This systematic review and meta-analysis assesses the association of treatment to lower blood pressure with death and cardiovascular disease at different blood pressure levels in randomized clinical trials.
Key Points
Question
What is the association between treatment to lower blood pressure and death and cardiovascular disease at different blood pressure levels?
Findings
In this systematic review and meta-analysis, including 74 trials and more than 300 000 patients, treatment to lower blood pressure was associated with a reduced risk for death and cardiovascular disease if baseline systolic blood pressure was 140 mm Hg or above. Below 140 mm Hg, the treatment effect was neutral in primary preventive trials, but with possible benefit on nonfatal cardiovascular events in trials of patients with coronary heart disease.
Meaning
Systolic blood pressure of 140 mm Hg or higher should be treated to prevent death and cardiovascular disease, whereas treatment may be considered in patients with coronary heart disease and systolic blood pressure below 140 mm Hg, but not for primary prevention.
Abstract
Importance
High blood pressure (BP) is the most important risk factor for death and cardiovascular disease (CVD) worldwide. The optimal cutoff for treatment of high BP is debated.
Objective
To assess the association between BP lowering treatment and death and CVD at different BP levels.
Data Sources
Previous systematic reviews were identified from PubMed, the Cochrane Database of Systematic Reviews, and the Database of Abstracts of Reviews of Effect. Reference lists of these reviews were searched for randomized clinical trials. Randomized clinical trials published after November 1, 2015, were also searched for in PubMed and the Cochrane Central Register for Controlled Trials during February 2017.
Study Selection
Randomized clinical trials with at least 1000 patient-years of follow-up, comparing BP-lowering drugs vs placebo or different BP goals were included.
Data Extraction and Synthesis
Data were extracted from original publications. Risk of bias was assessed using the Cochrane Collaborations assessment tool. Relative risks (RRs) were pooled in random-effects meta-analyses with Knapp-Hartung modification. Results are reported according to PRISMA guidelines.
Main Outcomes and Measures
Prespecified outcomes of interest were all-cause mortality, cardiovascular mortality, major cardiovascular events, coronary heart disease (CHD), stroke, heart failure, and end-stage renal disease.
Results
Seventy-four unique trials, representing 306 273 unique participants (39.9% women and 60.1% men; mean age, 63.6 years) and 1.2 million person-years, were included in the meta-analyses. In primary prevention, the association of BP-lowering treatment with major cardiovascular events was dependent on baseline systolic BP (SBP). In trials with baseline SBP 160 mm Hg or above, treatment was associated with reduced risk for death (RR, 0.93; 95% CI, 0.87-1.00) and a substantial reduction of major cardiovascular events (RR, 0.78; 95% CI, 0.70-0.87). If baseline SBP ranged from 140 to 159 mm Hg, the association of treatment with mortality was similar (RR, 0.87; 95% CI, 0.75-1.00), but the association with major cardiovascular events was less pronounced (RR, 0.88; 95% CI, 0.80-0.96). In trials with baseline SBP below 140 mm Hg, treatment was not associated with mortality (RR, 0.98; 95% CI, 0.90-1.06) and major cardiovascular events (RR, 0.97; 95% CI, 0.90-1.04). In trials including people with previous CHD and mean baseline SBP of 138 mm Hg, treatment was associated with reduced risk for major cardiovascular events (RR, 0.90; 95% CI, 0.84-0.97), but was not associated with survival (RR, 0.98; 95% CI, 0.89-1.07).
Conclusions and Relevance
Primary preventive BP lowering is associated with reduced risk for death and CVD if baseline SBP is 140 mm Hg or higher. At lower BP levels, treatment is not associated with any benefit in primary prevention but might offer additional protection in patients with CHD.
Introduction
High blood pressure (BP) is the most important risk factor for death and cardiovascular disease (CVD) worldwide. Guidelines uniformly recommend treating high BP to a systolic BP (SBP) goal below 140 mm Hg. Within the past 2 years, the Systolic Blood Pressure Intervention Trial (SPRINT) and a meta-analysis of BP lowering at different SBP levels were published. Both studies found beneficial effects of antihypertensive treatment in people already below current goals. Based on these results, many have argued for lower SBP goals in high-risk patients.
Contrary to this finding, we have previously shown that intensive BP lowering might be harmful in people with diabetes. Also, the results from SPRINT and the meta-analysis are difficult to interpret. The BP measurement method used in SPRINT results in lower values compared with standard methods, the difference between methods being between 10 and 20 mm Hg. The meta-analysis by Ettehad et al used a method of standardization that exaggerates treatment effect and distorts study weights. Also, a number of trials were missing from that meta-analysis.
We performed a systematic review and meta-analysis to estimate the treatment effect of BP lowering on death and CVD across SBP levels. We aimed to achieve a more comprehensive study inclusion than previous meta-analyses, and we used nonstandardized methods, with risk ratios and SEs derived from raw data.
Methods
We performed a systematic review and meta-analysis of randomized clinical trials to assess the effect of BP-lowering treatment on cardiovascular outcomes at different SBP levels. The review was not registered a priori, and no formal protocol was developed.
We included trials with at least 1000 patient-years of follow-up that compared drugs against placebo or different BP targets against each other. Trials comparing agents against each other were not eligible because they risk assessing BP-independent effects of agents. We excluded trials in patients with heart failure or left ventricular dysfunction and trials in the acute phase after myocardial infarction because other mechanisms than BP lowering might be responsible for treatment effects in these settings.
Data Sources and Searches
We identified relevant trials through a 2-step process following PRISMA guidelines (eFigure 1 in the Supplement). First, we searched PubMed, the Cochrane Database of Systematic Reviews (CDSR), and the Database of Abstracts of Reviews of Effect (DARE) to identify previous systematic reviews. Reference lists from relevant reviews were scrutinized for trials. Second, we searched PubMed and Cochrane Central Register for Controlled Trials (CENTRAL) to find trials published after the latest previous electronic search included in the reviews. Records were screened assessing titles and abstracts and thereafter retrieved in full text and judged according to eligibility criteria. Search strategies are described in eMethods 1 in the Supplement.
