Key Points
Question
Is use of inhaled corticosteroids associated with an increased risk of bone fracture in children with asthma?
Findings
In this population-based nested case-control study, no significant associations between current, recent, or past use of inhaled corticosteroids and first fracture after asthma diagnosis were observed in children with asthma, controlling for age, sex, age at asthma diagnosis, sociodemographic factors, and systemic corticosteroid use.
Meaning
Use of inhaled corticosteroids for the treatment of pediatric asthma should not be limited based on fear of fracture.
Abstract
Importance
Daily use of inhaled corticosteroids is a widely recommended treatment for mild persistent asthma in children. There is concern that, similar to systemic corticosteroids, inhaled corticosteroids may have adverse effects on bone health.
Objective
To determine whether there is an increased risk of bone fracture associated with inhaled corticosteroid use in children with asthma.
Design, Setting, and Participants
In this population-based nested case-control study, we used health administrative databases to identify a cohort of children aged 2 to 18 years with a physician diagnosis of asthma between April 1, 2003, and March 31, 2014, who were eligible for public drug coverage through the Ontario Drug Benefit Program (Ontario, Canada). We matched cases of first fracture after asthma diagnosis to fracture-free controls (ratio of 1 to 4) based on date of birth (within 1 year), sex, and age at asthma diagnosis (within 2 years). We used a 1-year lookback period to ascertain history of inhaled corticosteroid use. Multivariable conditional logistic regression was used to obtain an odds ratio (OR) with 95% confidence interval for fracture, comparing no inhaled corticosteroid use vs current, recent, and past use.
Exposures
Inhaled corticosteroid use during the child’s 1-year lookback period, measured as current user if the prescription was filled less than 90 days prior to the index date, recent user (91-180 days), past user (181-365 days), or no use.
Main Outcomes and Measures
First emergency department visit for fracture after asthma diagnosis, identified using International Statistical Classification of Diseases and Related Health Problems, 10th Revision codes.
Results
This study included 19 420 children (61.0% male; largest proportion of children, 31.5%, were aged 6-9 years at their index date). The multivariable regression results did not show a significant association between first fracture after asthma diagnosis and current use (OR, 1.07; 95% CI, 0.97-1.17), recent use (OR, 0.96; 95% CI, 0.86-1.07), or past use (OR, 1.00; 95% CI, 0.91-1.11) of inhaled corticosteroids, compared with no use, while adjusting for sociodemographic factors and other medication use. However, use of systemic corticosteroids in the 1-year lookback period resulted in greater odds of fracture (OR, 1.17; 95% CI, 1.04-1.33).
Conclusions and Relevance
Systemic corticosteroids, but not inhaled corticosteroids, were significantly associated with increased odds of fracture in the pediatric asthma population.
This population-based, case-control study investigates whether the use of inhaled corticosteroid contributes to bone fractures in children with asthma.
Introduction
Asthma is a common chronic respiratory disease, with onset usually occurring during childhood or adolescence. Inhaled corticosteroids (ICSs) are considered the gold-standard treatment for long-term management of mild persistent asthma in children. Inhaled corticosteroids are recommended as a daily treatment, administered through an inhaler, and prescribed at the lowest effective dose to reduce the risk of adverse effects. Research suggests that ICSs may have adverse effects on skeletal growth and development. Corticosteroids directly interfere with osteoclast and osteoblast functioning, resulting in decreased bone formation, increased resorption, and bone calcium loss. This can predispose corticosteroid users to osteoporosis, increasing their risk of bone fracture. Bone health is particularly important during childhood, leaving children with asthma a potentially vulnerable population for fractures. Concerns about the possible adverse effects of ICSs may also result in poor medication adherence, leading to more frequent and severe asthma exacerbations.
Few studies have quantified the association between ICS use and fracture risk in children with asthma. A systematic review and meta-analysis reported a lack of convincing evidence for an association; however, the authors were only able to pool the results of 2 studies, which had inconsistent results. A study in the United Kingdom in 2004 compared fracture risk in children receiving different types of corticosteroids and found a greater risk of fracture in the ICS group, compared with the nonsystemic corticosteroid group (topical, nasal, or ophthalmic corticosteroids). Conversely, another United Kingdom study in 2004 did not find a significant association between fractures and current use of ICSs in children. Studies on the association between ICS use and reduced bone mineral density, a risk factor for fracture, also present conflicting results. Clinical trials showed that regular ICS use resulted in decreased bone mineral density growth after 12 to 18 months, while observational studies showed ICS-associated bone mineral density loss in males only or showed no significant association.
