Key Points
Question
Are mutations in RTN4IP1 (OPA10), encoding a mitochondrial quinone oxidoreductase, solely causing recessive optic neuropathy?
Findings
In this study of 12 individuals with severe central nervous system diseases, novel RTN4IP1 mutations were identified, resulting in a spectrum of clinical presentations from isolated optic atrophy to severe early encephalopathies.
Meaning
RTN4IP1 is a candidate gene to consider in syndromes involving mainly central nervous system features and mitochondrial dysfunction.
Abstract
Importance
Neurologic disorders with isolated symptoms or complex syndromes are relatively frequent among mitochondrial inherited diseases. Recessive RTN4IP1 gene mutations have been shown to cause isolated and syndromic optic neuropathies.
Objective
To define the spectrum of clinical phenotypes associated with mutations in RTN4IP1 encoding a mitochondrial quinone oxidoreductase.
Design, Setting, and Participants
This study involved 12 individuals from 11 families with severe central nervous system diseases and optic atrophy. Targeted and whole-exome sequencing were performed—at Hospital Angers (France), Institute of Neurology Milan (Italy), Imagine Institute Paris (France), Helmoltz Zentrum of Munich (Germany), and Beijing Genomics Institute (China)—to clarify the molecular diagnosis of patients. Each patient’s neurologic, ophthalmologic, magnetic resonance imaging, and biochemical features were investigated. This study was conducted from May 1, 2014, to June 30, 2016.
Main Outcomes and Measures
Recessive mutations in RTN4IP1 were identified. Clinical presentations ranged from isolated optic atrophy to severe encephalopathies.
Results
Of the 12 individuals in the study, 6 (50%) were male and 6 (50%) were female. They ranged in age from 5 months to 32 years. Of the 11 families, 6 (5 of whom were consanguineous) had a member or members who presented isolated optic atrophy with the already reported p.Arg103His or the novel p.Ile362Phe, p.Met43Ile, and p.Tyr51Cys amino acid changes. The 5 other families had a member or members who presented severe neurologic syndromes with a common core of symptoms, including optic atrophy, seizure, intellectual disability, growth retardation, and elevated lactate levels. Additional clinical features of those affected were deafness, abnormalities on magnetic resonance images of the brain, stridor, and abnormal electroencephalographic patterns, all of which eventually led to death before age 3 years. In these patients, novel and very rare homozygous and compound heterozygous mutations were identified that led to the absence of the protein and complex I disassembly as well as mild mitochondrial network fragmentation.
Conclusions and Relevance
A broad clinical spectrum of neurologic features, ranging from isolated optic atrophy to severe early-onset encephalopathies, is associated with RTN4IP1 biallelic mutations and should prompt RTN4IP1 screening in both syndromic neurologic presentations and nonsyndromic recessive optic neuropathies.
This case series describes the RTN4IP1 mutations found in 12 children and young adults with severe central nervous system diseases and describes the association of these mutations with neurologic phenotypes.
Introduction
Mitochondrial inherited diseases are frequent causes of neurologic disorders that may consist of either isolated symptoms (eg, optic neuropathy, deafness, and myopathy) or complex syndromes (eg, Leigh syndrome, mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes, and neurogenic weakness with ataxia and retinitis pigmentosa) associated with many debilitating symptoms and these diseases and often lead to premature death because of severe encephalopathies. Recently, the first mutations in the RTN4IP1 (OMIM 610502) were described in individuals with isolated recessive optic atrophy-10 (OPA10) in 3 families with a founder effect as well as in 2 sisters from an unrelated family, who were affected with a syndromic form associating the optic atrophy with cerebellar ataxia, mild intellectual disability, and seizures. The RTN4IP1 gene encodes a mitochondrial targeted protein with a quinone oxidoreductase activity, involving 2 domains—an amino-terminal region with an alcohol dehydrogenase GroES-like signature (ADH_N) and a carboxy-terminal domain including a zinc-binding motif (ADH_Zinc). Mutations in this gene were not reported to affect mitochondrial network morphologic features, as is frequently the case in cells from individuals with dominant optic atrophy, but result in a mild deficit in mitochondrial complexes I and IV enzymatic activities. Together, these data led us to consider RTN4IP1 sequence integrity in cohorts of individuals with inherited optic atrophy and complex I deficiency by using either targeted or whole-exome sequencing. In this article, we report on the identification of 11 novel families with RTN4IP1 mutations and describe their association with various neurologic phenotypes.