Data Extraction and Quality Assessment
The risk of bias in all eligible trials was assessed using Cochrane Collaborations risk for bias tool by both of us separately. Studies judged to be at high risk of selection bias, performance bias, or detection bias were excluded from the meta-analyses (eTable 1 in the Supplement). Attrition bias was generally judged on study level for all outcomes. In 1 study, mortality data were collected to a large extent, whereas many participants were lost to follow-up with respect to cardiovascular events. Hence, this study was included in the mortality analyses but not in analyses of other outcomes. Selective reporting of study outcomes impairs the validity of meta-analyses on outcome level. Therefore, trials judged to be at high risk of selective reporting were not excluded from any analyses, but instead the possible lack of data should be held in mind interpreting the results of such analyses. We systematically searched for early termination, baseline imbalances, protocol changes, and sponsor involvement as other potential sources of bias.
Data were extracted separately by both of us from original publications into a specially designed spreadsheet (Excel, version 14.7.0; Microsoft Corp). In case of disagreement, we revisited the original publications and resolved any ambiguity by discussion. For 6 trials, we used additional data from previous reviews or Clinical Trials in Hypertension. In 4 cases, this concerned BP values, and in 2 cases, outcome data. In both cases of outcome data, the review authors were also coauthors of the original publication, and hence we judge these data as valid. Data on clinical characteristics, comorbidities, and interventions were collected on study level. The numbers of participants, all-cause deaths, cardiovascular deaths, major cardiovascular events (MACE), coronary heart disease (CHD) events, strokes, heart failure events, and end-stage renal disease events were collected for each treatment arm separately. Outcome definitions are given in eMethods 2 in the Supplement. We extracted SBP values at baseline for all participants together and the follow-up SBP values for each treatment arm separately. The difference in SBP between treatment arms was calculated from follow-up SBP values in each arm if not presented separately.
Data Synthesis and Analysis
Trials were classified as primary preventive if less than 50% of patients had clinically established CVD or atherosclerotic disease documented through angiography or ultrasonography. If more than 50% of patients had previous CVD, trials were classified as CHD trials, poststroke trials, or mixed CVD trials based on which type of CVD was most prevalent. We analyzed trial categories separately because trials in different categories were judged to be clinically heterogeneous, representing different patient populations and diverse clinical situations.
We calculated relative risks (RRs) in treated patients vs controls for all outcomes in all trials. Risks were calculated using data from original publications, according to the intention-to-treat principle. Originally, we planned to perform meta-analyses stratified by baseline SBP to assess treatment effect at different BP levels and meta-regression analyses to explore the potential interaction between baseline SBP and treatment effect, for each category of trials and each outcome separately. For CHD trials, 9 of 12 studies, including more than 90% of patients, had baseline SBPs ranging from 129 to 141 mm Hg. Poststroke trials included 6 studies and mixed CVD trials included 5 studies. For the above reasons, neither stratified meta-analyses or meta-regression analyses were considered suitable in these trial categories. Results were instead pooled using nonstratified random-effects meta-analyses with Knapp-Hartung modification. We used the Knapp-Hartung modification to better estimate imprecision in meta-analyses with heterogeneity and few included trials.
In primary preventive trials, we performed stratified meta-analyses, using BP categories below 140 mm Hg, 140 to 159 mm Hg, and 160 mm Hg or above. These categories were chosen to represent different stages of hypertension, where below 140 mm Hg is classified as normotension; 140 to 159 mm Hg, as mild hypertension; and 160 mm Hg or above, as moderate to severe hypertension. Interaction between baseline SBP and treatment effect was estimated using random-effects meta-regression. First, we performed univariate meta-regression with baseline SBP as the independent variable and treatment effect as the dependent variable. Second, we performed multivariate meta-regression, adding mean age, sex (percentage female), diabetes (dichotomous at ≥50% or <50%), and treatment duration (mean or median) as covariates. Figure 1 includes multivariate estimates; univariate estimates are given in eTables 2 and 3 in the Supplement.
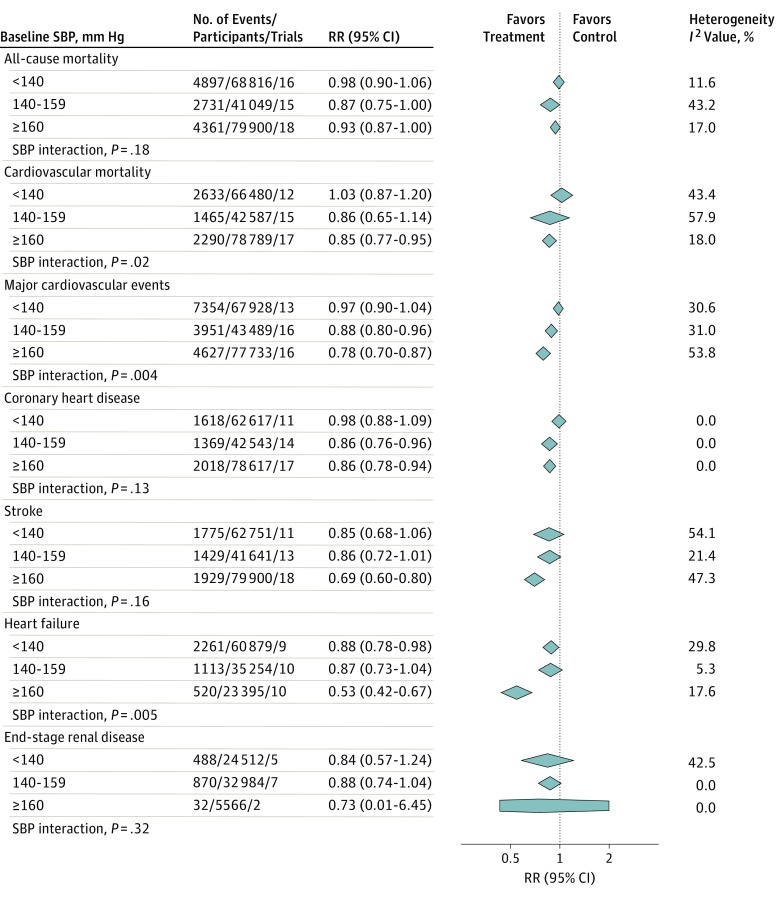
Figure 1. Effect of Treatment to Lower Blood Pressure (BP) at Different BP Levels in Primary Prevention.