Thus, further population-based research is needed to determine if there is an increased risk of fracture with ICS use in children with asthma to provide clarity about the safety of these widely used medications. The primary objective of this study was to determine the association between ICS use and first fracture after asthma diagnosis in children. This was examined in a population of 19 420 children with asthma who were eligible for public drug coverage through the Ontario Drug Benefit Program in Ontario, Canada.
Methods
Study Design and Population
The association between ICS use and fracture risk was investigated using a nested case-control design, with a 1-year lookback period for exposure status. The study cohort, which formed the population for case and control selection, included Ontario children aged 2 to 18 years with a physician diagnosis of asthma between April 1, 2003, and March 31, 2014. In addition, to be eligible for the study, children had to fill at least 1 prescription through a publicly funded drug plan during their 1-year lookback period. Public drug coverage is available in Ontario through the Ontario Drug Benefit Program for those aged 65 years and older and those registered in social assistance programs (Trillium Benefit, Ontario Works, and Ontario Disability Support Program), along with their dependents. Thus, most eligible children were from low-income families or had high drug costs relative to their family income. Children were excluded from the cohort if they were ever diagnosed with cancer or diabetes mellitus or had ever had an organ transplant. Children were also excluded if they did not have data on age, an Ontario residence code, or a valid Ontario health card number.
Cases were defined as children aged 2 to 18 years who had a bone fracture after asthma diagnosis between April 1, 2003, and March 31, 2015. Cases were matched to controls in a 1:4 ratio, based on year of birth (within 2 years), sex, and age at asthma diagnosis (within 1 year). The index date for the 1-year lookback period was the date of fracture for cases. Controls were given the same index date as their matched case’s date of fracture. Controls were all fracture free since asthma diagnosis at the time of matching to cases.
Ethics approval was obtained from the Hospital for Sick Children Research Ethics Board. Individual consent was not required for this study, as only deidentified health administrative records were used. Under Ontario’s Personal Health Information Protection Act, the Institute for Clinical Evaluative Sciences has access to health administrative data for research purposes under strict privacy guidelines.
Data Sources
This study used routinely collected health administrative data for Ontario. In Ontario, there is a publicly funded single-payer health care system. Health administrative data were linked using unique encoded identifiers at the Institute for Clinical Evaluative Sciences. Data on emergency department visits were captured through the National Ambulatory Care Reporting System and coded using the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). Data on prescriptions filled were captured through the Ontario Drug Benefit database and specific drugs were identified using their unique Drug Identification Number. Data on patient characteristics, such as age, sex, residence postal code, income, and date of death (if applicable), were captured through the Provincial Registered Persons Database.
Date of asthma diagnosis was captured through the Ontario Asthma Surveillance Information System. The Ontario Asthma Surveillance Information System contains a cohort of Ontario residents aged 0 to 99 years with a physician diagnosis of asthma between April 1, 1996, and March 31, 2014, based on the administrative case definition at least 1 hospitalization for asthma ever or 2 or more outpatient visits for asthma in 2 consecutive years. This definition has been validated in Ontario children, with a sensitivity of 91.4% and a specificity of 82.9%. Children with cancer or diabetes were identified using the Ontario Cancer Registry and the Ontario Diabetes Database, respectively. Children with a history of organ transplant were identified by their health care utilization for organ transplant in health administrative databases using ICD-10 codes (eTable in the Supplement).
Exposure and Outcome Definitions
The primary outcome was fracture, defined as the first emergency department visit for fracture after asthma diagnosis, identified using ICD-10 codes (eTable in the Supplement). Any type of bone fracture was considered as an outcome. For the primary exposure, cases and controls were defined as current users if they received a prescription for an ICS medication 0 to 90 days prior to their index date, recent users if they received a prescription 91 to 180 days prior to their index date, past users if they received a prescription 181 to 365 days prior to their index date, or no use if they had no record of any ICS prescriptions during the year prior to their index date. Secondarily, ICS use was also examined as a binary variable (yes or no), as the number of ICS prescriptions filled in the year prior to their index date (0, 1, 2, or ≥3 prescriptions), and as the estimated daily dose of the most recent fluticasone propionate prescription (or equivalent dose) filled in the year prior to their index date (none, low, moderate, or high dose).
