Key Points
Question
How accurate are the existing athlete-specific electrocardiographic interpretation criteria in elite basketball players?
Findings
In this observational study of the electrocardiographic findings of 519 National Basketball Association athletes, 81 players (15.6%) were observed to have results classified as abnormal under international criteria; abnormal T-wave inversions were the most prevalent abnormality. Higher rates of abnormalities were observed under previous athlete-specific criteria.
Meaning
The prevalence of abnormal electrocardiographic findings in National Basketball Association players is higher than in other studied athlete groups; continued work is required to investigate the importance of the prevalence of repolarization abnormalities in this elite athlete population.
This cohort study analyzes the electrocardiographic results of National Basketball Association athletes and assesses the accuracy of athlete-specific interpretation criteria as applied to the elite professional players of this sport.
Abstract
Importance
While it is known that long-term intensive athletic training is associated with cardiac structural changes that can be reflected on surface electrocardiograms (ECGs), there is a paucity of sport-specific ECG data. This study seeks to clarify the applicability of existing athlete ECG interpretation criteria to elite basketball players, an athlete group shown to develop significant athletic cardiac remodeling.
Objective
To generate normative ECG data for National Basketball Association (NBA) athletes and to assess the accuracy of athlete ECG interpretation criteria in this population.
Design, Setting, and Participants
The NBA has partnered with Columbia University Medical Center to annually perform a review of policy-mandated annual preseason ECGs and stress echocardiograms for all players and predraft participants. This observational study includes the preseason ECG examinations of NBA athletes who participated in the 2013-2014 and 2014-2015 seasons, plus all participants in the 2014 and 2015 NBA predraft combines. Examinations were performed from July 2013 to May 2015. Data analysis was performed between December 2015 and March 2017.
Exposures
Active roster or draft status in the NBA and routine preseason ECGs and echocardiograms.
Main Outcomes and Measures
Baseline quantitative ECG variables were measured and ECG data qualitatively analyzed using 3 existing, athlete-specific interpretation criteria: Seattle (2012), refined (2014), and international (2017). Abnormal ECG findings were compared with matched echocardiographic data.
Results
Of 519 male athletes, 409 (78.8%) were African American, 96 (18.5%) were white, and the remaining 14 (2.7%) were of other races/ethnicities; 115 were predraft combine participants, and the remaining 404 were on active rosters of NBA teams. The mean (SD) age was 24.8 (4.3) years. Physiologic, training-related changes were present in 462 (89.0%) athletes in the study. Under Seattle criteria, 131 (25.2%) had abnormal findings, compared with 108 (20.8%) and 81 (15.6%) under refined and international criteria, respectively. Increased age and increased left ventricular relative wall thickness (RWT) on echocardiogram were highly associated with abnormal ECG classifications; 17 of 186 athletes (9.1%) in the youngest age group (age 18-22 years) had abnormal ECGs compared with 36 of the 159 athletes (22.6%) in the oldest age group (age 27-39 years) (odds ratio, 2.9; 95% CI, 1.6-5.4; P < .001). Abnormal T-wave inversions (TWI) were present in 32 athletes (6.2%), and this was associated with smaller left ventricular cavity size and increased RWT. One of the 172 athletes (0.6%) in the lowest RWT group (range, 0.24-0.35) had TWIs compared with 24 of the 163 athletes (14.7%) in the highest RWT group (range, 0.41-0.57) (odds ratio, 29.5; 95% CI, 3.9-221.0; P < .001).
Conclusions and Relevance
Despite the improved specificity of the international recommendations over previous athlete-specific ECG criteria, abnormal ECG classification rates remain high in NBA athletes. The development of left ventricular concentric remodeling appears to have a significant influence on the prevalence of abnormal ECG classification and repolarization abnormalities in this athlete group.
