Key Points
Question
Can the association between LPA variants and aortic stenosis be detected using electronic health record–derived data?
Findings
In this case-control study, individuals with 2 LPA risk alleles in any combination had 2-fold or greater odds of developing aortic stenosis compared with individuals with no risk alleles. In addition, age significantly modified the association of the LPA variant rs10455872 with aortic stenosis.
Meaning
Variation at the LPA locus increases the odds of aortic stenosis, with 2-fold or greater odds in individuals with the highest genetic risk, but this association may be modified by risk factors.
Abstract
Importance
Elevated lipoprotein(a) levels are a risk factor for aortic stenosis (AS). However, a large-scale replication of associations between LPA variants and AS, their interactions with risk factors, and the effect of multiple risk alleles is not well established.
Objective
To replicate the association between LPA variants with AS and identify subgroups who are at higher risk of developing AS.
Design, Setting, and Participants
This case-control study of AS included 44 703 individuals (3469 cases) 55 years or older who were enrolled in the Genetic Epidemiology Research on Aging cohort and who were members of the Kaiser Permanente Northern California health care delivery system. The study leveraged the linkage of administrative health data, electronic medical records, genotypes, and self-reported questionnaire data. The 3469 AS cases were diagnosed between January 1996 and December 2015. Individuals with valvular congenital valvular heart disease were excluded.
Exposures
Two single-nucleotide polymorphisms in the LPA locus, rs10455872 and rs3798220, that are known to associate with circulating plasma lipoprotein(a) levels and an LPA risk score.
Main Outcomes and Measures
Aortic stenosis or aortic valve replacement.
Results
The 44 703 participants were of European ancestry, of whom 22 019 (49.3%) were men. The mean (SD) age for the control group was 69.3 (8.3) years and the mean (SD) age for AS cases was 74.6 (8.5) years. Both LPA variants were associated with AS, with a per risk allele odds ratio of 1.34 (95% CI, 1.23-1.47; P = 1.7 × 10−10) for rs10455872 and 1.31 (95% CI, 1.09-1.58; P = 3.6 × 10−3) for rs3798220 after adjusting for age, age2, and sex. The results remained significant after adjusting for risk factors. The estimates were similar for an LPA risk score. Individuals with 2 risk alleles had a 2-fold or greater odds of AS compared with individuals with no risk alleles (for rs10455872, homozygous odds ratio, 2.05; 95% CI, 1.37-3.07; P = 5.3 × 10−4; for rs3798220, homozygous odds ratio, 3.74; 95% CI, 1.03-13.62; P = .05; and for compound heterygotes, odds ratio, 2.00; 95% CI, 1.17-3.44; P = .01). For rs10455872, the odds ratio for AS was greatest in individuals aged 55 to 64 years and declined with age (interaction P = .03). Each rs10455872 risk allele was also associated with AS that was diagnosed 0.71 years earlier (95% CI, −1.42 to 0; P = .05).
Conclusions and Relevance
We provide a large-scale confirmation of the association between 2 LPA variants and AS, reaching genome-wide significance. In addition, individuals with 2 risk alleles have 2-fold or greater odds of developing AS. Age may modify these associations and identify subgroups who are at greater risk of developing AS.
This case-control study explores whether variants in the LPA locus are associated with aortic stenosis.
Introduction
Lipoprotein(a) (Lp[a]), a complex of apolipoprotein(a) and a low-density lipoprotein–like particle, is a risk factor for cardiovascular disease.1,2,3,4,5 Plasma levels of Lp(a) have a strong genetic basis, with more than half of the variation in Lp(a) levels attributable to the LPA gene, which encodes apolipoprotein(a).6 To date, LPA is also the only locus that has been robustly associated with valve calcification and aortic stenosis (AS),7 the most common valve disease in the United States.8 These associations, when combined with instrumental variables analyses, provide evidence that elevated Lp(a) levels promote AS.3,9 To our knowledge, no medical therapy exists that effectively slows or reverses the progression of AS, leading to interest in the LPA locus as a therapeutic target. Elevated levels of Lp(a) have recently been associated with a faster disease progression,10 highlighting the potential for Lp(a) as a target for medical therapy.
