Key Points
Question
Is prescription opioid use in one household member associated with increased risk of prescribed opioid use in other household members?
Findings
In a study comparing 12 695 280 commercial insurance beneficiaries with a household member who started a new prescription of opioids, to 6 359 639 beneficiaries with a household member who started a new prescription of nonopioid pain relievers, the 1-year risk of subsequent opioid use was 0.71% higher among individuals exposed to opioids through a household member’s prescription.
Meaning
Prescription opioid use may spread within households.
This cohort study investigates whether individuals living in a household with a prescription opioid user vs prescription nonsteroidal anti-inflammatory drug user are more likely to initiate prescription opioids themselves.
Abstract
Importance
Increases in prescription opioid use in the United States have been attributed to changing prescribing guidelines and attitudes toward pain relief; however, the spread of opioid use within households through drug diversion may also be a contributing factor.
Objective
To investigate whether individuals living in a household with a prescription opioid user are more likely to initiate prescription opioids themselves, compared with individuals in households with a prescription nonsteroidal anti-inflammatory drug (NSAID) user.
Design, Setting, and Participants
This was a retrospective cohort study using administrative health care claims data from 2000 to 2014 of commercial insurance beneficiaries sharing a health plan with continuous prescription drug coverage, without opioid or NSAID use in the prior year. Enrollees were followed from the date of the first prescription filled by a household member for an eligible opioid or NSAID until initiation of prescription opioids, disenrollment, or administrative censoring after 1 year or the end of follow-up on December 31, 2014. Risk of opioid initiation was derived from inverse probability-weighted (IPW) Kaplan-Meier estimators that adjusted for potential confounders, prognostic factors, and predictors of censoring.
Exposure
Outpatient pharmacy dispensing of a prescription opioid or prescription NSAID.
Main Outcomes and Measures
Incident outpatient pharmacy fill for a prescription opioid by a household member.
Results
From 2000 to 2014, 12 695 280 individuals were exposed to prescription opioids and 6 359 639 to prescription NSAIDS through an index prescription to a household member. The IPW estimated risk of opioid initiation in the subsequent year was 11.83% (95% CI, 11.81%-11.85%) among individuals exposed to prescription opioids in the household, compared with 11.11% (95% CI, 11.09%-11.14%) among individuals exposed to prescription NSAIDs, resulting in a risk difference of 0.71% (95% CI, 0.68%-0.74%). An unmeasured confounder that is modestly associated with the exposure (eg, prevalence difference = 0.9%) and the outcome (eg, risk difference = 0.9) after adjustment for all measured variables could explain our observed estimate of the overall risk difference (0.71%).
Conclusions and Relevance
Living in a household with a prescription opioid user may increase risk of prescription opioid use, which may reflect both increased access to these products as well as shared risk factors, such as prescriber preference and prescription drug monitoring.
Introduction
Opioid prescribing in the US increased 300% from 1991 to 2009 and totaled 246 million dispensed prescriptions in 2015. Globally, the United States continues to be the largest consumer of the world’s supply of hydrocodone (99%) and oxycodone (81%), with hydrocodone-acetaminophen being the leading prescription drug dispensed by US retail pharmacies. This increase in opioid prescribing has been attributed to changes in prescribing guidelines, attitudes toward pain relief, aggressive pharmaceutical marketing, and the liberalization of laws governing the ability of physicians to prescribe opioids without training in pain management. Opioid adverse effects include QT interval prolongation and respiratory depression, which can lead to opioid-related emergency department visits, hospitalizations, and death. In 2011, prescription opioids were involved in more than 480 000 ER visits and 16 000 deaths, surpassing the mortality burden from firearms and motor vehicle accidents for Americans ages 35 to 54 years. Accidental ingestion of prescription opioids also caused more than 5000 ER visits among children 5 years or younger in 2011, which underscores the harms of the broad availability of opioids in US households.
Opioids are often prescribed in doses exceeding clinical guidelines for patients with non–cancer-related pain, and in large quantities, resulting in surpluses of opioids stored in household medicine cabinets. Unused medications create opportunities for nonprescribed use and drug sharing among friends and family members, who may perceive these medications to be low risk given their storage at home. For example, a third of veterans receiving prescription opioid therapy report sharing unused medications with family members, and more than 70% of non–medical opioid users obtain the drugs from family members and friends. Because prescription medication sharing is common, families and other social networks likely shape norms and behaviors surrounding the use of prescription opioids. Given the documented spread of substance use behaviors, such as heavy drinking within social networks, it is likely that prescription opioid use can also spread within networks, such as families. Although a previous study has examined the association between opioid abuse in one person and opioid abuse in another, the extent to which prescribed opioid use in one person is associated with increased risk of prescribed opioid use in another person remains unknown.
We quantified the association between potential access to opioids resulting from a new prescription started by a household member and subsequent new use of prescription opioids by other family members. Specifically, we compared the 1-year risk of opioid initiation due to the introduction of prescription opioids vs introduction of nonopioid analgesics (prescription nonsteroidal anti-inflammatory drugs [NSAIDs]) in households of commercial insurance beneficiaries that included employees, spouses, and dependents (hereinafter, opioid households and NSAID households). We further explored whether associations varied across subgroups of age, geographic region, potential indications, refill availability, days’ supply, and by year and opioid dose.