Introduction
Electrocardiographic (ECG) findings are frequently different in well-trained athletes than age-matched nonathletes. It can be challenging in an athlete to distinguish physiologic, training-related ECG changes from findings that may reflect an underlying cardiac disorder. Consequently, athlete-specific ECG interpretation criteria have been developed that appear to improve specificity. A limitation of these criteria is that they are not sport-specific, so they cannot encapsulate how the wide variations in hemodynamic demands of different sports and the varied baseline characteristics of athletes engaged in these sports might affect the ECG results. Because basketball is one of the leading sports in the world and elite male basketball players have been shown via echocardiographic findings to develop significant athletic cardiac remodeling, the generation of normative ECG data in this athlete group offers important reference value.
This study presents a comprehensive ECG analysis of 519 athletes from the National Basketball Association (NBA). By comparing ECG findings with matched echocardiographic data, the study additionally assesses which athletic remodeling changes were most closely associated with abnormal ECG classification under current athlete-specific interpretation criteria.
Methods
Study Population
The policies of the NBA mandate an ECG and stress echocardiogram for each athlete annually, including prior to league entrance, as part of a preseason medical evaluation. For this study, the results of ECGs and echocardiograms performed by NBA team-affiliated physicians in the 2013-2014 and 2014-2015 preseasons on active-roster athletes, as well as those performed as part of the 2014 and 2015 predraft combine, an annual event designed for the evaluation of prospective players, were sent to the investigators for core laboratory analysis via a league-wide electronic medical records system. The 519 athletes included in this study represent the total number of athletes who had paired ECGs and echocardiograms available for review. Subgroup analysis by race/ethnicity was performed because of the well-established data characterizing differences in ECG patterns between African American and white athletes, which have been incorporated into the current athlete-specific ECG interpretation criteria. The race/ethnicity classifications of included participants were determined by the investigators.
The NBA, the National Basketball Players Association, and the institutional review boards of Columbia University Medical Center and Northwestern University approved the study protocol. Pursuant to the institutional review board approvals at Columbia University Medical Center and Northwestern University, informed consent was not required for this study.
ECG Analysis
Quantitative ECG data were obtained through manual measurements by the investigators. Quantitative ECG measures included heart rate, PR interval, QRS duration, and QTc interval (Bazett correction). The qualitative characteristics of each ECG were analyzed using 3 existing, athlete-specific interpretation criteria (Seattle, refined, and international) to detect accepted training-related ECG findings as well as findings classified as abnormal (quantitative methods, ECG definitions, and sample ECG tracings can be found in the eMethods and eAppendix in the Supplement). Training-related findings and abnormal findings are listed in Table 1 and Table 2, respectively.
Table 1. Baseline Characteristics, Conventional Electrocardiographic (ECG) Measurements, and Training-Related ECG Findings.
| Characteristic | Mean (SD) | P Value | ||
|---|---|---|---|---|
| Total Athletes (n = 519) | Racial/Ethnic Subgroupsa | |||
| African American (n = 409) | White (n = 96) | |||
| Baseline | ||||
| Age, y | 24.8 (4.3) | 24.5 (4.2) | 25.8 (4.2) | .003 |
