Abstract
Importance
The prevalence of pathologic conditions of the brain associated with Alzheimer disease increases strongly with age. Little is known about the distribution and clinical significance of preclinical biomarker staging in the oldest old, when most individuals without dementia are likely to have positive biomarkers.
Objective
To compare the patterns of long-term cognitive decline in multiple domains by preclinical biomarker status in the oldest old without dementia.
Design, Setting, and Participants
A longitudinal observational study with a mean (SD) of 12.2 (2.2) years (range 7.2-15.1 years) of follow-up was conducted in an academic medical center from August 24, 2000, to January 14, 2016, including and extending observations from the Ginkgo Evaluation of Memory study. A total of 197 adults who had completed the Ginkgo Evaluation of Memory study, were free of dementia, and were able to undergo magnetic resonance imaging were eligible for a neuroimaging study in 2009. Of these patients, 175 were included in the present analyses; 140 (80%) were cognitively normal and 35 (20%) had mild cognitive impairment.
Main Outcomes and Measures
Biomarker groups included amyloid β negative (Aβ−)/neurodegeneration negative (ND−), amyloid β positive (Aβ+)/ND−, Aβ−/neurodegeneration positive (ND+), and Aβ+/ND+ based on Pittsburgh Compound B retention and hippocampal volume in 2009. Participants completed baseline neuropsychological testing from 2000 to 2002 and annual testing from 2004 to 2016. Domains included memory, executive function, language, visual-spatial reasoning, and attention and psychomotor speed. Slopes of decline were evaluated with linear mixed models adjusted for age, sex, and years of education.
Results
Of the 175 participants (71 women and 104 men), at imaging, mean (SD) age was 86.0 (2.9) years (range, 82-95 years). A total of 42 participants (24.0%) were Aβ−/ND−, 32 (18.3%) were Aβ+/ND−, 35 (20.0%) were Aβ−/ND+, and 66 (37.7%) were Aβ+/ND+. On all cognitive measures, the Aβ+/ND+ group showed the steepest decline. Compared with the Aβ−/ND− group, the amyloid deposition alone (Aβ+/ND−) group showed faster decline on tests of verbal and visual memory (–0.3513; 95% CI, –0.5269 to –0.1756), executive function (0.0158; 95% CI, 0.0013-0.0303), and language (–0.1934; 95% CI, –0.3520 to –0.0348). The Aβ−/ND+ group showed faster visual memory decline than the Aβ−/ND− reference group (–0.3007; 95% CI, –0.4736 to –0.1279).
Conclusions and Relevance
In the oldest old without dementia, presence of either or both Aβ and hippocampal atrophy is typical (>75%). Isolated hippocampal volume atrophy is associated only with greater decline in memory. However, isolated Aβ is associated with decline in memory plus language and executive functions. These findings suggest different underlying pathophysiologic processes in the Aβ+/ND− and Aβ−/ND+ groups.
This longitudinal study in a subgroup of the Ginkgo Evaluation of Memory study compares the patterns of long-term cognitive decline in multiple domains by preclinical biomarker status in the oldest old without dementia.
Key Points
Question
Do domain patterns of long-term cognitive decline in the oldest old differ by neuroimaging biomarker status?
Findings
In this longitudinal study, 175 adults without dementia who were older than 80 years were followed up a mean of 12.2 years, with positron emission tomography and magnetic resonance imaging completed in the middle of the observation period. Isolated hippocampal atrophy was associated only with greater decline in memory, while isolated amyloid β was associated with decline in memory plus language and executive functions.
Meaning
These findings suggest different pathophysiologic processes underlying preclinical biomarkers of Alzheimer disease in the oldest old.
Introduction
In recent years, studies of neuroimaging biomarkers of Alzheimer disease (AD) pathologic conditions have demonstrated the independence of amyloid β (Aβ) amyloidosis and neurodegeneration (ND) markers in the preclinical phase. Specifically, Jack et al proposed a 2-feature biomarker approach to classify clinically normal older individuals with Aβ accumulation and/or ND. The cross-tabulation results in the following 4 biomarker classifications: Aβ and ND negative (Aβ−/ND−), Aβ positive and ND negative (Aβ+/ND−), Aβ negative and ND positive (Aβ−/ND+), and Aβ positive and ND positive (Aβ+/ND+). Corresponding to the National Institute on Aging–Alzheimer’s Association staging of preclinical AD and as modified by Jack et al, Aβ−/ND− maps to stage 0, Aβ+/ND− to stage 1, and Aβ+/ND+ to stage 2/3; the Aβ−/ND+ classification has been termed suspected non-Alzheimer disease pathophysiology (SNAP). The 2-feature biomarker approach has been applied to study participants with normal cognition (NC) and mild cognitive impairment (MCI).
The characterization of SNAP has triggered debate about its pathophysiologic basis and its role in cognitive decline and progression to dementia. Cross-sectionally, cognitive profiles of groups with NC and SNAP do not differ from groups with NC and Aβ−/ND− and Aβ+/ND− at baseline. However, among individuals with MCI, SNAP and Aβ+/ND− groups show deficits compared with Aβ−/ND− groups. Regarding the risk of cognitive decline, studies consistently show that Aβ+/ND+ confers the highest risk of cognitive decline and Aβ−/ND− the lowest risk.
However, it is unclear whether the Aβ+/ND− and the SNAP groups show specific cognitive decline signatures. Several studies have examined longitudinal composite scores or global cognitive scores, but few have compared patterns of decline in various cognitive domains between SNAP and Aβ-positive participants. Furthermore, little is known about 2-feature biomarker classification in oldest old adults, when prevalence of amyloidosis and neurodegenerative pathologic conditions is at its highest. In this study, we applied the 2-feature biomarker grouping method to compare groups defined by Aβ deposition and evidence of ND (hippocampal atrophy) on cognitive decline across various cognitive domains during long-term follow-up, with a mean of 12.2 years.
