is no evidence in the literature that routinely following up these small aneurysms adds utility.
We assumed that all growths would be detected on MRA, and growth seen on results of imaging was assumed to be a true positive. The criteria used to define growth are widely variable in the literature. Sensitivity of MRA in detecting small changes in size or morphologic characteristics of tiny aneurysms could be questionable. Also, false-positive MRAs may require digital subtraction angiography for confirmation, which would make imaging surveillance even less effective. Computed tomographic angiography would have much higher spatial resolution, but would not be ideal for imaging surveillance owing to radiation concerns.
Limitations
We did not study the effect of age, sex, or aneurysm location in our model on the effectiveness of the different strategies. These factors may need further study to stratify strategies based on risk.
An inherent limitation of most studies on the natural history of UIAs is possible selection bias, with patients at higher risk undergoing treatment and the rate of rupture being underestimated in patients who underwent conservative treatment. Our sensitivity analysis indicates that the decision of frequency of imaging surveillance would be altered only if risk of rupture is higher than 30%, which is not close to the low rates of rupture reported in patients with tiny aneurysms.
It has been postulated that the risk of rupture may be higher shortly after diagnosis of UIA and that the risk may decline with time. This possibility might imply closer supervision of these aneurysms initially, with increased spacing of imaging over time. However, there is a lack of specific literature on dynamic growth patterns of small aneurysms. We did not factor this in our model but it might need consideration once more data are available.
The duration of follow-up imaging for unruptured aneurysms as well as aneurysms after coiling is not well understood. The frequency of follow-up in the first 5 years has been reported to be even more aggressive than that incorporated in this model, with many centers using multiple digital subtraction angiographies. In the only long-term study of the natural history of UIAs, Juvela et al found that the risk of bleeding from an unruptured aneurysm remained virtually constant during the first 25 years after diagnosis except for patients above 50 years of age.
Recent meta-analyses on UIAs show the wide variability in imaging modalities and parameters used to define growth of UIAs. Except for the study by Juvela et al, other studies assessing growth have a follow-up less than 5 years. Our study emphasizes the need for better, more consistent, and longer-term studies reporting the growth and rate of rupture of UIAs to better define the optimal management of small UIAs. Clinical decisions are currently being made based on the limited evidence in the literature.
Conclusions
Management of tiny (≤3 mm) UIAs is often a dilemma. Given the current literature, our study reveals that no treatment or imaging follow-up is the most effective strategy, resulting in better health outcomes and lower health care spending. More aggressive management strategies might be appropriate in selected high-risk patients. Clinicians should discuss with patients all the potential variables involved in decision making, and policy makers may want to consider the study findings for future guidelines.
eFigure 1. Simplified Tree Structure
eFigure 2. One-Way Sensitivity Analysis Varying the Rupture Risk of Growing Aneurysms (Imaging Strategies Only)
eFigure 3. Two-Way Sensitivity Analysis Varying the Proportion and Rupture Risk of Growing Aneurysms
eFigure 4. One-Way Sensitivity Analysis Varying the QALY of SAH
eFigure 5. One-Way Sensitivity Analysis Assess the Impact of Mortality from SAH After Aneurysmal Rupture
References
- 1.Qureshi AI, Suri MF, Nasar A, et al. Trends in hospitalization and mortality for subarachnoid hemorrhage and unruptured aneurysms in the United States. Neurosurgery. 2005;57(1):1-8. [DOI] [PubMed] [Google Scholar]
- 2.Murayama Y, Takao H, Ishibashi T, et al. Risk analysis of unruptured intracranial aneurysms: prospective 10-year cohort study. Stroke. 2016;47(2):365-371. [DOI] [PubMed] [Google Scholar]
- 3.Thompson BG, Brown RD Jr, Amin-Hanjani S, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention; American Heart Association; American Stroke Association . Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(8):2368-2400. [DOI] [PubMed] [Google Scholar]
