Key Points
Question
Do seizures and encephalopathy occur more commonly in myelin oligodendrocyte glycoprotein IgG disease than in aquaporin 4 IgG disease?
Findings
In this case series, 5 of 34 patients (14.7%) with myelin oligodendrocyte glycoprotein IgG disease had seizures compared with 1 of 100 patients with aquaporin 4 IgG neuromyelitis optica spectrum disorder. All 5 patients with myelin oligodendrocyte glycoprotein IgG disease had inflammatory cortical brain lesions associated with the seizures.
Meaning
Myelin oligodendrocyte glycoprotein IgG disease may be the cause for a proportion of autoimmune encephalitis-like presentations with seizures.
Abstract
Importance
Antibodies to myelin oligodendrocyte glycoprotein IgG (MOG-IgG) are increasingly detected in patients with non–multiple sclerosis–related demyelination, some of whom manifest a neuromyelitis optica (NMO) phenotype. Cortical involvement, encephalopathy, and seizures are rare in aquaporin 4 antibody (AQP4-IgG)–related NMO in the white European population. However, the authors encountered several patients with seizures associated with MOG-IgG disease.
Objective
To compare incidence of seizures and encephalitis-like presentation, or both between AQP4-IgG–positive and MOG-IgG–positive patients.
Design, Setting, and Participants
Retrospective case series of all patients who were seropositive for MOG-IgG (n = 34) and the last 100 patients with AQP4-IgG disease (NMO spectrum disorder) seen in the NMO service between January 2013 and December 2016, and analysis was completed January 4, 2017. All patients were seen in a tertiary neurological center, The Walton Centre NHS Foundation Trust in Liverpool, England.
Main Outcomes and Measures
The difference in seizure frequency between the AQP4-IgG–positive and MOG-IgG–positive patient groups was determined.
Results
Thirty-four patients with MOG-IgG disease (20 female) with a median age at analysis of 30.5 years (interquartile range [IQR], 15-69 years), and 100 AQP4-IgG–positive patients (86 female) with a median age at analysis of 54 years (IQR, 12-91 years) were studied. Most patients were of white race. Five of the 34 patients with MOG-IgG (14.7%) had seizures compared with 1 patient with AQP4-IgG (2-sided P < .008, Fisher test). On magnetic resonance imaging, all 5 MOG-IgG–positive patients had inflammatory cortical brain lesions associated with the seizures. In 3 of the 5 MOG-IgG–positive patients, seizures occurred as part of the index event. Four of the 5 presented with encephalopathy and seizures, and disease relapsed in all 5 patients. Four of these patients were receiving immunosuppressant medication at last follow-up, and 3 continued to take antiepileptic medication. In contrast, the only AQP4-IgG–positive patient with seizures had a diagnosis of complex partial epilepsy preceding the onset of NMO by several years and experienced no encephalitic illness; her magnetic resonance imaging results demonstrated no cortical, subcortical, or basal ganglia involvement.
Conclusions and Relevance
Patients with MOG-IgG–associated disease were more likely to have seizures and encephalitis-like presentation than patients with AQP4-IgG–associated disease.
The case series describes myelin oligodendrocyte glycoprotein IgG– and aquaporin 4 IgG–associated disease in 134 English patients who presented with seizure.