Covariates
Covariates hypothesized to confound the association of interest were derived from literature review. Socioeconomic status was measured by proxy, using the Ontario Marginalization Index. The Ontario Marginalization Index provided a measure of marginalization at the population level based on census information using 4 dimensions: material deprivation, residential instability, dependency, and ethnic concentration. Based on each participant’s residence postal code, he or she was assigned a score from 1 (least marginalized) to 5 (most marginalized) for each dimension. Residence was considered rural if the individual resided in a community with a population of 10 000 people or fewer, or urban if the opposite was true. Geographical differences in health care delivery were captured based on participants’ residence in one of the 14 Local Health Integration Networks in Ontario. Individuals’ income was measured by proxy using neighborhood-level income in quintiles. Each income quintile represented the distribution of income based on census dissemination area, with people in the lowest 20% falling into quintile 1, and the highest 20% falling into quintile 5. Systemic corticosteroid use (oral or intramuscular) was measured as the number of prescriptions filled during the lookback period (0, 1, or ≥2 prescriptions), and the estimated daily dose of the most recent prednisone prescription (or equivalent dose) filled during the lookback period (none, low, moderate, or high dose). A “missing” category was included for any variable missing data for more than 5 individuals; otherwise, participants with missing data were excluded.
Statistical Analysis
Statistical differences in baseline characteristics between cases and controls were examined using t tests for continuous variables and χ2 tests for categorical variables. A 2-tailed P < .05 was considered statistically significant for all analyses. Conditional logistic regression was used in univariable and multivariable analyses to account for the matched design. Conditional logistic regression takes each 1:4 case-control match as a stratum and produces regression coefficients for the independent variables that vary within the stratum. The unadjusted and adjusted odds ratios (ORs) for first fracture after asthma diagnosis based on ICS medication use during the lookback period served as the primary measures of effect. A forward model building approach was used to construct the multivariable model. Models were further stratified by sex and age group as subgroup analyses, decided a priori based on biological rationale. Forest plots were used to compare adjusted ORs with 95% confidence intervals across the population subgroups. All statistical analyses were conducted using SAS software version 9.3 (SAS Institute Inc), and forest plots were generated using the forestplot package in R statistical computing software version 3.3.3 (R Foundation).
Results
There were 391 641 children aged 2 to 18 years with a diagnosis of asthma between April 1, 2003, and March 31, 2014 (eFigure in the Supplement). Of these, 39 594 children had their first episode of fracture after asthma diagnosis between April 1, 2003, and March 31, 2015. After excluding children with chronic conditions, missing data, and ineligibility for public drug coverage during the 1-year lookback period, 3884 cases were eligible to be matched to controls. After applying the same exclusion criteria to controls, 4 controls from a pool of the remaining 351 941 children were randomly assigned to each case, matching on the aforementioned criteria. This resulted in a control sample size of 15 536 children and a total sample size of 19 420 children.
The population was 61.0% male, and approximately 67.4% met the definition for physician-diagnosed asthma when they were aged 5 years or younger (Table 1). The majority of the population (approximately 61.0%) had their first fracture after asthma diagnosis when they were younger than 10 years. Cases and controls were both largely from areas of the lowest income quintile (1728 cases [44.5%] and 7133 controls [45.9%]) and high marginalization index. More cases (333 [8.6%]) resided in rural locations than controls (1086 [7.0%]). A high proportion of children in the study were nonusers of ICS in the 1-year lookback period (1894 cases [48.8%] and 7643 controls [49.2%]). A total of 496 cases (12.8%) used systemic corticosteroids in the 1-year lookback period, compared with 1705 controls (11.0%).
Table 1. Characteristics of the Study Population.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| Cases (n = 3884) |
Controls (n = 15 536) |
||
| Demographic | |||