RR indicates relative risk; SBP, systolic BP. Different size markers indicate weight. Studies included in the analyses are given in eTable 7 in the Supplement.
Sensitivity analyses were performed to further test the robustness of our results. We performed analyses excluding trials that included people with heart failure, analyses restricted to true primary and secondary preventive trials, analyses excluding trials using automated BP measurement devices, and analyses excluding trials with a BP difference between treatment arms of less than 5 mm Hg.
The risk of publication bias across trials was assessed using funnel plots for primary preventive trials and CHD trials separately. The number of poststroke trials and mixed CVD trials was too small for funnel plots to be meaningful. We tested for funnel plot asymmetry with the Egger and Harbord tests. To assess how baseline SBP affects funnel plot asymmetry in primary preventive trials, we marked trials in the funnel plots by SBP strata. All analyses were performed using STATA software (version 12; StataCorp). P < .05 indicated significance.
Results
We found 86 potentially eligible trials. Eight of these were judged to be at high risk of bias and were excluded from all analyses. Four trials did not present BP or outcome data and could not be included (eTable 1 in the Supplement). The final analyses included 74 unique trials, corresponding to 306 273 unique participants (39.9% women and 60.1% men; mean age, 63.6 years) and 1.2 million patient-years of follow-up (eTable 4 in the Supplement for trial characteristics and eTables 5 and 6 in the Supplement for risk of bias assessment). Of these, 70 trials reported funding sources. Fifty-six trials were partially or fully sponsored by the industry, whereas 14 trials were fully funded by governmental grants or academia. Weighted mean completeness of follow-up was 97.3% for all-cause mortality.
Fifty-one trials, including 192 795 participants, were categorized as primary preventive. These trials included 46.7% women with a mean age of 63 years. The mean baseline SBP was 154 mm Hg; patients were followed up for a mean of 4.0 years; and mean SBP difference between treatment and control during follow-up was 6.6 mm Hg.
Results from stratified meta-analyses of primary preventive trials are shown in Figure 1 and eTable 7 in the Supplement. Treatment to lower BP reduced the risk for all-cause mortality if the baseline SBP ranged from 140 to 159 mm Hg (RR, 0.87; 95% CI, 0.75-1.00) or was 160 mm Hg or above (RR, 0.93; 95% CI, 0.87-1.00). For baseline SBP below 140 mm Hg, the point estimate indicates null treatment effect with CIs excluding more than 10% RR reduction (RR, 0.98; 95% CI, 0.90-1.06). Multivariate meta-regression analysis was not significant for an interaction between baseline SBP and treatment effect (P = .18). For cardiovascular mortality, on the other hand, meta-regression analysis found a linear association between baseline SBP and treatment effect (P = .02). Cardiovascular mortality was reduced by 15% if baseline SBP was 160 mm Hg or above (RR, 0.85; 95% CI, 0.77-0.95), whereas results were neutral below 140 mm Hg (RR, 1.03; 95% CI, 0.87-1.20).
Treatment association with MACE followed a linear pattern across SBP levels (P = .004). The composite end point was reduced by 22% if baseline SBP was 160 mm Hg or above (RR, 0.78; 95% CI, 0.70-0.87) and by 12% if baseline SBP was 140 to 159 mm Hg (RR, 0.88; 95% CI, 0.80-0.96), but remained unaffected if baseline SBP was below 140 mm Hg (RR, 0.97; 95% CI, 0.90-1.04). Point estimates for CHD and stroke showed similar patterns, although meta-regression analyses were not significant for individual outcomes. Of note, BP lowering was highly effective in reducing the risk for stroke at BP levels of 160 mm Hg or above, with a mean RR reduction of 31% (RR, 0.69; 95% CI, 0.60-0.80). The effect of treatment on heart failure is hard to interpret. The stratified analysis showed a significant risk reduction if baseline SBP was below 140 mm Hg (RR, 0.88; 95% CI, 0.78-0.98). However, the effect was nonsignificant at a baseline SBP of 140 to 159 mm Hg (RR, 0.87; 95% CI, 0.73-1.04), and meta-regression analysis indicated decreasing treatment effect at lower baseline SBPs (P = .005). The effect of BP lowering on end-stage renal disease showed no clear pattern across BP levels and was nonsignificant at all BP levels.
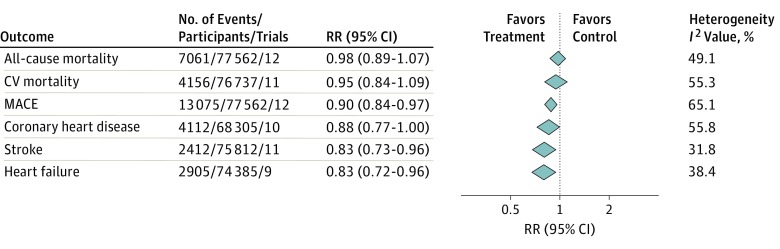
Twelve trials, including 77 562 participants, were categorized as CHD trials. These trials included only 23.8% women and had substantially lower baseline SBP (138 mm Hg) compared with primary preventive trials. Also, all CHD trials were partially or fully sponsored by the industry. Mean age was 64 years; patients were followed up for a mean of 4.5 years, with a mean difference of 3.8 mm Hg in SBP between treatment and control groups. Meta-analyses in CHD trials (Figure 2) found no association between treatment and all-cause (RR, 0.98; 95% CI, 0.89-1.07) or cardiovascular (RR, 0.95; 95% CI, 0.84-1.09) mortality, but reduced risk for MACE (RR, 0.90; 95% CI, 0.84-0.97), as well the individual components of CHD (RR, 0.88; 95% CI, 0.77-1.00), stroke (RR, 0.83; 95% CI, 0.73-0.96), and heart failure (RR, 0.83; 95% CI, 0.72-0.96). Of note, heterogeneity was high for mortality outcomes, MACE, and CHD. This finding could be explained by differences in baseline SBP, with numerically larger effect in trials with higher baseline SBP, although meta-regression analysis failed to confirm this.