Introduction
Antibody-associated central nervous system inflammation is increasingly recognized to cause a wide spectrum of relapsing neurologic diseases. Myelin oligodendrocyte glycoprotein (MOG), a membrane protein expressed on oligodendrocyte cell surfaces and on the outermost surface of myelin sheaths, is the target for one such antibody, MOG-IgG. Initially, MOG-IgG was linked to childhood-onset multiple sclerosis, but more recently it has been found in a proportion of patients who meet the clinical criteria for neuromyelitis optica spectrum disorder (NMOSD) but who lack antibodies against aquaporin 4 IgG (AQP4-IgG). Although the NMOSD phenotype appears common to these 2 antibodies, the pathogenesis is distinct, with AQP4-IgG triggering complement-mediated astrocyte death rather than targeting myelin and oligodendrocytes. Differences in phenotype are also emerging: patients with MOG-IgG are more likely to have a milder or less disabling clinical course compared with patients with AQP4-IgG and less likely to be female, and association with other autoimmune disorders is less common. In addition, and in spite of the broadening spectrum of NMOSD outlined in the 2015 International Panel for NMO Diagnosis criteria, some patients with MOG-IgG have limited or different phenotypes to typical AQP4-IgG NMOSD. Whether MOG-IgG–associated demyelination is part of an evolving NMOSD or a distinctive disease is hotly debated and highlights the potential importance of any clinical feature that appears unique to one or the other antibody. In our cohort of patients with NMOSD and similar disorders, we noticed that some with MOG-IgG antibodies had presented with seizures or an encephalitis-like illness that we had not observed in patients with AQP4-IgG–positive NMOSD. Review of the literature found isolated reports of patients with MOG-IgG–associated disease having seizures or encephalopathy (Table 1). Consequently, we considered it important to study this association further.
Table 1. Cases of MOG-IgG–Associated Seizures Identified on Literature Reviewa.
| Source | No. of Patients | Age, y/Sex | Ethnicity | Other Antibodies | Type of Seizure | Clinical Syndrome | Cognitive Changes | Disease Course | T2/FLAIR MRI Brain Changes and Location |
|---|---|---|---|---|---|---|---|---|---|
| Hino-Fukuyo et al, 2015 | 3 | 12/M; 14/M; 5/M | Japanese | None | NA | ADEM | NA | Monophasic; relapsing; monophasic | Multiple WM, L basal ganglia and bilateral thalami (1 mo after onset); multiple WM, bilateral; basal ganglia (at third attack); unknown |
| Tsuburaya et al, 2015 | 1 | 7/M | Japanese | None | Partial (eye deviation and L arm clonic seizures) | ADEM, ON | No | Relapsing | Plaque involving subcortical WM in the R frontal lobe |
| Ramberger et al, 2015 | 22b | NA | NA | None | NA | ADEM | NA | NA | NA |
| Titulaer et al, 2014 | 1 | 4/F | Hispanic | NMDAR | NA | Seizures, hemiparesis; later: mutism, chorea, and orofacial dyskinesia | Yes | Relapsing | Periventricular, basal ganglia, cerebellum, and pons |
| Ogawa et al, 2017 | 4 | 39/M; 36/M; 23/M; 38/M | Japanese? | None | GTC; GTC; GTC+ focal; GTC | Encephalopathy, ON; seizure, ON; encephalopathy; seizure, aphasia, and R hemiparesis | Yes; during seizure; yes; during seizure | All monophasic | R frontoparietal cortex; L hemisphere cortex |
| Fujimori et al, 2017 | 1 | 46/M | Japanese | None | Focal progressed to secondary generalized | Encephalopathy, paraplegia | Yes | Relapsing | Bilateral frontal cortex, corpus callosum |
Abbreviations: ADEM, acute disseminated encephalomyelitis; BG, basal ganglia; CBA, cell-based assay; F, female; FLAIR, fluid-attenuated inversion recovery; GTC, generalized tonic-clonic; L, left; M, male; MOG, myelin oligodendrocyte glycoprotein; MRI, magnetic resonance imaging; NA, not available; NMDAR, N-methyl-d-aspartate receptor; R, right; ON, optic neuritis; WM, white matter.
All studies used a cell-based assay for MOG antibody testing.
Represents patients who presented with 1 or a combination of cognitive impairments or seizures. It is unclear how many of these patients had seizures.