Methods
Genetic Investigations
All patients were born from asymptomatic parents, except the mother from family 2, who showed symptoms unrelated to the disease of the 2 affected daughters. In consanguineous families (families 1 to 5 as well as 7 and 8), we anticipated identifying homozygous mutations. In the 4 nonconsanguineous simplex families (families 6, 9, 10, and 11), we considered both recessive and dominant de novo mutations, especially in family 6, in which the affected girl was born to a father in his 50s. After extraction of genomic DNA from peripheral blood cells, we found homozygous or biallelic mutations in RTN4IP1 by resequencing panels of genes dedicated to the molecular diagnosis of inherited optic neuropathies (panel of 17 genes, families 1-5) or to mitochondrial inherited diseases (panel of 219 genes, family 9) or by whole-exome sequencing (performed at the Imagine Institute for family 6; at the Helmoltz Zentrum München for families 7, 10, and 11; and at the Beijing Genomics Institute for Family 8). We analyzed the results by applying various prioritization filters, which are described elsewhere. All identified nucleotide substitutions were absent or had a frequency lower than 1 in 1000 in public databases. Classification of mutation pathogenicity was performed according to the American College of Medical Genetics and Genomics criteria. The Iranian mutation was absent in ethically and geographically matched 450 exomes. With Sanger sequencing using primers, which are described elsewhere, we confirmed the identified mutations and tested the carrier status of the unaffected relatives when their DNA was available. This study was approved by the institutional review boards of Angers University Hospital in Angers, France; Neurological Institute “Besta” in Milan, Italy; Technical University of Munich in Munich, Germany; and King’s College in London, England. Written informed patient consent was obtained from each participant in this study or from the parents of participants who were younger than 18 years, according to approved protocols of the different institutions involved in this study and the Declaration of Helsinki. This study was conducted from May 1, 2014, to June 30, 2016.
Biochemical and Cellular Studies of Fibroblasts and Muscle Biopsies
Biochemical measurement of individual oxidative phosphorylation complex activities was performed by standard spectrophotometric assays in muscle homogenate and digitonin-treated skin fibroblasts. Assessments of RTN4IP1 abundance and the mitochondrial network structure of RTN4IP1-mutated and control fibroblast cell lines were performed according to the process described in Angebault et al. Quadruple immunofluorescence analysis and assessment of the assembly of the respiratory chain complexes of muscle biopsy specimens were performed according to the process described in Rocha et al. Blue native polyacrylamide gel electrophoresis on skeletal muscle samples was performed as described in Alston et al.
Results
Patient Presentations
Family 1
A teenaged boy was the first child born to Romany consanguineous parents. His sister and brother were healthy. Strabismus was detected at age 3 years and operated on at age 6 years. Nystagmus was present at age 6 years and was stable. His bilateral visual acuity was 20/250, and fundus examination revealed a temporal pale papilla. Optical coherence tomography (OCT) confirmed the optic atrophy. Magnetic resonance images (MRIs) of the brain were normal.
Family 2
Two girls—a preteen and a teenager—born to Romany consanguineous parents had optic atrophy and a visual acuity of 20/200 and 20/500, respectively. Their sister and brother were healthy. Their brain MRIs were normal. The mother had photophobia, a mild right ptosis, and balance disorder.
Family 3
This teenaged boy born to Romany consanguineous parents presented at age 4 years with progressive bilateral visual loss leading to visual acuity of 20/100 bilaterally. Visual evoked potential (VEP) showed cortical conduction delay with amplitude change, OCT confirmed the bilateral optic atrophy, and a brain MRI was normal. He had a healthy sister.
Family 4
This young man in his 20s born to Romany consanguineous parents presented with bilateral visual acuity loss (20/400) at age 5 years. The VEP and OCT confirmed the presence of a bilateral optic atrophy.
Family 5
This girl younger than 10 years was born to consanguineous Maghrebian parents and diagnosed at the age of 6 with an optic atrophy. She had a visual acuity of 3/20 bilaterally and a red-green dyschromatopsy, but she had no nystagmus.
Family 6
This girl younger than 10 years was the last and only affected child of 4 children born to an unrelated Mauritius father of Irish/Pakistani descent and a mother of Indian descent. There was no family history of visual deficiency. At conception, her father was almost 60 years old and her mother was in her early 30s. The girl was born full term after an uneventful pregnancy and delivery. The perinatal period was unremarkable. At age 2 years, she presented with strabismus, but the diagnosis of optic neuropathy was made at age 5 years when she experienced a sudden and severe decrease in visual acuity—from 20/32 OU to 20/400 OD and 20/200 OS in a 3-month period.
Ophthalmological examination revealed bilateral temporal optic atrophy at the fundus, loss of optic nerve fibers and normal macula at the OCT, severely altered VEP with normal electroretinogram, and dyschromatopsia with red-green axis. Neurologic examination and cerebral and medullar MRI were strictly normal.