- 4.Wardlaw JM, White PM. The detection and management of unruptured intracranial aneurysms. Brain. 2000;123(pt 2):205-221. [DOI] [PubMed] [Google Scholar]
- 5.Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998;29(1):251-256. [DOI] [PubMed] [Google Scholar]
- 6.Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G; European Stroke Organization . European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35(2):93-112. [DOI] [PubMed] [Google Scholar]
- 8.Wiebers DO, Whisnant JP, Huston J III, et al. ; International Study of Unruptured Intracranial Aneurysms Investigators . Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103-110. [DOI] [PubMed] [Google Scholar]
- 9.Wermer MJ, van der Schaaf IC, Algra A, Rinkel GJ. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke. 2007;38(4):1404-1410. [DOI] [PubMed] [Google Scholar]
- 10.Malhotra A, Wu X, Forman HP, et al. Growth and rupture risk of small unruptured intracranial aneurysms: a systematic review. Ann Intern Med. 2017;167(1):26-33. [DOI] [PubMed] [Google Scholar]
- 11.Sonobe M, Yamazaki T, Yonekura M, Kikuchi H. Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke. 2010;41(9):1969-1977. [DOI] [PubMed] [Google Scholar]
- 12.Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg. 2008;108(5):1052-1060. [DOI] [PubMed] [Google Scholar]
- 13.Ohashi Y, Horikoshi T, Sugita M, Yagishita T, Nukui H. Size of cerebral aneurysms and related factors in patients with subarachnoid hemorrhage. Surg Neurol. 2004;61(3):239-245. [DOI] [PubMed] [Google Scholar]
- 14.Yamaki VN, Brinjikji W, Murad MH, Lanzino G. Endovascular treatment of very small intracranial aneurysms: meta-analysis. AJNR Am J Neuroradiol. 2016;37(5):862-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morita A, Kirino T, Hashi K, et al. ; UCAS Japan Investigators . The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366(26):2474-2482. [DOI] [PubMed] [Google Scholar]
- 16.International Study of Unruptured Intracranial Aneurysms Investigators Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339(24):1725-1733. [DOI] [PubMed] [Google Scholar]
- 17.Güresir E, Vatter H, Schuss P, et al. Natural history of small unruptured anterior circulation aneurysms: a prospective cohort study. Stroke. 2013;44(11):3027-3031. [DOI] [PubMed] [Google Scholar]
- 18.Alshafai N, Falenchuk O, Cusimano MD. Practises and controversies in the management of asymptomatic aneurysms: results of an international survey. Br J Neurosurg. 2015;29(6):758-764. [DOI] [PubMed] [Google Scholar]
- 19.Fargen KM, Soriano-Baron HE, Rushing JT, et al. A survey of intracranial aneurysm treatment practices among United States physicians [published online February 9, 2017]. J Neurointerv Surg. [DOI] [PubMed] [Google Scholar]
- 20.US Dept of Veterans Affairs Cost-effectiveness analysis. https://www.herc.research.va.gov/include/page.asp?id=cost-effectiveness-analysis. Updated April 25, 2016. Accessed July 25, 2016.
- 21.Zali A, Khoshnood RJ, Zarghi A. De novo aneurysms in long-term follow-up computed tomographic angiography of patients with clipped intracranial aneurysms. World Neurosurg. 2014;82(5):722-725. [DOI] [PubMed] [Google Scholar]
- 22.Arias E. United States life tables, 2010. Natl Vital Stat Rep. 2014;63(7):1-63. [PubMed] [Google Scholar]
- 23.Huhtakangas J, Lehto H, Seppä K, et al. Long-term excess mortality after aneurysmal subarachnoid hemorrhage: patients with multiple aneurysms at risk. Stroke. 2015;46(7):1813-1818. [DOI] [PubMed] [Google Scholar]
- 24.Greving JP, Rinkel GJ, Buskens E, Algra A. Cost-effectiveness of preventive treatment of intracranial aneurysms: new data and uncertainties. Neurology. 2009;73(4):258-265. [DOI] [PubMed] [Google Scholar]
- 25.Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet. 2015;385(9969):691-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bor AS, Koffijberg H, Wermer MJ, Rinkel GJ. Optimal screening strategy for familial intracranial aneurysms: a cost-effectiveness analysis. Neurology. 2010;74(21):1671-1679. [DOI] [PubMed] [Google Scholar]
- 27.Chmayssani M, Rebeiz JG, Rebeiz TJ, Batjer HH, Bendok BR. Relationship of growth to aneurysm rupture in asymptomatic aneurysms ≤7 mm: a systematic analysis of the literature. Neurosurgery. 2011;68(5):1164-1171. [DOI] [PubMed] [Google Scholar]
