Abstract
Importance
Despite the introduction of combination antiretroviral therapy (cART), HIV-associated neurocognitive disorders continue to be a problem for treated HIV-positive individuals. The cause of this impairment remains unclear.
Objective
To determine if detectable brain changes occur during a 2-year period in HIV-positive individuals who were aviremic and treated with cART.
Design, Setting, and Participants
In this longitudinal case-control study, participants underwent neuroimaging and neuropsychological assessment approximately 2 years apart. Data were collected from October 26, 2011, to March 1, 2016. Data from 92 HIV-positive individuals were acquired at Washington University in St Louis from ongoing studies conducted in the infectious disease clinic and AIDS Clinical Trial Unit. A total of 55 HIV-negative control participants were recruited from the St Louis community and a research participant registry. A total of 48 HIV-positive individuals who were aviremic and treated with cART and 31 demographically similar HIV-negative controls met the study requirements and were included in the analyses.
Main Outcomes and Measures
Brain volumes were extracted with tensor-based and voxel-based morphometry and cortical modeling. Raw scores from neuropsychological tests quantified cognitive performance. Multivariable mixed-effects models assessed the effect of HIV serostatus on brain volumes and cognitive performance, and determined if HIV serostatus affected how these measures changed over time. With HIV-positive participants, linear regression models tested whether brain volumes and cognitive performance were associated with measures of infection severity and duration of infection.
Results
The 2 groups were demographically similar (HIV-positive group: 23 women and 25 men; mean [SD] age, 47.7 [13.2] years; mean [SD] educational level, 13.3 [3.4] years; and HIV-negative group, 16 women and 15 men; mean [SD] age, 51.2 [12.9] years; mean [SD] educational level, 14.5 [2.1] years). The HIV-positive participants had poorer neuropsychological test scores compared with controls on the Trail Making Test Part A (5.9 seconds; 95% CI, 1.5-10.3; P = .01), Trail Making Test Part B (27.3 seconds; 95% CI, 15.0-39.6; P < .001), Digit Symbol Substitution Task (–12.5 marks; 95% CI, –18.9 to –6.0; P < .001), Letter-Number Sequencing (–2.5 marks; 95% CI, –3.7 to –1.3; P < .001), Letter Fluency (–6.6 words; 95% CI, –11.5 to –1.6; P = .01), and Hopkins Verbal Learning Test–Revised immediate recall (–2.4 words; 95% CI, –4.4 to –0.4; P = .05), after adjusting for age, sex, and educational level. Only changes in Trail Making Test Part A significantly differed between the groups. Cortical thickness and subcortical volumes were smaller in HIV-positive individuals compared with controls. However, changes in brain volume over time were similar between the groups.
Conclusions and Relevance
These findings are consistent with the idea that cognitive and structural brain changes may occur early after seroconversion, and argue that maintaining aviremia with cART can prevent or minimize progressive brain injury.
This longitudinal case-control study examines whether detectable brain changes occur during a 2-year period in HIV-positive individuals who were aviremic and treated with combination antiretroviral therapy.
Key Points
Question
Do HIV-positive individuals receiving combination antiretroviral therapy with good viral suppression experience progressive brain atrophy and cognitive decline?
Findings
In this longitudinal case-control study that included 48 HIV-positive individuals who were aviremic and treated with combination antiretroviral therapy and 31 demographically similar controls, HIV-positive participants had reduced cortical thickness and subcortical brain volumes and poorer cognitive performance compared with controls. However, the changes in cognitive performance and brain volumes during a 2-year period were similar between the groups.
Meaning
Infection with HIV may result in reduced brain volumes and decreased cognitive performance, but early initiation of treatment and maintaining full viral suppression may minimize brain injury in HIV-positive individuals.
Introduction
The introduction of combination antiretroviral therapy (cART) has transformed HIV from a fatal disease to a chronic condition. However, HIV-associated neurocognitive disorders (HAND) are still prevalent, affecting up to 40% of HIV-positive individuals despite effective viral suppression. The possible cause of this mild brain dysfunction that limits quality of life remains unclear.
Recently, some studies have reported that, while HAND remains common, progressive worsening is uncommon, with only a small proportion of HIV-positive individuals whose treatment is stable with good viral suppression showing cognitive decline as assessed with repeated neuropsychological testing during a 3- to 4-year period. However, it is unclear whether effective viral suppression can mitigate the progression of brain atrophy. Previous neuroimaging studies have provided evidence for ongoing brain atrophy in HIV-positive individuals with advanced disease and poor viral control, but those results may not generalize to individuals treated with cART who have viral suppression. A recent neuroimaging study reported no longitudinal changes in cortical thickness, deep gray matter volumes, or white matter integrity in HIV-positive individuals treated with cART who had undetectable viral loads during a 2-year period. However, this study included only 21 participants, had no HIV-negative comparison group, and extracted brain measures only from predefined regions of interest.
In this longitudinal study, we sought evidence of ongoing brain atrophy during a 2-year period using structural magnetic resonance imaging (MRI) and neuropsychological assessment in a larger sample of HIV-positive individuals treated with cART who had well-controlled viral loads, compared with demographically similar HIV-negative controls. We characterized brain volumes as seen on MRI scans by applying multiple advanced neuroimaging processing methods (tensor-based morphometry [TBM], voxel-based morphometry [VBM], and cortical modeling) and assessed cognitive function with a standard battery of neuropsychological tests.