Family 7
This boy was born at term after a normal pregnancy by vaginal delivery to healthy, consanguineous parents from Pakistan. A younger and an older brother were healthy. Postnatal adaption and early development were reported as normal. As a toddler, he had a first seizure during an infection with a high temperature. Two weeks after this acute episode, twitching of the eyes and the mouth were observed. Sixteen months later, the first generalized tonic-clonic seizure occurred. Thereafter, he had seizures every 2 to 3 months that did not respond well to medication. Subsequently, he lost already acquired skills, such as chewing and expressive language (single words). On physical examination in the preteen years, his weight was 22.9 kg (<third centile), length was 116 cm (<third centile), and head circumference was 53 cm (21st centile). He was severely intellectually impaired, nonverbal, and unable to stand or walk unaided. A hearing impairment was suspected clinically, but no pediatric audiologic examination had been performed. Extensive laboratory testing was unremarkable, and only the plasma lactate concentration was elevated (28.8 mg/dL [to convert to millimoles per liter, multiply by 0.111]). Results of a brain MRI and cranial ultrasonography were normal. Testing of the POLG (OMIM *174763) gene did not reveal any pathogenic variants. No optic atrophy was mentioned in the results from cardiac MRI, and no explicit ophthalmologic examination was performed.
Family 8
The woman in her 30s was from a consanguineous family from south Iran. She has 3 sisters and 1 brother, all of whom are healthy. She was hypotonic at birth but otherwise was normal. All her growth measurements were within the normal range. She had psychomotor delay with walking difficulty characterized by unsteady ataxic gaits, poor balance, and frequent falls. She could not walk without assistance, and this condition got worse gradually; in her midteens, she had lost the ability to walk and became wheelchair-bound and bedridden. Her lower limbs were spastic, and she experienced severe pain in her lower legs.
She has poor language skills and speaks only single words. She displays severe intellectual disability, cannot live independently, and requires close supervision and support with self-care activities. Vertical nystagmus was noticed in infancy, and in her preteen years, her vision started to decline gradually, which eventually led to loss of vision in her mid-20s. Ophthalmologic examination showed progressive bilateral optic atrophy associated with nystagmus, photophobia, and color vision impairment. She experienced her first seizure suddenly before age 5 years. In her childhood, she experienced seizure only once a day, every 2 to 3 months, but this increased to be more severe with 5 to 6 attacks every 2 to 3 months that were not responsive to medication. Her bouts of seizure only remitted in her mid-20s. Currently, her seizure is under control with low doses of medication. Brain MRI and OCT have not been performed for this patient.
Family 9
This patient was a boy younger than 10 years and was the second child born to healthy, unrelated Italian parents. His family history was unremarkable. He was born at term after a regular pregnancy and had a normal perinatal period. During his first year of life, his parents noticed poor visual contact and delay in head control. First instrumental examinations were performed before 1 year of age: fundus examination disclosed bilateral optic atrophy, and a brain MRI showed normal findings. Clinical evolution confirmed psychomotor developmental delay: sitting position at older than 1 year, supported deambulation from age 5 years, and babbling at age 2 years (no other language skills were reported). At age 3 years, he presented with generalized seizures that became drug resistant in the following years and were associated with multifocal and subcontinous abnormalities on the electroencephalogram (EEG). Serial brain MRI disclosed bilateral T2 hyperintensities in the subthalamic nucleus and in the nucleus dentate and brainstem, brainstem auditory evoked potential revealed the absence of a v wave, and elevated serum lactate (2.9 mmol/L; reference range, 0.5-2.1 mmol/L) and serum pyruvate (150 μmol/L; reference range, 40-140 μmol/L) levels were detected. A mitochondrial disease was suspected, a muscle biopsy was performed, and the result of a respiratory chain analysis was normal. Analysis of the POLG, MTATP6 (OMIM +516060), OPA1 (OMIM *605290), SCA1 (OMIM 164400), SCA2 (OMIM 183090), and SCA6 (OMIM 183086) genes were negative for pathogenic variants.
This patient arrived for our observation before age 10 years. Results of a clinical examination showed spastic tetraparesis and dystonia at the upper limbs, with deambulation possible only with support and severe cognitive impairment (which was impossible to test). Fundus oculi confirmed bilateral optic atrophy, visual and brainstem auditory evoked potentials disclosed marked multisystem central abnormalities, and neuronography showed sensitive axonal neuropathy. A brain MRI disclosed T2 hyperintensities in the thalami as well as abnormalities in the subthalamic and dentate nuclei and in the brainstem. The lactate level (32.4 mg/dL; reference range, 5.2-18.9 mg/dL) and pyruvate level (1.7 mg/dL; reference range, 0.4-1.2 mg/dL [to convert to micromoles per liter, multiply by 113.56) were elevated in plasma; amino acid serum levels and urinary organic acid levels were normal. Treatment with thiamin hydrochloride, coenzyme Q 10, and riboflavin was started, but no clinical benefits were observed. Clinical evolution was stable except for worsening of epilepsy. The spectrophotometric determination of mitochondrial respiratory chain complex activities displayed an isolated reduction of complex I normalized to citrate synthase (12.6; reference range, 16-34) in skin fibroblasts. No further material from muscle biopsy was available.