Methods
Participants
Participants were a subgroup of the Ginkgo Evaluation of Memory (GEM) study, conducted from 2000 to 2008, who were enrolled in the subsequent Ginkgo Evaluation of Memory Study (GEMS) Imaging Sub-Study (details of which have been previously described). Of 966 participants of the GEM study at the Pittsburgh, Pennsylvania, site, 197 continued in 2009 with the GEMS Imaging Sub-Study, which included Pittsburgh Compound B positron emission tomography (PiB-PET) and magnetic resonance imaging (MRI). The study inclusion criterion was completion of the GEM parent study. Exclusion criteria were dementia at the completion of the GEM parent study and contraindications for neuroimaging. Of the 197 participants in the GEMS Imaging Sub-Study, 3 were excluded for technical issues with PiB-PET, 16 were further excluded for lack of T2 MRI imaging data, and 3 were excluded owing to a dementia diagnosis in 2009, resulting in 175 participants for this study. The 175 older adults had a mean (SD) age of 78.0 (2.8) years (range, 75.0-87.8 years) at the initial GEM study baseline visit between 2000 to 2002 and were followed up for a mean (SD) of 12.2 (2.2) years (range, 7.2-15.1 years). At the time of imaging in 2009, the mean (SD) age was 86.0 (2.9) years. Clinical and neuropsychological evaluations were completed annually through the GEM study and the GEMS Imaging Sub-Study. The mean (SD) length of follow-up from 2009 was 4.2 (2.2) years (range, 0-6.9 years). Therefore, in total, study procedures were conducted from August 24, 2000, to January 14, 2016. Participants provided written informed consent for all study procedures as approved by the University of Pittsburgh institutional review board.
Neuroimaging
Positron emission tomography data were acquired for 20 minutes (4 × 5-minute frames) beginning 50 minutes after injection of a mean (SD) of 15 (1.5) mCi of PiB on a Siemens/Computer Technology Imaging emission computerized axial tomography high-resolution plus scanner in 3-dimensional imaging mode (63 planes with slice width of 2.4 mm). Retention of PiB was measured over the 50- to 70-minute scan interval and scaled to the injected dose and body mass to generate standardized uptake values (SUVs). The SUVs were then normalized to the SUV of the cerebellum reference region to generate SUV ratio (SUVR) measures of PiB retention. Using an iterative mild outlier cutoff method based on an independent sample of 62 controls, we define Aβ-negative and Aβ-positive status by a global cortical measure (mean of the frontal, anterior cingulate, precuneus, parietal, and temporal cortices) with a cutoff of 1.57 SUVR units.
Structural 1.5-T MRI images (General Electric Signa) were acquired, from which the hippocampal volume (HV) was derived and normalized to total intracranial volume. w-Scores were calculated based on an independent reference group of 77 NC individuals (age range, 45-89 years) adjusted for sex and age. Neurodegeneration negative was defined as a w-score less than −0.9063, reflecting 85% sensitivity to AD generated from an independent sample of 51 individuals with AD. The 175 participants were stratified into the following groups: Aβ−/ND−, Aβ+/ND−, Aβ−/ND+ (SNAP), and Aβ+/ND+ based on PiB and MRI findings in 2009 (Figure 1).
Figure 1. Timing of Neuroimaging Relative to Neuropsychological Assessments.
Participants were evaluated longitudinally from 2000 to 2016. Annual cognitive testing was performed with the exception of a 3- to 4-year gap from 2000 to 2004. Classification of biomarker status was based on Pittsburgh Compound B positron emission tomography and magnetic resonance imaging performed in 2009.
Cognitive Assessment
Participants completed the following neuropsychological test battery at the initial visit (2000-2002) and annually starting in 2004. Memory: California Verbal Learning Test (CVLT), immediate and delayed recall, and the modified Rey-Osterrieth (R-O) figure test, immediate and delayed recall. Executive functions: Trail-Making Test part B (TMT-B) and the Trenerry Stroop Test, interference condition. Visual-spatial reasoning: Modified Wechsler Adult Intelligence Scale–Revised Block Design and copy condition of the modified R-O figure test. Language: letter verbal fluency, semantic (animals) verbal fluency, and the 30-item Boston Naming Test (BNT). Attention and psychomotor speed: Trail-Making Test part A (TMT-A) and the Trenerry Stroop Test, control condition. In 2009 at the time of neuroimaging, a reduced battery was administered, omitting Block Design, BNT, and the Stroop Test (see Figure 1 for study timeline).
The GEM study Cognitive Diagnostic Center completed adjudication in the Imaging Sub-Study in 2009, blind to PiB-PET results, taking into account historical serial cognitive assessments from the parent GEM study. Criteria for MCI included 1 to 3 tests impaired at cutoffs of 1.5 SD below means adjusted for age and educational level.
Statistical Analysis
Linear mixed models with a fixed factor of time, quadratic time, and biomarker group as well as their linear and quadratic interactions (when significant) were used to estimate the rate of cognitive decline in neuropsychological test scores for the entire observational period. A random intercept and slope were included with an unstructured covariance to account for the repeated observation structure of the data. The Satterthwaite method was used for computing degrees of freedom. Models were adjusted for age, sex, and years of education. Primary analyses included all 175 participants, adjusted for age, sex, and years of education. The Aβ/ND status group × time interaction term was used to reflect difference in the rates of cognitive performance decline over time relative to the reference group, which was Aβ−/ND− status. The addition of quadratic time and quadratic interaction effects were tested using likelihood ratio test between the models with and without these terms and compared with the corresponding χ2 degrees of freedom. The quadratic interactions reflect accelerating or decelerating decline rates compared with the reference group. Secondary analyses included only participants with normal cognitive status (n = 140) at the time of imaging. A sensitivity analysis examined shifting the baseline of cognitive trajectories to the time of imaging (2009). Since the number of cognitive measurements was reduced, time was modeled as a linear term only in these models.