Figure 2. Effect of Treatment to Lower Blood Pressure (BP) in Coronary Heart Disease Trials.
CV indicates cardiovascular; MACE, major cardiovascular events; and RR, relative risk. The following trials were included in the analysis: Poole-Wilson et al,19 Nissen et al,20 Fox and the EUROPA Investigators,21 Yusuf et al,22 Rouleau et al,85 the MACB Study Group (all outcomes except coronary heart disease and heart failure),86 Yusuf et al,23 Braunwald et al (all outcomes except coronary heart disease),24 Pitt et al (all outcomes except CV mortality),25 Pitt et al (all outcomes except stroke and heart failure),87 Teo et al (all outcomes except heart failure),26 and Yusuf et al.27
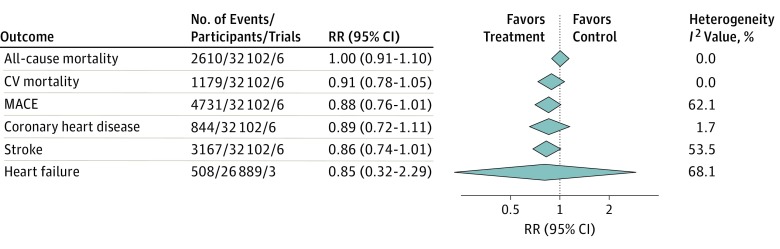
Six trials, including 32 102 patients, were classified as poststroke trials. These trials included 35.1% women, with a mean age of 65 years and mean baseline SBP of 146 mm Hg. Participants were followed up for a mean of 2.9 years, with a mean SBP difference of 5.9 mm Hg between treatment arms. Confidence intervals for meta-analyses were generally wide, indicating low power to detect treatment effect (Figure 3). We found a nonsignificant tendency toward decreased risk of MACE (RR, 0.88; 95% CI, 0.76-1.01) and recurrent stroke (RR, 0.86; 95% CI, 0.74-1.01), with inconclusive results for other outcomes. Heterogeneity was generally low but should be interpreted with caution owing to low power to detect heterogeneity in these analyses.
Figure 3. Effect of Treatment to Lower Blood Pressure (BP) in Poststroke Trials.
CV indicates cardiovascular; MACE, major cardiovascular events; and RR, relative risk. The following trials were included in all the analyses except for heart failure: the Dutch TIA Trial Study Group, Hypertension-Stroke Cooperative Study Group, Yusuf et al, MacMahon et al, Benavente et al, and Eriksson et al. The following trials were included in the heart failure analysis: Hypertension-Stroke Cooperative Study Group, Yusuf et al, and MacMahon et al.
Mixed CVD trials included heterogeneous patient populations, and meta-analyses were inconclusive for all outcomes. These analyses were judged to be noninformative and are therefore only reported in eFigure 2 in the Supplement.
Sensitivity analyses restricted to true primary or secondary preventive trials, excluding trials that included people with heart failure, excluding trials that used automated BP measurements, and excluding trials with 5 mm Hg or less within-trial BP difference, confirmed the null effect in primary prevention below 140 mm Hg, the beneficial effect in primary prevention of 160 mm Hg or above, and the reduced risk for MACE in people with previous CHD (eTables 8-11 in the Supplement). Some analyses had limited power, resulting in wide CIs.
Funnel plot asymmetry was found for MACE and stroke in primary preventive trials (eFigures 3-24 in Supplement). Visual inspection of stratified funnel plots suggested asymmetry within the highest SBP stratum, which was also confirmed in separate analyses.
Discussion
This systematic review with stratified meta-analyses shows that the primary preventive effect of BP-lowering treatment on CVD is attenuated with lower baseline SBP. Treatment was associated with reduced a risk for death and MACE if baseline SBP is 140 mm Hg or above, but it lacks effect if baseline SBP is below 140 mm Hg. These results refute the previous view that the relative benefit of treatment is the same across BP levels and that lower is always better. In CHD trials, the effect of baseline SBP on treatment effect could not be analyzed owing to lack of spread in BP values between trials. Mean baseline SBP in these trials was slightly below 140 mm Hg, with positive effects on several cardiovascular outcomes, indicating a possible benefit of lower treatment goals in this specific population.
The results presented herein are consistent with those from a recently published systematic review of BP-lowering treatment in people with diabetes. In the diabetes review, we found an interaction between mean baseline SBP in trials and treatment effect on all-cause mortality, cardiovascular mortality, and myocardial infarction. Also, the results from the recently published Heart Outcomes Prevention Evaluation 3 (HOPE-3) trial point in the same direction. In the HOPE-3 trial, people with intermediate cardiovascular risk were randomized to BP-lowering treatment and/or treatment to lower lipid levels vs placebo in a 2×2 factorial design. Although treatment to lower lipid levels was beneficial regardless of baseline low-density lipoprotein values, the effect of BP lowering significantly interacted with baseline SBP. In the highest SBP tertile, treatment reduced the risk for the primary composite cardiovascular outcome, whereas the lowest SBP tertile tended toward harm.
The results presented herein contrast those of 2 recent meta-analyses. Ettehad and collegues showed that the effect of BP lowering on mortality and cardiovascular events was independent of baseline SBP, with beneficial effects across all SBP strata. These findings were confirmed by Bundy et al in a recent network meta-analysis. The differences between our results and those of Ettehad et al and Bundy et al can be explained because our analyses are more comprehensive. We include 19 additional trials compared with Ettehad et al and 43 additional trials compared with Bundy et al. Adding to this, Bundy et al included heart failure trials and trials in the acute phase after myocardial infarction, in their analyses. Such trials will inevitably bias meta-analyses of BP lowering because treatment acts through different mechanisms in these conditions.
