Key Points
Question
Does a mechanically expanded valve result in noninferior safety and effectiveness outcomes compared with an established self-expanding valve in high risk patients with severe aortic stenosis undergoing transcatheter aortic valve replacement?
Findings
In this randomized trial (n = 912 patients), the primary safety end point (30-day mortality and major adverse clinical events) occurred in 20.3% patients who received mechanically expanded valves and in 17.2% of patients who received self-expanding valves, and the primary effectiveness end point (1-year mortality, disabling stroke, and paravalvular leak) occurred in 15.4% of patients with mechanically expanded valves and in 25.5% of patients with self-expanding valves, both meeting criteria for statistical noninferiority.
Meaning
The mechanically expanded transcatheter aortic valve replacement valve may be a useful addition for treating severe aortic stenosis.
Abstract
Importance
Transcatheter aortic valve replacement (TAVR) is established for selected patients with severe aortic stenosis. However, limitations such as suboptimal deployment, conduction disturbances, and paravalvular leak occur.
Objective
To evaluate if a mechanically expanded valve (MEV) is noninferior to an approved self-expanding valve (SEV) in high-risk patients with aortic stenosis undergoing TAVR.
Design, Setting, and Participants
The REPRISE III trial was conducted in 912 patients with high or extreme risk and severe, symptomatic aortic stenosis at 55 centers in North America, Europe, and Australia between September 22, 2014, and December 24, 2015, with final follow-up on March 8, 2017.
Interventions
Participants were randomized in a 2:1 ratio to receive either an MEV (n = 607) or an SEV (n = 305).
Main Outcomes and Measures
The primary safety end point was the 30-day composite of all-cause mortality, stroke, life-threatening or major bleeding, stage 2/3 acute kidney injury, and major vascular complications tested for noninferiority (margin, 10.5%). The primary effectiveness end point was the 1-year composite of all-cause mortality, disabling stroke, and moderate or greater paravalvular leak tested for noninferiority (margin, 9.5%). If noninferiority criteria were met, the secondary end point of 1-year moderate or greater paravalvular leak was tested for superiority in the full analysis data set.
Results
Among 912 randomized patients (mean age, 82.8 [SD, 7.3] years; 463 [51%] women; predicted risk of mortality, 6.8%), 874 (96%) were evaluable at 1 year. The primary safety composite end point at 30 days occurred in 20.3% of MEV patients and 17.2% of SEV patients (difference, 3.1%; Farrington-Manning 97.5% CI, −∞ to 8.3%; P = .003 for noninferiority). At 1 year, the primary effectiveness composite end point occurred in 15.4% with the MEV and 25.5% with the SEV (difference, −10.1%; Farrington-Manning 97.5% CI, −∞ to −4.4%; P<.001 for noninferiority). The 1-year rates of moderate or severe paravalvular leak were 0.9% for the MEV and 6.8% for the SEV (difference, −6.1%; 95% CI, −9.6% to −2.6%; P < .001). The superiority analysis for primary effectiveness was statistically significant (difference, −10.2%; 95% CI, −16.3% to −4.0%; P < .001). The MEV had higher rates of new pacemaker implants (35.5% vs 19.6%; P < .001) and valve thrombosis (1.5% vs 0%) but lower rates of repeat procedures (0.2% vs 2.0%), valve-in-valve deployments (0% vs 3.7%), and valve malpositioning (0% vs 2.7%).
Conclusions and Relevance
Among high-risk patients with aortic stenosis, use of the MEV compared with the SEV did not result in inferior outcomes for the primary safety end point or the primary effectiveness end point. These findings suggest that the MEV may be a useful addition for TAVR in high-risk patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT02202434
This randomized noninferiority trial compares the effect of mechanically expanded vs self-expanding transcatheter aortic valve replacement (TAVR) on mortality and major adverse clinical events in patients with aortic stenosis at high or extreme risk of adverse outcomes.
Introduction
Transcatheter aortic valve replacement (TAVR) for patients with symptomatic severe aortic stenosis at intermediate or greater surgical risk using balloon-expandable and self-expanding devices has gained US approval and widespread use in practice. This is a result of a series of randomized trials comparing these TAVR platforms with medical therapy and conventional surgery. Despite advances, existing TAVR devices have limitations, including the inability to implant without being able to retrieve or reposition after fully expanded, hemodynamic compromise during implantation and the requirement for rapid pacing during deployment. Valve malposition requiring a second TAVR valve has been associated with an increased procedural risk. The need for permanent pacemaker implantation is another important limitation of TAVR. Paravalvular leak (PVL) can occur. Compared with patients without leaks, there is a higher mortality associated with moderate or severe PVL. Although iterative improvements in TAVR devices and periprocedural technique have occurred, PVL has been reduced but not eliminated.