Results
Demographic and Clinical Status at Baseline and the Time of Imaging
At the time of imaging (2009), 42 participants (24.0%) were classified as Aβ−/ND−, 32 (18.3%) as Aβ+/ND−, 35 (20.0%) as Aβ−/ND+, and 66 (37.7%) as Aβ+/ND+. Shown in Table 1, the 4 groups stratified based on Aβ and ND status in 2009 did not differ in age, sex, race, and years of education, although the Aβ+/ND+ group showed a trend toward older age. The Aβ+/ND+ group had more APOE*4 carriers (22 of 62 [35.5%]) compared with the Aβ−/ND− group (2 of 40 [5.0%]) and Aβ−/ND+ group (2 of 34 [5.9%]), with the Aβ+/ND− group intermediate (5 of 27 [18.5%]). In 2009, most participants (140 [80.0%]) had NC and the remaining 35 (20.0%) had MCI. The proportion of participants with MCI within each group did not differ significantly. Table 1 also presents neuropsychological test scores at the time of imaging. The Aβ+/ND+ group performed the poorest. On tests of executive function and psychomotor speed (TMT-A and TMT-B), both Aβ+ groups performed comparably. At baseline of the observation period (2000-2002), there were significant differences among the imaging subgroups on Stroop interference (F3,159 = 2.89; P = .04) and control (F3,167 = 3.02; P = .03) conditions, with the Aβ+/ND− group showing the poorest performance. On all other baseline cognitive tests, there were no significant differences among imaging subgroups.
Table 1. Demographics and Neuropsychological Test Scores at the Time of Neuroimaginga .
| Characteristic | Biomarker Group | P Value | ||||
|---|---|---|---|---|---|---|
| Aβ−/ND−
(n = 42) |
Aβ+/ND− (n = 32) |
Aβ−/ND+
(n = 35) |
Aβ+/ND+ (n = 66) |
Total (n = 175) |
||
| Female sex, No. (%) | 15 (35.7) | 13 (40.6) | 13 (37.1) | 30 (45.5) | 71 (40.6) | .75 |
| Nonwhite race/ethnicity, No. (%) | 0 | 1 (3.1) | 2 (5.7) | 1 (1.5) | 4 (2.3) | |
| Age in 2009, mean (SD), y | 85.1 (2.0) | 86.8 (3.7) | 85.8 (2.8) | 86.2 (2.8) | 86.0 (2.9) | .08 |
| Education, mean (SD), y | 14.7 (2.8) | 13.9 (2.2) | 14.8 (2.8) | 15.0 (2.6) | 14.7 (2.6) | .30 |
| APOE*4 allele carrier, No. (%) | 2/40 (5.0) | 5/27 (18.5) | 2/34 (5.9) | 22/62 (35.5) | 31/163 (19.0) | <.001 |
| MCI in 2009, No. (%) | 4 (9.5) | 6 (18.8) | 7 (20.0) | 18 (27.3) | 35 (20) | .18 |
| Randomized to receive Ginkgo biloba, 2000-2008, No. (%) | 24 (57.1) | 18 (56.3) | 35 (53.0) | 11 (31.4) | 88 (50.3) | .09 |
| Memory, mean (SD), score | ||||||
| CVLT immediate recall (No. of words) | 46.71 (10.58) | 42.06 (11.90) | 42.14 (11.41) | 40.50 (12.55) | 42.60 (15) | .07 |
| CVLT delayed recall (No. of words) | 9.38 (3.19) | 8.38 (3.32) | 7.40 (3.87) | 7.57 (4.03) | 8.12 (3.74) | .05 |
| R-O figure immediate recall | 17.50 (3.41) | 16.84 (3.62) | 16.30 (3.82) | 14.83 (4.21) | 16.13 (3.97) | .004 |
| R-O figure delayed recall (maximum 24 points) | 16.85 (3.70) | 17.16 (4.17) | 15.64 (4.28) | 14.92 (4.42) | 15.93 (4.17) | .03 |
| Executive function, mean (SD), score | ||||||
| Trails B (s) | 100.86 (44.66) | 127.38 (52.33) | 105.94 (43.29) | 122.68 (53.60) | 114 (50.08) | .05 |
| Visual-spatial reasoning, mean (SD), score | ||||||
| R-O figure copy (maximum 24 points) | 21.00 (2.37) | 20.30 (2.17) | 20.53 (2.46) | 19.95 (2.56) | 20.38 (2.44) | .18 |
| Language, mean (SD), score | ||||||
| Semantic fluency (No. of words) | 27.38 (9.03) | 28.13 (7.15) | 26.85 (8.42) | 28.07 (7.90) | 27.67 (8.15) | .88 |
| Phonemic fluency (No. of words) | 16.50 (3.68) | 15.00 (3.62) | 15.11 (3.86) | 14.10 (4.07) | 15.05 (3.93) | .02 |
| Attention and psychomotor speed, mean (SD), score | ||||||
| Trails A (s) | 42.19 (13.21) | 47.06 (15.40) | 39.91 (12.74) | 50.60 (19.84) | 45.77 (16.75) | .007 |
Abbreviations: Aβ, amyloid β; CVLT, California Verbal Learning Test; MCI, mild cognitive impairment; ND, neurodegeneration; R-O, Rey-Osterrieth.
Neuroimaging was performed in 2009. A reduced battery was administered in 2009, omitting Block Design, Stroop Test, and Boston Naming Test.