Family 10
This boy was the first born after a normal pregnancy by vaginal delivery to nonconsanguineous Italian parents. In his first months of life, the first signs noticed were eyelid myoclonia with inconsolable stridor on awakening, impaired eye-to-eye contact, hypotonia, and developmental delay. At age 4 months, concomitant to the first vaccination, he manifested fever and seizures consisting of eyelid myoclonias and partial tonic-clonic fits that rapidly spread into generalized myoclonic epileptic refractory status. Later, his condition led to drug-resistant partial seizures and myoclonic status. An EEG showed a burst-suppression pattern. He developed a severe psychomotor delay with hypotonia and scarce visual contact. Results of a brain MRI were normal in the first few months of life but revealed severe cerebral atrophy with an increase in lactate at spectroscopy as he approached 1 year. Visual function investigation showed evidence of mild pale papilla, normal photopic, and absent (impossible to test) scotopic responses on an electroretinogram. Visual evoked potentials showed cortical conduction delay with amplitude change. Extensive metabolic and molecular workup did not reveal alteration in values for serum, cerebrospinal fluid, or urine amino acids; urine organic acids; sulfitest; clinitest; cerebrospinal fluid neurotransmitters; serum very-long-chain fatty acid; congenital defects of glycosylation; biotinidase; and copper and ceruloplasmin dosage. Comparative genomic hybridization array, CDKL5 (OMIM *300203) and ARX (OMIM *300382) expression, and POLG sequence and karyotype were normal. Pyruvate dehydrogenase complex activities were normal on examination of skin and muscle biopsy specimens as was palmitoyl protein thioesterase 1 dosage on fibroblasts. Analysis of mitochondrial respiratory chain complexes showed an isolated complex I reduction in muscle (value normalized to citrate synthase activity, 6.5; reference range, 13-28) and skin fibroblasts (value normalized to citrate synthase activity, 12.2; reference range, 13-30). The boy died of cardiorespiratory failure before age 5 years.
Family 11
This girl was the second child born to unrelated parents. There were decreased fetal movements during the pregnancy but no anomalies on the antenatal scans. She was born at term (birth weight, 4 kg; length, 59 cm; and head circumference, 36 cm) and was discharged home at 2 days but readmitted at age 1 week for poor feeding and stridor. Her weight gain was very poor, and nasogastric feedings were started at 3 weeks. Investigations showed persistently elevated plasma lactate levels (3.0 mmol/L). At age 3 weeks, she developed eye-rolling movements and was not as responsive; she was referred to a specialist center for further investigation. An elevated plasma lactate level (28.8 mg/dL) was noted at admission along with a raised alanine level (6.59 mg/dL [to convert to micromoles per liter, multiply by 112.2); the urinary organic acid profile was nonspecific; and results of very-long-chain fatty acids, acylcarnitine profile, and liver function tests were all normal. Brain MRI findings showed symmetrical T2 high-signal change with associated swelling in the posterolateral aspect of both putamina suggestive of a mitochondrial disorder (eFigure 1 in the Supplement). She had progressive stridor, and bronchoscopy showed short aryepiglottic folds and collapsing arytenoids. She had a tracheostomy. She developed frequent apneas, and her EEG showed an abnormal attenuated background with slow waves and low-voltage spikes and spike-and-wave discharges that were seen independently over the left centrotemporal and right posterior quadrants and suggestive of focal left temporofocal epilepsy. Her clinical status deteriorated progressively, and she died before age 1 year.
Molecular Investigations
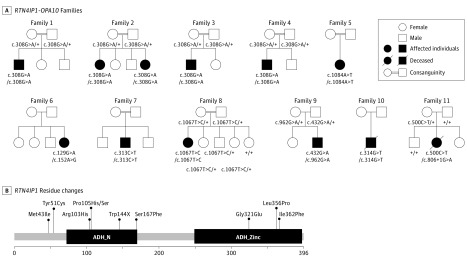
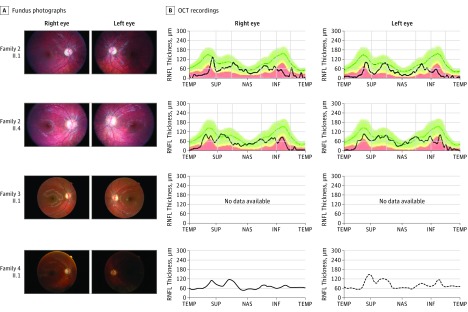
Using a dedicated gene panel covering all RTN4IP1 exons, we sequenced DNA from 300 patients with suspected inherited optic neuropathies that were negative for OPA1 mutations and the 3 main Leber hereditary optic neuropathy mutations. Five cases from consanguineous families (families 1-4) were identified with the homozygous c.308G>A mutation (Figure 1A) on a unique haplotype (described in Angebault et al), leading to the p.Arg103His residue change. These cases were all of Romany origin, suggesting a high prevalence of this mutation in that population. A patient with a homozygous c.1084A>T mutation (p.Ile362Phe) in a consanguineous Maghrebian family (family 5; Figure 1A) was also identified. The p.Arg103His and p.Ile362Phe mutations affect each of the 2 oxidoreductase conserved domains, ADH_N or ADH_Zinc, respectively (Figure 1B), and are considered to be deleterious according to the SIFT, Polyphen, and MutationTaster programs and the American College of Medical Genetics and Genomics criteria. A patient born to nonconsanguineous parents from Mauritius (family 6) was found to be compound heterozygous for the unreported c.129G>A (p.Trp51Cys) and c.152A>G (p.Met43Ile) mutations, which affect evolutionary conserved amino acids upstream of the ADH_N domain and are considered to be deleterious. All of these individuals presented severe, isolated, early-onset optic atrophy (Figure 2) and stable low visual acuity (Table).