| Sex | |||
| Male | 2368 (61.0) | 9472 (61.0) | >.99 |
| Female | 1516 (39.0) | 6064 (39.0) | |
| Age at asthma diagnosis, y | |||
| 0-5 | 2596 (66.8) | 10 492 (67.5) | .69 |
| 6-12 | 1060 (27.3) | 4167 (26.8) | |
| 13-18 | 228 (5.9) | 877 (5.6) | |
| Age at index date, y | |||
| 2-5 | 1157 (29.8) | 4690 (30.2) | .95 |
| 6-9 | 1224 (31.5) | 4896 (31.5) | |
| 10-13 | 1031 (26.5) | 4018 (25.9) | |
| 14-18 | 472 (12.2) | 1932 (12.4) | |
| Income quintile | |||
| 1, Lowest | 1728 (44.5) | 7133 (45.9) | .68 |
| 2 | 863 (22.2) | 3366 (21.7) | |
| 3 | 585 (15.1) | 2318 (14.9) | |
| 4 | 433 (11.2) | 1649 (10.6) | |
| 5, Highest | 266 (6.9) | 1041 (6.7) | |
| Missing | 9 (0.2) | 29 (0.2) | |
| Rural | |||
| Yes | 333 (8.6) | 1086 (7.0) | <.001 |
| No | 3551 (91.4) | 14 450 (93.0) | |
| Ontario Marginalization Index | |||
| Deprivation quintile | |||
| 1, Lowest | 312 (8.0) | 1266 (8.2) | .46 |
| 2 | 376 (9.7) | 1594 (10.3) | |
| 3 | 582 (15.0) | 2159 (13.9) | |
| 4 | 761 (19.6) | 2964 (19.1) | |
| 5, Highest | 1822 (46.9) | 7423 (47.8) | |
| Missing | 31 (0.8) | 130 (0.8) | |
| Dependency quintile | |||
| 1, Lowest | 1118 (28.8) | 4855 (31.3) | .07 |
| 2 | 894 (23.0) | 3512 (22.6) | |
| 3 | 701 (18.1) | 2739 (17.6) | |
| 4 | 595 (15.3) | 2290 (14.7) | |
| 5, Highest | 545 (14.0) | 2010 (12.9) | |
| Missing | 31 (0.8) | 130 (0.8) | |
| Ethnic concentration quintile | |||
| 1, Lowest | 410 (10.6) | 1423 (9.2) | <.001 |
| 2 | 423 (10.9) | 1501 (9.2) | |
| 3 | 566 (14.6) | 1837 (11.8) | |
| 4 | 789 (20.3) | 2905 (18.7) | |
| 5, Highest | 1665 (42.9) | 7740 (49.8) | |
| Missing | 31 (0.8) | 130 (0.8) | |
| Residential instability quintile | |||
| 1, Lowest | 386 (9.9) | 1747 (11.2) | .22 |
| 2 | 475 (12.2) | 1910 (12.3) | |
| 3 | 627 (16.1) | 2371 (15.3) | |
| 4 | 1023 (26.3) | 3993 (25.7) | |
| 5, Highest | 1342 (34.6) | 5385 (34.7) | |
| Missing | 31 (0.8) | 130 (0.8) | |
| Medication Use | |||
| ICSs | |||
| Current, <90 d from index date | 830 (21.4) | 3137 (20.2) | .35 |
| Recent, 91-180 d from index date | 483 (12.4) | 2033 (13.1) | |
| Past, 181-365 d from index date | 677 (17.4) | 2723 (17.5) | |
| None | 1894 (48.8) | 7643 (49.2) | |
| No. of ICS prescriptions | |||
| 0 | 1894 (48.8) | 7643 (49.2) | .47 |
| 1 | 982 (25.3) | 3987 (25.7) | |
| 2 | 432 (11.1) | 1755 (11.3) | |
| ≥3 | 576 (14.8) | 2151 (13.8) | |
| No. of systemic corticosteroid prescriptions | |||
| 0 | 3388 (87.2) | 13 831 (89.0) | .07 |
| 1 | 366 (9.4) | 1261 (8.1) | |
| ≥2 | 130 (3.4) | 444 (2.9) | |
| Fluticasone propionate or equivalent daily dose | |||
| None | 1894 (48.8) | 7643 (49.2) | .82 |
| Low, ≤250 µg | 568 (14.6) | 2209 (14.2) | |
| Moderate, >250 to 500 µg | 810 (20.9) | 3236 (20.8) | |
| High, >500 µg | 588 (15.1) | 2358 (15.2) | |
| Missinga | 24 (0.6) | 90 (0.6) | |
| Prednisone or equivalent daily dose | |||
| None | 3388 (87.2) | 13 831 (89.0) | .006 |
| Low, ≤10 mg | 74 (1.9) | 257 (1.7) | |
| Moderate, >10 to ≤30 mg | 301 (7.8) | 1102 (7.1) | |
| High, >30 mg | 114 (2.9) | 328 (2.1) | |
| Missinga | 7 (0.2) | 18 (0.1) | |
| Total No. of prescriptions, mean (SD) | 7.8 (12.0) | 7.0 (11.3) | <.001 |
Abbreviation: ICS, inhaled corticosteroid.
Daily dose values over the 99th percentile (data entry errors) or with unknown equivalency were set to missing.
In the multivariable conditional logistic regression analyses, current use (OR, 1.07; 95% CI, 0.97-1.17), recent use (OR, 0.96; 95% CI, 0.86-1.07), and past use (OR, 1.00; 95% CI, 0.91-1.11) of ICSs were not significantly associated with the odds of fracture, compared with no use (Table 2). These results did not change when examining ICS use as a binary variable, as the number of ICS prescriptions filled during the lookback period, or as the estimated daily dose of the most recent prescription filled during the lookback period. Models were adjusted for Ontario Marginalization Index (all domains), Local Health Integration Networks, rurality, and systemic corticosteroid use. The multivariable results showed that filling 1 prescription for a systemic corticosteroid was associated with a 17% increase in odds of fracture (OR, 1.17; 95% CI, 1.04-1.33), compared with never filling a prescription. Filling 2 or more prescriptions for systemic corticosteroids was not associated with significantly greater odds of fracture, although there were few children who filled more than 1 prescription (130 cases [3.4%] and 444 controls [2.9%]). The highest category of estimated prednisone daily dose or equivalent was associated with a 41% increase in odds of fracture (OR, 1.41; 95% CI, 1.14-1.76), compared with no use, while lower doses were not significantly associated.