Methods
We used the 2000-2014 Truven Health Analytics MarketScan Commercial Claims and Encounters databases covering the years 2000 to 2014. MarketScan contains standardized, deidentified, person-level information on enrollment, paid inpatient and outpatient services and procedures, and outpatient pharmacy dispensing claims of employer-sponsored commercial insurance beneficiaries, their spouses, and dependents. MarketScan is one of the largest, fully integrated, and most complete commercial insurance claims databases available for the United States, where beneficiaries can be tracked across insurance plans, health care sites, clinician types, and over time with median follow-up of 3 years. The database increased from 3.7 million enrollees to 47.2 million from 2000 to 2014. The Office of Human Research Ethics at the University of North Carolina at Chapel Hill deemed the study exempt from review.
Study Population
This retrospective cohort study included household members of patients who initiated use of prescription opioids or prescription NSAIDs based on outpatient pharmacy dispensing claims (henceforth, we refer to the index patient as “Patient 0”). Prescription NSAIDs were chosen as the active comparator group because they have similar indications for treating pain, which minimizes the potential for unmeasured confounding. New use was defined as the first pharmacy dispensing claim after a 365-day period of continuous enrollment without evidence of prescription opioid or prescription NSAID use in claims. We generalized the “new-user” design to “new households” by requiring all household members to have continuous prescription drug coverage with no record of prescription opioid or NSAID use during the baseline period. Household members entered either the opioid or NSAID cohort at the index date anchored to the date of initiation by Patient 0. Eligible index dates were those occurring between January 1, 2001, and December 31, 2014, to ensure observation of the 1-year baseline. To make comparisons between cohorts more rigorous and identify index patients with similar indications, household members of patients who had a diagnosis of malignant neoplasm, used hospice services during the baseline period, or initiated both prescription opioids and NSAIDs on the same day were excluded.
Opioid Medication Exposure
Pharmacy dispensing billing claims for the most commonly prescribed synthetic and semisynthetic opioids and NSAIDs were identified in the Outpatient Pharmaceutical Claims file by their generic string name. Opioids and NSAIDs were restricted to oral and transdermal formulations (see eTable 1 in the Supplement). We excluded claims for opioids used primarily to treat Parkinson disease (apomorphine), opioid dependence (methadone), and cough (potassium guaiacolsulfonate–hydrocodone bitartrate).
Outcome Assessment
Our primary outcome was initiation of prescription opioids by members of the index patient’s household. Initiation by a household member was assessed similarly to cohort eligibility defined by Patient 0 using the dispensing date for an eligible opioid in the Outpatient Pharmaceutical claims database.
Covariates
Baseline covariates for household members were assessed during the year prior to the index date defined by Patient 0. Potential confounders of the association between opioid receipt in Patient 0 and opioid initiation by household members were identified a priori using subject matter knowledge and causal diagrams, and included household size, composition (children <18 years, yes/no), region of residence (Northeast, North Central, South, West), calendar year of cohort entry, and history of substance use diagnosis for Patient 0 (yes/no). Baseline predictors of loss to follow-up (defined as disenrollment) and opioid initiation of at-risk household members included age, sex, history of chronic pain diagnosis, psychiatric comorbidity (dichotomous variables for yes/no), health care utilization (number of outpatient visits, continuous; emergency department visit in prior 7 or 30 days, yes/no), and use of scheduled prescription medications (dichotomous variables for yes/no for each drug class; see eTable 1 in the Supplement for definitions).
Statistical Analyses
We estimated the 1-year risk of opioid initiation by household members (excluding Patients 0) within each treatment group using the complement of the Kaplan-Meier estimator with time in days from the index date. Household members were individually censored at the earliest of an event, at 1 year of follow-up, disenrollment, or administrative censoring on December 31, 2014.
We estimated 1-year risks and risk differences (RDs) for opioid initiation using inverse probability-weighted Kaplan-Meier estimators adjusting for baseline confounders, prognostic factors, and dropout. Weights were the product of inverse probability of treatment and inverse probability of censoring weights (see the eMethods in the Supplement for details on the construction of weights). Age, number of outpatient visits during baseline, and household size were modeled using restricted quadratic splines and calendar year as a categorical variable with each year as a separate category. Estimates of precision were obtained using nonparametric bootstrap resampling with replacement.
We explored potential heterogeneity of the association between household opioid availability and opioid initiation across subgroups: age of at-risk household members (0-5, 6-11, 12-17, 18-25, 26-35, 36-45, 46-55, ≥56 years); region of residence; potential indication for Patients 0 (back pain [yes vs no]; fracture [yes vs no]; year of cohort entry; and characteristics of the index prescription (availability of refills [yes vs no]; days of supply [<30 days or ≥30 days]). Subgroup differences were examined by stratifying the original cohort and repeating the primary analysis described herein within each stratum. We further examined variations in risk by daily opioid dose by converting all index opioid prescriptions to morphine milligrams equivalents (MME) and categorized dosage as 0 to 19, 20 to 50, 51 to 89, or 90 or more MME/d.
We performed 2 sensitivity analyses. First, the primary analysis was performed after restricting to 2-adult (age >18 years) households to account for shared genetic factors that could give rise to opioid-seeking behavior. Second, we assessed the extent to which unmeasured differences between the opioid and NSAID cohorts could explain our results. Additional details are provided in the eMethods in the Supplement. All analyses were conducted using SAS statistical software (version 9.4; SAS Institute Inc).