Figure 1. Pedigree of the RTN4IP1 (OPA10) Families, Segregation of the Mutations, and Alterations of the Protein Primary Structure.
A, Pedigrees show the affected cases in the 11 families and the segregation of the mutations. Black symbols indicate affected patients. B, Localization of RTN4IP1 residue changes in the protein: the primary structure of RTN4IP1 protein (domains and amino acid positions) is described in the figure with all of the pathogenic mutations reported in this study. ADH_N indicates alcohol dehydrogenase GroES-like domain; ADH_Zinc, zinc-binding dehydrogenase domain.
Figure 2. Ophthalmological Examination of RTN4IP1 (OPA10) Patients.
Eye fundus of the right and left eyes from indicated patients (A) and the corresponding optical coherence tomographic (OCT) recordings (B) are shown, when available. INF indicates inferior; NAS, nasal; RNFL retinal nerve fiber layer; SUP, superior; and TEMP, temporal.
Table. Clinical Data of the Patients With the RTN4IP1 (OPA10) Genotype.
| Family No./Sex | OA/VA | Epilepsy | Brain MRI | Respiratory Chain | Digestive Symptom | ORF Mutation | Protein Change | Ref Seq No. | Frequency in the Exome Aggregation Consortium | Variant Classification |
|---|---|---|---|---|---|---|---|---|---|---|
| 1/M | 20/250 | No | Normal | Unknown | No | c.308G>A HM | p.Arg103His | rs372054380 | 4,12E-05 | Pathogenic |
| 2/F and 2/F |
20/200 | No | Normal | Unknown | No | c.308G>A HM | p.Arg103His | rs372054380 | 4,12E-05 | Pathogenic |
| 3/M | 20/100 | No | Normal | Unknown | No | c.308G>A HM | p.Arg103His | rs372054380 | 4,12E-05 | Pathogenic |
| 4/M | 20/400 | No | Unknown | Unknown | No | c.308G>A HM | p.Arg103His | rs372054380 | 4,12E-05 | Pathogenic |
| 5/F | 3/20 | No | Unknown | Unknown | No | c.1084A>T HM | p.Ile362Phe | Unknown | Unknown | Pathogenic |
| 6/F | 20/400 | No | Normal | Unknown | No | c.129G>A + c.152A>G |
p.Met43Ile + Tyr51Cys |
Unknown + unknown |
Unknown | Pathogenic Pathogenic |
| 7/M | Unknown | Yes | Normal | Unknown | Poor feeding | c.313C>T HM | p.Pro105Ser | Unknown | Unknown | Likely pathogenic |
| 8/F | OA | Yes | Unknown | Unknown | No | c.1067T>C HM | p.Leu356Pro | Unknown | Unknown | Pathogenic |
| 9/M | OA | Yes | Leigh pattern | CI deficit | No | c.432G>A + c.962G>A |
p.Trp144 + p.Gly321Glua |
rs755175825 + unknown |
8.24e-06 + unknown |
Pathogenic Pathogenic |
| 10/M | Pale papilla | Yes | Cerebral atrophy | CI deficit | No | c.314C>A HM | p.Pro105His | Unknown | Unknown | Pathogenic |
| 11/F | Eye rolling | Unknown | Swelling putamina | CI deficit | Poor feeding | c.500C>T + c.806 + 1G>Aa |
p.Ser167Phe + splice defect |
Unknown + rs772984484 |
Unknown + 1.6e-05 |
Pathogenic |
Abbreviations: CI, complex I; MRI, magnetic resonance imaging; OA, optic atrophy; ORF, open reading frame; Ref Seq, reference sequence; VA, visual acuity.
Indicates de novo mutation.