Table 2. Unadjusted and Adjusted Results of Conditional Logistic Regression (N = 19 420).
| Characteristics | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI)a | P Value | |
| Exposure | ||||
| ICS use | ||||
| None | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Current, <90 d from index date | 1.07 (0.97-1.17) | .16 | 1.07 (0.97-1.17) | .20 |
| Recent, 91-180 d from index date | 0.96 (0.86-1.07) | .47 | 0.96 (0.86-1.08) | .53 |
| Past, 181-365 d from index date | 1.00 (0.91-1.11) | .94 | 1.01 (0.91-1.12) | .86 |
| No. of ICS prescriptions | ||||
| 0 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 1 | 1.00 (0.91-1.09) | .91 | 1.01 (0.92-1.10) | .91 |
| 2 | 0.99 (0.88-1.12) | .92 | 0.99 (0.88-1.11) | .82 |
| ≥3 | 1.08 (0.97-1.20) | .15 | 1.07 (0.96-1.20) | .21 |
| Ever used ICSs | ||||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 1.02 (0.95-1.09) | .62 | 1.02 (0.95-1.10) | .63 |
| Fluticasone propionate or equivalent daily doseb | ||||
| None | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Low, ≤250 µg | 1.04 (0.93-1.17) | .48 | 1.07 (0.96-1.19) | .26 |
| Moderate, >250 to ≤500 µg | 1.01 (0.92-1.11) | .81 | 1.00 (0.91-1.10) | .94 |
| High, >500 µg | 1.01 (0.91-1.12) | .90 | 1.00 (0.90-1.11) | .99 |
| Covariate | ||||
| No. of systemic corticosteroid prescriptions | ||||
| 0 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 1 | 1.20 (1.05-1.35) | .006 | 1.17 (1.04-1.33) | .01 |
| ≥2 | 1.21 (0.99-1.48) | .07 | 1.19 (0.97-1.46) | .10 |
| Prednisone or equivalent daily doseb | ||||
| None | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Low, ≤10 mg | 1.19 (0.91-1.55) | .21 | 1.16 (0.88-1.51) | .29 |
| Moderate, >10 to ≤30 mg | 1.12 (0.98-1.28) | .10 | 1.12 (0.97-1.28) | .12 |
| High, >30 mg | 1.42 (1.14-1.76) | .002 | 1.41 (1.14-1.76) | .002 |
Abbreviations: ICS, inhaled corticosteroid; OR, odds ratio.
Adjusted for rurality, Ontario Marginalization Index, Local Health Integration Networks, and number of systemic corticosteroid prescriptions (or daily dose of systemic corticosteroids, when exposure was daily dose of ICS).
Estimates for missing category not shown (none significantly associated with outcome).
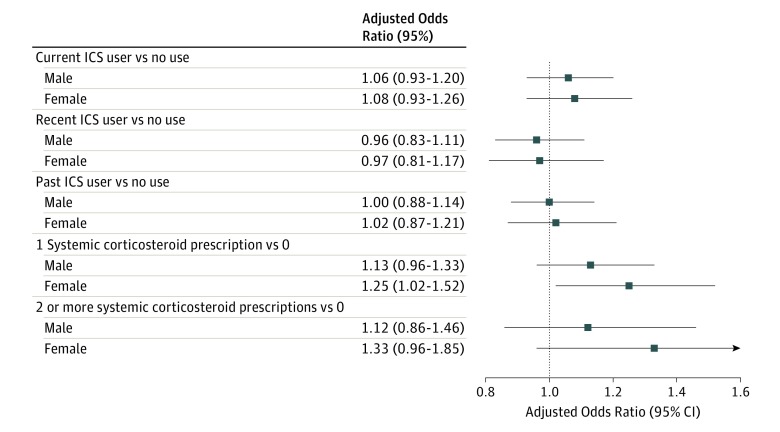
No significant associations were observed between ICS use and odds of fracture within male and female subpopulations (Figure 1), or within age-at-index-date subgroups (Figure 2). In girls, filling 1 prescription for a systemic corticosteroid was associated with 25% greater odds of fracture (OR, 1.25; 95% CI, 1.02-1.52), compared with no prescription. This effect was smaller in magnitude in boys and was not statistically significant. Filling 1 systemic corticosteroid prescription, compared with none, was associated with a 66% increase in odds of fracture in children aged 10 to 13 years (OR, 1.66; 95% CI, 1.26-2.20). Filling 2 or more systemic corticosteroid prescriptions (not shown) was not associated with any statistically significant effects in any age groups.