Methods
Participants
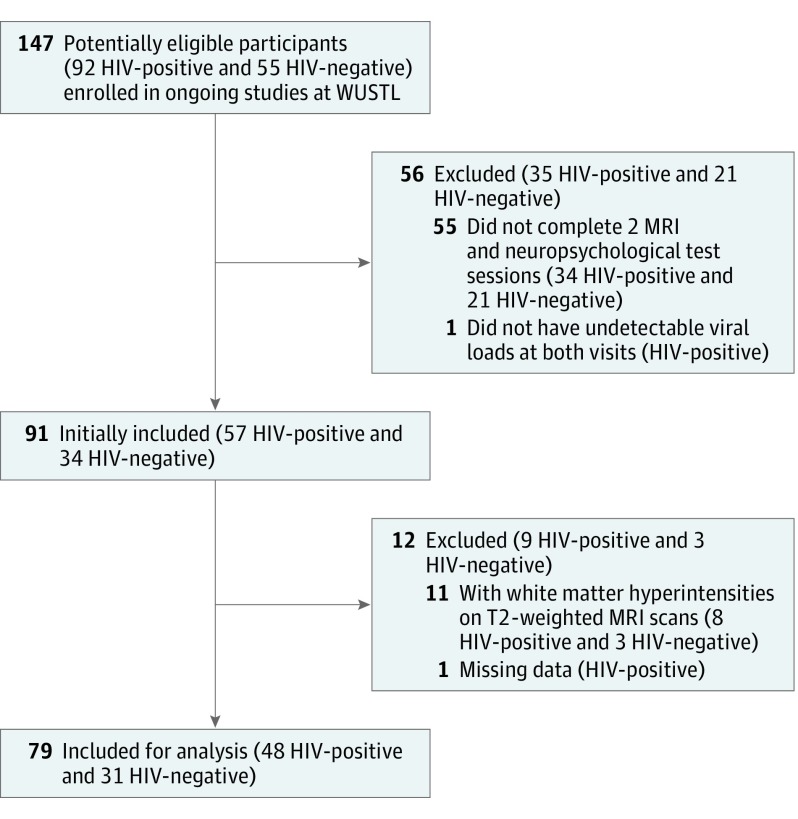
The HIV-positive participants were selected from ongoing studies conducted in the infectious disease clinic and the AIDS Clinical Trial Unit at Washington University in St Louis from October 26, 2011, to March 1, 2016. Demographically similar HIV-negative control participants were recruited from the St Louis community by leaflets and a research participant registry at Washington University in St Louis. Participants were not eligible to enter the studies at Washington University in St Louis if they had a history of confounding neurologic disorders, current or past opportunistic central nervous system infection, traumatic brain injury (loss of consciousness >30 minutes), major psychiatric disorders, or an active substance abuse and dependence diagnosis according to Diagnostic and Statistical Manual of Mental Disorders (fourth edition) criteria. The present study included HIV-positive and HIV-negative participants who had completed 2 MRI and neuropsychological testing sessions at least 1.5 years apart. The HIV-positive participants were receiving stable cART and had undetectable viral loads (<50 copies/mL) at baseline and follow-up visits. Participants were excluded if they had extensive white matter hyperintensities on T2-weighted MRI scans as defined by an expert neurologist (B.M.A.). All participants who met these criteria were included in the analysis, yielding 48 HIV-positive and 31 HIV-negative participants. A CONSORT diagram showing the participant selection process is provided in Figure 1. For all HIV-positive participants, a central nervous system penetration effectiveness (CPE) score was generated based on previous methods. The Washington University in St Louis institutional review board approved the study. Written informed consent was obtained from all participants.
Figure 1. Study CONSORT Diagram.
MRI indicates magnetic resonance imaging; and WUSTL, Washington University in St Louis.
Neuropsychological Testing
All participants underwent a neuropsychological assessment at both visits that consisted of 8 standard tests recommended to assess HAND: Trail Making Test Part A and B, Digit Symbol Substitution Task, Letter-Number Sequencing, Letter Fluency, Action (verb naming) Fluency, and Hopkins Verbal Learning Test–Revised immediate and delayed recall. All participants also completed the Wide Range Achievement Test reading subtest to estimate premorbid intellectual ability. Functional limitation in activities of daily living was not assessed.
MRI Acquisition
All participants at both visits underwent MRI using the same 3-T Siemens Tim TRIO whole-body magnetic resonance scanner (Siemens) with a 12-channel transmit and receive head coil at Washington University in St Louis. The scanning protocol included T1-weighted 3-dimensional magnetization-prepared rapid acquisition gradient echo sequence (repetition time [TR]/echo time [TE]/inversion time [TI] = 2400/3.16/1000 milliseconds; voxel = 1.0 mm3) and T2-weighted Fast Spin Echo sequence (TR/TE = 3200/460 milliseconds; voxel = 1.0 mm3).
MRI Processing
T1-weighted data were processed using a longitudinal pipeline, as previously described. Preprocessing included denoising, intensity inhomogeneity removal, and brain masking. Images were linearly registered to the Montreal Neurological Institute International Consortium for Brain Mapping (ICBM) 152 template using a 9-parameter affine transform to correct for variations in head size and orientation. To ensure the registrations to the ICBM152 space were consistent across all time points, a participant-specific template was created using an unbiased template creation approach. This participant-specific template creation process yields nonlinear transformations that maps each visit to the ICBM152 space in a consistent manner reducing the intra-participant variability in brain volume measures across visits, increasing the statistical power to detect changes within participants. All data were carefully inspected for unacceptable processing outcomes. All data passed visual quality control, and were available for TBM, VBM, and cortical modeling.