Results
Characteristics of the Study Sample
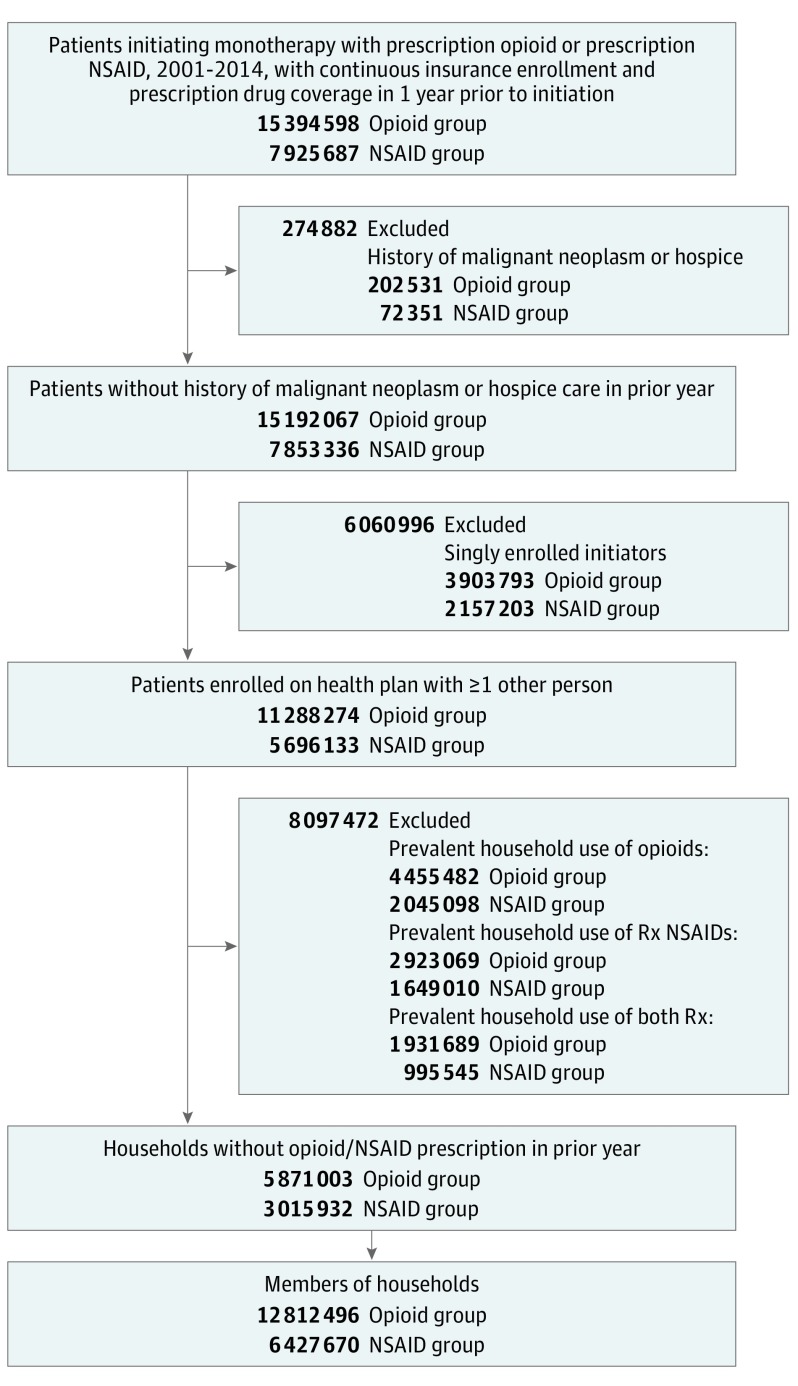
The study sample comprised 12 695 280 members of 5 871 003 opioid households and 6 359 639 members of 3 015 932 NSAID households, after excluding 84 810 households (1%) without information on geographic region (Figure 1). Opioid and NSAID households were of similar size (median, 3 enrollees; interquartile range [IQR], 2-4 enrollees), but opioid households were more likely to reside in the South than NSAID households (Table 1). Demographic characteristics were similar between cohorts: the median age of household members was 22 years (IQR, 11-45 years), and less than half were female. Overall, baseline covariates were balanced between household exposure groups. However, compared with members of NSAID households, slightly greater proportions of members of opioid households had used attention-deficit/hyperactivity disorder medications, antibiotics, benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), and sleep medications, but not β-blockers, statins, or muscle relaxants during baseline. Members of opioid households were also more likely than members of NSAID households to have a history of back and neck pain, migraine, fractures, depression, substance abuse, and cancer screening, and less likely to have diabetes mellitus and arthritis. However, utilization of outpatient and inpatient services and emergency department visits were similar between household exposure groups.
Figure 1. Derivation of Cohort in the MarketScan Commercial Claims and Encounters Databases, 2000-2014.
NSAID indicates nonsteroidal anti-inflammatory drug; Rx, prescription.
Table 1. Baseline Characteristics of Household Members of Prescription Analgesic Initiators, MarketScan Commercial Claims and Encounters, 2000-2014a.