Figure 1. Adjusted Odds Ratios for Fracture From Conditional Logistic Regression for Boys and Girls.
Models were adjusted for rurality, Ontario Marginalization Index, Local Health Integration Networks, and number of systemic corticosteroid prescriptions. Data are shown for boys (n = 11 840) and girls (n = 7580). ICS indicates inhaled corticosteroid.
Figure 2. Adjusted Odds Ratios for Fracture From Conditional Logistic Regression.
Models were adjusted for rurality, Ontario Marginalization Index, Local Health Integration Networks, and number of systemic corticosteroid prescriptions. Data are shown for participants aged 2 to 5 years (n = 5847), 6 to 9 years (n = 6120), 10 to 13 years (n = 5049), and 14 to 18 years (n = 2404) at the index date. ICS indicates inhaled corticosteroid.
Discussion
This population-based study of children with asthma did not show statistically significant associations between cases of first fracture after asthma diagnosis and use of ICSs. Odds of fracture were not statistically increased for children receiving ICSs near the time of fracture, for children with frequent ICS use in the 1-year lookback period (≥3 prescriptions), or for children with a higher estimated ICS daily dose, compared with children with no recorded ICS use. However, odds of fracture were significantly higher for children who filled 1 prescription for a systemic corticosteroid in the 1-year lookback period compared with those who did not. There was also evidence of a potential dose effect of systemic corticosteroids, as only those children in the highest dose category had increased odds of fracture compared with no use.
These results are fairly consistent with the limited published pediatric literature. Schlienger et al reported no significant association between first episode of fracture and current ICS use among children aged 5 to 17 years using a similar nested case-control design. When incorporating systemic corticosteroid use, they reported an OR close to significance (OR, 1.21; 95% CI, 0.99-1.49). Similarly, a small prospective cohort study of children with asthma aged 5 to 12 years at baseline found no association between inhaled or oral corticosteroid use and fracture. However, that study was limited by the small number of fractures within their population during the study period.
The largest prospective study to date on ICSs and fracture in children aged 4 to 17 years found an 18% increased risk of fracture for children who received ICSs compared with those who used other nonsystemic corticosteroids. The authors attributed this effect primarily to severity of disease, as opposed to medication use, because the same association was seen in children who used bronchodilators, and the dose-response effects of ICSs disappeared after adjustment for disease severity. The authors hypothesized that greater disease severity would reduce a child’s physical activity level, decreasing bone strength and predisposing a child for fracture. However, they were unable to determine this from their study data alone.
In the current study, use of systemic corticosteroids could also be seen as a measure of asthma severity. We excluded some conditions that might require use of systemic corticosteroids (cancer and organ transplant); thus, a large proportion of the systemic corticosteroid use in our population is likely attributable to asthma exacerbations. Therefore, some of the association between systemic corticosteroids and fracture may be attributed to more severe asthma. However, there is also the strong potential that systemic corticosteroids significantly increase the risk of fracture independent of disease severity, as these medications have a much higher circulating bioavailability of corticosteroids than ICSs. There is a moderate body of evidence from adult studies that links high doses of systemic corticosteroids to an increased risk of bone fracture. There is limited research on this topic in pediatric populations; however, one other study has demonstrated an increased risk of fracture with oral corticosteroid use.
Our results have further implications when considering the low use of ICSs in our study population of children with asthma. Almost half the children failed to fill a single ICS prescription during the 1-year lookback period, despite having prior physician-diagnosed asthma. We used an objective measure of pharmacy claims to measure ICS use, which research has indicated is more accurate than self-reports or parental reports of ICS use. Other studies using objective measures are consistent with our finding, with average ICS adherence ranging from 51% to 70% among children with asthma. Low adherence to ICSs may lead to poorly controlled asthma, eventually resulting in asthma attacks. Severe asthma attacks may require short-term use of systemic corticosteroids, which are associated with adverse effects.
Limitations
There are several limitations to this study owing to the nature of the databases used. First, we were unable to assess true medication use from pharmacy claims data alone; thus, estimates of daily dose may not correspond to exact doses received by children. Furthermore, because public drug coverage for individuals younger than 65 years is only available through social assistance programs in Ontario, our study population was restricted to those who were eligible for these programs and their dependents. This may limit the generalizability of the findings, especially to populations of moderate to high socioeconomic status because our population was generally of low socioeconomic status. Despite this, there is strong biological rationale for the association between corticosteroids and fracture, and a lack of evidence that different socioeconomic subgroups would experience different effects. Using health administrative data also did not allow for the measurement of individual-level risk factors, such as nutrition (vitamin D and calcium levels), physical activity, and disease severity. However, it allowed for complete participant follow-up, a large sample size, and high power, which was needed to detect an effect on a rare outcome, such as fracture.