Tensor-Based Morphometry
Tensor-based morphometry provides a voxelwise estimate of brain structure volume relative to the ICBM152 template. Structural volumes were calculated by taking the Jacobian determinant of the deformation field from the nonlinear transform.
Voxel-Based Morphometry
Voxel-based morphometry provides a voxelwise estimate of the amount of gray matter, white matter, and cerebrospinal fluid. After spatial normalization to the ICBM152 space, each voxel was identified as gray matter, white matter, or cerebrospinal fluid. The tissue maps were then modulated by the Jacobian determinants of the nonlinear transform. Resulting modulated tissue maps were smoothed with an 8-mm full width at half maximum gaussian kernel.
Cortical Modeling
Cortical modeling provides a quantitative measure of cortical thickness. Cortical thickness estimates were extracted with fast accurate cortical extraction by deforming polygonal meshes to fit the gray-white matter and pial surface boundaries. Thickness estimates were mapped to the ICBM152 mean cortical template using an iterative feature–based registration algorithm and blurred with a 20-mm surface-based kernel.
Statistical Analysis
Multivariable mixed-effects models were used to assess neuropsychological test performance, while voxelwise and vertexwise mixed-effects models were used to assess regional brain volumes, estimated with TBM, VBM, and cortical modeling, from all available data at both visits. To compare neuropsychological scores and brain volumes by HIV serostatus, and to determine if changes in these measures during a 2-year period were significantly different between the HIV-positive and HIV-negative groups, a mixed-effects model included fixed effects for HIV serostatus, time (years from baseline visit), mean age (mean of age at baseline and follow-up), sex, and HIV serostatus by time interaction, as well as a participant-specific random intercept. Within each group, independent mixed-effects frameworks modeled time, age, and sex as fixed effects, along with participant-specific random intercepts, to test if significant changes in test scores and brain volumes occurred between visits. Within the HIV-positive group, linear regressions were used to explore the association between neuropsychological scores and brain volumes with the following HIV-related factors: current and nadir CD4 cell counts, CPE score, and duration of infection. These models were applied to only baseline data. Additional linear regressions tested whether baseline current CD4 cell counts and CPE score were associated with neuropsychological scores and brain volumes at follow-up. Statistical significance was set at P < .05 (2-sided) for all models that assessed neuropsychological performance. Whole-brain statistical maps were corrected for multiple comparisons using the standard false discovery rate with a false-positive rate of 5%. Additional information on model structures can be found in the eAppendix in the Supplement.
Results
Participants
Table 1 summarizes the baseline demographic and clinical characteristics of study participants. The HIV-positive and HIV-negative participants were comparable with respect to age, sex, educational level, race/ethnicity, and history of drug use. The HIV-positive group tended to have lower Wide Range Achievement Test reading scores compared with HIV-negative controls, although these differences did not reach statistical significance after controlling for age, sex, and educational level (raw score, –2.4; 95% CI, –3.9 to –0.9; P = .09). Both groups had similar mean (SD) time periods between visits (HIV-positive, 2.1 [0.08] years; HIV-negative, 1.9 [0.3] years). All HIV-positive participants were receiving stable cART throughout the study period.
Table 1. Baseline Demographic and Clinical Characteristics of Study Participants.
| Characteristic | HIV-Positive Participants (n = 48) |
HIV-Negative Participants (n = 31) |
P Valuea |
|---|---|---|---|
| Age, mean (SD), y | 47.7 (13.2) | 51.2 (12.9) | .25 |
| Male sex, No. (%) | 25 (52) | 15 (48) | .93 |
| Race/ethnicity, No. (%) | |||
| White | 15 (31) | 16 (52) | .12 |
| African American | 33 (69) | 15 (48) | |
| Educational level, mean (SD), y | 13.3 (3.4) | 14.5 (2.1) | .09 |
| WRAT-3 reading score, mean (SD) | 43.8 (8.9) | 48.1 (6.1) | .09b |
| Current CD4 count, median (IQR), cells/μL | 630 (486, 881) | NA | NA |
| Nadir CD4 count, median (IQR), cells/μLc | 190 (57-300) | NA | NA |
| Estimated duration of HIV infection, median (IQR), yd | 13.5 (5.2-20) | NA | NA |
| Hepatitis C coinfection, No. (%) | 1 (2) | 0 | .30 |
| CPE score, median (range) | 7.5 (5-13) | NA | NA |
| Past substance use, No. (%)d | |||
| Marijuana | 5 (10) | 3 (10) | .90 |
| Methamphetamines | 1 (2) | 1 (3) | .80 |
| Opiates | 1 (2) | 1 (3) | .80 |
Abbreviations: CPE, Central Nervous System Penetration Effectiveness; IQR, interquartile range; NA, not applicable; WRAT-3, Wide Range Achievement Test.
P value determined using t test (age and education) or χ2 test (sex, race/ethnicity, past substance use, and hepatitis C coinfection), unless otherwise stated.
P value computed from a linear regression model. The WRAT-3 reading score was the dependent variable and HIV serostatus, age, sex, and educational level were independent variables.
A total of 7 participants were missing nadir CD4 count.
Based on patient’s self-report.