Prior genetic studies of AS have been limited to smaller cohorts with limited power to provide a detailed examination of these genetic associations with clinical AS, including the association of multiple alleles at the LPA locus and possible interactions. An improved understanding of these associations could identify vulnerable groups who are at higher risk for Lp(a)-mediated AS, which could better inform the specific targeting of therapeutics. Accordingly, we used electronic health records (EHRs) from a large health care organization in northern California to establish a case-control study of AS. We performed a large-scale replication of the association between LPA single-nucleotide polymorphisms (SNPs) and AS and investigated the interaction of the LPA SNPs rs10455872 and rs3798220 with age, sex, and traditional cardiovascular risk factors.
Methods
The Genetic Epidemiology Research on Aging (GERA) cohort is a population-based cohort of more than 100 000 adults who are living in northern California. All participants are members of the Kaiser Permanente Northern California integrated health care delivery system who provided written, informed consent (database of Genotypes and Phenotypes study accession phs000674.v2.p2). The study was approved by the relevant internal review boards at Kaiser Permanente Northern California and the McGill University Health Centre.
Participants completed a detailed, self-administered survey of behavioral and demographic variables. Using DNA extracted from saliva, genome-wide genotyping was performed on customized, ethnicity-specific Axiom Genotyping Solution (Affymetrix) arrays that have been described elsewhere.11,12 Responses from the Kaiser Permanente Research Program on Genes, Environment, and Health (2007-2010) or the California Men’s Health Study (2002-2003) questionnaires, genotypes, and EHRs were subsequently linked using an anonymized, unique patient identifier to protect the privacy of the participants (database of Genotypes and Phenotypes study accession phs000788.v1.p2). Aortic stenosis cases, determined through extracting EHR data from January 1996 to December 2015, inclusive, were defined based on the presence of either: (1) an International Classification of Diseases, Ninth Revision (ICD-9) code for AS (ICD-9 424.1), or (2) a procedure code for a prior aortic valve replacement (AVR), an approach that has been previously validated to have a positive predictive value of more than 90%.7 Coronary artery disease (CAD) cases were defined using a diagnosis of myocardial infarction or CAD (ICD-9 410-414); procedure codes for percutaneous coronary intervention or coronary artery bypass surgery; or self-reported angina, myocardial infarction, or revascularization. Individuals with congenital valvular heart disease (ICD-9 746-747) were excluded. Dyslipidemia was defined as 2 or more diagnoses of disorders of lipid metabolism (ICD-9 272) and 1 or more prescriptions for a statin, as noted in the Kaiser Permanente prescriptions database. Smoking (ever/never), diabetes mellitus, and hypertension were self-reported in the questionnaire. Ages older than 89 years were rounded down to 90 to further protect the privacy of those participants (n = 389). Controls were GERA participants without an ICD-9 code for AS or a procedure code for AVR. For this case-control study of AS we included individuals aged 55 years or older with known AS disease status from January 1996 to December 2015, inclusive (n = 44 703). We restricted our analyses to participants who self-reported only European descent, as there were insufficient numbers of individuals available of other races/ethnicities.
We selected for study the LPA SNPs rs10455872 and rs3798220, which are strongly associated with Lp(a) levels13 and have been recently examined for their association with AS in studies with smaller collections of AS cases (n < 1800).14,15 The SNP rs10455872 had been genotyped with the Axiom Genotyping Solution (Affymetrix) array while the SNP rs3798220 required imputation. Following a standard quality control of the genotyped data, we imputed rs10455872 (to fill in any missing genotypes) and rs3798220 using SHAPEIT216 (University of Oxford) and IMPUTE217,18 (Univerity of Oxford) with the 1000 Genomes Project19 serving as the reference panel. A continuous dosage value was created for each individual by adding 2 times the probability of the homozygous minor allele genotype with the probability of the heterozygous genotype. Thus, dosage values ranged from 0 to 2. Imputed dosages were complete for rs10455872 and rs3798220. When necessary, the dosages were converted to hard calls by rounding dosages of 0.5 or less to 0 and dosages of 1.5 or more to 2, with the remaining dosages being set to 1. The carriers of a variant were defined as individuals with at least 1 risk allele. An LPA risk score was constructed by adding the number of rs10455872 and rs3798220 risk alleles (minor allele G for rs10455872 and minor allele C for rs3798220) that a participant possessed.