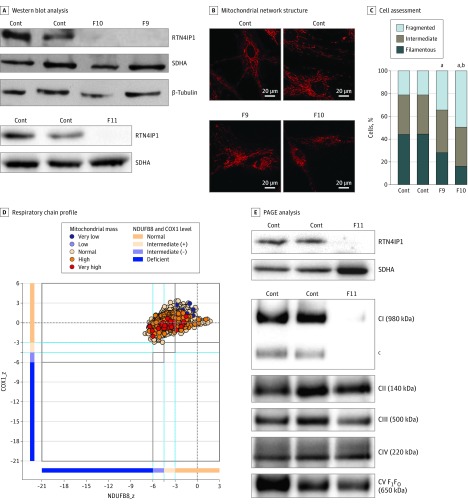
Using a resequencing panel of 219 mitochondrial genes or by whole-exome sequencing, we identified novel pathogenic RTN4IP1 mutations in 5 individuals with severe early-onset encephalopathy associated with optic atrophy (Figure 1A). Three homozygous mutations were found in families: c.313C>T in family 7, c.314C>A in family 8, and c.1067T>C in family 10. These mutations are anticipated to change a proline to either a serine or lysine (p.Pro105Ser/Lys) in the ADH_N domain or a leucine to a proline (p.Leu356Pro) in the ADH_Zinc domain, thus possibly destabilizing the protein structure or impairing its function (Figure 1B). In the 2 heterozygous composite cases from families 9 and 11 (Figure 1A), 1 mutated allele led to a loss of function corresponding to either a stop codon (p.Tyr144*) or to a splicing defect (de novo c.806 + 1G>A mutation; eFigure 2 in the Supplement), while the other mutated allele induced a rare damaging missense mutation (p.Gly321Glu or p.Ser167Phe, respectively). All these changes altered either of the 2 conserved ADH enzymatic domains of RTN4IP1 (Figure 1B). In these affected individuals, a core of common symptoms was identified that consisted of optic atrophy (although not investigated by an ophthalmologist in family 7), seizures, and various severity of global developmental delay (Table) as reported in a previous study. In addition, in the most affected cases (families 9-11), the associated clinical features were deafness, brain MRI abnormalities (eFigure 1 in the Supplement), stridor, and abnormal EEG, all of which eventually led to premature death. In these cases, we disclosed a complete absence of RTN4IP1 protein in fibroblasts and muscle biopsy (Figure 3A and C), which was associated with the fragmentation of the mitochondrial network (Figure 3B) in fibroblasts from the index cases of families 9 and 10 and with the drastic effect on NDUFB8 (OMIM *602140) (complex I) protein levels and mitochondrial complex I assembly in the muscle biopsy from the index case of family 11 (Figure 3C). Furthermore, increased lactate concentrations in blood and brain were revealed by MRI spectroscopy in these severely affected patients.
Figure 3. Association of Decreased RTN4IP1 Levels With Mitochondrial Network Structure Muscle of Severely Affected Patients.
A, Western blot analysis of RTN4IP1 protein levels in fibroblasts from controls (Cont) and RTN4IP1 index cases of families 9 (F9), 10 (F10), and 11 (F11). Succinate dehydrogenase and β-tubulin antibodies were used as loading Cont. B, Assessment of mitochondrial network structure from 2 Cont fibroblast cell lines and from fibroblasts of the index cases of F9 and F10 (MitoTracker stain; Thermo Fisher Scientific). C, Assessment of the percentage of cells presenting a fragmented, intermediate, or filamentous network. D, Respiratory chain profile following quadruple oxidative phosphorylation immunofluorescence analysis of cryosectioned muscle from the index case of F11, confirming the presence of fibers lacking complex I (NDUFB8) protein but with normal complex IV (COX1) expression. (Experimental procedures per the protocols of Rocha et al.) Each dot represents the measurement from an individual muscle fiber, color coded according to its mitochondrial mass. Gray dashed lines indicate SD limits for the classification of fibers; lines next to x- and y-axes, the levels (SDs from the average of Cont fibers after normalization to porin/VDAC1 levels) of NDUFB8 and COX1, respectively; and _z, the z score for COX1 or NDUFB8. (For formulas and full description of statistics involved, see the Methods section of Rocha et al.) Blue dotted lines indicate the mean expression level observed in respiratory normal fibers. E, One-dimensional blue native polyacrylamide gel electrophoresis (PAGE) (4%-16% gradient) analysis showing a specific complex I assembly defect in skeletal muscle from the index case of F11 and age-matched Cont. Individual oxidative phosphorylation complexes were detected by immunoblotting using subunit-specific antibodies (complex I [C1] [NDUFB8], CII [SDHA], CIII [UQCRC2], CIV [COX1], and CV [ATP5A]). Complex V comprises the F1 and FO subcomplexes. F1FO denotes that the band is the complete assembled form of adenosine triphosphate synthase (CV). aP < .001 compared with Cont. bP < .05 compared with F9. cIndicates the presence of an additional, partially assembled CI intermediate also detected with the NDUFB8 antibody, absent in the patient sample, and likely corresponding to the approximately 650-kDa Iβ subcomplex of the hydrophobic membrane arm.