Methods
All patients in this case series were under the care of the NMO UK Service, a specialist multidisciplinary clinic for patients with NMOSD and similar non–multiple-sclerosis–related demyelination based at The Walton Centre NHS Foundation Trust, Liverpool, England, between January 2013 and December 2016. We reviewed the clinical and T2-weighted magnetic resonance imaging (MRI) data of all patients with MOG-IgG antibodies (n = 34) (all of white race) seen at the center and the 100 most recent AQP4-IgG–positive patients (74% white, 16% Asian, 7% African or Afro-Caribbean, and 3% mixed or other race/ethnicity). Both AQP4-IgG and MOG-IgG were detected in patients’ serum using a validated live cell–based assay with high specificity (developed at John Radcliffe Hospital, Oxford, England). For titration purposes, we used antihuman MOG-IgG1 (heavy and light chain) secondary assay. Neuromyelitis optica spectrum disorder was diagnosed in all 100 AQP4-IgG–positive patients and in 17 (50%) of MOG-IgG–positive patients according to the 2015 International Panel for NMO Diagnosis criteria. Data analysis was completed January 4, 2017. This study was approved by the Research Ethics Service, NRES Committee London. All patients provided written informed consent.
Results
Thirty-four patients with MOG-IgG disease (20 female) with a median age at analysis of 30.5 years (interquartile range [IQR], 15-69 years) and 100 AQP4-IgG–positive patients (86 female) with a median age at analysis of 54 years (IQR, 12-91 years) were studied. Most patients were of white race. One of the 100 AQP4-IgG–positive patients (1%) experienced seizures. This patient experienced her first focal seizure 5 years before her presentation with NMOSD. The patient experienced additional focal seizures and was diagnosed as having focal epilepsy. Magnetic resonance images of the brain were normal. Her AQP4-IgG titer was 1:1600. Five of the 34 MOG-IgG–positive patients (14.7%) presented with seizures at the time of a major episode of central nervous system inflammation, based on both clinical and radiological findings. All 35 MOG-IgG–positive patients were AQP4-IgG negative. Four of these 5 patients had clinical encephalopathy during these particular events. Demographic, clinical, and immunologic profiles for the 5 patients are summarized in Table 2 and described below.
Table 2. Demographic and Clinical Features of the 5 Patients With MOG-IgG–Associated Seizures.
| Patient Characteristic | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Sex | F | M | M | M | F |
| Age (decade) at onset, y | 10s | 40s | 10s | 10s | 30s |
| Disease duration, y | 5 | 12 | 2 | 4 | 2 |
| Disease course/No. of attacks | R/3 | R/7 | R/4 | R/2 | R/2 |
| Clinical phenotype (No. of attacks)a | Cerebral syndrome (1), ON (3), LETM (1) | ON (2), TM (1), brainstem (1), cerebral syndrome (5) | ON (2), TM (1), brainstem (1), cerebral syndrome (5) | Cerebral syndrome (2) | Cerebral syndrome (1), ON (1) |
| Seizure type | GTC | GTC | GTC | PS and GTC | CPS |
| Altered sensorium/encephalopathy along with seizures | Yes | Yes | No | Yes | Yes |
| Interval between index event and second demyelinating event | 3 mo | 7 y | 2 mo | 4 y | 7 mo |
| Recurrence of unprovoked seizures (epilepsy) | No | Yes | No | Yes, including 1 episode of status epilepticus | No |
| Residual neurologic impairment at last follow-up | Unilateral pale optic disc | Aphasia right hemiparesis, cognitive impairment, right eye impaired vision | Impaired color vision | Impaired memory and mild cognitive dysfunction | None |
| Last EDSS score | 1 | 5 | 1.5 | 1.5 | 0 |
| CSF findings | |||||
| OCB | Negative | Positive | Negative | Negative | Positive |
| WBCs, /μL | 50 | NA | 154 | 550 | High |
| MOG-IgG titers | NA | 300 | 300, 400 | 800 | 400 |
| Brain MRI findings | Bilateral cortical and hemispheric WM changes | Brainstem, cortical, and subcortical extensive demyelination | Cortical lesion extending to deep WM along long tracts and large area of signal changes in the brainstem and both sides of midbrain | Temporoparietal cortical and subcortical lesion | Widespread WM changes in right hemisphere and involving basal ganglia and cortex |
| Residual brain MRI changes | None | Atrophy occipital gliosis | None | Temporal gliosis | WM lesions |
| Current treatment | Rituximab and MMF | MMF and corticosteroid, levetiracetam | MMF | Corticosteroid, levetiracetam, carbamazepine | MMF |
Abbreviations: CPS, complex partial seizures; CSF, cerebrospinal fluid; EDSS, Expanded Disability Status Scale; F, female; GTC, generalized tonic-clonic; L, left; LETM, longitudinal extensive transverse myelitis; M, male; MMF, mycophenolate mofetil; MOG, myelin oligodendrocyte glycoprotein; MRI, magnetic resonance imaging; NA, not available; OCB, oligoclonal bands; ON, optic neuritis; PS, partial seizures; R, relapsing; TM, transverse myelitis; WBCs, white blood cells; WM, white matter.