Statistical analyses were conducted using R, version 3.3.0 (R Foundation).20 The differences between the AS cases and controls were calculated using the Welch t test for continuous traits and the Pearson χ2 test for categorical traits. All logistic and linear regression models were adjusted for age, age2, and sex, except when age was the outcome. Multivariable models were further adjusted for dyslipidemia, smoking, diabetes, and hypertension. Additionally, we adjusted the multivariable models for CAD status to examine whether the observed associations were an epiphenomenon because of the high prevalence of concomitant CAD in AS cases. To assess whether the association of the LPA locus with AS is modified by clinical risk factors, interactions of the 2 LPA variants and the LPA risk score with age, sex, dyslipidemia, smoking, diabetes, and hypertension were separately modeled by introducing multiplicative interaction terms in the models. Models with interaction terms were only adjusted for age, age2, and sex due to the similar estimates that arose from models of the main effects with and without adjustment for the additional clinical risk factors. No multiple-testing corrections were applied because of the hypothesis-generating nature of the analyses. Two-tailed tests with P < .05 were considered significant.
Results
Descriptive characteristics of the study participants are shown in Table 1. Compared with the controls, AS cases (n = 3469) were older (mean [SD] of 74.6 [8.5] in cases vs 69.3 [8.3] in controls; P < .001) and were more likely to be male (56% [n = 1943] of cases vs 49% [n = 20 076] of controls; P < .001). As expected, a greater proportion of cases had dyslipidemia or comorbid CAD and more likely to be hypertensive, diabetic, and a past or present smoker (Table 1).
Table 1. Characteristics of the GERA Cohort.
| Characteristic | Aortic Stenosis | ||
|---|---|---|---|
| No. (%) | P Value | ||
| Controls | Cases | ||
| No. | 41 234 | 3469 | NA |
| Male | 20 076 (49) | 1943 (56) | <.001 |
| Age, mean (SD), y | 69.3 (8.3) | 74.6 (8.5) | <.001 |
| Body mass index (calculated as weight in kilograms divided by height in meters squared),a mean (SD) | 26.8 (4.9) | 27.4 (5.4) | <.001 |
| Dyslipidemia | 23 926 (58) | 2666 (77) | <.001 |
| Coronary artery disease | 10 007 (24) | 2131 (61) | <.001 |
| Hypertension | 16 961 (41) | 1945 (56) | <.001 |
| Ever smokedb | 20 247 (52) | 1834 (56) | <.001 |
| Diabetes mellitus | 4491 (11) | 606 (17) | <.001 |
| LPA rs10455872, carrier | 5240 (13) | 563 (16) | <.001 |
| LPA rs3798220, carrier | 1330 (3) | 137 (4) | .03 |
| Aortic valve replacement | NA | 400 (12) | NA |
Abbreviations: GERA, Genetic Epidemiology Research on Aging; NA, not applicable.
Data on body mass index were available for 42 962 participants.
Data on smoking were available for 42 535 participants.
The minor allele frequencies for rs10455872 and rs3798220 were 0.07 and 0.02, respectively. Both SNPs were in Hardy-Weinberg equilibrium (P ≥ .31). The theoretical range for the LPA risk score that combined both SNPs was 0 to 4, but we observed a range of 0 to 2. That is, individuals possessed at most 2 risk alleles, either from the same SNP (homozygous) or 1 from each SNP (compound heterozygous).
Association of LPA Variants and the LPA Risk Score With AS
Both LPA SNPs and the LPA risk score were significantly associated with AS in logistic regression models that were adjusted for age, age2, and sex (Table 2). Per risk allele, the odds ratio (OR) was 1.34 (95% CI, 1.23-1.47; P = 1.7 × 10−10) for rs10455872, 1.31 (95% CI, 1.09-1.58; P = 3.6 × 10−3) for rs3798220, and 1.35 (95% CI, 1.24-1.46; P = 1.3 × 10−12) for the risk score. Both the LPA SNPs and the risk score remained significant after adjusting for additional clinical risk factors. The magnitude of these associations did not materially differ when further adjusted for the presence of CAD (eTable 1 in the Supplement).