The mechanically expanded valve (MEV) was designed to function early during deployment and is repositionable and retrievable when in final position. The MEV also includes an adaptive seal (which is a thin polyurethane layer that folds up on itself and fills space between the valve frame and the native annulus) to minimize PVL. Accordingly, the MEV was evaluated by conducting a randomized noninferiority comparison with an approved self-expanding valve (SEV) in patients at high or extreme surgical risk.
Methods
Study Design and Oversight
The Repositionable Percutaneous Replacement of Stenotic Aortic Valve Through Implantation of Lotus Valve System–Randomized Clinical Evaluation (REPRISE III) trial is a multicenter randomized clinical trial conducted at 55 sites globally (eTable 1 in Supplement 1). Patients were randomized 2:1 to receive the mechanically expanded Lotus Valve System (MEV; Boston Scientific Corp) or the commercially available self-expanding CoreValve (either the SEV CoreValve Classic or the SEV-E CoreValve EvolutR; Medtronic).
Institutional review boards at each site approved the protocol (available in Supplement 2). All patients provided written informed consent.
Patient Selection and Follow-up
Patients were screened by the case review committee (eTable 2 in Supplement 1) and randomized 2:1 between September 22, 2014, and December 24, 2015, at 55 centers in North America, Europe, and Australia. Eligible symptomatic patients had severe native aortic stenosis with a valve area of 1.0 cm2 or less (or an aortic valve area index ≤0.6 cm2/m2) and a mean pressure gradient of at least 40 mm Hg or a jet velocity of at least 4.0 m/s. Patients were required to have a Society of Thoracic Surgeons (STS) predicted risk of mortality of at least 8% or another indicator of high or extreme risk. Agreement by the local heart team (including an interventional cardiologist and a cardiac surgeon) regarding risk and suitability for TAVR was required. An aortic annulus size of 20 mm or larger and 27 mm or smaller (based on computed tomography) and eligibility for an available size of both valves were required. All patients were reviewed by the REPRISE III Case Review Committee to confirm eligibility. Additional inclusion and exclusion criteria are provided in eTables 3 and 4 in Supplement 1.
Randomization and Masking
A computerized pseudorandom number generator randomized patients stratified by center and high or extreme risk status. A 2:1 ratio was chosen to capture more information on the investigational device. Random permuted blocks (size 3 or 6, each with 50% chance, in a ratio of 2:1) were used to ensure approximate balance of treatment allocation within each stratum. Packaging and design of the MEV and SEV are different; therefore, the investigators performing the procedure were not blinded to the assigned treatment.
Study Device and Procedure
The MEV consists of 3 bovine pericardial tissue valve leaflets and a braided nitinol frame with a polycarbonate-based urethane adaptive seal. Investigators completed comprehensive training on the MEV. Centers without previous experience implanting MEVs performed at least 2 roll-in procedures and received on-site proctorship during their initial implant procedures. The device was introduced via the femoral artery using conventional techniques. Balloon aortic valvuloplasty was followed by advancement of the MEV through the aorta and aortic arch. The valve was mechanically expanded and its position and function assessed. Repositioning (or retrieval) of the MEV could be performed if needed. Once a satisfactory position was achieved, the valve was locked and released. Postdeployment aortography of the ascending aorta was required. Mechanically expanded valve sizes included 23-mm, 25-mm, and 27-mm diameters.
The SEV consists of a self-expanding nitinol frame and trileaflet porcine pericardial valve. The valve was introduced via the femoral artery per the instructions for use. Balloon aortic valvuloplasty was required. All centers were approved and proctor free for SEV implants before enrolling patients. Self-expanding valve sizes included 26-mm, 29-mm, and 31-mm diameters.
All data were analyzed by independent core laboratories (blinded to treatment allocation), and a clinical events committee adjudicated all major clinical events. An independent data and safety monitoring board provided study oversight (eTable 2 in Supplement 1). Clinical follow-up occurred at discharge or 7 days after the procedure (whichever came first), then at 30 days, 6 months, and 1 year. Annual follow-up will occur through 5 years. Patients were treated with aspirin and a thienopyridine before and for at least 1 month following TAVR. Patients receiving long-term anticoagulation treatment could be treated with a single antiplatelet drug.