Change Over Time in Neuropsychological Test Scores
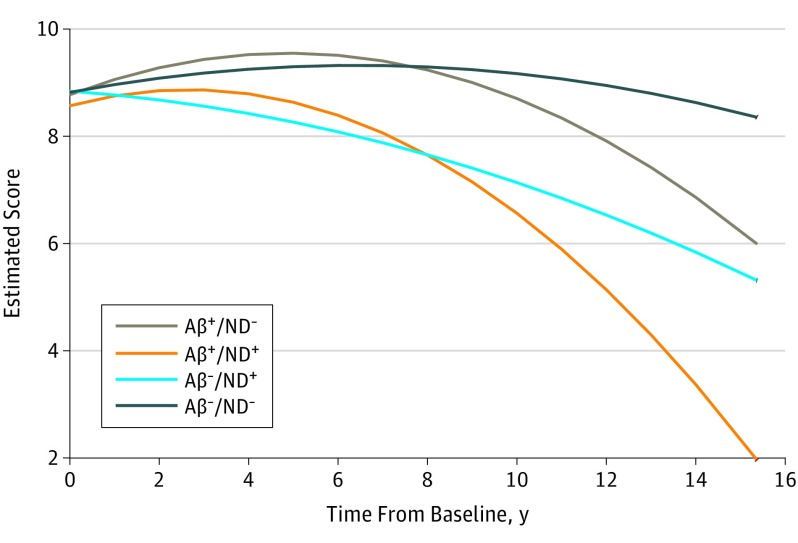
Table 2 shows the relative rates of annualized cognitive test score change among the Aβ+/ND−, Aβ−/ND+, and Aβ+/ND+ groups compared with Aβ−/ND− as the reference group. The Aβ+/ND+ group showed significantly steeper decline in all neuropsychological tests except for phonemic fluency and the control condition (ie, word reading) of the Stroop test. All 3 biomarker-positive groups showed steeper decline in R-O figure immediate (eFigure 1 in the Supplement) and delayed recalls. In addition, the Aβ+/ND− group showed steeper decline in CVLT delayed recall (Figure 2), TMT-B (eFigure 2 in the Supplement), BNT, and semantic fluency compared with the Aβ−/ND− group. In contrast, the Aβ−/ND+ group showed steeper decline only in R-O figure immediate and delayed recall compared with the Aβ−/ND− reference group.
Table 2. Estimates of the Difference in Rates of Annualized or Accelerated Cognitive Test Score Change, by Biomarker Group Relative to Aβ−/ND− Groupa,b,c.
| Cognitive Outcome | Group × Time Interaction: Difference in Rates of Change (95% CI) | |||||
|---|---|---|---|---|---|---|
| Aβ+/ND−
(n = 32) |
P Value | Aβ−/ND+
(n = 35) |
P Value | Aβ+/ND+
(n = 66) |
P Value | |
| Memory | ||||||
| CVLT sum of learning trials (No. of words)a | −0.0620 (−0.1260 to 0.0020) |
.06 | 0.0019 (−0.0598 to 0.0636) |
.95 | −0.0908 (−0.1452 to −0.0364) |
.001 |
| CVLT delayed recall (No. of words)a | −0.0203 (−0.0395 to −0.0011) |
.04 | 0.0011 (−0.0176 to 0.0198) |
.91 | −0.0306 (−0.0469 to −0.0142) |
<.001 |
| R-O figure immediate recall (24 points) | −0.3513 (−0.5269 to −0.1756) |
<.001 | −0.3007 (−0.4736 to −0.1279) |
<.001 | −0.4621 (−0.6119 to −0.3124) |
<.001 |
| R-O figure delayed recall (24 points) | −0.3341 (−0.5170 to −0.1512) |
<.001 | −0.2896 (−0.4661 to −0.1131) |
.001 | −0.4513 (−0.6070 to −0.2956) |
<.001 |
| Executive functions | ||||||
| Trails B (log seconds) | 0.0158 (0.0013 to 0.0303) |
.03 | 0.0074 (−0.0068 to 0.0216) |
.31 | 0.0254 (0.0131 to 0.0378) |
<.001 |
| Stroop color-word interference (No. of items) | −0.5026 (−1.4292 to 0.4240) |
.29 | −0.6985 (−1.5925 to 0.1955) |
.13 | −1.1773 (−1.9616 to −0.3929) |
.003 |
| Visual-spatial reasoning | ||||||
| Block Design (24 points) | −0.1093 (−0.2407 to 0.0221) |
.10 | −0.0829 (−0.2128 to 0.04706) |
.21 | −0.1665 (−0.2789 to −0.0540) |
.004 |
| R-O figure copy (24 points) | −0.0922 (−0.2081 to 0.0238) |
.12 | −0.0698 (−0.1836 to 0.0440) |
.23 | −0.1347 (−0.2337 to −0.0357) |
.008 |
| Language | ||||||
| Semantic fluency (No. of words) | −0.1934 (−0.3520 to −0.0348) |
.02 | −0.0523 (−0.2095 to 0.1048) |
.51 | −0.2619 (−0.3971 to −0.1266) |
<.001 |
| Phonemic fluency (No. of words) | −0.0922 (−0.3640 to 0.1795) |
.51 | −0.1349 (−0.4024 to 0.1326) |
.32 | −0.1437 (−0.3747 to 0.0873) |
.22 |
| Boston Naming Test (30 points)a | −0.0218 (−0.0405 to −0.0031) |
.02 | −0.0031 (−0.0214 to 0.0152) |
.74 | −0.0306 (−0.0467 to −0.0146) |
<.001 |
| Attention and psychomotor speed | ||||||
| Trails A (log seconds) | 0.0096 (−0.0026 to 0.0217) |
.12 | 0.0012 (−0.0108 to 0.0131) |
.85 | 0.0159 (0.0056 to 0.0262) |
.003 |
| Stroop word reading (No. of words) | 0.6834 (−0.9779 to 2.3447) |
.41 | 0.0977 (−1.5281 to 1.7235) |
.91 | −0.7297 (−2.1529 to 0.6935) |
.32 |
Abbreviations: Aβ, amyloid β; CVLT, California Verbal Learning Test; ND, neurodegeneration; R-O, Rey-Osterrieth.
For 3 neuropsychological test outcomes (CVLT learning trials, CVLT delayed recall, and Boston Naming Test), estimates are from the Time2 × group interaction term.
Estimates adjusted for age, sex, and years of education.