Neuropsychological Performance
The HIV-positive participants had lower neuropsychological scores compared with HIV-negative participants on the Trail Making Test Part A (5.9 seconds; 95% CI, 1.5-10.3; P = .01), Trail Making Test Part B (27.3 seconds; 95% CI, 15.0-39.6; P < .001), Digit Symbol Substitution Task (–12.5 marks; 95% CI, –18.9 to –6.0; P < .001), Letter-Number Sequencing (–2.5 marks; 95% CI, –3.7 to –1.3; P < .001), Letter Fluency (–6.6 words; 95% CI, –11.5 to –1.6; P = .01), and Hopkins Verbal Learning Test–Revised immediate recall (–2.4 words; 95% CI, –4.4 to –0.4; P = .05), after adjusting for age, sex, and educational level (Table 2). No differences in Hopkins Verbal Learning Test–Revised delayed recall or Action Fluency were observed.
Table 2. Neuropsychological Test Scores at Baseline and Follow-up.
| Neuropsychological Test | HIV-Positive Participants | HIV-Negative Participants | P Value for Group Statistics | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | P Value for Change Over Timea | Mean (SD) | P Value for Change Over Timea | HIV-Positive vs HIV-Negative Participantsa | Group × Timea |
|||
| Baseline | Follow-up | Baseline | Follow-up | |||||
| Trail Making Test A, time to completion, sb | 32.5 (11.0) | 30.9 (12.4) | .08 | 26.0 (7.6) | 27.9 (9.5) | .14 | .01 | .03 |
| Trail Making Test B, time to completion, sb | 93.7 (38.0) | 84.9 (34.1) | .21 | 62.4 (16.4) | 65.2 (21.1) | .20 | <.001 | .14 |
| HVLT-R: immediate recall, No. of correct wordsc | 20.8 (4.7) | 21.6 (5.22) | .29 | 23.4 (4.7) | 23.4 (5.4) | .90 | .05 | .56 |
| HVLT-R: delayed recall, No. of correct wordsc | 7.5 (2.4) | 7.1 (4.1) | .60 | 8.5 (3.1) | 8.1 (2.8) | .43 | .24 | .91 |
| Digit Symbol Substitution Task, No. of correct marksc | 65.3 (15.3) | 67.5 (16.9) | .02 | 76.6 (15.9) | 77.3 (13.1) | .73 | <.001 | .27 |
| Letter-Number Sequencing, No. of correct marksc | 8.3 (3.2) | 8.6 (2.3) | .70 | 10.9 (2.7) | 10.9 (3.0) | .78 | <.001 | .49 |
| Action Fluency, No. of verbsc | 12.6 (4.9) | 13.0 (5.7) | .74 | 15.6 (7.4) | 15.0 (7.5) | .21 | .10 | .31 |
| Letter Fluency, No. of wordsc | 33.4 (11.2) | 36.6 (10.2) | .003 | 40.1 (12.7) | 43.6 (12.2) | .02 | .01 | .76 |
Abbreviation: HVLT-R, Hopkins Verbal Learning Test–Revised.
Statistical models included all available data from both time points adjusting for age, sex, and educational level with mixed-effects modeling. Participant level intercept was included as a random effect. P value derived from likelihood ratio testing.
Lower scores indicate better performance.
Higher scores indicate better performance.
The primary analysis compared changes in neuropsychological scores over time by HIV serostatus. Improvements in test scores were observed in Letter Fluency in HIV-positive (1.4 words per year; 95% CI, 0.5-2.3; P = .003) and HIV-negative participants (1.7 words per year; 95% CI, 0.2-3.3; P = .02) and Digit Symbol Substitution Task in the HIV-positive group (1.1 marks per year; 95% CI, 0.3-2.0; P = .02). Only changes in Trail Making Test Part A scores differed between the groups (–1.9 seconds per year; 95% CI, –3.8 to –0.02; P = .03): the HIV-positive group had greater improvements compared with HIV-negative individuals over time. No significant interactions between HIV serostatus and time were detected in other neuropsychological test results.
Neuropsychological scores did not correlate with current CD4 count, nadir CD4 count, CPE score, or duration of infection. In addition, baseline current CD4 and CPE scores did not predict neuropsychological performance at follow-up.