Cerebral syndrome is defined as seizure or hemiparesis or cognitive changes.
Case 1
A preteen girl presented with generalized tonic-clonic seizures (GTCSs) preceded by fatigue, headache, photophobia, confusion, and vomiting. Magnetic resonance imaging demonstrated bilateral hemispheric abnormalities (Figure 1A and B). She concurrently had right optic neuritis and transverse myelitis. Eight weeks after initial symptoms, MOG-IgG was reported to be positive. She was treated with methylprednisolone administered intravenously and plasmapheresis. Shortly thereafter, she developed optic neuritis affecting the left eye, which was treated with mycophenolate mofetil, 1.25 g/d. She was relapse free for 2 years but then developed optic neuritis in the right eye. Mycophenolate was withdrawn and treatment with rituximab was commenced. At last follow-up, she was in remission for 17 months and no longer receiving antiepileptic treatment. Her MOG-IgG results were persistently positive during the 5 years since her initial presentation. No MOG-IgG titers are available for this patient because samples are not available at present.
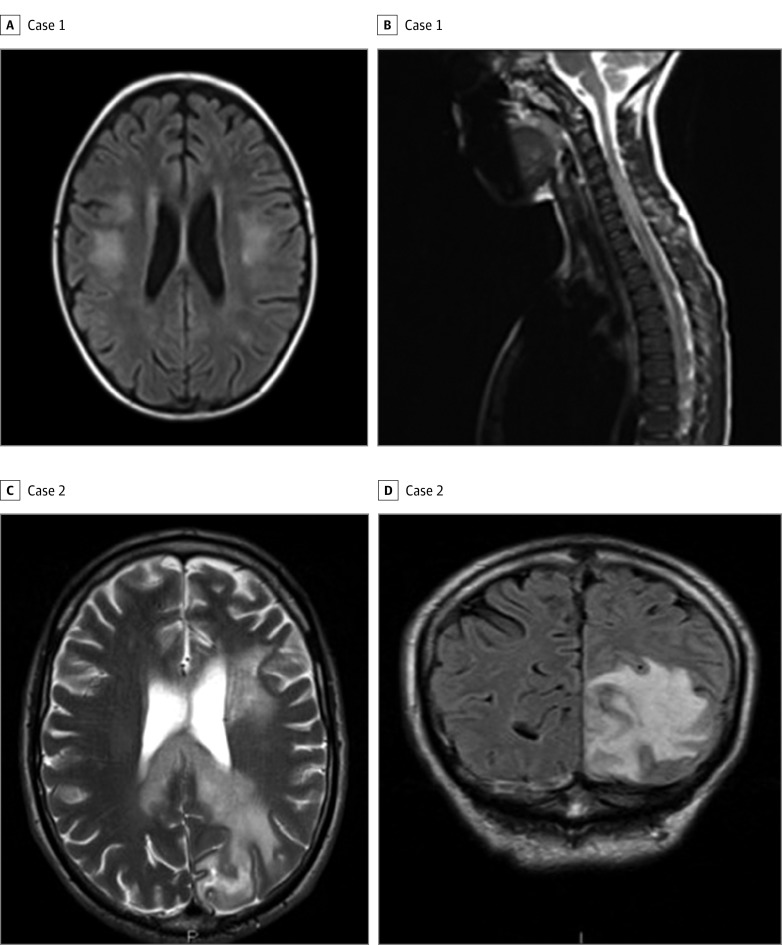
Figure 1. Magnetic Resonance Imaging (MRI) of the Brain and Spinal Cord, Cases 1 and 2.