Ettehad et al standardized the effect estimate and the weight in the included trials to an SBP reduction of 10 mm Hg. Standardization of effect estimates assumes that treatment effects on clinical outcomes are associated with SBP reduction in a linear way across all SBP levels. The results of our meta-analyses suggest that this is not the case, with substantial risk reductions at high SBPs but no effect or a small effect at low SBPs. Standardization of study weights distorts the association between number of events and weight, so that large trials with modest BP reductions may be overshadowed by smaller trials with greater BP reduction. For example, the Hypertension Optimal Treatment (HOT) trial, including 18 792 participants, is given 0.6% weight in the all-cause mortality analysis, whereas the European Working Party on High Blood Pressure in the Elderly (EWPHE) trial, including 840 participants, is given 7.3% weight.
Two recent reviews focusing on trials comparing different BP targets have found that more intensive treatment is beneficial compared to less intensive treatment. Further, neither of these reviews have found an interaction between SBP level and treatment effect. Although these analyses are useful to test the concept of BP targets, they lack power to test for interaction between treatment effect and SBP level. For example, Xie et al included 3 trials with baseline SBP below 140 mm Hg in their interaction analysis, compared with 16 trials with similar SBPs in our meta-regression analyses.
At first glance, our results also contrast those of SPRINT. In SPRINT, participants with a mean baseline SBP of 139.7 mm Hg were randomized to an SBP goal below 120 mm Hg compared with below 140 mm Hg. The trial was stopped preterm because the treatment effect on mortality and composite cardiovascular events was dramatic. However, BP in SPRINT was measured using self-operated automated BP measurement devices. This unattended measuring has previously been shown to give SBP values that are 10 to 20 mm Hg lower compared with attended office measurements, meaning that if BP had been measured using the same method in SPRINT as in other trials, it would most likely have appeared in the strata of 140 to 159 mm Hg.
Limitations
This systematic review has 2 major limitations. First, we only had access to study-level data. Therefore, the stratified analyses and the meta-regression analyses are susceptible to ecological bias, with the potential for other intertrial differences, except baseline SBP levels, to affect results. We performed separate analyses for primary preventive trials, CHD trials, poststroke trials, and mixed CVD trials to minimize bias from previous vascular diseases. We also included age, sex, diabetes, and treatment duration as covariates in our meta-regression analyses to account for differences in these potential effect modifiers. None of these factors showed any significant association with treatment effect in separate regression analyses. Another potential source of ecological bias is that trials with high baseline SBP often achieve large BP reductions, whereas trials with lower baseline SBP achieve more modest reductions. The SBP difference between treatment and control was 5.4 mm Hg in the stratum with baseline SBP below 140 mm Hg; 4.6 mm Hg, with baseline SBP of 140 to 159 mm Hg; and 8.6 mm Hg, with baseline SBP of 160 mm Hg or above. The difference in RR for MACE can hardly be accounted for by the small BP difference between the strata below 140 mm Hg (RR, 0.97; 95% CI, 0.90-1.04) and 160 mm Hg or above (RR, 0.78; 95% CI, 0.70-0.87).
The second major limitation is that the classification of trials as primary or secondary preventive is very rough, using a dichotomous approach based on the proportion of participants in each trial with previous CVD. We chose this approach because we wanted to include all trials, not discarding mixed trial populations, and because more detailed stratification would have resulted in dimensionality problems with simultaneous stratification on SBP. Only 13 trials were truly primary preventive, and 17 trials truly secondary preventive, in the sense that none or all of the included patients had previous CVD. This definition leaves 44 trials with mixed populations, 38 of which were distributed continuously from 0 to 50%. Although trials were separated dichotomously, the mean proportion of patients with previous CVD was 16% in the primary preventive group and 92% in the secondary preventive groups combined. We believe that these analyses are sufficient to test the concept of interaction, but recognize that this interaction has to be evaluated further, preferentially using individual patient-data meta-analyses.
The evidence base included in this review also has its limitations. First, most of the trials were fully or partially sponsored by the industry. For CHD trials, none of the included studies were independent. Industry sponsoring is associated with higher rate of favorable outcomes. We cannot exclude industry bias in our analyses, and treatment effects might therefore be exaggerated, especially in CHD trials. Second, women are underrepresented in secondary preventive trials in this review. One-fourth of participants in CHD trials and one-third of participants in poststroke trials were women, compared with one-half of people with prevalent CVD in the United States. Third, the mean age in our analyses was 63.6 years. The prevalence of hypertension increases with age, approaching 80% in people 75 years or older, meaning that for a large portion of patients, the applicability of our results is questionable. Last, we found funnel plot asymmetry for MACE and stroke within the highest SBP category for primary preventive trials. The trials in this stratum are old and small compared with those in other strata. We cannot exclude that other small trials, conducted before the era of trial registration, failed to be published owing to negative results. This situation could in turn lead to an overestimation of the treatment effect on MACE and stroke in the SBP stratum of 160 mm Hg or above.
Conclusions
Treatment to lower BP is associated with a reduced risk for death and MACE if SBP is 140 mm Hg or above. If SBP is below 140 mm Hg, treatment is not associated with any benefit in primary prevention, but may reduce the risk for several cardiovascular outcomes in people with previous CHD. These results do not support lower BP goals in general, but they opt for potentially lower targets in CHD secondary prevention.