| Height, cm | 199.9 (8.6) | 198.9 (8.1) | 204.0 (9.1) | .001 |
| Weight, kg | 100.0 (12.0) | 99.1 (11.7) | 103.6 (12.4) | .001 |
| Body surface area,b m2 | 2.37 (0.18) | 2.35 (0.18) | 2.44 (0.19) | .001 |
| Conventional ECG measurements | ||||
| Heart rate, bpm | 55.7 (10.5) | 55.6 (10.4) | 56.4 (11.4) | .49 |
| PR interval, ms | 180 (30) | 181 (30) | 176 (31) | .11 |
| QRS duration, ms | 99 (13) | 98 (13) | 102 (11) | .01 |
| QTc interval, ms | 418 (31) | 418 (31) | 419 (29) | .79 |
| Max voltage, mm | ||||
| Limb leads R | 16.2 (6.1) | 16.4 (6.1) | 15.8 (5.9) | .40 |
| V1-V3 S | 17.9 (7.6) | 18.5 (7.8) | 15.9 (6.5) | .003 |
| V5-V6 R | 21.7 (7.0) | 22.1 (7.2) | 20.5 (6.3) | .05 |
| Training-related ECG findings | ||||
| Sinus bradycardiac | 149 (28.7) | 118 (28.9) | 27 (28.1) | .99 |
| PR Interval >200 ms | 100 (19.3) | 83 (20.3) | 15 (15.6) | .32 |
| Mobitz type I second-degree atrioventricular nodal block (Wenckebach) | 3 (0.6) | 3 (0.7) | 0 (0) | .93 |
| Junctional rhythm | 3 (0.6) | 3 (0.7) | 0 (0) | .93 |
| Ectopic atrial rhythm | 14 (2.7) | 14 (3.4) | 0 (0) | .08 |
| Premature atrial contractions | 21 (4.0) | 17 (4.2) | 3 (3.1) | .78 |
| Isolated premature ventricular contractions | 3 (0.6) | 3 (0.7) | 0 (0) | .93 |
| Incomplete right bundle branch block | 90 (17.3) | 63 (15.4) | 25 (26.0) | .001 |
| Early repolarization | 362 (69.7) | 297 (72.6) | 56 (58.3) | .009 |
| Convex ST elevationd | 23 (4.4) | 22 (5.4) | 1 (1.0) | .10 |
| Right ventricular hypertrophye | 59 (11.4) | 51 (12.5) | 8 (8.3) | .29 |
| Left ventricular hypertrophy (Sokolow-Lyon)f | 183 (35.3) | 162 (39.6) | 21 (21.9) | .001 |
| Left ventricular hypertrophy (Cornell)f | 38 (7.3) | 33 (8.1) | 5 (5.2) | .40 |
Abbreviation: bpm, beats per minute.
Athletes identified as neither white nor African American are omitted from racial/ethnic subgroup analysis because small numbers (n = 14) precluded meaningful comparisons.
Body surface area is measured by the equation √([height (cm) × weight (kg)]/3600).
Sinus bradycardia is defined as a heart rate less than 50 bpm.
As measured via leads V1 through V4.
Right ventricular hypertrophy defined as R in V1 + S in V5 ≥ 10.5 mm.
Left ventricular hypertrophy (Sokolow-Lyon) is defined as S in V1 + R in lead V5 or V6 ≥ 35 mm, whereas left ventricular hypertrophy (Cornell) S in V3 + R in aVL≥28 mm.
Table 2. Abnormal Electrocardiographic (ECG) Findings.
| Abnormal ECG Classification | No. (%) | P Value | ||
|---|---|---|---|---|
| Total Athletes (n = 519) | Racial/Ethnic Subgroups | |||
| African American (n = 409) | White (n = 96) | |||
| Seattle criteria | 131 (25.2) | 103 (25.2) | 23 (24.0) | .90 |
| Refined criteria | 108 (20.8) | 87 (21.2) | 16 (16.6) | .33 |
| International recommendations | 81 (15.6) | 65 (15.8) | 11 (11.5) | .34 |
| Abnormal ECG findings | ||||
| Short QT Interval (QTc <320 ms) | 0 | 0 | 0 | .99 |
| Long QT Interval (QTc >470 ms) | 25 (4.8) | 20 (4.9) | 4 (4.2) | .98 |
| Left bundle branch block | 1 (0.2) | 1 (0.2) | 0 | .99 |
| Intraventricular conduction delaya | 5 (1.0) | 4 (1.0) | 0 | .74 |
| Q wavesb | 7 (1.4) | 4 (1.0) | 2 (2.1) | .32 |
| ST-segment depressionc | 9 (1.7) | 9 (2.2) | 0 | .22 |
| Abnormal T-wave inversiond | 32 (6.2) | 27 (6.6) | 3 (3.1) | .24 |
| Ventricular preexcitatione | 1 (0.2) | 1 (0.2) | 0 | .99 |
| Frequent premature ventricular contraction (>2) | 2 (0.4) | 2 (0.5) | 0 | .99 |
| ≥2 Borderline findings | 29 (5.6) | 22 (5.4) | 6 (6.3) | .91 |
| Borderline ECG findingsf | ||||
| Left atrial enlargement | 69 (13.3) | 53 (13.0) | 13 (13.5) | .87 |
| Right atrial enlargement | 46 (8.9) | 40 (9.8) | 6 (6.3) | .33 |
| QRS axis deviation | 21 (4.0) | 15 (3.7) | 6 (6.3) | .26 |
| Right bundle branch block | 25 (4.8) | 20 (4.9) | 4 (4.2) | .99 |
Intraventricular conduction delay is defined as any QRS duration >140 milliseconds.