A, Axial T2-weighted fluid-attenuated inversion recovery image of the brain shows bilateral hemispheric lesions. B, Sagittal T2-weighted MRI of the spinal cord shows long myelitis. C, Axial T2-weighted MRI shows involvement of the frontal and occipital cortex and transcallosal lesion. D, An extensive occipital lesion can be seen.
Case 2
A white man in his 50s presented with 8 GTCSs, each lasting 3 to 4 minutes following 5 episodes of demyelination across 10 years (2 brainstem events, 1 transverse myelitis, 1 cerebral event, and optic neuritis, after which he was discovered to be MOG-IgG positive, with a titer of 1:300). He was taking prednisolone, 10 mg/d, which had been tapered from a 60-mg/d dosage commenced during his last relapse, 5 months previously. He had started treatment with oral azathioprine 1 month before this episode. An MRI of the brain showed residual inflammatory lesions in the left frontal, temporal, and occipital lobes (Figure 1C and D). Six weeks later, he had a GTCS in association with severe optic neuritis. No new lesions were present on MRI. His immunosuppressive therapy was switched to mycophenolate, antiepileptic drugs were optimized, and, at last follow-up, he had remained stable for the past 12 months. However, he showed significant residual cognitive damage, including severe expressive aphasia. He remained MOG-IgG positive at subsequent testing across 3 years.
Case 3
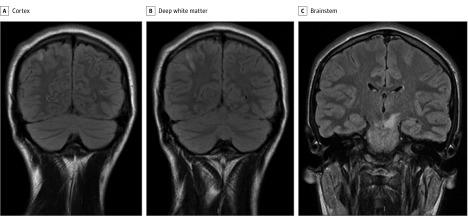
A man in his early 20s experienced his first neurological event with optic neuritis and brainstem demyelination and was treated with methylprednisolone and immunoglobulin, both intravenously. Six weeks later, he presented with a cluster of GTCSs, the first during sleep and the second 4 days later. Magnetic resonance imaging showed a new inflammatory lesion (Figure 2); this time, he was found to be MOG-IgG positive, with a titer of 1:300. He received methylprednisolone intravenously, followed by oral prednisolone, 60 mg/d, and levetiracetam, 1 g/d, for a year, after which it was withdrawn with no recurrence of seizures. His MOG-IgG test remained positive, with a titer of 1:300 twelve months after initial testing.
Figure 2. Magnetic Resonance Imaging of the Brain, Case 3.
A and B, A lesion extends from the cortex to deep white matter. C, A lesion can be seen in the brainstem.
Case 4
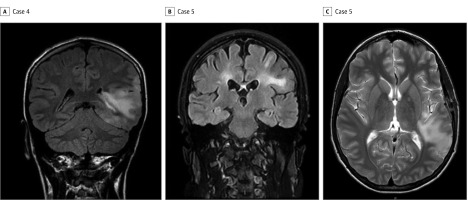
A boy in his early teens presented with fluctuating level of consciousness, extensor posturing of his limbs, and a 4-minute focal seizure affecting predominantly his head, the right side of his face, and the right arm. Magnetic resonance imaging showed marked signal abnormality in the left temporal lobe, particularly within the gray matter (Figure 3). He made a good initial recovery after receiving methylprednisolone intravenously. However, he was readmitted 2 weeks later with severe left-sided retro-orbital and forehead pain; within 24 hours, he experienced 2 focal seizures affecting the left side of his face that progressed to a GTCS. An MRI scan performed at this time showed progression of the previous lesion. His symptoms resolved completely following methylprednisolone delivered intravenously and a tapering course of oral prednisolone, and he continued on a regimen of levetiracetam 2 g/d.