eFigure 1. PRISMA Flow Chart
eFigure 2. Meta-Analyses for Mixed Cardiovascular Disease Trials
eFigures 3-24. Funnel Plots
eMethods 1. Search Strategy
eMethods 2. Outcome Definitions
eTable 1. Excluded trials With Reasons for Exclusion
eTable 2. Results From Univariate Metaregression
eTable 3. Results From Multivariate Metaregression
eTable 4. Characteristics of Included Trials
eTables 5 and 6. Risk of Bias
eTable 7. Studies in Included in the Treatment Effect Analyses
eTables 8-11. Sensitivity Analyses
References
- 1.Lim SS, Vos T, Flaxman AD, et al. . A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber MA, Schiffrin EL, White WB, et al. . Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32(1):3-15. [DOI] [PubMed] [Google Scholar]
- 3.Mancia G, Fagard R, Narkiewicz K, et al. ; Task Force Members . 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281-1357. [DOI] [PubMed] [Google Scholar]
- 4.James PA, Oparil S, Carter BL, et al. . 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. [DOI] [PubMed] [Google Scholar]
- 5.Wright JT Jr, Williamson JD, Whelton PK, et al. ; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ettehad D, Emdin CA, Kiran A, et al. . Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957-967. [DOI] [PubMed] [Google Scholar]
- 7.Perkovic V, Rodgers A. Redefining blood-pressure targets–SPRINT starts the marathon. N Engl J Med. 2015;373(22):2175-2178. [DOI] [PubMed] [Google Scholar]
- 8.Laurent S, Boutouyrie P. Blood pressure lowering trials: wrapping up the topic? Lancet. 2016;387(10022):923-924. [DOI] [PubMed] [Google Scholar]
- 9.Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kjeldsen SE, Lund-Johansen P, Nilsson PM, Mancia G. Unattended blood pressure measurements in the systolic blood pressure intervention trial: implications for entry and achieved blood pressure values compared with other trials. Hypertension. 2016;67(5):808-812. [DOI] [PubMed] [Google Scholar]
- 11.Filipovský J, Seidlerová J, Kratochvíl Z, Karnosová P, Hronová M, Mayer O Jr. Automated compared to manual office blood pressure and to home blood pressure in hypertensive patients. Blood Press. 2016;25(4):228-234. [DOI] [PubMed] [Google Scholar]
- 12.Brunström M, Carlberg B. Standardization according to blood pressure lowering in meta-analyses of antihypertensive trials: comparison of three methodological approaches. J Hypertens. doi: 10.1097/HJH.0000000000001574 [DOI] [PubMed] [Google Scholar]
- 13.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension, 4: effects of various classes of antihypertensive drugs–overview and meta-analyses. J Hypertens. 2015;33(2):195-211. [DOI] [PubMed] [Google Scholar]
- 14.McMurray JJV, Adamopoulos S, Anker SD, et al. ; ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology, developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787-1847. [DOI] [PubMed] [Google Scholar]
- 15.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amery A, Birkenhäger W, Brixko P, et al. . Mortality and morbidity results from the European Working Party on High Blood-Pressure in the Elderly trial. Lancet. 1985;1(8442):1349-1354. [DOI] [PubMed] [Google Scholar]
- 18.Black HR, ed. Clinical Trials in Hypertension. New York, NY: Marcel Dekker Inc; 2001. [Google Scholar]
- 19.Poole-Wilson PA, Lubsen J, Kirwan BA, et al. ; Coronary disease Trial Investigating Outcome with Nifedipine gastrointestinal therapeutic system investigators . Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet. 2004;364(9437):849-857. [DOI] [PubMed] [Google Scholar]
- 20.Nissen SE, Tuzcu EM, Libby P, et al. ; CAMELOT Investigators . Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA. 2004;292(18):2217-2225. [DOI] [PubMed] [Google Scholar]
- 21.Fox KM; EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators . Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet. 2003;362(9386):782-788. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G; Heart Outcomes Prevention Evaluation Study Investigators . Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342(3):145-153. [DOI] [PubMed] [Google Scholar]
- 23.Yusuf S, Teo KK, Pogue J, et al. ; ONTARGET Investigators . Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547-1559. [DOI] [PubMed] [Google Scholar]
- 24.Braunwald E, Domanski MJ, Fowler SE, et al. ; PEACE Trial Investigators . Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351(20):2058-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitt B, Byington RP, Furberg CD, et al. ; PREVENT Investigators . Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. Circulation. 2000;102(13):1503-1510. [DOI] [PubMed] [Google Scholar]
- 26.Teo KK, Burton JR, Buller CE, et al. . Long-term effects of cholesterol lowering and angiotensin-converting enzyme inhibition on coronary atherosclerosis: The Simvastatin/Enalapril Coronary Atherosclerosis Trial (SCAT). Circulation. 2000;102(15):1748-1754. [DOI] [PubMed] [Google Scholar]
- 27.Yusuf S, Teo K, Anderson C, et al. ; Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators . Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372(9644):1174-1183. [DOI] [PubMed] [Google Scholar]
- 28.The Dutch TIA Trial Study Group Trial of secondary prevention with atenolol after transient ischemic attack or nondisabling ischemic stroke. Stroke. 1993;24(4):543-548. [DOI] [PubMed] [Google Scholar]
- 29.Hypertension-Stroke Cooperative Study Group Effect of antihypertensive treatment on stroke recurrence. JAMA. 1974;229(4):409-418. [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S, Diener HC, Sacco RL, et al. ; PRoFESS Study Group . Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359(12):1225-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacMahon S, Neal B, Tzourio C, et al. ; PROGRESS Collaborative Group . Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358(9287):1033-1041. [DOI] [PubMed] [Google Scholar]
- 32.Benavente OR, Coffey CS, Conwit R, et al. ; SPS3 Study Group . Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eriksson S, Olofsson BO, Wester PO. Atenolol in secondary prevention after stroke. Cerebrovasc Dis. 1995;5(1):21-25. [Google Scholar]
- 34.Wright JT Jr, Bakris G, Greene T, et al. ; African American Study of Kidney Disease and Hypertension Study Group . Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421-2431. [DOI] [PubMed] [Google Scholar]
- 35.Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care. 2000;23(suppl 2):B54-B64. [PubMed] [Google Scholar]
- 36.Hedblad B, Wikstrand J, Janzon L, Wedel H, Berglund G. Low-dose metoprolol CR/XL and fluvastatin slow progression of carotid intima-media thickness: Main results from the β-Blocker Cholesterol-Lowering Asymptomatic Plaque Study (BCAPS). Circulation. 2001;103(13):1721-1726. [DOI] [PubMed] [Google Scholar]