Table 2. Association of LPA Variants With ASa.
| LPA Variant | Adjusted for Age and Sex (n = 44 703) |
Adjusted for Age, Sex, and Risk Factors (n = 42 535) |
||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| rs10455872 | 1.34 (1.23-1.47) | 1.7 × 10−10 | 1.30 (1.18-1.43) | 4.9 × 10−8 |
| rs3798220 | 1.31 (1.09-1.58) | 3.6 × 10−3 | 1.26 (1.04-1.52) | 0.02 |
| Risk score | 1.35 (1.24-1.46) | 1.3 × 10−12 | 1.30 (1.19-1.42) | 1.7 × 10−9 |
Abbreviations: AS, aortic stenosis; OR, odds ratio.
Multivariable models were adjusted for dyslipidemia, smoking, diabetes mellitus, and hypertension in addition to age and sex. Odds ratios are per risk allele.
Possessing 2 risk alleles in any combination led to a 2-fold or greater increase in the odds of developing AS relative to individuals without any risk alleles (Table 3). The OR for individuals who were homozygous for rs10455872 was 2.05 (95% CI, 1.37-3.07; P = 5.3 × 10−4), and for rs3798220 was 3.74 (95% CI, 1.03-13.62; P = .05). For compound heterozygotes, the OR was 2.00 (95% CI, 1.17-3.44; P = .01).
Table 3. LPA Risk Allele Combinations and Their Corresponding Associations With ASa.
| No. of Risk Alleles | No. | OR (95% CI) | P Value | ||
|---|---|---|---|---|---|
| rs10455872 | rs3798220 | Total | Cases | ||
| 0 | 0 | 37 554 | 2785 | 1 [Reference] | 1 [Reference] |
| Reference vs any carrier | |||||
| 1+ | 0 | 5682 | 547 | 1.35 (1.22-1.49) | 2.3 × 10−9 |
| 0 | 1+ | 1346 | 121 | 1.26 (1.04-1.53) | .02 |
| Reference vs 1 risk allele | |||||
| 1 | 0 | 5477 | 518 | 1.32 (1.20-1.49) | 4.4 × 10−8 |
| 0 | 1 | 1330 | 118 | 1.24 (1.02-1.51) | .03 |
| Reference vs 2 risk alleles | |||||
| 2 | 0 | 205 | 29 | 2.05 (1.37-3.07) | 5.3 × 10−4 |
| 1 | 1 | 121 | 16 | 2.00 (1.17-3.44) | .01 |
| 0 | 2 | 16 | 3 | 3.74 (1.03-13.62) | .05 |
Abbreviations: AS, aortic stenosis; OR, odds ratio.
Models were adjusted for age, age2, and sex.
In sensitivity analyses, we evaluated the association of the LPA SNPs and the risk score with AVR. Both rs10455872 and the LPA risk score were significantly associated with AVR; per risk allele, the OR for rs10455872 was 1.57 (95% CI, 1.24-1.99; P = 1.5 × 10−4) and for the risk score was 1.53 (95% CI, 1.23-1.90; P = 1.2 × 10−4). The SNP rs3798220 did not reach statistical significance for association with AVR (per risk allele OR, 1.27; 95% CI, 0.75-2.13; P = .37).
Interaction of LPA Variants With Risk Factors for AS
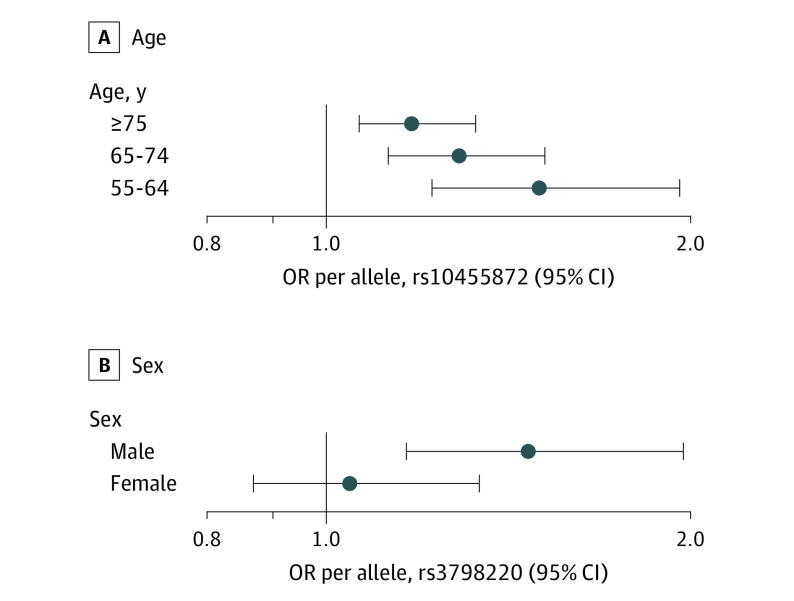
The association between rs10455872 and AS was modified by age (interaction P = .03) (Figure). In participants aged 55 to 64 years, the per risk allele OR for rs10455872 was 1.59 (95% CI, 1.29-1.97; P = 1.7 × 10−5). In participants aged 65 to 74 years, the OR decreased to 1.37 (95% CI, 1.17-1.60; P = 9.1 × 10−5), and in participants aged 75 years or older, the OR was 1.24 (95% CI, 1.09-1.41; P = 1.1 × 10−3). No age interaction was observed for either rs3798220 or the LPA risk score (data not shown).