Negative estimates indicate steeper decline compared to the reference group except for Trails A and B, for which positive estimates indicate declining performance over time.
Figure 2. California Verbal Learning Test Delayed Recall (Verbal Memory) Performance Over Time, by Biomarker Group.
Decline in California Verbal Learning Test delayed recall performance over the follow-up period by the 4 biomarker groups. Higher score is better. Aβ indicates amyloid β; ND, neurodegeneration.
When excluding participants with MCI at the time of imaging, results were highly similar, as shown in Table 3. A total of 38 of the 140 patients with NC (27.1%) were classified as Aβ−/ND−, 26 (18.6%) were classified as Aβ+/ND−, 28 (20.0%) were classified as Aβ−/ND+, and 48 (34.3%) were classified as Aβ+/ND+. The Aβ+/ND+ group showed steeper decline in most of the neuropsychological tests compared with the Aβ−/ND− group (exceptions were: R-O figure copy condition, phonemic fluency, and Stroop control condition). As in the primary analyses, all 3 groups showed steeper decline in R-O figure immediate and delayed recall. In addition, the Aβ+/ND− group showed steeper decline in TMT B, CVLT learning trials and delayed recall, and semantic fluency.
Table 3. Estimates of the Difference in Rates of Annualized or Accelerated Cognitive Test Score Change, by Biomarker Group Relative to the Aβ−/ND− Group in Participants With Normal Cognition at Neuroimaginga,b,c.
| Cognitive Outcome | Group × Time Interaction: Difference in Rates of Change (95% CI) | |||||
|---|---|---|---|---|---|---|
| Aβ+/ND− (n = 26) |
P Value | Aβ−/ND+ (n = 28) |
P Value | Aβ+/ND+ (n = 48) |
P Value | |
| Memory | ||||||
| CVLT sum of learning trials (No. of words)a | −0.0756 (−0.1324 to −0.0188) |
.009 | 0.0122 (−0.0411 to 0.0656) |
.65 | −0.1003 (−0.1490 to −0.0515) |
<.001 |
| CVLT delayed recall (No. of words)a | −0.0219 (−0.0382 to −0.0056) |
.008 | 0.0066 (−0.0087 to 0.0219) |
.40 | −0.0305 (−0.0445 to −0.0165) |
<.001 |
| R-O figure immediate recall (24 points) | −0.3159 (−0.4838 to −0.1481) |
<.001 | −0.2230 (−0.3872 to −0.0588) |
.008 | −0.3604 (−0.5056 to −0.2153) |
<.001 |
| R-O figure delayed recall (24 points) | −0.2951 (−0.4784 to −0.1119) |
.002 | −0.2308 (−0.4070 to −0.0546) |
.01 | −0.3459 (−0.5052 to −0.1867) |
<.001 |
| Executive functions | ||||||
| Trails B (log seconds) | 0.0159 (0.0012 to 0.0307) |
.04 | 0.0048 (−0.0096 to 0.0191) |
.51 | 0.0217 (0.0089 to 0.0345) |
<.001 |
| Stroop color-word interference (No. of items) | −0.2083 (−1.1853 to 0.7687) |
.68 | −0.4867 (−1.4259 to 0.4525) |
.31 | −0.9495 (−1.7859 to −0.1130) |
.03 |
| Visual-spatial reasoning | ||||||
| Block Design (24 points) | −0.0432 (−0.1824 to 0.0960) |
.54 | −0.0278 (−0.1635 to 0.1079) |
.69 | −0.1455 (−0.2666 to −0.0244) |
.02 |
| R-O figure copy (24 points) | −0.0721 (−0.1915 to 0.0474) |
.24 | −0.0570 (−0.1738 to 0.05970) |
.34 | −0.0920 (−0.1954 to 0.0114) |
.08 |
| Language | ||||||
| Semantic fluency (No. of words)a | −0.0256 (−0.0506 to −0.0007) |
.04 | −0.0221 (−0.0456 to 0.0014) |
.07 | −0.0291 (−0.0505 to −0.0076) |
.008 |
| Phonemic fluency (No. of words) | −0.1295 (−0.4156 to 0.1565) |
.38 | −0.0930 (−0.3723 to 0.1863) |
.51 | −0.0766 (−0.3229 to 0.1698) |
.54 |
| Boston Naming Test (30 points)a | −0.0134 (−0.0284 to 0.0017) |
.08 | 0.0011 (−0.0130 to 0.0153) |
.88 | −0.0271 (−0.0402 to −0.0141) |
<.001 |
| Attention and psychomotor speed | ||||||
| Trails A (log seconds) | 0.0101 (−0.0020 to 0.0221) |
.10 | 0.0026 (−0.0091 to 0.0144) |
.66 | 0.0138 (0.0034 to 0.0243) |
.009 |
| Stroop word reading (No. of words) | 1.0568 (−0.7555 to 2.8691) |
.25 | 0.1213 (−1.6351 to 1.8777) |
.89 | −0.4097 (−1.9861 to 1.1666) |
.61 |
Abbreviations: Aβ, amyloid β; CVLT, California Verbal Learning Test; ND, neurodegeneration; R-O, Rey-Osterrieth.
For 4 neuropsychological test outcomes (CVLT learning trials, CVLT delayed recall, semantic fluency, and Boston Naming Test), estimates are from the Time2 × group interaction term.
Estimates adjusted for age, sex, and educational level.
Negative estimates indicate steeper decline compared to the reference group except for Trails A and B, for which positive estimates indicate declining performance over time.
In the sensitivity analysis shifting the baseline of cognitive trajectories to the time of imaging, patterns of results were generally similar but with fewer significant contrasts. Differences to the primary analyses (Table 2) were as follows: CVLT delayed recall did not decline more for the Aβ+/ND− group; R-O figure delayed recall did not decline more for the Aβ+/ND− and Aβ−/ND+ groups; TMT-A did not decline more for the Aβ+/ND+ group; TMT-B did not decline more for the Aβ+/ND− group; Stroop color-word interference, R-O copy, and Block Design did not decline more for the Aβ+/ND+ group; BNT did not decline more for the Aβ+/ND− group; and phonemic fluency declined more for the Aβ+/ND+ group (eTable in the Supplement).