Figure 3. Magnetic Resonance Imaging of the Brain, Cases 4 and 5.
A, Coronal T2-weighted fluid-attenuated inversion recovery (FLAIR) image shows an extensive lesion affecting both the left temporal and parietal lobes. B, Coronal T2-weighted FLAIR image shows bilateral hemispheric white matter changes. C, Axial T2-weighted magnetic resonance imaging illustrates cortical involvement.
Three years later, seizures recurred. A severe headache associated with pallor and profuse vomiting developed, followed by rhythmic twitching of his head and eyes to the right, without loss of consciousness. No new changes were found on MRI. He was treated by increasing his levetiracetam dosage to 3 g/d. Despite this treatment, he presented with additional clusters of focal seizures with secondary generalization, occurring almost every 4 weeks. The MRI was repeated and showed a new parietal lobe lesion. Following consultation at our center (4 years from initial presentation), MOG-IgG was checked and was positive. His titer was 1:800, and follow-up samples were positive 1 year later. The patient opted not to take long-term immunosuppressant therapy. At last follow-up, he continued to take a combination of levetiracetam and carbamazepine and had been seizure free for 8 months.
Case 5
A white woman in her early 40s experienced a seizure associated with her first demyelinating event. Her symptoms started with lethargy, confusion, and altered sense of smell and taste. She was noted to behave oddly and had left-sided weakness. She experienced a focal seizure that was recorded during an electroencephalogram. An MRI showed widespread white matter changes, particularly confluent over the right hemisphere and involving both basal ganglia (Figure 3B and C), and her MOG-IgG test results returned positive 4 weeks after presentation. She received methylprednisolone intravenously and by the third day was able to walk. Her cognition improved gradually and, while taking levetiracetam, she experienced no further seizures for approximately 2 years. A second episode of optic neuritis occurred during a gradual withdrawal of prednisolone treatment, which she had been taking orally. Mycophenolate therapy was introduced, and no further neurological events occurred over the ensuing 20 months. She continued to test positive for MOG-IgG, and her titer was 1:400 when retested 22 months after the second episode.
None of the 5 patients described here had any other identified cause, including infective or drug related, for their focal or generalized seizures. There was no known family history of epilepsy. None of these patients tested positive for other antineuronal antibodies, including anti-N-methyl-d-aspartate receptor and anti–voltage-gated potassium channel antibodies; leucine-rich, glioma inactivated 1 (LGI1); contactin-associated protein 2 (CASPR2); glutamic acid decarboxylase (GAD); and paraneoplastic antibodies (antibodies against Hu, Yo, Ri, Tr, CV2, and amphiphysin).
Discussion
We describe 5 patients with MOG-IgG–associated inflammatory central nervous system disease and seizures. All had brain cortex involvement, and 4 of the 5 had encephalopathy. Viral or autoimmune encephalitis was the initial diagnosis in these 4 patients. Four of the 5 patients meet the 2015 diagnostic criteria for NMOSD without AQP4-IgG. The main clinical spectrum for MOG-IgG–positive disorders is optic neuritis, transverse myelitis, acute disseminated encephalomyelitis, clinically isolated syndrome, pediatric multiple sclerosis, and NMO. Acute disseminated encephalomyelitis-like disorders, including encephalopathy, associated with MOG-IgG have previously been reported. A recent report identified 4 cases of MOG-IgG–associated encephalitis from a cohort of 24 cases of steroid-responsive encephalitis. These patients were all men with unilateral and “benign” lesions with full resolution. Our case reports suggest that this is not always true. Two of the 5 patients were women. The encephalitis can be severe with lasting damage in some (case 2). Unprovoked seizure recurrence (epilepsy) occurred in 2 of these patients, indicating possible underlying gliosis. Follow-up imaging showed gliosis atrophy or persistent T2-weighted lesions in 3 patients.