| Characteristic | Opioid Households (n = 12 695 280) |
NSAID Households (n = 6 359 639) |
|---|---|---|
| Demographics | ||
| Female sex | 48.5 | 48.1 |
| Age, median (IQR), y | 22 (11-45) | 21 (11-45) |
| Age group, y | ||
| <6 | 10.8 | 10.4 |
| 6-11 | 15.1 | 15.6 |
| 12-17 | 15.7 | 16.5 |
| 18-25 | 11.8 | 13.1 |
| 26-35 | 7.4 | 6.3 |
| 36-45 | 14.8 | 13.7 |
| 46-55 | 16.2 | 15.8 |
| ≥56 | 8.3 | 8.7 |
| Baseline mediation use | ||
| Benzodiazepines | 3.1 | 2.8 |
| ADHD medications | 1.5 | 1.4 |
| Antibiotics | 36.0 | 35.8 |
| Muscle relaxants | 1.0 | 1.0 |
| Sleep medications | 1.8 | 1.6 |
| SSRIs | 4.6 | 4.1 |
| Statins | 6.0 | 6.2 |
| Preexisting pain conditions | ||
| Back and neck pain | 7.6 | 7.0 |
| Back pain | 3.7 | 3.5 |
| Back disorder | 5.9 | 5.7 |
| Headache | 2.3 | 2.4 |
| Migraine | 1.0 | 1.0 |
| Arthritis | 6.3 | 6.3 |
| Osteoarthritis | 0.9 | 0.9 |
| Rheumatoid arthritis | 0.4 | 0.4 |
| Fibromyalgia | 0.9 | 0.8 |
| Fractures | 1.7 | 1.7 |
| Preexisting comorbidities | ||
| Depression | 2.8 | 2.6 |
| Substance abuse | 1.0 | 1.0 |
| Alcohol use | 0.3 | 0.3 |
| Gastrointestinal bleeding | 0.5 | 0.5 |
| COPD | 0.4 | 0.4 |
| Health care utilization | ||
| ER visit in past 30 d | 1.1 | 1.1 |
| ER visit in past 7 d | 0.4 | 0.3 |
| Outpatient visits, mean (SD) | 2.3 (2.95) | 2.4 (3.03) |
| Inpatient days, mean (SD) | 0.1 (1.40) | 0.1 (1.42) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; COPD, chronic obstructive pulmonary disease; ER, emergency department; IQR, interquartile range; SSRIs, selective serotonin reuptake inhibitors.
Data are given as percentages except where noted. Covariates are defined in eTable 1 in the Supplement. Baseline characteristics were assessed in the 1 y prior to the index date.
Unadjusted Analysis
Members of opioid households and NSAID households were followed for a median of 1 year (IQR, 0.6-1.0-year). During 14 846 450 person-years of follow-up, 1 786 014 individuals initiated use of prescription opioids and 4 187 048 individuals disenrolled from their health plan (21% in NSAID households, 22% in opioid households). Overall, the 1-year risk of prescription opioid initiation by a second household member was 11.32% (95% CI, 11.30%-11.34%). The 1-year unadjusted risk of prescription opioid initiation was 11.68% (95% CI, 11.66%-11.70%) within opioid households and 10.60% (95% CI, 10.57%-10.63%) within NSAID households.
Inverse Probability-Weighted Analysis
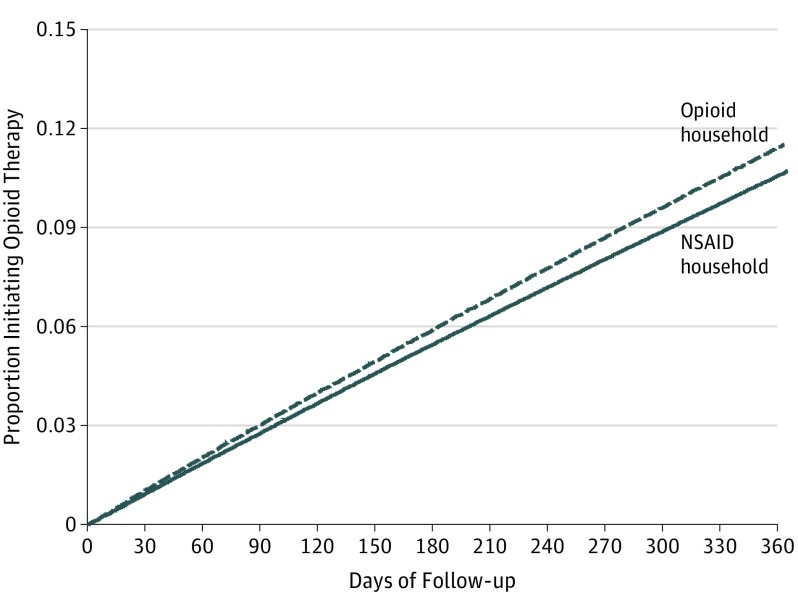
All potential confounders and predictors of opioid initiation or dropout identified a priori were included in corresponding propensity score models for treatment and censoring weights. The means of the treatment and censoring weights were 1.00 (range, 0.36-6.06) and 0.98 (range, 0.36-3.59), respectively. Cumulative incidence curves for opioid initiation over 1 year of follow-up by household opioid availability are presented in Figure 2. The 1-year risk of opioid initiation was 11.83% (95% CI, 11.81%-11.85%) among individuals in an opioid household, compared with 11.11% (95% CI, 11.09%-11.14%) among individuals in an NSAID household (Table 2). The adjusted 1-year RD in opioid initiation due to potential access to household opioids was 0.71% (95% CI, 0.68%-0.74%).
Figure 2. Inverse Probability-Weighted Cumulative Incidence Curves for Opioid Initiation by Household Availability of Prescription Opioids, MarketScan Commercial Claims and Encounters, 2000-2014.
Estimates adjusted for household size, region, year of cohort entry, family history of substance abuse, age, sex, baseline pain and psychiatric co-morbidities, use of scheduled and unscheduled prescription medications, and health care utilization. NSAID indicates nonsteroidal anti-inflammatory drug.