- 37.MacMahon S, Sharpe N, Gamble G, et al. ; PART-2 Collaborative Research Group . Randomized, placebo-controlled trial of the angiotensin-converting enzyme inhibitor, ramipril, in patients with coronary or other occlusive arterial disease. PART-2 Collaborative Research Group. Prevention of Atherosclerosis with Ramipril. J Am Coll Cardiol. 2000;36(2):438-443. [DOI] [PubMed] [Google Scholar]
- 38.Cornell JE, Mulrow CD, Localio R, et al. . Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med. 2014;160(4):267-270. [DOI] [PubMed] [Google Scholar]
- 39.Sterne JA, Sutton AJ, Ioannidis JP, et al. . Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 40.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443-3457. [DOI] [PubMed] [Google Scholar]
- 42.Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61(3):1086-1097. [DOI] [PubMed] [Google Scholar]
- 43.Cushman WC, Evans GW, Byington RP, et al. ; ACCORD Study Group . Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yusuf S, Healey JS, Pogue J, et al. ; ACTIVE I Investigators . Irbesartan in patients with atrial fibrillation. N Engl J Med. 2011;364(10):928-938. [DOI] [PubMed] [Google Scholar]
- 45.Patel A, MacMahon S, Chalmers J, et al. ; ADVANCE Collaborative Group . Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829-840. [DOI] [PubMed] [Google Scholar]
- 46.Maschio G, Alberti D, Janin G, et al. ; The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group . Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med. 1996;334(15):939-945. [DOI] [PubMed] [Google Scholar]
- 47.Parving HH, Brenner BM, McMurray JJV, et al. ; ALTITUDE Investigators . Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367(23):2204-2213. [DOI] [PubMed] [Google Scholar]
- 48.The Australian therapeutic trial in mild hypertension. Report by the Management Committee. Lancet. 1980;1(8181):1261-1267. [PubMed] [Google Scholar]
- 49.Hansson L. The BBB Study: the effect of intensified antihypertensive treatment on the level of blood pressure, side-effects, morbidity and mortality in "well-treated" hypertensive patients: Behandla Blodtryck Bättre. Blood Press. 1994;3(4):248-254. [DOI] [PubMed] [Google Scholar]
- 50.Ruggenenti P, Fassi A, Ilieva AP, et al. ; Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) Investigators . Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351(19):1941-1951. [DOI] [PubMed] [Google Scholar]
- 51.Ruggenenti P, Fassi A, Ilieva A, et al. ; BENEDICT-B Study Investigators . Effects of verapamil added-on trandolapril therapy in hypertensive type 2 diabetes patients with microalbuminuria: the BENEDICT-B randomized trial. J Hypertens. 2011;29(2):207-216. [DOI] [PubMed] [Google Scholar]
- 52.Verdecchia P, Staessen JA, Angeli F, et al. ; Cardio-Sis investigators . Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374(9689):525-533. [DOI] [PubMed] [Google Scholar]
- 53.Ruggenenti P, Lauria G, Iliev IP, et al. ; DEMAND Study Investigators . Effects of manidipine and delapril in hypertensive patients with type 2 diabetes mellitus: the delapril and manidipine for nephroprotection in diabetes (DEMAND) randomized clinical trial. Hypertension. 2011;58(5):776-783. [DOI] [PubMed] [Google Scholar]
- 54.Marre M, Lievre M, Chatellier G, Mann JF, Passa P, Ménard J; DIABHYCAR Study Investigators . Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: randomised, double blind, placebo controlled trial (the DIABHYCAR study). BMJ. 2004;328(7438):495-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bosch J, Yusuf S, Gerstein HC, et al. ; DREAM Trial Investigators . Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355(15):1551-1562. [DOI] [PubMed] [Google Scholar]
- 56.Liu L, Zhang Y, Liu G, Li W, Zhang X, Zanchetti A; FEVER Study Group . The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens. 2005;23(12):2157-2172. [DOI] [PubMed] [Google Scholar]
- 57.Fogari R, Preti P, Zoppi A, et al. . Effects of amlodipine fosinopril combination on microalbuminuria in hypertensive type 2 diabetic patients. Am J Hypertens. 2002;15(12):1042-1049. [DOI] [PubMed] [Google Scholar]
- 58.Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. BMJ (Clin Res Ed). 1986;293(6555):1145-1151. Clin Res Ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asayama K, Ohkubo T, Metoki H, et al. ; Hypertension Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure (HOMED-BP) . Cardiovascular outcomes in the first trial of antihypertensive therapy guided by self-measured home blood pressure. Hypertens Res. 2012;35(11):1102-1110. [DOI] [PubMed] [Google Scholar]
- 60.Lonn EM, Bosch J, López-Jaramillo P, et al. ; HOPE-3 Investigators . Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374(21):2009-2020. [DOI] [PubMed] [Google Scholar]
- 61.Hansson L, Zanchetti A, Carruthers SG, et al. ; HOT Study Group . Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351(9118):1755-1762. [DOI] [PubMed] [Google Scholar]
- 62.Beckett NS, Peters R, Fletcher AE, et al. ; HYVET Study Group . Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887-1898. [DOI] [PubMed] [Google Scholar]
- 63.Bulpitt CJ, Beckett NS, Cooke J, et al. ; Hypertension in the Very Elderly Trial Working Group . Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens. 2003;21(12):2409-2417. [DOI] [PubMed] [Google Scholar]
- 64.Lewis EJ, Hunsicker LG, Clarke WR, et al. ; Collaborative Study Group . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851-860. [DOI] [PubMed] [Google Scholar]
- 65.The IPPPSH Collaborative Group Cardiovascular risk and risk factors in a randomized trial of treatment based on the beta-blocker oxprenolol: the International Prospective Primary Prevention Study in Hypertension (IPPPSH). J Hypertens. 1985;3(4):379-392. [DOI] [PubMed] [Google Scholar]
- 66.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P; Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group . The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870-878. [DOI] [PubMed] [Google Scholar]
- 67.JATOS Study Group Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31(12):2115-2127. [DOI] [PubMed] [Google Scholar]
- 68.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD; The Collaborative Study Group . The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329(20):1456-1462. [DOI] [PubMed] [Google Scholar]