In contrast, only 4 studies in the English-language literature reported seizures among AQP4-IgG–positive patients with NMOSD. One is a Japanese study describing 3 of 31 (9.6%) AQP4-IgG–positive patients with NMO who had seizures. One of them had evident seizures associated with an inflammatory event, and it is unclear whether antibody testing was conducted by enzyme-linked immunosorbent assay or cell-based assay. The other 3 are pediatric studies: McKeon et al studied 88 AQP4-IgG–positive, mainly nonwhite children with NMO and reported that 2 (2%) had focal seizures. Lotze et al reported 2 Latin American children who had NMO and NMO antibody and presented with seizures. A third study also reports seizures but is unclear about the serostatus of the patients described. In our cohort, it was striking that none of the 100 AQP4-IgG–positive patients experienced seizures as part of an inflammatory event. The difference between our AQP4-IgG–positive and MOG-IgG–positive patients in terms of seizure occurrence was statistically significant (1 of 100 AQP4-IgG–positive patients vs 5 of 34 MOG-IgG–positive patients; 2-sided P < .008, Fisher exact test).
The encephalopathic disorders and seizures that occurred in these patients were both likely triggered by an episode of demyelination caused by MOG-IgG. There was no evidence that infective encephalitis or other associated autoimmune antibodies (eg, anti-N-methyl-d-aspartate receptor or anti–voltage-gated potassium channel antibodies) were responsible for the seizures. A study of N-methyl-d-aspartate receptor antibodies in 215 patients with a range of inflammatory demyelinating diseases, including 22 MOG-IgG–positive patients with cognitive problems, seizures, or both, concluded that double seropositivity is rare. Jarius et al described an MOG-IgG–positive patient who experienced seizures complicated with cerebral venous thrombosis and localized brain edema following intravenous treatment with a high dose of methylprednisolone. In our cohort, there was no evidence of treatment-induced seizures.
Limitations
There are several limitations to this study. This was a single-center, cross-sectional study with retrospective collection of data. The clinic is highly specialized and serves as a national referral center. It is possible that we have encountered only the severe forms of MOG-IgG disease and that, perhaps in a larger cohort including milder cases, seizures may be rarer. There could have been recall bias about details of seizures, although we relied on hospital notes whenever possible. Larger studies on MOG-IgG disease that specifically ask for the presence of seizures as a clinical feature will be needed to validate this observation further.
Conclusions
In this cohort, patients with MOG-IgG–associated disease were more likely to present with an encephalopathic disorder and seizures compared with AQP4-IgG–positive cases. The spectrum of MOG-IgG–associated disease continues to expand and includes atypical cerebral inflammatory lesions, which may have been previously characterized as relapsing steroid-responsive autoimmune encephalitis, acute disseminated encephalomyelitis, atypical multiple sclerosis, or central nervous system vasculitis. Myelin oligodendrocyte glycoprotein IgG–associated demyelinating disease is not always benign and can have a relapsing course and cause significant residual damage. Our study further supports the view that AQP4-IgG– and MOG-IgG–associated central nervous system inflammation are 2 different diseases with some overlapping phenotypes, particularly opticospinal inflammation. We recommend that testing for MOG-IgG be considered in patients with atypical inflammatory brain lesions, particularly those with an encephalitis-like presentation.