Discussion
This study compares long-term cognitive trajectories for a mean of 12 years of biomarker groups classified by Aβ deposition and hippocampal atrophy near the middle of the cognitive trajectories. Although the study design is not typical, the duration of neuropsychological observation is among the longest, to our knowledge. Furthermore, the participants were among the oldest old; thus, the study expands the current literature investigating ND and Aβ deposition in older adults without dementia.
The distribution of the biomarker groups is generally consistent with previous observations and prediction. At the time of imaging, the mean (SD) age of this study cohort was 86.0 (2.9) years, and the proportions of the 4 groups were 24.0% Aβ−/ND−, 18.3% Aβ+/ND−, 20.0% Aβ−/ND+, and 37.7% Aβ+/ND+. Jack et al reported a similar distribution in 80 individuals who were 85 to 89 years of age: 21% were Aβ−/ND−, 20% were Aβ+/ND−, 25% were Aβ−/ND+, and 34% were Aβ+/ND+. Compared with younger old cohorts, our finding of 37.7% Aβ+/ND+ is higher than in many previous studies (9% in the study by Burnham et al, 17% in the study by Mormino et al, 13% in the study by Soldan et al, and 16% in the study by Jack et al). Consistent with results of postmortem and imaging studies, advanced aging is associated with increased Aβ accumulation and hippocampal ND. Other studies observed Aβ−/ND+ (ie, SNAP) in approximately 23% of individuals with NC and approximately 25% of individuals with MCI, slightly higher than the 20.0% we report here. Many of these studies defined ND as [F] fluorodeoxyglucose (FDG) abnormality on PET results or hippocampal atrophy on MRI findings, encompassing more individuals in the Aβ−/ND+ group than in our study, which operationalized ND using only hippocampal atrophy. Although HV reduction is not unique to AD (other possible causes include hippocampal sclerosis, frontotemporal lobar degeneration, argyrophilic grain disease, and ischemia), it is predictive of cognitive status in AD.
At baseline, we did not observe significant cognitive differences among the 4 groups on most tests, consistent with Mormino et al. However, other studies found lower baseline cognition, mostly in memory, for Aβ+/ND+, and another study reported lower baseline global cognitive measures for SNAP. Regarding cognitive decline, our findings show that in the oldest old, presence of both Aβ and ND markers together confers the fastest rate of cognitive decline across domains, consistent with results of previous studies and consistent with the National Institute on Aging–Alzheimer’s Association preclinical stage model. We also observed that Aβ deposition without ND (Aβ+/ND−) is associated with decline in memory, executive functions, and language, while isolated hippocampal atrophy (Aβ−/ND+; SNAP) was associated only with decline in memory. Our findings are consistent with those of a study by Burnham et al, in which Aβ+/ND− showed a steeper decline trajectory for 8 years than did Aβ−/ND− in verbal memory, while SNAP did not. Owing to the unique length of follow-up in our study, we found the quadratic interaction fit for time can better reflect the accelerated cognitive decline pattern over years, unlike the linear fit used in previous studies to approximate cognitive decline trajectories. This finding suggests that cognitive decline often does not follow a linear decline pattern over time. Although a previous study demonstrated diminished practice effect in the Aβ+/ND− and Aβ−/ND+ groups compared with the Aβ−/ND− group, the extended length of follow-up and advanced age of participants in this study allows us to observe absolute and relative cognitive decline on most tests.
A previous study reported that Aβ+ participants show steeper decline in visual memory, semantic fluency, and executive function 8 years prior to imaging compared with Aβ− participants. The present study confirms and extends these findings in that both of the Aβ+ groups (Aβ+/ND− and Aβ+/ND+) exhibit steeper decline for a longer observation period, including 8 years of prospective follow-up, on these cognitive measures. The association between Aβ and long-term decline in extramemory cognitive processes, particularly executive functions and language, is consistent with an established literature showing that executive function and semantic fluency deficits are very early predictors of AD.
This study included individuals with both NC and MCI at the time of imaging. When restricting the sample to participants with NC in 2009, however, the results were highly similar, indicating that the larger cohort findings were not driven by the MCI group. Because the imaging performed in 2009 lagged behind the initial cognitive assessment performed between 2000 and 2002, biomarker status at initial intake was unknown. In a sensitivity analysis that excluded all cognitive assessments prior to imaging, the patterns of effects were similar, although there were fewer significant group contrasts, likely owing to reduced power. This analysis conveys the power of baseline imaging to estimate subsequent cognitive decline, most significantly for the oldest old individuals with Aβ+/ND+.
Operationalization with different ND markers contributes significantly to variability among studies of cognitive decline. For example, differences in relative risk for cognitive decline in Aβ+/ND− compared with SNAP depend on whether cerebrospinal fluid τ or imaging (HV or FDG) was used to define SNAP. Because HV and FDG hypometabolism are not completely concordant, this study simplifies the complexity of ND biomarkers by focusing only on HV. Regarding the underlying pathologic findings of SNAP, Crary et al noted similarities between primary age-related tauopathy and SNAP and proposed a correspondence. Like SNAP, primary age-related tauopathy has a lower prevalence of APOE*4 (OMIM 107741), its prevalence increases with age, and it prominently involves medial temporal lobe pathologic findings. However, Mormino et al reported comparable τ PET imaging results between SNAP (classified based on HV and FDG hypometabolism) and Aβ−/ND− groups, challenging the hypothesis of a close correspondence between SNAP and primary age-related tauopathy. Gordon et al, investigating longitudinal changes in PiB and HV, found that SNAP was similar to Aβ−/ND− in the rate of accumulation of Aβ and rate of hippocampal atrophy; thus, they concluded that SNAP is more likely to reflect inherent variability in brain structure than early AD. Our cognitive slope findings support the notion that SNAP is dissimilar to early AD in that no domains other than memory decline in SNAP, whereas isolated Aβ is associated with extramemory decline (namely, language and executive function). We observed some evidence of a progressive process in SNAP (ie, visual memory decline greater than the nonpathologic reference), whereas in the younger cohort (mean age, 66 years), SNAP was not associated with faster rate of hippocampal atrophy than other groups.