Q waves are defined as 40 milliseconds or more or 25% or greater of height of ensuing R wave.
ST-segment depression is defined as 0.5 mm or greater in 2 or more leads.
Abnormal T-wave inversion is defined as 1 mm or greater in 2 or more leads (excluding III, aVR,V1, and T-wave inversion in V1-V4 in African American athletes when preceded by J point and/or ST elevation).
Ventricular preexcitation is defined as PR<120 milliseconds with delta wave and widened QRS.
Borderline ECG findings, when present in isolation, are considered unlikely to represent cardiac pathology in athletes, but 2 or more borderline findings are classified as abnormal.
Each ECG was reviewed by at least 2 investigators, cardiologists with expertise in sports cardiology and/or electrophysiology (M.P.W., W.W., D.J.E., R.K.M.), using a uniform protocol and worksheet containing all definitions of training-related and abnormal ECG findings under the 3 athlete criteria sets. All abnormal ECG findings were subsequently rereviewed by 2 investigators (M.P.W., D.J.E.) to adjudicate the final classification.
Echocardiograms
Echocardiograms were performed and analyzed as described previously (eMethods in the Supplement). Two-dimensional transthoracic echocardiograms were performed using commercially available systems in use at each of the 30 NBA team training sites. Left ventricular dimensions, including the interventricular septum thickness, posterior wall thickness, and left ventricular end-diastolic diameter (LVEDD), were measured as per American Society of Echocardiography recommendations. Left ventricular mass was calculated with a validated method and indexed by body surface area. Left ventricular relative wall thickness (RWT) was calculated as 2-fold the posterior wall thickness, divided by LVEDD.
Statistical Analysis
Differences between the means of baseline characteristics and quantitative ECG and echocardiographic variables in African American and white athletes were assessed via 2-tailed t test. Hispanic and Asian athletes were not included in the subgroup analyses because of the small number of representative athletes (14; 2.7%). A Fisher exact test was used in the subgroup analysis of abnormal ECG findings. Group tertiles were formed on the basis of biometric and echocardiographic parameters and analyzed with the ECG interpretation criteria. Differences among tertiles were evaluated using an analysis of variance model. For all statistical analyses, a 2-tailed P value of < .05 was considered significant.
Results
Athlete Characteristics and Training-Related ECG Findings
The mean (SD) athlete age was 24.8 (4.3) years (range, 18-39 years) (Table 1). The mean (SD) height was 199.9 (8.6) cm and mean (SD) body surface area was 2.37 (0.18) m2. African American participants composed 78.8% of the athlete group. White athletes were older than African American athletes, with a mean (SD) age of 25.8 (4.2) years vs 24.5 (4.2) years (P = .003), and white athletes had increased mean (SD) body surface area compared with African American athletes (2.44 [0.19] and 2.35 [0.18] m2, respectively; P = .001). Precordial QRS voltages were greater in African American athletes than white athletes, with a V1-V3 maximum S-wave voltage mean (SD) of 18.5 (7.8) mm compared with a mean (SD) of 15.9 (6.5) mm in white athletes (P = .003), but there were no differences in limb lead voltages. Early repolarization was the most prevalent training-related ECG finding; it was present in 362 athletes (69.7%) and occurred in 297 African American athletes (72.6%) compared with 56 white athletes (58.3%) (odds ratio [OR], 1.9; 95% CI, 1.2-3.0; P < .01). Convex ST elevation combined with T-wave inversion (TWI) in ECG leads V1 through V4, a well-recognized training-related ECG finding in African American athletes, was present in 22 African American athletes (5.4%) and 1 white athlete (1.0%). Most athletes (n = 462; 89%) had at least 1 training-related ECG change, and 327 (63.0%) demonstrated 2 or more such changes. African American and white athletes were equally likely to have at least 1 training-related ECG finding, with 360 African American athletes (88%) affected vs 79 white athletes (82%) (OR, 1.6; 95% CI, 0.9-2.9; P = .08). However, African American athletes were more likely to have 2 or more of these findings, with 270 African American athletes (66%) affected vs 47, or 49%, of white athletes (OR, 2.00; 95% CI, 1.3-3.2; P = .002). Further details are presented in Table 1.