Conclusions
This population-based study demonstrated no clinically important association between ICSs and fracture among children with asthma, but did find a significantly increased risk of fracture associated with systemic corticosteroid use. This effect was even larger when analyzing girls only. Clinicians using ICSs to optimize the control of childhood asthma should be reassured by the lack of association with fractures; fear of fracture is not a reason to limit the therapeutic use of ICSs. Furthermore, asthma control with ICSs might decrease the likelihood of asthma exacerbations requiring systemic corticosteroid use, so wider appropriate use of ICSs may potentially lead to a reduced fracture risk. Future research should investigate how severity of asthma may play a role in the risk of fracture, as some of the increased risk associated with systemic corticosteroids may be due to the underlying illness itself.
eTable. ICD-10 Codes Used to Define Fracture, Asthma and Organ Transplant
eFigure. Study Flow Diagram Showing Case and Control Selection From Original Study Cohort
References
- 1.Ismaila AS, Sayani AP, Marin M, Su Z. Clinical, economic, and humanistic burden of asthma in Canada: a systematic review. BMC Pulm Med. 2013;13(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousquet J, Mantzouranis E, Cruz AA, et al. . Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization consultation on severe asthma. J Allergy Clin Immunol. 2010;126(5):926-938. [DOI] [PubMed] [Google Scholar]
- 3.Lougheed MD, Lemiere C, Ducharme FM, et al. ; Canadian Thoracic Society Asthma Clinical Assembly . Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can Respir J. 2012;19(2):127-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loke YK, Gilbert D, Thavarajah M, Blanco P, Wilson AM. Bone mineral density and fracture risk with long-term use of inhaled corticosteroids in patients with asthma: systematic review and meta-analysis. BMJ Open. 2015;5(11):e008554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly HW, Van Natta ML, Covar RA, Tonascia J, Green RP, Strunk RC; CAMP Research Group . Effect of long-term corticosteroid use on bone mineral density in children: a prospective longitudinal assessment in the childhood asthma management program (CAMP) study. Pediatrics. 2008;122(1):e53-e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melton LJ III, Patel A, Achenbach SJ, Oberg AL, Yunginger JW. Long-term fracture risk among children with asthma: a population-based study. J Bone Miner Res. 2005;20(4):564-570. [DOI] [PubMed] [Google Scholar]
- 7.van Staa T-P, Bishop N, Leufkens HG, Cooper C. Are inhaled corticosteroids associated with an increased risk of fracture in children? Osteoporos Int. 2004;15(10):785-791. [DOI] [PubMed] [Google Scholar]
- 8.Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100(8):1307-1317. [DOI] [PubMed] [Google Scholar]
- 9.Greer FR, Krebs NF; American Academy of Pediatrics Committee on Nutrition . Optimizing bone health and calcium intakes of infants, children, and adolescents. Pediatrics. 2006;117(2):578-585. [DOI] [PubMed] [Google Scholar]
- 10.Schlienger RG, Jick SS, Meier CR. Inhaled corticosteroids and the risk of fractures in children and adolescents. Pediatrics. 2004;114(2):469-473. [DOI] [PubMed] [Google Scholar]
- 11.Sidoroff VH, Ylinen MK, Kröger LM, Kröger HP, Korppi MO. Inhaled corticosteroids and bone mineral density at school age: a follow-up study after early childhood wheezing. Pediatr Pulmonol. 2015;50(1):1-7. [DOI] [PubMed] [Google Scholar]
- 12.Jung J-W, Kang H-R, Kim J-Y, Lee S-H, Kim SS, Cho SH. Are asthmatic patients prone to bone loss? Ann Allergy Asthma Immunol. 2014;112(5):426-431. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson AC, Van Bever HP, Teper AM, Lasytsya O, Goldfrad CH, Whitehead PJ. A comparison of the relative growth velocities with budesonide and fluticasone propionate in children with asthma. Respir Med. 2007;101(1):118-129. [DOI] [PubMed] [Google Scholar]
- 14.Turpeinen M, Pelkonen AS, Nikander K, et al. . Bone mineral density in children treated with daily or periodical inhaled budesonide: the Helsinki Early Intervention Childhood Asthma Study. Pediatr Res. 2010;68(2):169-173. [DOI] [PubMed] [Google Scholar]
- 15.Agertoft L, Pedersen S. Bone mineral density in children with asthma receiving long-term treatment with inhaled budesonide. Am J Respir Crit Care Med. 1998;157(1):178-183. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization International Statistical Classification of Diseases, Tenth Revision (ICD-10). Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 17.To T, Dell S, Dick PT, et al. . Case verification of children with asthma in Ontario. Pediatr Allergy Immunol. 2006;17(1):69-76. [DOI] [PubMed] [Google Scholar]