Brain Volumes
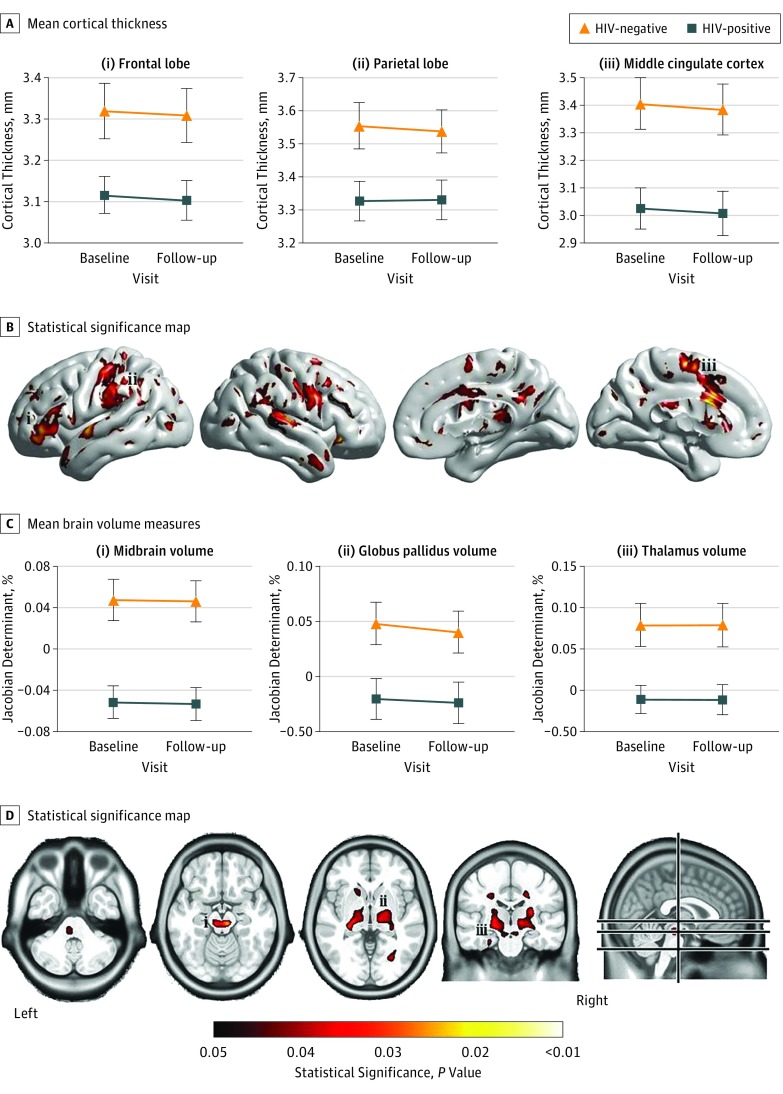
Comparing brain volumes revealed reduced cortical thickness and smaller subcortical volumes in the HIV-positive group compared with controls (Figure 2; eFigure 1 in the Supplement). Cortical thickness differences were detected in the bilateral primary sensory and motor cortex, superior temporal gyrus and poles, middle and posterior cingulate cortex, and left frontal lobe (Figure 2A). Tensor-based morphometry revealed significantly smaller subcortical volumes, and VBM showed significantly reduced white matter volumes in the thalamus, caudate, putamen, globus pallidus, brainstem, and midbrain of HIV-positive participants (Figure 2B). Modeling brain volumes over time did not reveal significant differences in the changes in regional volume or cortical thickness between the groups. The changes in these brain volume estimates over time were similar between the groups.
Figure 2. Cortical Thickness and Subcortical Volume Reductions in HIV-Positive and HIV-Negative Participants.
A, Mean cortical thickness estimates at baseline and follow-up visits in the frontal lobe, parietal lobe, and middle cingulate cortex in HIV-positive and HIV-negative control participants. B, Statistical significance map of brain regions that had significantly reduced cortical thickness estimates in the HIV-positive group compared with HIV-negative controls. C, Mean brain volume measures with tensor-based morphometry (TBM) at baseline and follow-up in the midbrain, globus pallidus, and thalamus in HIV-positive and HIV-negative control participants. D, Statistical significance map of brain regions that had significantly smaller volumes in the HIV-positive group compared with HIV-negative controls with TBM (voxel-based morphometry results shown in eFigure 1 in the Supplement). Error bars indicate SE. The values under the color spectrum are P values.
Power calculations were performed to aid in interpreting the absence of detectable differences in brain volume change. This analysis showed that differences in loss of brain volume between the groups ranging from 0.1% to 6.0% per year (median, 0.90% per year [interquartile range, 0.71%-1.12%]) could have been detected, if present, when brain volumes were estimated with TBM or VBM (eFigure 2 in the Supplement). Likewise, differences in cortical thickness changes between groups ranging from 0.01 to 0.5 mm per year (median, 0.08 mm per year [interquartile range, 0.07-0.1]) could have been detected, if present, using cortical modeling (eFigure 3 in the Supplement).
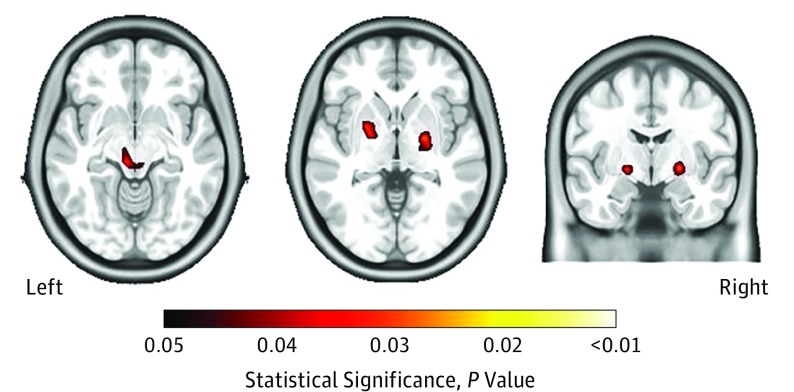
Lower nadir CD4 counts were significantly correlated with reduced white matter volumes and smaller brain volumes in the putamen, globus pallidus, and thalamus, as revealed with VBM and TBM (Figure 3). In contrast, no correlations between nadir CD4 counts and cortical thickness estimates were observed. The remaining HIV-associated factors (current CD4 count, CPE score, and duration of infection) were not associated with any brain volume estimates. Baseline current CD4 count and CPE score was not significantly associated with brain volumes at follow-up.
Figure 3. Smaller Subcortical Brain Regions and Lower Nadir CD4 Counts.
Smaller brain volumes in the putamen, globus pallidus, and thalamus, as revealed with tensor-based morphometry, correlated with lower nadir CD4 cell counts. The left image shows the thalamus, the center image shows the putamen and globus pallidus, and the right image shows the putamen and globus pallidus.