Abnormal ECG Classification
The study found that rates of abnormal ECG classification in the NBA athlete group varied under existing athlete-specific ECG interpretation criteria. Of the total 519 athletes, 131 (25.2%) had abnormal ECG findings under the Seattle criteria compared with 108 (20.8%) under the refined criteria and 81 (15.6%) under the international criteria. There was no difference in the prevalence of abnormal ECG findings between African American and white athletes. Abnormal TWIs were the most prevalent ECG abnormality in this athlete group, present in 32 athletes (6.2%). The distribution of TWIs included 12 athletes (2.3%) with inferolateral TWIs and 20 athletes (3.9%) with TWIs confined to anterior or inferior leads. There was no difference in the prevalence of TWIs in African American compared with white athletes. Further findings are given in Table 2.
Relation of Abnormal ECG Findings With Biometric and Echocardiographic Parameters
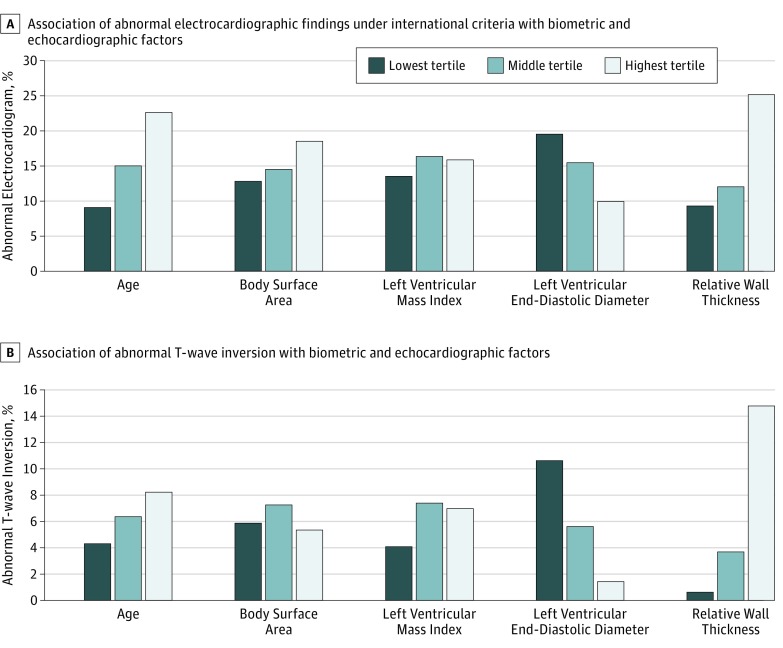
More ECG abnormalities were observed in the oldest group of athletes (age range, 27-39 years) compared with the youngest group in the cohort (age range, 18-22 years); 36 of 159 older athletes (22.6%) were affected vs 17 of 186 younger athletes (9.1%) (OR, 2.9; 95% CI, 1.6-5.4; P < .001). The prevalence of abnormal ECG findings did not relate to athlete height or body surface area. Abnormal ECG classifications did not relate to the mass or cavity size of the left ventricle. However, there was a statistically significant relationship between RWT and abnormal ECG classification; 41 of 163 athletes (25.2%) in the highest tertile of RWT had abnormal ECGs compared with 16 of 172 athletes (9.3%) in the group with the lowest RWT (OR, 3.3; 95% CI, 1.8-6.1; P < .001). In addition, mean RWT was higher in those athletes with abnormal ECG findings than those with normal ECG classifications (0.41 [95% CI, 0.40-0.42] and 0.38 [95% CI, 0.38-0.39], respectively; P < .001). These findings are summarized in the Figure.