Table 2. Cumulative Incidence of Opioid Initiation Among Household Members of Prescription Opioid vs Prescription NSAID Initiators Overall and by Age, Region, and Potential Indication for Opioid Initiation by Patient 0, MarketScan Commercial Claims and Encounters, 2000-2014.
| Opioid Initiation | 1-Year Risk, % (95% CI)a | Risk Difference, % (95% CI)b | Risk Ratio (95% CI) | |
|---|---|---|---|---|
| Opioid | NSAID | |||
| Unadjusted | 11.68 (11.66-11.70) | 10.60 (10.57-10.63) | 1.08 (1.05-1.11) | 1.10 (1.10-1.11) |
| Inverse-probability weighted | 11.83 (11.81-11.85) | 11.11 (11.09-11.14) | 0.71 (0.68-0.74) | 1.06 (1.06-1.07) |
| Subgroup analyses | ||||
| Age group, y | ||||
| 0-5 | 3.6 (3.5-3.6) | 3.2 (3.1-3.2) | 0.41 (0.35-0.48) | 1.13 (1.11-1.15) |
| 6-11 | 3.7 (3.7-3.8) | 3.3 (3.3-3.4) | 0.41 (0.36-0.45) | 1.12 (1.11-1.14) |
| 12-17 | 10.2 (10.2-10.3) | 9.5 (9.4-9.5) | 0.77 (0.70-0.86) | 1.08 (1.07-1.09) |
| 18-25 | 13.7 (13.6-13.7) | 12.8 (12.7-12.8) | 0.91 (0.81-1.01) | 1.07 (1.06-1.08) |
| 26-35 | 18.8 (18.7-18.9) | 17.5 (17.4-17.7) | 1.26 (1.08-1.43) | 1.07 (1.06-1.08) |
| 36-45 | 16.0 (16.0-16.1) | 15.3 (15.2-15.4) | 0.72 (0.62-0.80) | 1.05 (1.04-1.05) |
| 46-55 | 15.7 (15.6-15.7) | 15.1 (15.0-15.1) | 0.62 (0.52-0.71) | 1.04 (1.03-1.05) |
| ≥56 | 17.1 (17.0-17.2) | 16.3 (16.1-16.4) | 0.85 (0.73-1.00) | 1.05 (1.04-1.06) |
| Region | ||||
| Northeast | 9.6 (9.6-9.7) | 9.1 (9.0-9.1) | 0.57 (0.47-0.66) | 1.12 (1.11-1.14) |
| North Central | 11.5 (11.4-11.5) | 11.0 (11.0-11.1) | 0.44 (0.38-0.51) | 1.08 (1.07-1.09) |
| South | 13.2 (13.1-13.2) | 12.4 (12.3-12.4) | 0.76 (0.71-0.81) | 1.07 (1.06-1.08) |
| West | 11.2 (11.2-11.3) | 10.3 (10.2-10.3) | 0.95 (0.88-1.02) | 1.07 (1.06-1.08) |
| Family history (Patient 0) | ||||
| Back and neck pain | 12.1 (12.0-12.1) | 11.3 (11.3-11.4) | 0.78 (0.71-0.85) | 1.07 (1.06-1.08) |
| No back and neck pain | 11.8 (11.8-11.8) | 11.0 (11.0-11.0) | 0.81 (0.77-0.84) | 1.07 (1.07-1.08) |
| Fracture | 12.1 (12.0-12.2) | 11.7 (11.5-11.9) | 0.42 (0.21-0.61) | 1.04 (1.02-1.05) |
| No fracture | 11.8 (11.8-11.9) | 11.0 (11.0-11.0) | 0.82 (0.79-0.85) | 1.07 (1.07-1.08) |
| Sensitivity analyses, 2-adult householdsc | 17.7 (17.6- 17.9) | 16.6 (16.5- 16.8) | 1.08 (0.90- 1.32) | 1.06 (1.05- 1.08) |
Abbreviation: NSAID, nonsteroidal anti-inflammatory drugs.
Risks, risk differences, and risk ratios adjusted for household size, household composition, year of cohort entry, region and/or age, sex, family history of substance abuse, pain and psychiatric comorbidities, use of scheduled and unscheduled prescription medications, and health care utilization.
For risk difference homogeneity, P < .001 for all comparisons.
Households with 2 adults older than 18 y.
Subgroup Analyses
The association between household opioid availability and opioid initiation varied across subgroups of age, geographic region, and potential indication of Patient 0 (Table 2). The RD among younger adults ages 18 to 25 years was 0.91% (95% CI, 0.81%-1.01%), compared with 1.26% (95% CI, 1.08%-1.43%) among those ages 26 to 35 years. In the North Central region, the RD was 0.44% (95% CI, 0.38%-0.51%), compared with 0.95% (95% CI, 0.88%-1.02%) in the West. Risk differences did not vary by history of back and neck pain in Patient 0, but RDs were markedly smaller among household members of patients with a history of fracture (RD, 0.42% [95% CI, 0.21%-0.61%]) than those without (RD, 0.82% [95% CI, 0.79%-0.85%]).
Results varied across characteristics of the index prescription (Table 3). The RD for opioid initiation was 0.53% (95% CI, 0.49%-0.57%) in households where the index supply was less than 30 days, compared with 1.14% (95% CI, 0.97%-1.34%) in households with at least a 30-day supply. The RD was 0.44% (95% CI, 0.40%-0.47%) in households with a single fill of the index prescription, compared with 1.43% (95% CI, 1.33%-1.53%) in households with refills. Compared with NSAID households, the RD for opioid initiation in households with an index opioid prescription for less than 20 MME/d was 1.44% (95% CI, 1.39%-1.50%), 0.81% (95% CI, 0.77%-0.85%) for 20-50 MME/d, 0.78% (95% CI, 0.72%-0.83%) for 51-89 MME/d, and 1.02% (95% CI, 0.95%-1.07%) for ≥90 MME/d. Risk differences did not vary appreciably over the years of the study (see eFigures 1 and 2 in the Supplement).