References
- 1.Ramanathan S, Dale RC, Brilot F. Anti-MOG antibody: the history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun Rev. 2016;15(4):307-324. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin KA, Chitnis T, Newcombe J, et al. Age-dependent B cell autoimmunity to a myelin surface antigen in pediatric multiple sclerosis. J Immunol. 2009;183(6):4067-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitley J, Woodhall M, Waters P, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79(12):1273-1277. [DOI] [PubMed] [Google Scholar]
- 4.Mader S, Gredler V, Schanda K, et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation. 2011;8:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reindl M, Rostasy K. MOG antibody–associated diseases. Neurol Neuroimmunol Neuroinflamm. 2015;2(1):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarius S, Kleiter I, Ruprecht K, et al. ; Neuromyelitis Optica Study Group (NEMOS) . MOG-IgG in NMO and related disorders: a multicenter study of 50 patients: part 3, brainstem involvement—frequency, presentation and outcome. J Neuroinflammation. 2016;13(1):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wingerchuk DM, Banwell B, Bennett JL, et al. ; International Panel for NMO Diagnosis . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamid SH, Elsone L, Mutch K, Solomon T, Jacob A. The impact of 2015 neuromyelitis optica spectrum disorders criteria on diagnostic rates. Mult Scler. 2017;23(2):228-233. [DOI] [PubMed] [Google Scholar]
- 9.Hino-Fukuyo N, Haginoya K, Nakashima I, et al. Clinical features and long-term outcome of a group of Japanese children with inflammatory central nervous system disorders and seropositivity to myelin-oligodendrocyte glycoprotein antibodies. Brain Dev. 2015;37(9):849-852. [DOI] [PubMed] [Google Scholar]
- 10.Tsuburaya RS, Miki N, Tanaka K, et al. Anti-myelin oligodendrocyte glycoprotein (MOG) antibodies in a Japanese boy with recurrent optic neuritis. Brain Dev. 2015;37(1):145-148. [DOI] [PubMed] [Google Scholar]
- 11.Ramberger M, Bsteh G, Schanda K, et al. NMDA receptor antibodies: a rare association in inflammatory demyelinating diseases. Neurol Neuroimmunol Neuroinflamm. 2015;2(5):e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Titulaer MJ, Höftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti–N-methyl-d-aspartate receptor encephalitis. Ann Neurol. 2014;75(3):411-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa R, Nakashima I, Takahashi T, et al. MOG antibody–positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm. 2017;4(2):e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimori J, Takai Y, Nakashima I, et al. Bilateral frontal cortex encephalitis and paraparesis in a patient with anti-MOG antibodies. J Neurol Neurosurg Psychiatry. 2017;88(6):534-536. [DOI] [PubMed] [Google Scholar]
- 15.Numa S, Kasai T, Kondo T, et al. An adult case of anti-myelin oligodendrocyte glycoprotein (MOG) antibody-associated multiphasic acute disseminated encephalomyelitis at 33-year intervals. Intern Med. 2016;55(6):699-702. [DOI] [PubMed] [Google Scholar]
- 16.Jarius S, Ruprecht K, Kleiter I, et al. ; Optica Study Group (NEMOS) . MOG-IgG in NMO and related disorders: a multicenter study of 50 patients: part 2, epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13(1):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waters P, Woodhall M, O’Connor KC, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waters PJ, McKeon A, Leite MI, et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4–IgG assays. Neurology. 2012;78(9):665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hacohen Y, Absoud M, Deiva K, et al. Myelin oligodendrocyte glycoprotein antibodies are associated with a non-MS course in children. Neurol Neuroimmunol Neuroinflamm. 2015;2(2):e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakano H, Tanaka M, Kinoshita M, et al. Epileptic seizures in Japanese patients with multiple sclerosis and neuromyelitis optica. Epilepsy Res. 2013;104(1-2):175-180. [DOI] [PubMed] [Google Scholar]
- 21.McKeon A, Lennon VA, Lotze T, et al. CNS aquaporin-4 autoimmunity in children. Neurology. 2008;71(2):93-100. [DOI] [PubMed] [Google Scholar]
- 22.Lotze TE, Northrop JL, Hutton GJ, Ross B, Schiffman JS, Hunter JV. Spectrum of pediatric neuromyelitis optica. Pediatrics. 2008;122(5):e1039-e1047. [DOI] [PubMed] [Google Scholar]
- 23.Chitnis T, Ness J, Krupp L, et al. Clinical features of neuromyelitis optica in children: US Network of Pediatric MS Centers report. Neurology. 2016;86(3):245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]