Figure. Interactions of LPA Variants With Clinical Risk Factors.
Interaction for aortic stenosis of LPA variants with age (P = .03) (A) and sex (P = .05) (B). Odds ratios (ORs) are presented per risk allele.
Consistent with these analyses, rs10455872 was significantly associated with the age that a patient received an AS diagnosis, with each risk allele corresponding to a reduction of 0.71 years in the age at disease diagnosis (95% CI, −1.42 to 0; P = .05). Neither rs3798220 nor the LPA risk score were significantly associated with age at disease diagnosis (eTable 2 in the Supplement).
We also observed an interaction between sex and rs3798220 for AS (interaction P = .05) (Figure). Among men, each risk allele of rs3798220 was associated with an OR of 1.55 (95% CI, 1.22-1.98; P = 3.4 × 10−4). In women, the association was not significant (OR per risk allele, 1.07; 95% CI, 0.80-1.42; P = .66). No sex interaction was observed for either rs10455872 or the LPA risk score (data not shown). No significant interactions were identified between the LPA variants or the risk score and dyslipidemia, smoking, diabetes mellitus, or hypertension for AS (eTable 3 in the Supplement).
Discussion
In this case-control study of 44 703 individuals, we have confirmed that variants in the LPA locus are strongly associated with AS. Our data provide a large-scale replication of the rs10455872 association, reaching genome-wide significance, by using a single large cohort based on EHR-derived diagnostic and procedure codes. The associations were similar even when we restricted our analysis to AVR, a robust valve outcome, providing strong validation of this association. In addition, we provide new evidence that rs3798220, a rare variant in the LPA locus for which limited data exist, is also associated with AS. Given the large sample size, we were able to extend these associations to evaluate individuals who possessed 2 risk alleles of either rs10455872 or rs3798220 (including compound heterozygotes that possessed 1 risk allele from each SNP) and demonstrated a 2-fold or greater increase in their odds of developing AS relative to individuals who possessed no risk alleles. These results suggest that homozygous (or compound heterozygous) individuals at the LPA locus have a markedly elevated risk for AS, likely because of higher circulating Lp(a) levels,3,13,14,21 and may therefore be candidates for future Lp(a)-lowering therapies. We further demonstrated that the association of rs10455872 with AS was modified by age but not by other clinical risk factors. Indeed, the association between rs10455872 and AS declined with increasing age, suggesting that an increased Lp(a) level may be more relevant in younger cases of AS and that other etiologies may predominate at older ages. We also observed that rs3798220 was associated with AS only in men, but this requires validation in other cohorts. Finally, our confirmation of the association between LPA and AS using EHRs provides an important validation of our methods for determining AS disease statuses, based on diagnostic (ICD-9) codes for AS and procedure codes for AVR in the EHRs of participants. This validation supports the construction of phenotypes from health records and points to health care organizations as being valuable sources of data for answering questions about the genetic etiology of complex diseases that may have a relatively low prevalence among the general population, such as AS.