Discussion
Although HAND persists, recent studies have reported that neuropsychological performance does not deteriorate during durations of 3 to 4 years in most HIV-positive individuals who were aviremic and treated with cART. Whether stable treatment and effective viral suppression also prevents progressive structural brain atrophy is unclear. We observed significant differences in cortical thickness, subcortical volumes, and cognitive performance in HIV-positive participants compared with demographically similar HIV-negative controls at both visits. However, the changes in cognition and brain volumes during the 2-year period were similar between the HIV-positive and HIV-negative groups.
We applied multiple neuroimaging processing methods capable of detecting small changes in cortical thickness and subcortical volumes. Post hoc power analysis demonstrated that differences in annual loss of brain volume between the groups as small as 0.1% per year in subcortical regions and 0.01 mm per year in the cortex could be detected, if present. Changes of greater magnitude were reported in an HIV-positive group, 33% of whom had detectable viral loads, with 3.2% more volume loss detected in the temporal lobe compared with an HIV-negative group. In other conditions with mild cognitive impairment, such as prodromal Alzheimer disease, thinning rates were 0.01 mm per year greater in individuals who progressed to mild cognitive impairment compared with those who maintained cognitive health. Although the absence of detectable cortical thinning and subcortical volume loss in our study is not proof of the absence of ongoing brain atrophy in HIV-positive individuals, the power analysis demonstrates that clinically meaningful changes could have been detected, if present. These findings are consistent with those of a recent smaller longitudinal study, which likewise found no significant changes in mean cortical thickness and deep gray matter volumes during a 2-year period in HIV-positive participants with undetectable viral loads receiving treatment with cART. Collectively, these findings support the hypothesis that effective viral suppression with stable cART could halt the previously reported progression of brain atrophy in HIV.
Improvements in neuropsychological test scores were observed to a similar degree in both groups in the Letter Fluency and Digit Symbol Substitution tasks. Improvement was also seen in Trail Making Test Part A scores, with the HIV-positive group showing greater improvements than controls. On average, these improvements were less than 0.5 SD from baseline, a threshold generally considered to indicate clinically meaningful change. The observed improvements in test scores likely reflect imperfect test-retest reliability and practice effects; in any case, the findings argue against substantial cognitive decline. However, a caveat of mixed-effects modeling is the assumption that patterns of longitudinal change are the same for all individuals, which may not be true. It is possible that the mixed-effects models masked unique cognitive trajectories that have clinical meaning. Future studies should consider alternative approaches such as group-based trajectory analysis, which identifies distinct cognitive trajectories. This approach was applied to a large sample of HIV-positive participants drawn from the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) cohort. Consistent with our findings, a decline in scores of even 1 neuropsychological test was uncommon, with most HIV-positive participants remaining cognitively stable during a 3-year period.
The absence of worsening cognitive function also agrees with a recent Multicenter AIDS Cohort study and another CHARTER study. These studies found that stability was the rule, with only small subsets of HIV-positive participants having deterioration in neuropsychological performance or HAND status. Those studies supported the possibility that cardiovascular disease contributed to cognitive decline, with lower high-density lipoprotein concentrations and hypercholesterolemia predicting decline. We focused on individuals who were aviremic and treated with cART, excluding those with evidence of white matter hyperintensities attributable to microvascular injury a priori, to test the hypothesis that HIV, rather than vascular comorbidities, causes brain injury. Future studies should evaluate brain volumes and neuropsychological performance in HIV-positive individuals over a longer period to clarify whether very subtle progressive effects continue. Studies specifically concentrating on older HIV-positive individuals (>55 years) are also needed, as increasing age and HIV infection may have synergistic effects on brain structure and function.
Smaller cortical and subcortical volumes and poorer cognitive performance in the HIV-positive group may reflect brain injury that occurred soon after seroconversion, possibly during the time of untreated infection. Viral markers and markers of immune activation are elevated in the cerebrospinal fluid during this phase of the infection. These viral and immunopathogenic changes are thought to be associated with neuronal damage. If the infection is left untreated, high levels of HIV replication continue, leading to increased production of toxic viral proteins and neuroinflammatory responses, resulting in potentially permanent damage. Supporting this hypothesis, cross-sectional studies investigating brain volumes and cognitive function in primary HIV infection (defined as <1 year after exposure) have reported reduced putamen and cortical gray matter volumes, and poorer cognitive performance in tasks involving executive function, attention and working memory, language, and speed of information processing. This finding demonstrates that neuronal injury is present early in the infection. Our results are also consistent with a large body of cross-sectional work with individuals with chronic HIV infection (defined as >1 year after exposure) who reported volume reductions throughout the subcortical regions; cortical thickness reductions in the primary sensory and motor cortices, temporal lobe, and middle cingulate cortex; and weaker performance on neuropsychological tests compared with controls.
Supporting the idea that these differences are associated with events prior to initiation of cART, previous studies have demonstrated that a history of more severe immunosuppression, indexed by nadir CD4 cell counts, is associated with smaller brain volumes and poorer neuropsychological performance. We observed a significant correlation between lower nadir CD4 counts and smaller subcortical volumes, but not with cortical thickness or neuropsychological performance. Although this finding corresponds with previous work, the literature is not consistent because smaller cortical volumes and poorer neuropsychological performance have been previously linked with lower nadir CD4 counts. Discrepancies between the results most likely reflect differences in sample size, disease severity, and neuropsychological test selection. Taken together, our results could support the hypothesis that neurobiological changes occurring early in infection may be responsible for the cognitive impairment found in chronic HIV-positive individuals. This finding suggests that early initiation of cART may have neurocognitive benefits. However, future longitudinal work assessing brain structure and function in primary HIV infection is required to verify this hypothesis.