Study End Points
The primary safety end point was the composite of all-cause mortality, stroke, life-threatening and major bleeding events, stage 2/3 acute kidney injury, and major vascular complications at 30 days. The primary effectiveness end point was the 1-year composite rate of all-cause mortality, disabling stroke, and moderate or greater PVL based on core laboratory assessment. The secondary end point was moderate or greater PVL at 1 year. Additional end points were based on the Valve Academic Research Consortium (VARC) end points and definitions. Additional exploratory analyses related to device performance, procedural success, and functional, neurological, and health status are described and shown in eTables 5, 10, and 11 in Supplement 1. Clinical procedural and device success were assessed at 30 days (eTable 5 in Supplement 1). Additional valve parameters were measured by transthoracic echocardiogram and assessed by an independent core laboratory. Functional status was evaluated by gait speed and New York Heart Association classification. Neurological status was determined by the National Institutes of Health Stroke Scale and the Modified Rankin Scale. The analysis of permanent pacemaker implantation, quality-of-life, and resource utilization data will be reported separately.
Statistical Analysis
Details of the statistical analyses are provided in the eAppendix in Supplement 1. Briefly, testing of primary and secondary end points was performed hierarchically to control for type I error. First, the 30-day primary safety and 1-year primary effectiveness end points were tested. If the null hypothesis for both end points was rejected to show noninferiority of the MEV to the SEV, the secondary end point was tested for superiority. If the null hypothesis for the secondary end point was rejected to show superiority of the MEV to the SEV, then the primary effectiveness end point was tested for superiority of the MEV to the SEV. The prespecified population for the primary noninferiority analyses was the implanted analysis set (Figure 1), which included consenting, enrolled patients treated with the assigned valve. Farrington-Manning tests were used to test noninferiority of the MEV vs the SEV. For the primary safety end point, an assumed composite event rate of 40% in each group and an absolute noninferiority margin of 10.5% required 912 patients (2:1 MEV:SEV) to provide 85% power (assuming 5% attrition). For the primary effectiveness end point, an expected composite event rate of 32% in the MEV and SEV groups and an absolute noninferiority margin of 9.5% required 912 patients to provide 80% power to detect noninferiority (assuming 10% attrition). For both primary end points, noninferiority of the MEV vs the SEV was concluded if P < .025, corresponding to a 1-sided upper 97.5% confidence interval on the difference in observed rates between groups being less than the noninferiority margin. Expected rates were derived from the CoreValve High Risk and CoreValve US trials, as the current trial was designed to enroll patients similar to the ones enrolled in those trials. Noninferiority margins were based on available data at the time of protocol development and were considered clinically reasonable. For both primary composite end points, if any individual component was missing, then the composite end point was set as missing. Missing data sensitivity analyses using the tipping point approach were performed for the primary end points by imputing missing data in both treatment groups with all possible combinations of failures to identify tipping points that might result in a change of statistical conclusion.
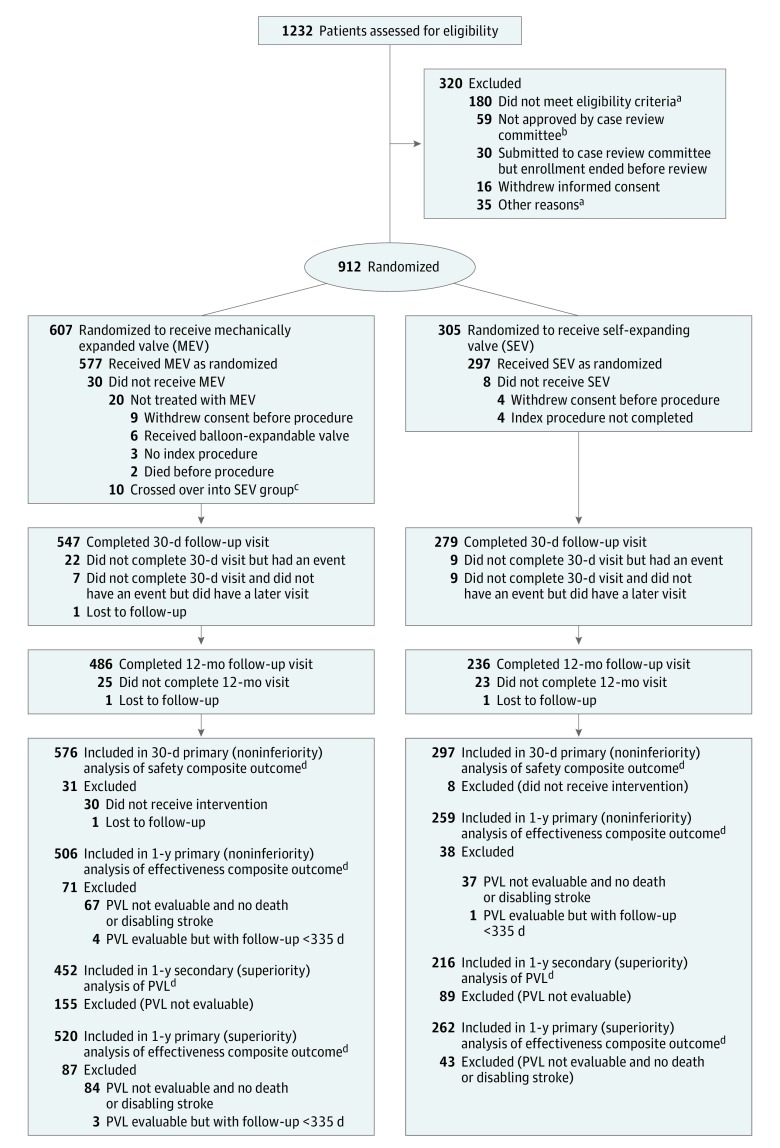
Figure 1. Patient Flow in the REPRISE III Randomized Clinical Trial.