Discussion
A large spectrum of neurologic features, ranging from isolated optic atrophy to severe early-onset encephalopathy, can be associated with biallelic mutations in RTN4IP1. A first group of nonsyndromic cases comprised patients who presented severe, early optic atrophy with stable, low visual acuity but no additional symptoms (Table). Their clinical presentations overlapped with those of previously reported families; the common Romany origin and the shared p.Arg103His mutation confirm a founder effect in this ethnic group and a strict genotype-phenotype correlation for this variant. Nevertheless, the same phenotype was observed in 2 cases (families 5 and 6) with different origins and mutations. In family 6, heterozygous composite mutations affecting amino acids 43 and 51 located out of the 2 conserved ADH domains might interfere with the structure of the mitochondrial targeting sequence, which is predicted to encompass amino acids 1 to 49. In the syndromic cases, harboring various novel mutations, a core of common symptoms consisted of optic atrophy, seizures, and various severity of mental and growth retardation (Table), as already reported in the last family of our previous study. Nevertheless, the most severely affected individuals presented features of deafness, brain MRI abnormalities, stridor, and abnormal EEG findings, which were systematically associated with drastically reduced RTN4IP1 abundance, complex I activity, and assembly and increased lactate concentrations in blood, revealing a major impairment of the oxidative phosphorylation function, which is reported for many diseases with complex I defect, such as the Leigh syndrome.
Most novel RTN4IP1 mutations affect either of the 2 conserved enzymatic domains of RTN4IP1 protein, but there is no evident correlation between the severity of the clinical presentation and the affected domain. For instance, mutations affecting very close amino acids (ie, Arg103 and Pro105) led to highly different phenotypes. However, analysis of amino acid changes reveals that, in nonsyndromic cases, mutations in ADH domains led to conservative changes, substituting arginine to histidine (2 positively charged residues) and isoleucine to phenylalanine (2 hydrophobic residues). Similarly, the 2 mutations affecting the N-terminal domain located upstream of the ADH_N domain, changed methionine to isoleucine and tyrosine to cysteine, which are also conservative.
Conversely, in syndromic individuals, all the missense mutations change the biochemical properties of the residues, probably destabilizing the protein structure, often by introducing or deleting a proline. Two other mutations found in the most severe cases are predicted to cause the truncation of the protein; thus, these alterations are likely more deleterious on RTN4IP1 activity or structure than those leading to isolated optic atrophy. Nevertheless, the mitochondrial function of RTN4IP1 remains poorly described, particularly the characterization of its enzymatic activity, prompting future pathophysiological challenges.
The gradual severity associated with the different mutations in a single gene is a phenomenon increasingly reported in the past few years, particularly for mitochondrial diseases affecting primarily the central nervous system. RTN4IP1 is the third gene responsible for recessive isolated optic atrophy, after the identification of TMEM126A (OMIM *612988) and ACO2 (OMIM *100850), and is the most frequently mutated, as we have now reported a total of 9 RTN4IP1 families with this restricted ophthalmological presentation, whereas only 5 were reported with TMEM126A mutations and 1 with ACO2 mutations. Auditory neuropathy and encephalopathy associated with cerebellar ataxia have also been reported in association with the optic nerve atrophy in individuals bearing mutations in TMEM126A and ACO2. In this respect, many other syndromic presentations with recessive or dominant traits involve an alteration of the optic nerve, and an increasing number of mild mutations in causative genes that manifest syndromic disorders have been shown to be responsible for isolated optic atrophy, emphasizing the frailty of the retinal ganglion cells to mitochondrial dysfunction. This finding is further highlighted by the spectrum of complex I diseases that can result either in isolated optic neuropathy, such as Leber hereditary optic neuropathy, or in severe syndromic diseases, such as Leigh syndrome, which leads to clinical presentations quite similar to those described here.
Limitations
This study has several limitations. First, deep-intronic variants were not screened in our sample of individuals with isolated or syndromic optic neuropathies, possibly leading to an underestimation of RTN4IP1 involvement. Second, biochemical and mitochondrial investigations were not performed in all affected individuals, limiting the correlation that could be drawn between clinical and biochemical phenotypes.