Table 3. Cumulative Incidence of Opioid Initiation Among Household Members of Prescription Opioid vs Prescription NSAID Initiators by Characteristics of Index Prescription, MarketScan Commercial Claims and Encounters, 2000-2014a.
| Characteristic of Index Prescription | Opioid Household, 1-y Risk, % (95% CI)a | NSAID Household, 1-y Risk, % (95% CI) | Risk Difference, % (95% CI) | Risk Ratio (95% CI) |
|---|---|---|---|---|
| Overall | 11.8 (11.8-11.8) | 11.1 (11.1-11.1) | 0.71 (0.68-0.74) | 1.06 (1.06-1.07) |
| Supply, d | ||||
| <30 | 12.0 (12.0-12.0) | 11.5 (12.0-11.5) | 0.53 (0.49-0.57) | 1.05 (1.04-1.05) |
| ≥30b | 12.8 (12.7-13.0) | 11.7 (13.0-11.8) | 1.14 (0.97-1.34) | 1.10 (1.08-1.11) |
| Refills | ||||
| No | 11.8 (11.8-11.9) | 11.4 (11.9-11.4) | 0.44 (0.40-0.47) | 1.04 (1.03-1.04) |
| Yesb | 13.5 (13.5-13.6) | 12.1 (13.6-12.2) | 1.43 (1.33-1.53) | 1.12 (1.11-1.13) |
Abbreviation: NSAID, nonsteroidal anti-inflammatory drugs.
Risks, risk differences, and risk ratios adjusted for household size, household composition, year of cohort entry, region and/or age, sex, family history of substance abuse, pain and psychiatric comorbidities, use of scheduled and unscheduled prescription medications, and health care utilization.
For RD homogeneity, P < .001 for all comparisons.
Sensitivity Analyses
In 2-adult households (n = 2 706 922), an opioid prescription to 1 adult was associated with increased risk of opioid initiation in the other (RD, 1.08% [95% CI, 0.90%-1.39%]). Although we controlled for a number of potential confounders, we also assessed the sensitivity of our findings to unmeasured baseline differences between the opioid and NSAID groups (eTable 2 in the Supplement). We found that an unmeasured confounder that is modestly associated with the exposure (eg, prevalence difference = 0.9%) and the outcome (eg, RD = 0.9) after adjustment for all measured variables could explain our observed estimate of the RD (0.71%). Alternative scenarios are presented in eTable 2 in the Supplement.
Discussion
We conducted a large, retrospective, cohort study comparing the risk of opioid initiation among commercial insurance beneficiaries who were newly exposed to prescription opioids vs prescription NSAIDs through a household member’s prescription. We observed a 0.71% absolute higher 1-year risk of prescription opioid use among individuals in opioid households compared with prescription NSAID households, which could reflect increased access to these products or unmeasured risk factors. Associations varied across age, region, potential indication, and characteristics of the index prescription.
Previous studies on prescription opioids have used MarketScan, but none examined patterns of use within families. Prior research within families focused on disentangling genetic vs environmental transmission of drug abuse; however, opiate abuse can develop from opioids prescribed for pain management. Because medication sharing cannot be captured in administrative data, understanding patterns of prescribed opioid use within households may be a critical first step toward addressing the opioid epidemic. If associations across households and social networks are validated in future studies, even small risk differences may be relevant on a population level, considering the broad environmental availability of prescription opioids.
We assessed whether our results could be explained by potential unmeasured differences between members of opioid and NSAID households in sensitivity analyses. Unmeasured variables include whether household members see the same health care professional with a preference for prescribing opioids. We found that the magnitude of such an effect need not be large to explain our findings. A previous study estimated physician preference for prescribing the same analgesic to be more than 20%; thus, it is plausible that our results could be explained by unmeasured health care professional preference if household members see the same provider (eg, physician, dentist). Alternatively, unmeasured environmental or system-level factors or household socioeconomic status may have given rise to confounding.
The association between potential access to household opioids and opioid initiation could be explained by multiple mechanisms. First, family members may share their medications with other members, which may lead to one seeking his or her own prescription. Second, household opioid use may shape attitudes and norms of opioid use. Third, spousal correspondence of opioid use may be due to homophily, which is defined as the inclination of similar individuals to associate. Although we could not disentangle potential mechanisms, interventions that address the opioid epidemic could include initiatives promoting safe medication storage within households and safe disposal of unused medications. Clinicians may need to consider the context within which medications will be used to assess the risks and benefits of prescribing opioids. For certain patients, prescribing in smaller quantities or without refills may help mitigate the risk of subsequent opioid use by a household member.
Strengths and Limitations
We generalized the “new-user” design to leverage information on households in large administrative databases and restrict to enrollees who had no prior access to opioids or NSAIDs in the household. The benefit of our “new-household” approach is the exclusion of households with prevalent use of prescription opioids, which may be affected by differential attrition, adherence, physiologic adaptation, or early adverse effects. We used households with prescription NSAIDs as an active comparator group to contextualize households with prescription opioids, which have similar indications and minimize potential bias owing to differential allocation of opioids to certain patients. However, restriction to households limits the generalizability of our inferences and applicability to real-world pharmacotherapy decisions. Moreover, MarketScan oversamples the South and has poor coverage of the West; thus, our study may not directly generalize to the US commercially insured population.