The effect of treatment central nervous system penetration effectiveness, indexed by CPE score, on brain volumes and neuropsychological test performance was also explored. No correlations with any brain volume measures or neuropsychological test scores were observed. This outcome suggests that treatment regimens with higher penetration do not influence brain structure or function. However, given the limited number of treatment regimens prescribed (CPE score range, 5-13), this sample of HIV-positive participants was not appropriate to demonstrate the potential neuroprotective or neurotoxic effects of cART.
Limitations
This study has limitations. First, recent evidence has suggested that cardiovascular risk factors are more common in HIV-positive individuals and are associated with cognitive deficits. Although we excluded participants with overt evidence of cerebrovascular disease on results of imaging, data on vascular risk factors were not acquired. We cannot definitively exclude that vascular injury contributes to the smaller brain volumes and cognitive deficits. Second, this study focused on the direct effects of HIV, by including HIV-positive participants who were aviremic and treated with cART who had minimal comorbidities and no white matter hyperintensities. This selection limits the generalizability to individuals with similar characteristics. Indeed, HIV-positive individuals with lesions or other comorbid conditions may be more likely to experience ongoing brain injury despite full viral suppression. Finally, although the HIV-positive group performed more poorly on neuropsychological tests than the HIV-negative controls, we did not collect information on the functional limitations of daily living needed to categorize participants with respect to the HAND categories; our focus was on the change within the individual over time. The ability to detect change in neuropsychological performance depends on the tests used. It is possible that different tests would yield different results. For example, our neuropsychological assessment did not include measures of nonverbal learning and memory or tests of abstractions. However, no evidence of deterioration on the Trail Making Test Part B were observed, which was demonstrated to be the cognitive test most likely to show decline during a 36-month period across a battery of 15 neuropsychological tests administered to 701 HIV-positive individuals in a longitudinal CHARTER cohort. In that study, as well as ours, improvements in some test scores were observed, presumably owing to practice effects. These effects could yield stable performance despite underlying progressive brain injury, but the absence of detectably poorer brain volume loss in the same HIV-positive sample here argues against this interpretation.
Conclusions
We used multiple neuroimaging methods to assess brain structure and cognitive function in a cohort of HIV-positive participants with good virologic control who were treated with cART and demographically similar HIV-negative controls. Although we observed smaller cortical thickness and subcortical volumes and poorer cognitive function in the HIV-positive group, there was no significant brain volume loss or neuropsychological decline during a 2-year period. These findings support the hypothesis that brain injury due to HIV could occur principally during untreated infection. This finding suggests that early initiation of cART and full viral suppression may preserve long-term brain health.
eAppendix. Methods
eFigure 1. White Matter Tissue Reductions in HIV-Positive Patients Compared With Controls Revealed With Voxel-Based Morphometry
eFigure 2. Minimum Detectable Difference Between Groups in Brain Volume Changes Over Time
eFigure 3. Minimum Detectable Difference Between Groups in Cortical Thickness Changes Over Time
eReferences
References
- 1.Heaton RK, Clifford DB, Franklin DR Jr, et al. ; CHARTER Group . HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen MAM, Koopmans PP, Kessels RPC. Cognitive decline in relation to psychological wellbeing and HIV disease- and treatment characteristics in HIV-infected patients on cART: a one-year follow-up study. AIDS Behav. 2017;21(6):1728-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaton RK, Franklin DR Jr, Deutsch R, et al. ; CHARTER Group . Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis. 2015;60(3):473-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouillette MJ, Yuen T, Fellows LK, Cysique LA, Heaton RK, Mayo NE. Identifying neurocognitive decline at 36 months among HIV-positive participants in the CHARTER cohort using group-based trajectory analysis. PLoS One. 2016;11(5):e0155766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacktor N, Skolasky RL, Seaberg E, et al. . Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology. 2016;86(4):334-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stout JC, Ellis RJ, Jernigan TL, et al. ; HIV Neurobehavioral Research Center Group . Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. Arch Neurol. 1998;55(2):161-168. [DOI] [PubMed] [Google Scholar]
- 7.Cardenas VA, Meyerhoff DJ, Studholme C, et al. . Evidence for ongoing brain injury in human immunodeficiency virus–positive patients treated with antiretroviral therapy. J Neurovirol. 2009;15(4):324-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfefferbaum A, Rogosa DA, Rosenbloom MJ, et al. . Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiol Aging. 2014;35(7):1755-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrêa DG, Zimmermann N, Tukamoto G, et al. . Longitudinal assessment of subcortical gray matter volume, cortical thickness, and white matter integrity in HIV-positive patients. J Magn Reson Imaging. 2016;44(5):1262-1269. [DOI] [PubMed] [Google Scholar]