VARC indicates Valve Academic Research Consortium; PVL, paravalvular leak.
aNo additional information is available.
bThe clinical review committee was responsible for the review of patient screening data to confirm eligibility given the increased surgical risk of the patient population being studied and to ensure consistency of patients enrolled across study centers; 24 patients had aortic structures that were too large, 12 patients had aortic structures that were too small, 12 patients had peripheral vessels that were too small, and the rest were a mix of patients who had bicuspid valve, had excessive aortic tortuosity, or did not meet the risk criteria.
cPatients who crossed over were included in the full analysis data set but were not included in the implanted analysis data set; the implanted patient population was the prespecified analysis population for noninferiority testing and includes all patients who signed an informed consent form, were enrolled in the trial, and underwent implantation of the study device they were randomized to receive (excludes crossover patients). Event rates were calculated after the index procedure. Patients who withdrew consent were included in the denominator if they had an event before withdrawal of consent.
dSee Methods section of text for end-point definitions.
For the secondary end point of moderate or greater PVL at 1 year, superiority was tested with a χ2 test in the full analysis data set. The expected rates were 1.1% with the MEV and 5.3% with the SEV. Superiority was concluded if P<.05 and the 2-sided upper 95% confidence interval for the difference between groups (MEV − SEV) was less than 0. Given enrollment of 912 patients and 25% attrition, there was 86% power to show superiority. If the secondary end point was met, superiority testing of the primary effectiveness end point was prespecified (eAppendix in Supplement 1).
Continuous variables were estimated as mean with standard deviation and compared using the t test. Discrete variables were reported as counts with percentages and differences were assessed using χ2 or Fisher exact tests. Time-to-event analyses were performed using the Kaplan-Meier method and compared using the log-rank test. No adjustment for multiple tests was performed other than the hierarchical testing strategy used for the primary and secondary end points; analyses other than the primary and secondary analysis should be considered exploratory. Because the rate of PVL might be lower with the SEV-E, which was introduced during the course of the study, a post hoc analysis of the primary effectiveness end point, secondary end point, and PVL over time was conducted in patients treated with the SEV-E compared with the MEV. This was an underpowered post hoc analysis and should be considered hypothesis generating only. Statistical analyses were performed with SAS software, version 9.2 or later (SAS Institute Inc).
Results
A total of 1232 patients were screened by the case review committee; 912 patients were randomized. One-year clinical follow-up for the last patient occurred on March 8, 2017. The full analysis set included 607 patients randomized to receive the MEV and 305 randomized to receive the SEV (Figure 1). Among randomized patients, 30 MEV patients 8 SEV patients did not undergo the assigned procedure leading to a population with implants of 577 in the MEV group and 297 in the SEV group (Figure 1). The 1-year full analysis data set included the 96.7% of MEV patients and 97.4% of SEV patients who had either a VARC event or clinical follow-up after 335 days. Transthoracic echocardiogram assessment at 1 year occurred in 89.8% of MEV patients and 85.7% of SEV patients alive at 1 year. The 2 groups were well balanced for baseline clinical, procedural, and echocardiographic characteristics (Table 1). In the full analysis population, the mean age was 82.8 (SD, 7.3) years and 51% of patients were women. The mean STS risk was 6.7% in the MEV group and 6.9% in the SEV group; 23% of patients were considered at extreme risk. The first-generation MEV was used throughout the study while the second-generation SEV was introduced midway in the study, leading to use of the SEV in 153 (51.5%) and the SEV-E in 144 (48.5%) of 297 SEV patients. Procedure time was higher but x-ray contrast use was lower with the MEV (Table 1). Antiplatelet and anticoagulant medication use was similar between groups (eTable 6 in Supplement 1).