Despite our attempt to improve the internal validity of our estimates, we could not verify coresidence of household members or other sources of opioids, such as coresidents with public insurance or another health plan. Misclassification of prior exposure could introduce prevalent user bias, whereas underascertainment of true events will bias the RD even with perfect specificity and nondifferential sensitivity. Other databases with residential addresses to link patients or with clinician or system characteristics could help address exposure and outcome misclassification.
The cohort and primary outcome were identified using pharmacy dispensing billing claims. Electronic pharmacy dispensing claims are considered the gold standard of prescription drug exposure compared with self-reported medication use or outpatient medical records because insurance reimbursement is based on complete and accurate claims. Although opioids paid for in cash or received in inpatient settings were not captured in our data, approximately 15% of opioid prescriptions in 2008 were paid for in cash and 90% of opioids are dispensed from retail pharmacies. Similarly, pharmacy dispensing billing claims were used to identify prescription NSAIDs. Although we expect the sensitivity of prescription NSAID exposure to be high, we lack information on over-the-counter (OTC) NSAID use. However, OTC NSAID exposure is not expected to be a substantial source of bias in our study because sensitivity analyses have shown that prescription claims data can provide valid estimates of drug-outcome associations even when a large proportion of drug use is OTC.
Conclusions
Although we cannot rule out shared risk factors, such as clinician preference, prescription drug monitoring, or genetic predilection, our results suggest that opioid prescribing decisions may need to take into account the context within which medications will be used and the potential risk of subsequent opioid initiation by other individuals. Addressing the opioid use epidemic will require comprehensive solutions for all aspects contributing to this public health issue, including the potential for medication sharing and other unintended consequences of prescription opioid use in households.
eTable 1. List of Diagnostic Codes Used for Defining Inclusion and Exclusion Criteria, Covariates, and Outcome.
eTable 2. Bias analysis examining risk difference of opioid initiation by household opioid availability assuming different distributions of an unmeasured binary confounder.
eTable 3. Distribution of index prescriptions for opioids.
eFigure 1. Inverse probability weighted 1-year opioid initiation risk differences and 95% confidence intervals among household members by year of cohort entry, MarketScan Commercial Claims and Encounters 2000-2014.
eFigure 2. Inverse Probability-Weighted 1-Year Opioid Initiation Risk Differences and 95% CIs Among Household Members by Opioid Daily Dose MarketScan Commercial Claims and Encounters, 2000-2014
eMethods. Inverse probability of treatment and censoring weights.
eMethods. Sensitivity Analysis to Assess Potential Influence of Unmeasured Confounder.
References
- 1.Manchikanti L, Helm S II, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15(3)(suppl):ES9-ES38. [PubMed] [Google Scholar]
- 2.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107. [DOI] [PubMed] [Google Scholar]
- 3.Pezalla EJ, Rosen D, Erensen JG, Haddox JD, Mayne TJ. Secular trends in opioid prescribing in the USA. J Pain Res. 2017;10:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noble M, Tregear SJ, Treadwell JR, Schoelles K. Long-term opioid therapy for chronic noncancer pain: a systematic review and meta-analysis of efficacy and safety. J Pain Symptom Manage. 2008;35(2):214-228. [DOI] [PubMed] [Google Scholar]
- 5.IMS Institute for Health Care Informatics The Use of Medicines in the United States: Review of 2011. Parsippany, NJ: IMS Institute for Health Care Informatics; 2012. [Google Scholar]
- 6.The use of opioids for the treatment of chronic pain: a consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13(1):6-8. [PubMed] [Google Scholar]
- 7.Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg. 2007;105(1):205-221. [DOI] [PubMed] [Google Scholar]
- 8.Van Zee A. The promotion and marketing of OxyContin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joranson DE, Gilson AM, Dahl JL, Haddox JD. Pain management, controlled substances, and state medical board policy: a decade of change. J Pain Symptom Manage. 2002;23(2):138-147. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa K, Espinola JA, Brown DF, Camargo CA Jr. Trends in U.S. emergency department visits for opioid overdose, 1993-2010. Pain Med. 2014;15(10):1765-1770. [DOI] [PubMed] [Google Scholar]
- 11.Braden JB, Russo J, Fan M-Y, et al. Emergency department visits among recipients of chronic opioid therapy. Arch Intern Med. 2010;170(16):1425-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coben JH, Davis SM, Furbee PM, Sikora RD, Tillotson RD, Bossarte RM. Hospitalizations for poisoning by prescription opioids, sedatives, and tranquilizers. Am J Prev Med. 2010;38(5):517-524. [DOI] [PubMed] [Google Scholar]
- 13.Calcaterra S, Glanz J, Binswanger IA. National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999-2009. Drug Alcohol Depend. 2013;131(3):263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Vital signs: overdoses of prescription opioid pain relievers: United States, 1999-2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487-1492. [PubMed] [Google Scholar]
- 15.Substance Abuse and Mental Health Services Administration Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- 16.Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796-801. [DOI] [PubMed] [Google Scholar]
- 17.Shrank WH. Our bulging medicine cabinets: the other side of medication nonadherence. N Engl J Med. 2011;364(17):1591-1593. [DOI] [PubMed] [Google Scholar]
- 18.Lewis ET, Cucciare MA, Trafton JA. What do patients do with unused opioid medications? Clin J Pain. 2014;30(8):654-662. [DOI] [PubMed] [Google Scholar]