Although prior studies that evaluated LPA and calcific aortic valve disease have used computed tomographic measures of valve calcium and/or echocardiographic data for diagnosing AS, our estimates for the association between LPA variants and AS, using diagnostic and procedural codes, are consistent with these prior studies and add to the evidence that this association is robust and consistent.5,7,9,14 While LPA variants have been linked at a genome-wide significance level with aortic valve calcium,7 associations with clinical AS and valve replacement have been limited by small samples, precluding the evaluation of rare variants, such as rs3798220. We demonstrate that rs3798220, which is strongly associated with Lp(a) levels,3,13,14,21 is also associated with AS,14 with homozygous individuals possessing a 3.7-fold increase in the odds of developing AS. These results are concordant with a recent meta-analysis that demonstrated a significant association between rs3798220 and AS in a limited number of participants.15 To date, we are not aware of other studies that have evaluated age or sex interactions with LPA variants for AS, and these results will require validation by independent cohorts.
Given the consistent association between LPA variants, Lp(a) levels, and AS, mendelian randomization studies suggest that elevated Lp(a) levels contribute to the development of AS.3,7,9,14 Recent data also suggest that Lp(a) is involved in disease progression, with individuals who have mild to moderate AS and high Lp(a) levels possessing a more than 2-fold increase in their odds of AVR or cardiac death.10 Although limited treatment options currently exist, Lp(a)-lowering therapies are in development.22,23 Identifying which patients to target with such therapies in future randomized clinical trials will be an important consideration. Our results, if validated by others, would suggest that younger patients (younger than 65 years) and those with markedly high Lp(a) levels (eg, homozygous or compound heterozygotes for LPA variants) are at highest risk for developing AS, and would therefore likely derive the greatest benefit from Lp(a)-lowering therapy.
Strengths and Limitations
Our study has several strengths, including having one of the largest samples of patients with AS and genome-wide genotyping worldwide, as well as being clinically representative of a large US integrated health care delivery organization. Nonetheless, our study has several limitations. First, AS was defined based on ICD-9 codes and prior AVR rather than a detailed echocardiographic assessment, which is the gold standard. However, we have previously validated the ICD-9 code for AS with echocardiography, showing a positive predictive value more than 90%.7 We acknowledge that the accuracy of diagnostic codes may vary between different health care delivery systems and the misclassification of AS status in our study may be greater than previously reported. In addition, the inclusion of bicuspid aortic valve and/or other etiologies (eg, radiation-associated AS) among cases remains possible, but would represent only a small proportion of the included cases. Nonetheless, any such misclassification of disease status would be nondifferential by LPA status and would tend to bias our reported associations toward the null. Additionally, sensitivity analyses using AVR, a more robust valve outcome than ICD-9 coding, demonstrated consistent results. Second, quantitative data on other risk factors for AS, such as low-density lipoprotein cholesterol levels and blood pressure, were unavailable. However, adjustments for available clinical risk factors, including the presence of hypertension, dyslipidemia, diabetes, or smoking, did not materially change the estimates of effect, which was expected because LPA variants are not known to confer AS risk through any other risk factor except genetically elevated Lp(a) levels.3,9,14 Third, participants with AS were typically older than the controls, allowing for the possibility that some of the controls may subsequently develop AS. However, this would have led to an underestimation of the association that we observed between LPA variants and AS. Fourth, as is frequently performed for large-scale genetic studies, our analysis was conducted as a case-control study and therefore longitudinal estimates of absolute risks cannot be directly calculated; nonetheless, given that the prevalence of AS is less than 10% in the general population, our odds ratios approximate risk ratios. Lastly, our study was limited to individuals of European ancestry. However, the rs10455872 risk allele is rare in East Asian and African populations (allele frequency, <0.01), and owing to the greatly reduced numbers of non-European participants in the GERA cohort, those analyses would have low statistical power.
Conclusions
In this large-scale, case-control study of AS we confirmed the association between LPA SNPs rs10455872 and rs3798220 with AS and demonstrated that individuals with 2 risk alleles have a 2-fold or greater odds of developing AS. Age may modify these associations and could identify subgroups who are at greatest risk of developing AS and the most likely to benefit from Lp(a)-lowering therapies.
eTable 1. Association of LPA Variants With AS in Multivariable Models Adjusted for Cardiovascular Risk Factors and CAD.
eTable 2. Association of LPA Variants With Age of Diagnosis of AS.
eTable 3. Interaction of LPA Variants With Clinical Risk Factors For Associations With AS.