Table 1. Baseline Clinical, Procedural, and Echocardiographic Characteristics.
| Characteristics | Mechanically Expanded Valve (n = 607) |
Self-Expanding Valve (n = 305) |
|---|---|---|
| Age, mean (SD), y | 82.8 (7.1) | 82.9 (7.6) |
| Female, No. (%) | 304 (50.1) | 159 (52.1) |
| Race/ethnicity, No. (%) | ||
| White | 537 (88.5) | 271 (88.9) |
| Hispanic or Latino | 18 (3.0) | 2 (0.7) |
| Black, of African heritage | 17 (2.8) | 11 (3.6) |
| Other | 9 (1.5) | 7 (2.3) |
| Not disclosed | 28 (4.6) | 14 (4.6) |
| Extreme risk, No. (%)a | 140 (23.1) | 66 (21.6) |
| High risk, No. (%)b | 467 (76.9) | 239 (78.4) |
| EuroSCORE, mean (SD), %c | 6.4 (5.5) | 6.4 (5.5) |
| Society of Thoracic Surgeons scored | ||
| Mean (SD) | 6.7 (4.0) | 6.9 (4.1) |
| ≥8%, No. (%) | 188 (31.0) | 90 (29.5) |
| <8%, No./total (%)e | 419 (69.0) | 215 (70.5) |
| Porcelain aortaf | 19/419 (4.5) | 7/215 (3.3) |
| Severe pulmonary hypertension | 34/419 (8.1) | 18/215 (8.4) |
| Orthopedic diseaseg | 78/419 (18.6) | 27/215 (12.6) |
| Neuromuscular disease | 6/419 (1.4) | 5/215 (2.3) |
| Prior chest radiation therapy | 17/419 (4.1) | 8/215 (3.7) |
| Hostile chesth | 17/419 (4.1) | 10/215 (4.7) |
| Severe lung disease | 64/419 (15.3) | 30/215 (14.0) |
| CABG, at risk with reoperationi | 67/419 (16.0) | 43/215 (20.0) |
| Childs-Pugh class A or B liver diseasej | 7/419 (1.7) | 4/215 (1.9) |
| Frailty | 304/419 (72.6) | 152/215 (70.7) |
| Age ≥90 y | 42/419 (10.0) | 27/215 (12.6) |
| Otherk | 17/419 (4.1) | 15/215 (7.0) |
| Gait speed, walking 5 m, mean (SD), s | 8.7 (5.2) (n = 565) | 8.7 (4.2) (n = 285) |
| Maximal grip strength, mean (SD), kg | 21.1 (10.1) (n = 605) | 20.4 (9.7) (n = 303) |
| Katz Index Activities of Daily Living Score, mean (SD)l | 5.6 (0.9) (n = 605) | 5.6 (1.0) |
| Mini–Cognitive Assessment for Dementia Score, mean (SD)m | 3.6 (1.4) (n = 599) | 3.7 (1.4) (n = 304) |
| New York Heart Association class, No. (%)n | ||
| I | 0 | 0 |
| II | 174 (28.7) | 98 (32.1) |
| III | 386 (63.6) | 186 (61.0) |
| IV | 47 (7.7) | 21 (6.9) |
| Medically treated diabetes mellitus, No./total (%) | 187/606 (30.9) | 99/304 (32.6) |
| History of coronary artery disease, No./total (%) | 433/606 (71.5) | 224 (73.4) |
| History of myocardial infarction, No./total (%) | 109/597 (18.3) | 58 (19.0) |
| History of cerebrovascular accident, No./total (%) | 68/603 (11.3) | 44/304 (14.5) |
| History of peripheral vascular disease, No./total (%) | 187/602 (31.1) | 78/304 (25.7) |
| History of chronic obstructive pulmonary disease, No./total (%) | 191/599 (31.9) | 93/303 (30.7) |
| Chronic obstructive pulmonary disease (supplemental oxygen dependence), No/total (%) | 39/599 (6.5) | 19/303 (6.3) |
| History of atrial fibrillation, No./total (%) | 213/606 (35.1) | 96/304 (31.6) |
| Prior pacemaker implant, No. (%) | 108 (17.8) | 58 (19.0) |
| Baseline echocardiographic findings | ||
| Aortic valve area, mean (SD), cm2 | 0.69 (0.19) (n = 541) | 0.70 (0.19) (n = 280) |
| Mean aortic valve gradient, mean (SD), mm Hg | 44.64 (13.35) (n = 575) | 43.85 (12.31) (n = 294) |
| Moderate or greater aortic regurgitation, No./total (%)o | 0 | 1.0/290 (0.3) |
| Moderate or greater mitral regurgitation, No./total (%)o | 59/554 (10.6) | 33/283 (11.7) |
| Procedural characteristics | ||
| Total procedure time, mean (SD), min | 86.8 (41.8) (n = 596) | 76.7 (40.6) (n = 299) |
| Total fluoroscopy time, mean (SD), min | 27.1 (10.8) (n = 595) | 22.2 (12.2) (n = 299) |
| Total contrast used for procedure, mean (SD), mL | 110.6 (62.3) (n = 593) | 120.9 (64.6) (n = 299) |
| Postimplantation valve balloon dilatation, No./total (%) | 9/596 (1.5) | 94/301 (31.2) |
| Depth of implant from left coronary sinus, mean (SD), mm | 6.3 (2.3) (n = 524) | 6.9 (2.9) (n = 258) |
| Depth of implant from posterior aortic sinus of the ascending aorta, mean (SD), mm | 5.3 (2.5) (n = 519) | 5.5 (3.1) (n = 253) |
Extreme risk categorization was based on heart team assessment and was defined as predicted risk of operative mortality or serious, irreversible morbidity of 50% or more by 30 days.