Figure. Association of Electrocardiographic Abnormalities With Biometric and Echocardiographic Parameters.
A, The prevalence of abnormal findings in the highest and lowest age tertiles differed significantly under international electrocardiographic interpretation criteria (P < .001), as did the highest and lowest relative wall thickness tertiles (P < .001); full statistics, including additional significant differences, are available in the Supplement. (Similar results were obtained using the Seattle and refined criteria.) B, The prevalence of T-wave inversion in the highest and lowest left ventricular end-diastolic diameter tertiles differed significantly (P < .001), as did the highest and lowest relative wall thickness tertiles (P < .001); full statistics, including significant differences, are available in the Supplement.
We additionally examined the relationship between TWI, echocardiographic data, and biometric data in this athlete group (Figure). T-wave inversion did not relate to athlete age, height, body surface area, or left ventricular mass. A significant relationship between TWI and both LVEDD and RWT was observed. As left ventricular cavity size decreased and RWT increased, there was an increase in the prevalence of TWIs. T-wave inversions were present in 18 of 164 athletes (11.0%) in the lowest tertile of LVEDD (range, 44–54 mm) compared with 2 of 142 athletes (1.4%) in the highest tertile of LVEDD (range, 60-71 mm) (OR, 8.6; 95% CI, 2.0-37.9; P <.001). T-wave inversions were present in 1 of 172 athletes (0.6%) in the lowest tertile for RWT (range, 0.24-0.35) compared with 24 of 163 athletes (14.7%) in the highest tertile (range, 0.41-0.57) (OR, 29.5; 95% CI, 3.9-221.0; P <.001). Mean RWT was significantly higher in athletes with TWIs (0.44; 95% CI, 0.42-0.46) than those without TWIs (0.38; 95% CI, 0.376-0.384)(P < .001). Raw data for each athlete tertile are provided in eTables 1 and 2 in the Supplement.
Discussion
We observed a higher prevalence of abnormal ECG findings in NBA players compared with other athlete groups in a wide variety of sports from amateur to professional levels whose ECG findings have been published. The NBA players in this study were generally older (mean age, 24.8 years; range, 18-39 years) than most athletes included in other published studies of athlete groups, and this fact, combined with the finding of more ECG abnormalities in the oldest group of NBA athletes in the present study, might demonstrate that athlete age and cumulative years of intense training are important factors that influence athletic ECG changes. However, athlete height, body surface area, and race were not significant contributing factors to the prevalence of abnormal ECG findings within this athlete group.
Abnormal TWIs were the most common ECG abnormality in the NBA athlete group, appearing in 6.2% of NBA athletes. This abnormality did not relate to athlete age, height, body surface area, race/ethnicity, or left ventricular mass but rather was most prevalent in those athletes with the smallest left ventricular cavity size and the highest RWT. This finding emphasizes the importance of these factors of left ventricular geometry and this particular left ventricular concentric geometric pattern, which appears to have a significant influence on the surface ECG. The prolongation of QTc (as defined by the international criteria) was the third most prevalent ECG abnormality in the NBA group (4.8%) and similar to TWI, was more prevalent in NBA athletes than in other, previously studied athlete groups. Follow-up ECG data collection and analysis is planned to assess temporal changes in all abnormal ECG patterns observed in these athletes.
Limitations
While these data correlate current cross-sectional ECG and echocardiographic findings in individual NBA players, serial follow-up data for those athletes with abnormal ECGs are currently lacking. The findings in this study have direct application for the cardiac evaluation of elite basketball players, but care must be taken in extrapolating the results of this study to athletes in other sports and to youth basketball players. A further limitation of this study includes a lack of comparative ECG data for nonathletes with similar biometrics to the NBA cohort.