These findings further the understanding of AD pathophysiologic conditions in the oldest old. Postmortem studies indicate that after 80 years of age, presence of Aβ plaques (and neurofibrillary tangles) no longer discriminates between clinical dementia and nondementia cases. Furthermore, pathologic heterogeneity of dementia increases with advanced age. Weakening of associations between Aβ and cognition with advanced age suggests that AD may be a different disease above 80 years of age than below. If true, the argument has been made that Aβ is likely a misguided therapeutic target, as it may reflect an inherent process of advanced aging. As reported previously in this same oldest old cohort, Aβ deposition was not associated with the incidence of dementia during 2 years among the highest-risk participants with MCI (although it was associated with incidence of dementia among all participants). However, the more sensitive cognitive outcomes we now report for a 12-year follow-up indicate that, despite frequent occurrence of Aβ deposition at this age (98 of 175 [56.0%]), Aβ is associated with long-term cognitive decline compared with its absence. The same finding was observed for the frequent occurrence of hippocampal volume reduction (101 of 175 [57.7%]) vs its absence, although cognitive decline was restricted to visual memory. Both biomarkers appear to have measurable cognitive consequences and are hallmarks of decline, even among the oldest old. Although this study does not refute the notion of a diminished role of Aβ with advanced age, results support the hypothesis that Aβ remains functionally consequential in advanced aging and thus remains an important, if not sufficient, pathophysiologic process.
Limitations
This study has some limitations. Because the imaging lagged behind the initial cognitive assessment, biomarker status at initial intake was unknown. In addition, ND was operationalized only by HV, simplifying the complexity of ND biomarkers. Finally, the participants were relatively highly educated and mostly of white European descent; the findings may not generalize to other populations.
Conclusions
In the oldest old, the presence of both Aβ deposition and reduced hippocampal volume is common and confers the greatest risk for cognitive decline across domains. Neither biomarker abnormality is benign in the ninth and 10th decades of life. Isolated Aβ deposition is associated with decline in memory, executive functions, and some aspects of language, while isolated hippocampal atrophy is associated only with decline in memory. Suspected non-Alzheimer disease pathophysiology and isolated Aβ exhibit distinct cognitive decline profiles, which suggests different underlying pathophysiologic processes.
eTable. Estimates of the Difference (95% CI) in Rates of Prospective Annualized Cognitive Test Score Change, by Biomarker Group, Relative to Aβ– and ND–
eFigure 1. Incidental Immediate Recall of the Modified Rey-Osterrieth Figure (Visual Memory) Over Time (Higher is Better) by Biomarker Group
eFigure 2. Log Transformed Score of Trail Making Test Part B Over Time (Higher Is Worse) by Biomarker Group
References
- 1.Jack CR Jr, Wiste HJ, Knopman DS, et al. Rates of β-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology. 2014;82(18):1605-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging–Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71(6):765-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toledo JB, Weiner MW, Wolk DA, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Neuronal injury biomarkers and prognosis in ADNI subjects with normal cognition. Acta Neuropathol Commun. 2014;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen RC, Aisen P, Boeve BF, et al. Mild cognitive impairment due to Alzheimer disease in the community. Ann Neurol. 2013;74(2):199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caroli A, Prestia A, Galluzzi S, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Mild cognitive impairment with suspected nonamyloid pathology (SNAP): prediction of progression. Neurology. 2015;84(5):508-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mormino EC, Betensky RA, Hedden T, et al. Synergistic effect of β-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 2014;71(11):1379-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vos SJ, Verhey F, Frölich L, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain. 2015;138(pt 5):1327-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roe CM, Fagan AM, Grant EA, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80(19):1784-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wirth M, Villeneuve S, Haase CM, et al. Associations between Alzheimer disease biomarkers, neurodegeneration, and cognition in cognitively normal older people. JAMA Neurol. 2013;70(12):1512-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack CR Jr, Knopman DS, Chételat G, et al. Suspected non-Alzheimer disease pathophysiology—concept and controversy. Nat Rev Neurol. 2016;12(2):117-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12(10):957-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Harten AC, Smits LL, Teunissen CE, et al. Preclinical AD predicts decline in memory and executive functions in subjective complaints. Neurology. 2013;81(16):1409-1416. [DOI] [PubMed] [Google Scholar]
- 14.Wisse LEM, Butala N, Das SR, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Suspected non-AD pathology in mild cognitive impairment. Neurobiol Aging. 2015;36(12):3152-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prestia A, Caroli A, van der Flier WM, et al. Prediction of dementia in MCI patients based on core diagnostic markers for Alzheimer disease. Neurology. 2013;80(11):1048-1056. [DOI] [PubMed] [Google Scholar]
- 16.Knopman DS, Jack CR Jr, Wiste HJ, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78(20):1576-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soldan A, Pettigrew C, Cai Q, et al. ; BIOCARD Research Team . Hypothetical preclinical Alzheimer disease groups and longitudinal cognitive change. JAMA Neurol. 2016;73(6):698-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burnham SC, Bourgeat P, Doré V, et al. ; AIBL Research Group . Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer’s disease pathophysiology (SNAP) or Alzheimer’s disease pathology: a longitudinal study. Lancet Neurol. 2016;15(10):1044-1053. [DOI] [PubMed] [Google Scholar]