High risk categorization was based on heart team assessment and was defined as predicted risk of operative mortality or serious, irreversible morbidity of 15% or more by 30 days.
An estimate of the potential for operative mortality (range, 0%-100%); a higher score indicates an increased risk (http://www.euroSCORE.org).
An estimate of the potential for operative mortality (range, 0%-100%); a higher score indicates an increased risk.
A patient with a Society of Thoracic Surgeons score of <8% was required to have 1 of the conditions listed in the 12 rows below the score.
Extensive calcification of the ascending aorta or aortic arch.
Orthopedic disease that creates risk with patient mobilization or rehabilitation after surgical aortic valve replacement.
Hostile chest includes any of the following or other reasons that make reoperation through sternotomy or right anterior thoracotomy prohibitively hazardous: (1) abnormal chest wall anatomy due to severe kyphoscoliosis or other skeletal abnormalities; (2) complications of prior surgery; (3) evidence of severe radiation damage; or (4) history of multiple recurrent pleural effusions causing internal adhesions.
A high risk of injuring either the heart itself or previous coronary artery bypass grafts (CABG) with reentry into the chest.
The Childs-Pugh score for cirrhosis mortality includes 5 clinical measures of liver disease (total bilirubin, serum albumin, prothrombin time prolongation, ascites, and hepatic encephalopathy); points for severity of each item are added and, according to the sum of these points, patients can be categorized into Child-Pugh grades A (5-6 points), B (7-9 points), or C (10-15 points).
Other evidence that a patient is at high or extreme risk in the absence of other listed markers.
A score for independence in performance of bathing, dressing, toileting, transferring, continence, and feeding (1 point each); a score of 6 indicates full function; a score of ≤2 indicates severe functional impairment.
A score to differentiate patients with dementia based on a clock drawing distractor test (0-2 points) and recall of words (0-3 points); scores above 3 (of 5) are considered negative for dementia.
The New York Heart Association functional classification categorizes patients based on how much they are limited during physical activity (I, no limitation; IV, symptoms at rest).
Aortic and mitral regurgitation was graded based on Zhogbi.