References
- 1.Nordestgaard BG, Chapman MJ, Ray K, et al. ; European Atherosclerosis Society Consensus Panel . Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31(23):2844-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hopewell JC, Seedorf U, Farrall M, et al. ; PROCARDIS Consortium . Impact of lipoprotein(a) levels and apolipoprotein(a) isoform size on risk of coronary heart disease. J Intern Med. 2014;276(3):260-268. [DOI] [PubMed] [Google Scholar]
- 3.Langsted A, Varbo A, Kamstrup PR, Nordestgaard BG. Elevated lipoprotein(a) does not cause low-grade inflammation despite causal association with aortic valve stenosis and myocardial infarction: a study of 100 578 individuals from the general population. J Clin Endocrinol Metab. 2015;100(7):2690-2699. [DOI] [PubMed] [Google Scholar]
- 4.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331-2339. [DOI] [PubMed] [Google Scholar]
- 5.Emdin CA, Khera AV, Natarajan P, et al. ; CHARGE–Heart Failure Consortium; CARDIoGRAM Exome Consortium . Phenotypic characterization of genetically lowered human lipoprotein(a) levels. J Am Coll Cardiol. 2016;68(25):2761-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boerwinkle E, Leffert CC, Lin J, Lackner C, Chiesa G, Hobbs HH. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J Clin Invest. 1992;90(1):52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thanassoulis G, Campbell CY, Owens DS, et al. ; CHARGE Extracoronary Calcium Working Group . Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368(6):503-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005-1011. [DOI] [PubMed] [Google Scholar]
- 9.Arsenault BJ, Boekholdt SM, Dubé MP, et al. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: a prospective Mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet. 2014;7(3):304-310. [DOI] [PubMed] [Google Scholar]
- 10.Capoulade R, Chan KL, Yeang C, et al. Oxidized phospholipids,l(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66(11):1236-1246. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann TJ, Kvale MN, Hesselson SE, et al. Next generation genome-wide association tool: design and coverage of a high-throughput European-optimized SNP array. Genomics. 2011;98(2):79-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann TJ, Zhan Y, Kvale MN, et al. Design and coverage of high throughput genotyping arrays optimized for individuals of East Asian, African American, and Latino race/ethnicity using imputation and a novel hybrid SNP selection algorithm. Genomics. 2011;98(6):422-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke R, Peden JF, Hopewell JC, et al. ; PROCARDIS Consortium . Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518-2528. [DOI] [PubMed] [Google Scholar]
- 14.Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63(5):470-477. [DOI] [PubMed] [Google Scholar]
- 15.Cairns BJ, Coffey S, Travis RC, et al. A replicated, genome-wide significant association of aortic stenosis with a genetic variant for lipoprotein(a): meta-analysis of published and novel data. Circulation. 2017;135(12):1181-1183. [DOI] [PubMed] [Google Scholar]
- 16.Delaneau O, Marchini J; 1000 Genomes Project Consortium . Integrating sequence and array data to create an improved 1000 Genomes Project haplotype reference panel. Nat Commun. 2014;5:3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howie B, Marchini J, Stephens M. Genotype imputation with thousands of genomes. G3 (Bethesda). 2011;1(6):457-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5(6):e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auton A, Brooks LD, Durbin RM, et al. ; 1000 Genomes Project Consortium . A global reference for human genetic variation. Nature. 2015;526(7571):68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 21.Lu W, Cheng YC, Chen K, et al. Evidence for several independent genetic variants affecting lipoprotein (a) cholesterol levels. Hum Mol Genet. 2015;24(8):2390-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsimikas S, Viney NJ, Hughes SG, et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386(10002):1472-1483. [DOI] [PubMed] [Google Scholar]
- 23.Stein EA, Raal F. Future directions to establish lipoprotein(a) as a treatment for atherosclerotic cardiovascular disease. Cardiovasc Drugs Ther. 2016;30(1):101-108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Association of LPA Variants With AS in Multivariable Models Adjusted for Cardiovascular Risk Factors and CAD.
eTable 2. Association of LPA Variants With Age of Diagnosis of AS.
eTable 3. Interaction of LPA Variants With Clinical Risk Factors For Associations With AS.