- 18.Hossny E, Rosario N, Lee BW, et al. . The use of inhaled corticosteroids in pediatric asthma: update. World Allergy Organ J. 2016;9(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortiz-Alvarez O, Mikrogianakis A; Canadian Paediatric Society, Acute Care Committee . Managing the paediatric patient with an acute asthma exacerbation. Paediatr Child Health. 2012;17(5):251-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matheson FI, Dunn JR, Smith KL, Moineddin R, Glazier RH. Development of the Canadian marginalization index: a new tool for the study of inequality. Can J Public Health. 2012;103(8)(suppl 2):12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Statistics Canada Health Indicators. Ottawa, ON: Statistics Canada; 2016. [Google Scholar]
- 22.Liu D, Ahmet A, Ward L, et al. . A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosmer DW, Lemeshow S. Logistic regression for matched case-control studies In: Applied Logistic Regression. 2nd ed Hoboken, NJ: John Wiley & Sons; 2004:223-259. [Google Scholar]
- 24.Hosmer DW Jr, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed Hoboken, NJ: Vol 398 John Wiley & Sons; 2013. [Google Scholar]
- 25.Escott B. Childhood Fracture Begets Childhood Fracture: A Population-Based Study of Longitudinal Fracture Patterns in Ontario Children. Toronto, ON: Institute for Health Policy and Management, University of Toronto; 2012. [Google Scholar]
- 26.forestplot: Advanced Forest Plot Using 'grid' Graphics [computer program]. Version 1.7. https://CRAN.R-project.org/package=forestplot. Accessed April 17, 2017.
- 27.Hansen KE, Kleker B, Safdar N, Bartels CM. A systematic review and meta-analysis of glucocorticoid-induced osteoporosis in children. Semin Arthritis Rheum. 2014; 44 (1): 47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanis JA, Johansson H, Oden A, et al. . A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19(6):893-899. [DOI] [PubMed] [Google Scholar]
- 29.van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford). 2000;39(12):1383-1389. [DOI] [PubMed] [Google Scholar]
- 30.Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15(6):993-1000. [DOI] [PubMed] [Google Scholar]
- 31.Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15(4):323-328. [DOI] [PubMed] [Google Scholar]
- 32.Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186. [DOI] [PubMed] [Google Scholar]
- 33.van Staa TP, Cooper C, Leufkens HG, Bishop N. Children and the risk of fractures caused by oral corticosteroids. J Bone Miner Res. 2003;18(5):913-918. [DOI] [PubMed] [Google Scholar]
- 34.Krishnan JA, Bender BG, Wamboldt FS, et al. ; Adherence Ancillary Study Group . Adherence to inhaled corticosteroids: an ancillary study of the Childhood Asthma Management Program clinical trial. J Allergy Clin Immunol. 2012;129(1):112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Dellen QM, Stronks K, Bindels PJ, Öry FG, van Aalderen WM; PEACE Study Group . Adherence to inhaled corticosteroids in children with asthma and their parents. Respir Med. 2008;102(5):755-763. [DOI] [PubMed] [Google Scholar]
- 36.Bender B, Wamboldt FS, O’Connor SL, et al. . Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol. 2000;85(5):416-421. [DOI] [PubMed] [Google Scholar]
- 37.Carter ER, Ananthakrishnan M. Adherence to montelukast versus inhaled corticosteroids in children with asthma. Pediatr Pulmonol. 2003;36(4):301-304. [DOI] [PubMed] [Google Scholar]
- 38.Ducharme FM, Noya FJ, Allen-Ramey FC, Maiese EM, Gingras J, Blais L. Clinical effectiveness of inhaled corticosteroids versus montelukast in children with asthma: prescription patterns and patient adherence as key factors. Curr Med Res Opin. 2012;28(1):111-119. [DOI] [PubMed] [Google Scholar]
- 39.Schuh S, Reisman J, Alshehri M, et al. . A comparison of inhaled fluticasone and oral prednisone for children with severe acute asthma. N Engl J Med. 2000;343(10):689-694. [DOI] [PubMed] [Google Scholar]
- 40.Clark EM, Ness AR, Tobias JH. Vigorous physical activity increases fracture risk in children irrespective of bone mass: a prospective study of the independent risk factors for fractures in healthy children. J Bone Miner Res. 2008;23(7):1012-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. ICD-10 Codes Used to Define Fracture, Asthma and Organ Transplant
eFigure. Study Flow Diagram Showing Case and Control Selection From Original Study Cohort