Conclusions
The athlete-specific international criteria improved ECG specificity over previous ECG interpretation criteria in NBA players, yet abnormal ECG classification remained high. Continued work is required to understand the precise importance of the higher prevalence of repolarization abnormalities in this elite athlete population.
eMethods. Quantative ECG data collection, abnormal ECG classification, and echocardiograms.
eAppendix 1. Sample ECG tracings.
eTable 1. Raw data for athlete tertiles in relationship to abnormal T-waves.
eTable 2. Raw data for athlete tertiles in relationship to abnormal ECG.
References
- 1.Pelliccia A, Maron BJ, Culasso F, et al. Clinical significance of abnormal electrocardiographic patterns in trained athletes. Circulation. 2000;102(3):278-284. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S, Whyte G, Elliott P, et al. Electrocardiographic changes in 1000 highly trained junior elite athletes. Br J Sports Med. 1999;33(5):319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drezner JA, Ackerman MJ, Anderson J, et al. Electrocardiographic interpretation in athletes: the “Seattle criteria.” Br J Sports Med. 2013;47(3):122-124. [DOI] [PubMed] [Google Scholar]
- 4.Sheikh N, Papadakis M, Ghani S, et al. Comparison of electrocardiographic criteria for the detection of cardiac abnormalities in elite black and white athletes. Circulation. 2014;129(16):1637-1649. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, Drezner JA, Baggish A, et al. International recommendations for electrocardiographic interpretation in athletes. J Am Coll Cardiol. 2017;69(8):1057-1075. [DOI] [PubMed] [Google Scholar]
- 6.Engel DJ, Schwartz A, Homma S. Athletic cardiac remodeling in US professional basketball players. JAMA Cardiol. 2016;1(1):80-87. [DOI] [PubMed] [Google Scholar]
- 7.Rawlins J, Carre F, Kervio G, et al. Ethnic differences in physiological cardiac adaptation to intense physical exercise in highly trained female athletes. Circulation. 2010;121(9):1078-1085. [DOI] [PubMed] [Google Scholar]
- 8.Papadakis M, Carre F, Kervio G, et al. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro-Caribbean origin. Eur Heart J. 2011;32(18):2304-2313. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39. [DOI] [PubMed] [Google Scholar]
- 10.Magalski A, Maron BJ, Main ML, et al. Relation of race to electrocardiographic patterns in elite American football players. J Am Coll Cardiol. 2008;51(23):2250-2255. [DOI] [PubMed] [Google Scholar]
- 11.Drezner JA, Owens DS, Prutkin JM, et al. Electrocardiographic screening in National Collegiate Athletic Association athletes. Am J Cardiol. 2016;118(5):754-759. [DOI] [PubMed] [Google Scholar]
- 12.Fuller C, Scott C, Hug-English C, Yang W, Pasternak A. Five-year experience with screening electrocardiograms in National Collegiate Athletic Association Division I athletes. Clin J Sport Med. 2016;26(5):369-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magalski A, McCoy M, Zabel M, et al. Cardiovascular screening with electrocardiography and echocardiography in collegiate athletes. Am J Med. 2011;124(6):511-518. [DOI] [PubMed] [Google Scholar]
- 14.Choo JK, Abernethy WB III, Hutter AM Jr. Electrocardiographic observations in professional football players. Am J Cardiol. 2002;90(2):198-200. [DOI] [PubMed] [Google Scholar]
- 15.Wasfy MM, DeLuca J, Wang F, et al. ECG findings in competitive rowers: normative data and the prevalence of abnormalities using contemporary screening recommendations. Br J Sports Med. 2015;49(3):200-206. [DOI] [PubMed] [Google Scholar]
- 16.Marek J, Bufalino V, Davis J, et al. Feasibility and findings of large-scale electrocardiographic screening in young adults: data from 32,561 subjects. Heart Rhythm. 2011;8(10):1555-1559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Quantative ECG data collection, abnormal ECG classification, and echocardiograms.
eAppendix 1. Sample ECG tracings.
eTable 1. Raw data for athlete tertiles in relationship to abnormal T-waves.
eTable 2. Raw data for athlete tertiles in relationship to abnormal ECG.