- 10.Letendre S, Marquie-Beck J, Capparelli E, et al. ; CHARTER Group . Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65(1):65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antinori A, Arendt G, Becker JT, et al. . Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casaletto KB, Cattie J, Franklin DR, et al. ; HNRP Group . The Wide Range Achievement Test-4 Reading subtest “holds” in HIV-infected individuals. J Clin Exp Neuropsychol. 2014;36(9):992-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guizard N, Fonov VS, García-Lorenzo D, Nakamura K, Aubert-Broche B, Collins DL. Spatio-temporal regularization for longitudinal registration to subject-specific 3d template. PLoS One. 2015;10(8):e0133352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans Med Imaging. 2008;27(4):425-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87-97. [DOI] [PubMed] [Google Scholar]
- 16.Eskildsen SF, Coupé P, Fonov V, et al. ; Alzheimer’s Disease Neuroimaging Initiative . BEaST: brain extraction based on nonlocal segmentation technique. Neuroimage. 2012;59(3):2362-2373. [DOI] [PubMed] [Google Scholar]
- 17.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18(2):192-205. [PubMed] [Google Scholar]
- 18.Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL; Brain Development Cooperative Group . Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54(1):313-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3-D model–based neuroanatomical segmentation. Hum Brain Mapp. 1995;3(3):190-208. doi: 10.1002/hbm.460030304 [DOI] [Google Scholar]
- 20.Aubert-Broche B, Fonov VS, García-Lorenzo D, et al. . A new method for structural volume analysis of longitudinal brain MRI data and its application in studying the growth trajectories of anatomical brain structures in childhood. Neuroimage. 2013;82(15):393-402. [DOI] [PubMed] [Google Scholar]
- 21.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11(6, pt 1):805-821. [DOI] [PubMed] [Google Scholar]
- 22.Eskildsen SF, Østergaard LR Active surface approach for extraction of the human cerebral cortex from MRI. Paper presented at: Medical Image Computing and Computer-Assisted Intervention; October 1, 2006; Copenhagen, Denmark. [PubMed] [Google Scholar]
- 23.Eskildsen SF, Østergaard LR Evaluation of five algorithms for mapping brain cortical surfaces. Paper presented at: 2008 XXI Brazilian Symposium on Computer Graphics and Image Processing; Campo Grandy, Brazil; October 12, 2008. [Google Scholar]
- 24.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870-878. [DOI] [PubMed] [Google Scholar]
- 25.Pacheco J, Goh JO, Kraut MA, Ferrucci L, Resnick SM. Greater cortical thinning in normal older adults predicts later cognitive impairment. Neurobiol Aging. 2015;36(2):903-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582-592. [DOI] [PubMed] [Google Scholar]
- 27.González-Scarano F, Martín-García J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69-81. [DOI] [PubMed] [Google Scholar]
- 28.Wright PW, Pyakurel A, Vaida FF, et al. . Putamen volume and its clinical and neurological correlates in primary HIV infection. AIDS. 2016;30(11):1789-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ragin AB, Du H, Ochs R, et al. . Structural brain alterations can be detected early in HIV infection. Neurology. 2012;79(24):2328-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ragin AB, Wu Y, Gao Y, et al. . Brain alterations within the first 100 days of HIV infection. Ann Clin Transl Neurol. 2015;2(1):12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prakash A, Hou J, Liu L, Gao Y, Kettering C, Ragin AB. Cognitive function in early HIV infection. J Neurovirol. 2017;23(2):273-282. [DOI] [PubMed] [Google Scholar]
- 32.Chiang MC, Dutton RA, Hayashi KM, et al. . 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. Neuroimage. 2007;34(1):44-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen RA, Harezlak J, Schifitto G, et al. . Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol. 2010;16(1):25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker JT, Sanders J, Madsen SK, et al. ; Multicenter AIDS Cohort Study . Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav. 2011;5(2):77-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr. 2012;59(5):469-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kallianpur KJ, Shikuma C, Kirk GR, et al. . Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology. 2013;80(19):1792-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janssen MAM, Meulenbroek O, Steens SCA, et al. . Cognitive functioning, wellbeing and brain correlates in HIV-1 infected patients on long-term combination antiretroviral therapy. AIDS. 2015;29(16):2139-2148. [DOI] [PubMed] [Google Scholar]
- 38.Sanford R, Fernandez Cruz AL, Scott SC, et al. . Regionally specific brain volumetric and cortical thickness changes in HIV-infected patients in the HAART era. J Acquir Immune Defic Syndr. 2017;74(5):563-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson PM, Dutton RA, Hayashi KM, et al. . Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A. 2005;102(43):15647-15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kallianpur KJ, Kirk GR, Sailasuta N, et al. . Regional cortical thinning associated with detectable levels of HIV DNA. Cereb Cortex. 2012;22(9):2065-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellis RJ, Badiee J, Vaida F, et al. ; CHARTER Group . CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25(14):1747-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hua X, Boyle CP, Harezlak J, et al. ; HIV Neuroimaging Consortium . Disrupted cerebral metabolite levels and lower nadir CD4+ counts are linked to brain volume deficits in 210 HIV-infected patients on stable treatment. Neuroimage Clin. 2013;3:132-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brouillette MJ, Mayo N, Fellows LK, et al. . A better screening tool for HIV-associated neurocognitive disorders: is it what clinicians need? AIDS. 2015;29(8):895-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su T, Wit FW, Caan MW, et al. . White matter hyperintensities in relation to cognition in HIV-infected men with sustained suppressed viral load on combination antiretroviral therapy. AIDS. 2016;30(15):2329-2339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Methods
eFigure 1. White Matter Tissue Reductions in HIV-Positive Patients Compared With Controls Revealed With Voxel-Based Morphometry
eFigure 2. Minimum Detectable Difference Between Groups in Brain Volume Changes Over Time
eFigure 3. Minimum Detectable Difference Between Groups in Cortical Thickness Changes Over Time
eReferences