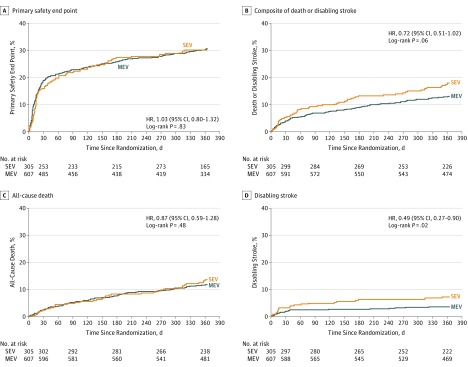
Testing of primary and secondary end points was performed hierarchically. The 30-day primary safety end point (all-cause mortality, stroke, life-threatening or major bleeding, stage 2/3 acute kidney injury, and major vascular complications) occurred in 20.3% of patients in the MEV group and in 17.2% of patients in the SEV group (difference, 3.1%; 1-sided Farrington-Manning 97.5% CI, −∞ to 8.3%; P = .003 for noninferiority) (Table 2). The Kaplan-Meier time-to-event curves for the primary safety end point for the MEV and SEV were similar at 1 year (Figure 2). The 1-year primary effectiveness end point (composite of all-cause mortality, disabling stroke, and moderate or greater PVL) occurred in 15.4% in the MEV group and in 25.5% in the SEV group (difference, −10.1%; 1-sided Farrington-Manning 97.5% CI, −∞ to −4.41%; P < .001 for noninferiority) (Table 2). Both noninferiority tests were performed in the implanted analysis set; similar results for both primary end points were observed in other analysis sets including the full analysis set (primary safety end point: 19.0% with the MEV and 16.2% with the SEV; difference, 2.8%, 1-sided 97.5% CI, −∞ to 7.8%; P = .001 for noninferiority; primary effectiveness end point: 15.8% with the MEV and 26.0% with the SEV; difference, −10.2%; 1-sided 97.5% CI, −∞ to −4.5%; P < .001 for noninferiority) (eTables 7 and 8 in Supplement 1). Tipping-point analyses suggested that it would be highly unlikely that the conclusions of the noninferiority and superiority testing of the primary end points would have been different if missing data were available (eFigures 1 and 2 in Supplement 1). The 1-year secondary end point of moderate or severe PVL occurred in 0.9% in the MEV group and 6.9% in the SEV group (difference, −6.1%; 95% CI, −9.6% to −2.6%; P < .001 for superiority) (Table 2 and eFigure 3 in Supplement 1). In superiority testing in the full analysis set, the between-group difference for the primary effectiveness end point also was significant (difference, −10.2%; 95% CI, −16.3% to −4.0%; P < .001 for superiority) (Table 2). Additional echocardiographic findings are shown in Table 2 and eTable 8 in Supplement 1. Effective orifice area was larger and mean aortic valve gradient was smaller in patients receiving the SEV compared with the MEV (Table 3).
Table 2. Hierarchical Testing of the Prespecified Safety and Effectiveness End Pointsa.
| End Points | No./Total (%) | Difference, % (97.5% Upper Confidence Bound) [95% CI]b | P Value | |
|---|---|---|---|---|
| Mechanically Expanded Valve | Self-Expanding Valve | |||
| Noninferiority testing of primary end points | ||||
| 30-d composite primary safety end pointc | 117/576 (20.3) | 51/297 (17.2) | 3.1 (8.3) [−2.3 to 8.5] | .003 |
| 1-y composite primary effectiveness end pointd | 78/506 (15.4) | 66/259 (25.5) | −10.1 (−4.4) [−16.2 to −3.9] | <.001 |
| Superiority testing of secondary end point | ||||
| 1-y moderate or greater PVLe | 4/452 (0.9) | 15/216 (6.9) | −6.1 [−9.6 to −2.6] | <.001 |
| Superiority testing of primary effectiveness end point | ||||
| 1-y composite primary effectiveness end pointd,f | 82/520 (15.8) | 68/262 (26.0) | −10.2 [−16.3 to −4.0] | <.001 |
Hierarchical testing of the primary and secondary end points was prespecified. If the null hypotheses for the primary safety and effectiveness end points were both rejected to show noninferiority of the mechanically expanded valve (MEV) to the self-expanding valve (SEV), then superiority of the secondary end point was tested. If the null hypothesis was rejected to show superiority of the MEV to the SEV, then superiority of the MEV to the SEV for the primary effectiveness end point could be tested. The primary analysis set for noninferiority testing was the population receiving implants (includes all patients who signed an informed consent form, were enrolled in the trial, and underwent implantation of the study device they were randomized to receive (excludes crossover patients); event rates were calculated after index procedure. The primary analysis set for superiority testing was the full analysis patient population (includes all patients who signed an informed consent form, were enrolled in the trial, and were randomized, regardless of whether an assigned study device was implanted); event rates were calculated after randomization.
Differences were calculated as MEV−SEV (1-sided 97.5% Farrington-Manning upper confidence bound).
Composite of all-cause mortality, stroke, life-threatening and major bleeding, stage 2/3 acute kidney injury, and major vascular complications through 30 days in the population receiving implants. The P value was calculated using the Farrington-Manning test and is based on the standard normal distribution; the between-group difference was 3.1% and the noninferiority margin was 10.5%.
Composite of all-cause mortality, disabling stroke, and moderate or greater paravalvular aortic regurgitation through 1 year. The P value was calculated using the Farrington-Manning test and is based on the standard normal distribution for noninferiority testing; the between-group difference was −4.41% and the noninferiority margin was 9.5%.
Moderate or greater paravalvular leak (PVL) based on core laboratory assessment. The P value was calculated using the χ2 test for superiority testing, and patients with echocardiogram findings of less than moderate total aortic regurgitation and visible PVL that was not gradable were included in the group with less than moderate PVL.