Conclusions
We expect that future works will identify novel mutations in tens of genes that will illustrate a spectrum of clinical presentations, ranging from isolated optic atrophy to very complex and severe syndromes, as exemplified here for RTN4IP1. In this respect, there is a need to generate novel databases that aggregate genotype-phenotype associations for genes involved in inherited mitochondrial diseases, such as the one we designed for OPA1-related isolated and syndromic optic neuropathies.
eFigure 1. Brain MRI of the RTN4IP1 Index Case of Family 11
eFigure 2. Aberrant RTN4IP1RNA Splicing Induced by the De novo c.806 + 1G>A Mutation
References
- 1.DiMauro S. Mitochondrial diseases. Biochim Biophys Acta. 2004;1658(1-2):80-88. [DOI] [PubMed] [Google Scholar]
- 2.Munnich A, Rustin P. Clinical spectrum and diagnosis of mitochondrial disorders. Am J Med Genet. 2001;106(1):4-17. [DOI] [PubMed] [Google Scholar]
- 3.Angebault C, Guichet PO, Talmat-Amar Y, et al. Recessive mutations in RTN4IP1 cause isolated and syndromic optic neuropathies. Am J Hum Genet. 2015;97(5):754-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu WH, Hausmann ON, Yan MS, Walters WM, Wong PK, Bethea JR. Identification and characterization of a novel Nogo-interacting mitochondrial protein (NIMP). J Neurochem. 2002;81(1):36-45. [DOI] [PubMed] [Google Scholar]
- 5.Lenaers G, Hamel C, Delettre C, et al. Dominant optic atrophy. Orphanet J Rare Dis. 2012;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao de la Barca JM, Prunier-Mirebeau D, Amati-Bonneau P, et al. OPA1-related disorders: diversity of clinical expression, modes of inheritance and pathophysiology. Neurobiol Dis. 2016;90:20-26. [DOI] [PubMed] [Google Scholar]
- 7.Legati A, Reyes A, Nasca A, et al. New genes and pathomechanisms in mitochondrial disorders unraveled by NGS technologies. Biochim Biophys Acta. 2016;1857(8):1326-1335. [DOI] [PubMed] [Google Scholar]
- 8.Colin E, Daniel J, Ziegler A, et al. ; FREX Consortium . Biallelic variants in UBA5 reveal that disruption of the UFM1 cascade can result in early-onset encephalopathy. Am J Hum Genet. 2016;99(3):695-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 11.Bugiani M, Invernizzi F, Alberio S, et al. Clinical and molecular findings in children with complex I deficiency. Biochim Biophys Acta. 2004;1659(2-3):136-147. [DOI] [PubMed] [Google Scholar]
- 12.Rocha MC, Grady JP, Grünewald A, et al. A novel immunofluorescent assay to investigate oxidative phosphorylation deficiency in mitochondrial myopathy: understanding mechanisms and improving diagnosis. Sci Rep. 2015;5:15037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alston CL, Howard C, Oláhová M, et al. A recurrent mitochondrial p.Trp22Arg NDUFB3 variant causes a distinctive facial appearance, short stature and a mild biochemical and clinical phenotype. J Med Genet. 2016;53(9):634-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angebault C, Charif M, Guegen N, et al. Mutation in NDUFA13/GRIM19 leads to early onset hypotonia, dyskinesia and sensorial deficiencies, and mitochondrial complex I instability. Hum Mol Genet. 2015;24(14):3948-3955. [DOI] [PubMed] [Google Scholar]
- 15.Fassone E, Rahman S. Complex I deficiency: clinical features, biochemistry and molecular genetics. J Med Genet. 2012;49(9):578-590. [DOI] [PubMed] [Google Scholar]
- 16.Lebre AS, Rio M, Faivre d’Arcier L, et al. A common pattern of brain MRI imaging in mitochondrial diseases with complex I deficiency. J Med Genet. 2011;48(1):16-23. [DOI] [PubMed] [Google Scholar]
- 17.Metodiev MD, Gerber S, Hubert L, et al. Mutations in the tricarboxylic acid cycle enzyme, aconitase 2, cause either isolated or syndromic optic neuropathy with encephalopathy and cerebellar atrophy. J Med Genet. 2014;51(12):834-838. [DOI] [PubMed] [Google Scholar]
- 18.Hanein S, Perrault I, Roche O, et al. TMEM126A, encoding a mitochondrial protein, is mutated in autosomal-recessive nonsyndromic optic atrophy. Am J Hum Genet. 2009;84(4):493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charif M, Roubertie A, Salime S, et al. A novel mutation of AFG3L2 might cause dominant optic atrophy in patients with mild intellectual disability. Front Genet. 2015;6:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klebe S, Depienne C, Gerber S, et al. Spastic paraplegia gene 7 in patients with spasticity and/or optic neuropathy. Brain. 2012;135(pt 10):2980-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouzier C, Bannwarth S, Chaussenot A, et al. The MFN2 gene is responsible for mitochondrial DNA instability and optic atrophy “plus” phenotype. Brain. 2012;135(Pt 1):23-34. [DOI] [PubMed] [Google Scholar]
- 22.Ferré M, Caignard A, Milea D, et al. Improved locus-specific database for OPA1 mutations allows inclusion of advanced clinical data. Hum Mutat. 2015;36(1):20-25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Brain MRI of the RTN4IP1 Index Case of Family 11
eFigure 2. Aberrant RTN4IP1RNA Splicing Induced by the De novo c.806 + 1G>A Mutation