- 19.Mormino EC, Papp KV, Rentz DM, et al. Heterogeneity in suspected non–Alzheimer disease pathophysiology among clinically normal older individuals. JAMA Neurol. 2016;73(10):1185-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837-1844. [DOI] [PubMed] [Google Scholar]
- 21.Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65(11):1509-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonnen JA, Santa Cruz K, Hemmy LS, et al. Ecology of the aging human brain. Arch Neurol. 2011;68(8):1049-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeKosky ST, Williamson JD, Fitzpatrick AL, et al. ; Ginkgo Evaluation of Memory (GEM) Study Investigators . Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300(19):2253-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeKosky ST, Fitzpatrick A, Ives DG, et al. ; GEMS Investigators . The Ginkgo Evaluation of Memory (GEM) study: design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp Clin Trials. 2006;27(3):238-253. [DOI] [PubMed] [Google Scholar]
- 25.Snitz BE, Weissfeld LA, Lopez OL, et al. Cognitive trajectories associated with β-amyloid deposition in the oldest-old without dementia. Neurology. 2013;80(15):1378-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed New York, NY: Oxford University Press; 2004. [Google Scholar]
- 27.Becker JT, Boller F, Saxton J, McGonigle-Gibson KL. Normal rates of forgetting of verbal and non-verbal material in Alzheimer’s disease. Cortex. 1987;23(1):59-72. [DOI] [PubMed] [Google Scholar]
- 28.Trenerry MR, Crosson B, DeBoe J, Leber WR. Stroop Neuropsychological Screening Test. Odessa, FL: Psychological Assessment Resources; 1989. [Google Scholar]
- 29.Lopez OL, Becker JT, Jagust WJ, et al. Neuropsychological characteristics of mild cognitive impairment subgroups. J Neurol Neurosurg Psychiatry. 2006;77(2):159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saxton J, Ratcliff G, Munro CA, et al. Normative data on the Boston Naming Test and two equivalent 30-item short forms. Clin Neuropsychol. 2000;14(4):526-534. [DOI] [PubMed] [Google Scholar]
- 31.Fai AH-T, Cornelius PL. Approximate F-tests of multiple degree of freedom hypotheses in generalized least squares analyses of unbalanced split-plot experiments. J Stat Comput Simul. 1996;54(4):363-378. [Google Scholar]
- 32.Wirth M, Oh H, Mormino EC, Markley C, Landau SM, Jagust WJ. The effect of amyloid β on cognitive decline is modulated by neural integrity in cognitively normal elderly. Alzheimers Dement. 2013;9(6):687-698.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jack CR Jr, Wiste HJ, Weigand SD, et al. Age-specific population frequencies of cerebral β-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: a cross-sectional study. Lancet Neurol. 2014;13(10):997-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18(4):351-357. [DOI] [PubMed] [Google Scholar]
- 35.Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676-1689. [DOI] [PubMed] [Google Scholar]
- 36.Jack CR Jr, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58(5):750-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitwell JL, Jack CR Jr, Parisi JE, et al. Does TDP-43 type confer a distinct pattern of atrophy in frontotemporal lobar degeneration? Neurology. 2010;75(24):2212-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jack CR., Jr PART and SNAP. Acta Neuropathol. 2014;128(6):773-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Paola M, Caltagirone C, Fadda L, Sabatini U, Serra L, Carlesimo GA. Hippocampal atrophy is the critical brain change in patients with hypoxic amnesia. Hippocampus. 2008;18(7):719-728. [DOI] [PubMed] [Google Scholar]
- 40.Mormino EC, Papp KV. Cognitive decline in preclinical stage 2 Alzheimer disease and implications for prevention trials. JAMA Neurol. 2016;73(6):640-642. [DOI] [PubMed] [Google Scholar]
- 41.Bäckman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology. 2005;19(4):520-531. [DOI] [PubMed] [Google Scholar]
- 42.Saxton J, Lopez OL, Ratcliff G, et al. Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to onset. Neurology. 2004;63(12):2341-2347. [DOI] [PubMed] [Google Scholar]
- 43.Masur DM, Sliwinski M, Lipton RB, Blau AD, Crystal HA. Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology. 1994;44(8):1427-1432. [DOI] [PubMed] [Google Scholar]
- 44.Klunk WE, Perani D. Amyloid and neurodegeneration: converging and diverging paths. Neurology. 2013;81(20):1728-1729. [DOI] [PubMed] [Google Scholar]
- 45.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128(6):755-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon BA, Blazey T, Su Y, et al. Longitudinal β-amyloid deposition and hippocampal volume in preclinical Alzheimer disease and suspected non–Alzheimer disease pathophysiology. JAMA Neurol. 2016;73(10):1192-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C; Medical Research Council Cognitive Function and Ageing Study . Age, neuropathology, and dementia. N Engl J Med. 2009;360(22):2302-2309. [DOI] [PubMed] [Google Scholar]
- 48.Haroutunian V, Schnaider-Beeri M, Schmeidler J, et al. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol. 2008;65(9):1211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: the 90+ Study. Neurology. 2015;85(6):535-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richard E, Schmand B, Eikelenboom P, Westendorp RG, Van Gool WA. The Alzheimer myth and biomarker research in dementia. J Alzheimers Dis. 2012;31(suppl 3):S203-S209. [DOI] [PubMed] [Google Scholar]
- 51.Kuller LH, Lopez OL. Dementia and Alzheimer’s disease: a new direction: the 2010 Jay L. Foster Memorial Lecture. Alzheimers Dement. 2011;7(5):540-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez OL, Klunk WE, Mathis C, et al. Amyloid, neurodegeneration, and small vessel disease as predictors of dementia in the oldest-old. Neurology. 2014;83(20):1804-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Estimates of the Difference (95% CI) in Rates of Prospective Annualized Cognitive Test Score Change, by Biomarker Group, Relative to Aβ– and ND–
eFigure 1. Incidental Immediate Recall of the Modified Rey-Osterrieth Figure (Visual Memory) Over Time (Higher is Better) by Biomarker Group
eFigure 2. Log Transformed Score of Trail Making Test Part B Over Time (Higher Is Worse) by Biomarker Group