- 19.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Secondary School Students. Vol I Ann Arbor: Institute for Social Research, The University of Michigan; 2011. Monitoring the Future national survey results on drug use, 1975–2010. [Google Scholar]
- 20.Substance Abuse and Mental Health Services Administration Results From the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: SMA; 2013:13-4795. [Google Scholar]
- 21.Goldsworthy RC, Schwartz NC, Mayhorn CB. Beyond abuse and exposure: framing the impact of prescription-medication sharing. Am J Public Health. 2008;98(6):1115-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. N Engl J Med. 2008;358(21):2249-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenquist JN, Murabito J, Fowler JH, Christakis NA. The spread of alcohol consumption behavior in a large social network. Ann Intern Med. 2010;152(7):426-433, W141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mednick SC, Christakis NA, Fowler JH. The spread of sleep loss influences drug use in adolescent social networks. PLoS One. 2010;5(3):e9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kendler KS, Ohlsson H, Sundquist K, Sundquist J. Within-family environmental transmission of drug abuse: a Swedish national study. JAMA Psychiatry. 2013;70(2):235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rigg KK, Murphy JW. Understanding the etiology of prescription opioid abuse: implications for prevention and treatment. Qual Health Res. 2013;23(7):963-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adamson DM, Chang S, Hansen LG. Health Research Data for the Real World: The MarketScan Databases. New York, NY: Thompson Healthcare; 2008. [Google Scholar]
- 28.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915-920. [DOI] [PubMed] [Google Scholar]
- 29.Pearl J. Causal diagrams for empirical research. Biometrika. 1995;82(4):669-688. [Google Scholar]
- 30.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45-49. [DOI] [PubMed] [Google Scholar]
- 31.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ Jr. Splines for trend analysis and continuous confounder control. Epidemiology. 2011;22(6):874-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CDC compilation of benzodiazepines, muscle relaxants, stimulants, zolpidem, and opioid analgesics with oral morphine milligram equivalent conversion factors, 2016 version. Centers for Disease Control and Prevention; 2016. http://www.pdmpassist.org/pdf/BJA_performance_measure_aid_MME_conversion.pdf. Accessed July 8, 2017.
- 33.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain: United States, 2016. JAMA. 2016;315(15):1624-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VanderWeele TJ. Sensitivity analysis for contagion effects in social networks. Sociol Methods Res. 2011;40(2):240-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanderweele TJ, Arah OA. Bias formulas for sensitivity analysis of unmeasured confounding for general outcomes, treatments, and confounders. Epidemiology. 2011;22(1):42-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mack KA, Zhang K, Paulozzi L, Jones C. Prescription practices involving opioid analgesics among Americans with Medicaid, 2010. J Health Care Poor Underserved. 2015;26(1):182-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birt J, Johnston J, Nelson D. Exploration of claims-based utilization measures for detecting potential nonmedical use of prescription drugs. J Manag Care Spec Pharm. 2014;20(6):639-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Logan J, Liu Y, Paulozzi L, Zhang K, Jones C. Opioid prescribing in emergency departments: the prevalence of potentially inappropriate prescribing and misuse. Med Care. 2013;51(8):646-653. [DOI] [PubMed] [Google Scholar]
- 39.Paulozzi LJ, Zhang K, Jones CM, Mack KA. Risk of adverse health outcomes with increasing duration and regularity of opioid therapy. J Am Board Fam Med. 2014;27(3):329-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volkow ND, McLellan AT. Opioid abuse in chronic pain: misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253-1263. [DOI] [PubMed] [Google Scholar]
- 41.Brookhart MA, Wang PS, Solomon DH, Schneeweiss S. Evaluating short-term drug effects using a physician-specific prescribing preference as an instrumental variable. Epidemiology. 2006;17(3):268-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shalizi CR, Thomas AC. Homophily and contagion are generically confounded in observational social network studies. Sociol Methods Res. 2011;40(2):211-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothman KJ. Modern Epidemiology. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 44.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323-337. [DOI] [PubMed] [Google Scholar]
- 45.McDonald DC, Carlson KE. The ecology of prescription opioid abuse in the USA: geographic variation in patients’ use of multiple prescribers (“doctor shopping”). Pharmacoepidemiol Drug Saf. 2014;23(12):1258-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.US Department of Justice Automation of Reports and Consolidated Orders System (ARCOS), Retail Drug Summary. https://www.deadiversion.usdoj.gov/arcos. Accessed January 13, 2017.
- 47.Yood MU, Campbell UB, Rothman KJ, et al. Using prescription claims data for drugs available over-the-counter (OTC). Pharmacoepidemiol Drug Saf. 2007;16(9):961-968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. List of Diagnostic Codes Used for Defining Inclusion and Exclusion Criteria, Covariates, and Outcome.
eTable 2. Bias analysis examining risk difference of opioid initiation by household opioid availability assuming different distributions of an unmeasured binary confounder.
eTable 3. Distribution of index prescriptions for opioids.
eFigure 1. Inverse probability weighted 1-year opioid initiation risk differences and 95% confidence intervals among household members by year of cohort entry, MarketScan Commercial Claims and Encounters 2000-2014.
eFigure 2. Inverse Probability-Weighted 1-Year Opioid Initiation Risk Differences and 95% CIs Among Household Members by Opioid Daily Dose MarketScan Commercial Claims and Encounters, 2000-2014
eMethods. Inverse probability of treatment and censoring weights.
eMethods. Sensitivity Analysis to Assess Potential Influence of Unmeasured Confounder.