- 69.Medical Research Council Working Party MRC trial of treatment of mild hypertension: principal results. BMJ (Clin Res Ed). 1985;291(6488):97-104. Clin Res Ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MRC Working Party Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ. 1992;304(6824):405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McMurray JJ, Holman RR, Haffner SM, et al. ; NAVIGATOR Study Group . Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362(16):1477-1490. [DOI] [PubMed] [Google Scholar]
- 72.Imai E, Chan JCN, Ito S, et al. ; ORIENT study investigators . Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia. 2011;54(12):2978-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Helgeland A. Treatment of mild hypertension: a five year controlled drug trial. The Oslo study. Am J Med. 1980;69(5):725-732. [DOI] [PubMed] [Google Scholar]
- 74.Lüders S, Schrader J, Berger J, et al. ; PHARAO Study Group . The PHARAO study: prevention of hypertension with the angiotensin-converting enzyme inhibitor ramipril in patients with high-normal blood pressure: a prospective, randomized, controlled prevention trial of the German Hypertension League. J Hypertens. 2008;26(7):1487-1496. [DOI] [PubMed] [Google Scholar]
- 75.Asselbergs FW, Diercks GF, Hillege HL, et al. ; Prevention of Renal and Vascular Endstage Disease Intervention Trial (PREVEND IT) Investigators . Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110(18):2809-2816. [DOI] [PubMed] [Google Scholar]
- 76.Fuchs SC, Poli-de-Figueiredo CE, Figueiredo Neto JA, et al. . Effectiveness of chlorthalidone plus amiloride for the prevention of hypertension: the PREVER-Prevention randomized clinical trial. J Am Heart Assoc. 2016;5(12):e004248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mauer M, Zinman B, Gardiner R, et al. . Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361(1):40-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ravid M, Brosh D, Levi Z, Bar-Dayan Y, Ravid D, Rachmani R. Use of enalapril to attenuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1998;128(12 Pt 1):982-988. [DOI] [PubMed] [Google Scholar]
- 79.Brenner BM, Cooper ME, de Zeeuw D, et al. ; RENAAL Study Investigators . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861-869. [DOI] [PubMed] [Google Scholar]
- 80.Haller H, Ito S, Izzo JL Jr, et al. ; ROADMAP Trial Investigators . Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364(10):907-917. [DOI] [PubMed] [Google Scholar]
- 81.Lithell H, Hansson L, Skoog I, et al. ; SCOPE Study Group . The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21(5):875-886. [DOI] [PubMed] [Google Scholar]
- 82.SHEP Cooperative Research Group Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265(24):3255-3264. [PubMed] [Google Scholar]
- 83.Perry HM Jr, Smith WM, McDonald RH, et al. . Morbidity and mortality in the Systolic Hypertension in the Elderly Program (SHEP) pilot study. Stroke. 1989;20(1):4-13. [DOI] [PubMed] [Google Scholar]
- 84.Wei Y, Jin Z, Shen G, et al. . Effects of intensive antihypertensive treatment on Chinese hypertensive patients older than 70 years. J Clin Hypertens (Greenwich). 2013;15(6):420-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rouleau JL, Warnica WJ, Baillot R, et al. ; IMAGINE (Ischemia Management with Accupril post-bypass Graft via Inhibition of the coNverting Enzyme) Investigators . Effects of angiotensin-converting enzyme inhibition in low-risk patients early after coronary artery bypass surgery. Circulation. 2008;117(1):24-31. [DOI] [PubMed] [Google Scholar]
- 86.The MACB Study Group Effect of metoprolol on death and cardiac events during a 2-year period after coronary artery bypass grafting. Eur Heart J. 1995;16(12):1825-1832. [PubMed] [Google Scholar]
- 87.Pitt B, O’Neill B, Feldman R, et al. ; QUIET Study Group . The QUinapril Ischemic Event Trial (QUIET): evaluation of chronic ACE inhibitor therapy in patients with ischemic heart disease and preserved left ventricular function. Am J Cardiol. 2001;87(9):1058-1063. [DOI] [PubMed] [Google Scholar]
- 88.Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet. 1991;338(8778):1281-1285. [DOI] [PubMed] [Google Scholar]
- 89.Staessen JA, Fagard R, Thijs L, et al. ; The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators . Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet. 1997;350(9080):757-764. [DOI] [PubMed] [Google Scholar]
- 90.Neaton JD, Grimm RH Jr, Prineas RJ, et al. ; Treatment of Mild Hypertension Study Research Group . Treatment of Mild Hypertension Study. Final results. JAMA. 1993;270(6):713-724. [PubMed] [Google Scholar]
- 91.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703-713. [PMC free article] [PubMed] [Google Scholar]
- 92.Fried LF, Emanuele N, Zhang JH, et al. ; VA NEPHRON-D Investigators . Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369(20):1892-1903. [DOI] [PubMed] [Google Scholar]
- 93.Veterans Administration Cooperative Study Group on Antihypertensive Agents Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213(7):1143-1152. [PubMed] [Google Scholar]
- 94.Ogihara T, Saruta T, Rakugi H, et al. ; Valsartan in Elderly Isolated Systolic Hypertension Study Group . Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56(2):196-202. [DOI] [PubMed] [Google Scholar]
- 95.Yusuf S, Bosch J, Dagenais G, et al. ; HOPE-3 Investigators . Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374(21):2021-2031. [DOI] [PubMed] [Google Scholar]
- 96.Bundy JD, Li C, Stuchlik P, et al. . Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2(7):775-781. doi: 10.1001/jamacardio.2017.1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xie X, Atkins E, Lv J, et al. . Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387(10017):435-443. [DOI] [PubMed] [Google Scholar]
- 98.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension, 7: effects of more vs less intensive blood pressure lowering and different achieved blood pressure levels—updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34(4):613-622. [DOI] [PubMed] [Google Scholar]
- 99.Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. 2017;2(2):MR000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Benjamin EJ, Blaha MJ, Chiuve SE, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. PRISMA Flow Chart
eFigure 2. Meta-Analyses for Mixed Cardiovascular Disease Trials
eFigures 3-24. Funnel Plots
eMethods 1. Search Strategy
eMethods 2. Outcome Definitions
eTable 1. Excluded trials With Reasons for Exclusion
eTable 2. Results From Univariate Metaregression
eTable 3. Results From Multivariate Metaregression
eTable 4. Characteristics of Included Trials
eTables 5 and 6. Risk of Bias
eTable 7. Studies in Included in the Treatment Effect Analyses
eTables 8-11. Sensitivity Analyses