- 28.Villablanca JP, Duckwiler GR, Jahan R, et al. Natural history of asymptomatic unruptured cerebral aneurysms evaluated at CT angiography: growth and rupture incidence and correlation with epidemiologic risk factors. Radiology. 2013;269(1):258-265. [DOI] [PubMed] [Google Scholar]
- 29.Kassell NF, Torner JC. Size of intracranial aneurysms. Neurosurgery. 1983;12(3):291-297. [DOI] [PubMed] [Google Scholar]
- 30.Brinjikji W, Zhu YQ, Lanzino G, et al. Risk factors for growth of intracranial aneurysms: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2016;37(4):615-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruneau M, Amin-Hanjani S, Koroknay-Pal P, et al. Surgical clipping of very small unruptured intracranial aneurysms: a multicenter international study. Neurosurgery. 2016;78(1):47-52. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26317673&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 32.van der Schaaf IC, Wermer MJ, Velthuis BK, Buskens E, Bossuyt PM, Rinkel GJ. Psychosocial impact of finding small aneurysms that are left untreated in patients previously operated on for ruptured aneurysms. J Neurol Neurosurg Psychiatry. 2006;77(6):748-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston SC, Gress DR, Kahn JG. Which unruptured cerebral aneurysms should be treated? a cost-utility analysis. Neurology. 1999;52(9):1806-1815. [DOI] [PubMed] [Google Scholar]
- 34.Nahed BV, DiLuna ML, Morgan T, et al. Hypertension, age, and location predict rupture of small intracranial aneurysms. Neurosurgery. 2005;57(4):676-683. [PubMed] [Google Scholar]
- 35.Bijlenga P, Ebeling C, Jaegersberg M, et al. ; @neurIST Investigators . Risk of rupture of small anterior communicating artery aneurysms is similar to posterior circulation aneurysms. Stroke. 2013;44(11):3018-3026. [DOI] [PubMed] [Google Scholar]
- 36.Carter BS, Sheth S, Chang E, Sethl M, Ogilvy CS. Epidemiology of the size distribution of intracranial bifurcation aneurysms: smaller size of distal aneurysms and increasing size of unruptured aneurysms with age. Neurosurgery. 2006;58(2):217-223. [DOI] [PubMed] [Google Scholar]
- 37.Romano DG, Cioni S, Vinci SL, et al. Thromboaspiration technique as first approach for endovascular treatment of acute ischemic stroke: initial experience at nine Italian stroke centers. J Neurointerv Surg. 2017;9(1):6-10. [DOI] [PubMed] [Google Scholar]
- 38.Kashiwazaki D, Kuroda S; Sapporo SAH Study Group . Size ratio can highly predict rupture risk in intracranial small (<5 mm) aneurysms. Stroke. 2013;44(8):2169-2173. [DOI] [PubMed] [Google Scholar]
- 39.Backes D, Rinkel GJ, Laban KG, Algra A, Vergouwen MD. Patient- and aneurysm-specific risk factors for intracranial aneurysm growth: a systematic review and meta-analysis. Stroke. 2016;47(4):951-957. [DOI] [PubMed] [Google Scholar]
- 40.Schwab KE, Gailloud P, Wyse G, Tamargo RJ. Limitations of magnetic resonance imaging and magnetic resonance angiography in the diagnosis of intracranial aneurysms. Neurosurgery. 2008;63(1):29-34. [DOI] [PubMed] [Google Scholar]
- 41.Suh SH, Cloft HJ, Huston J III, Han KH, Kallmes DF. Interobserver variability of aneurysm morphology: discrimination of the daughter sac. J Neurointerv Surg. 2016;8(1):38-41. [DOI] [PubMed] [Google Scholar]
- 42.Sato K, Yoshimoto Y. Risk profile of intracranial aneurysms: rupture rate is not constant after formation. Stroke. 2011;42(12):3376-3381. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell P, Jakubowski J. Estimate of the maximum time interval between formation of cerebral aneurysm and rupture. J Neurol Neurosurg Psychiatry. 2000;69(6):760-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta R, Griessenauer CJ, Adeeb N, et al. Evaluating imaging follow-up strategies and costs of unruptured intracranial aneurysms treated with endovascular techniques: a survey of academic neurovascular centers in the United States. World Neurosurg. 2016;94:360-367. [DOI] [PubMed] [Google Scholar]
- 45.Juvela S, Poussa K, Lehto H, Porras M. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. Stroke. 2013;44(9):2414-2421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Simplified Tree Structure
eFigure 2. One-Way Sensitivity Analysis Varying the Rupture Risk of Growing Aneurysms (Imaging Strategies Only)
eFigure 3. Two-Way Sensitivity Analysis Varying the Proportion and Rupture Risk of Growing Aneurysms
eFigure 4. One-Way Sensitivity Analysis Varying the QALY of SAH
eFigure 5. One-Way Sensitivity Analysis Assess the Impact of Mortality from SAH After Aneurysmal Rupture