χ2 Test for superiority in the full analysis patient population.
Figure 2. Kaplan-Meier Time-to-Event Curves in the Full Analysis Data Set.
HR indicates hazard ratio; MEV, mechanically expanded valve; SEV, self-expanding valve. The primary safety end point was a composite of all-cause mortality, stroke, life-threatening or major bleeding, stage 2/3 acute kidney injury, and major vascular complications through 30 days in the patient population undergoing implantation of the device they were randomized to receive. Median follow-up for the MEV group was 365 days (interquartile range, 369-391 days) and for the SEV group was 365 days (interquartile range, 346-387 days).
Table 3. Clinical Outcomes at 30-Day and 1-Year Follow-upa.
| Outcomes | 30 Days | 1 Year | ||||
|---|---|---|---|---|---|---|
| Mechanically Expanded Valve (n = 601) |
Self-Expanding Valve (n = 303) |
Difference, % (95% CI) | Mechanically Expanded Valve (n = 587) |
Self-Expanding Valve (n = 297) |
Difference, % (95% CI) | |
| All-cause mortality or disabling stroke, No. (%) | 24 (4.0) | 16 (5.3) | −1.3 (−4.3 to 1.7) | 78 (13.3) | 53 (17.8) | −4.6 (−9.7 to 0.6) |
| Cardiac death or disabling stroke, No. (%) | 23 (3.8) | 16 (5.3) | −1.5 (−4.4 to 1.5) | 56 (9.5) | 44 (14.8) | −5.3 (−10.0 to −0.6) |
| All-cause mortality, No. (%) | 15 (2.5) | 7 (2.3) | 0.2 (−1.9 to 2.3) | 70 (11.9) | 40 (13.5) | −1.5 (−6.2 to 3.1) |
| Cardiovascular | 14 (2.3) | 7 (2.3) | 0.0 (−2.1 to 2.1) | 45 (7.7) | 29 (9.8) | −2.1 (−6.1 to 1.9) |
| Noncardiovascular | 1 (0.2) | 0 | 0.2 (−0.2 to 0.5) | 25 (4.3) | 11 (3.7) | 0.6 (−2.1 to 3.3) |
| Stroke, No. (%) | 29 (4.8) | 13 (4.3) | 0.5 (−2.3 to 3.4) | 41 (7.0) | 28 (9.4) | −2.4 (−6.4 to 1.5) |
| Disabling | 12 (2.0) | 10 (3.3) | −1.3 (−3.6 to 1.0) | 21 (3.6) | 21 (7.1) | −3.5 (−6.8 to −0.2) |
| Nondisabling | 17 (2.8) | 3 (1.0) | 1.8 (0.1 to 3.6) | 21 (3.6) | 7 (2.4) | 1.2 (−1.1 to 3.5) |
| Myocardial infarction, No. (%) | 4 (0.7) | 4 (1.3) | −0.7 (−2.1 to 0.8) | 19 (3.2) | 13 (4.4) | −1.1 (−3.9 to 1.6) |
| Periprocedural | 3 (0.5) | 3 (1.0) | −0.5 (−1.7 to 0.8) | 3 (0.5) | 4 (1.3) | −0.8 (−2.3 to 0.6) |
| Spontaneous | 1 (0.2) | 1 (0.3) | −0.2 (−0.9 to 0.6) | 16 (2.7) | 10 (3.4) | −0.6 (−3.1 to 1.8) |
| Bleeding, No. (%) | 77 (12.8) | 33 (10.9) | 1.9 (−2.5 to 6.3) | 106 (18.1) | 53 (17.8) | 0.2 (−5.1 to 5.6) |
| Life-threatening or disabling | 48 (8.0) | 15 (5.0) | 3.0 (−0.2 to 6.3) | 58 (9.9) | 29 (9.8) | 0.1 (−4.0 to 4.3) |
| Major | 29 (4.8) | 18 (5.9) | −1.1 (−4.3 to 2.1) | 49 (8.3) | 25 (8.4) | −0.1 (−3.9 to 3.8) |
| Acute kidney injury, No. (%)b | 15 (2.5) | 11 (3.6) | −1.1 (−3.6 to 1.3) | 15 (2.6) | 11 (3.7) | −1.1 (−3.6 to 1.4) |
| Major vascular complications, No. (%) | 42 (7.0) | 16 (5.3) | 1.7 (−1.5 to 4.9) | 45 (7.7) | 18 (6.1) | 1.6 (−1.9 to 5.1) |
| Repeat procedure for valve-related dysfunction, No. (%) | 0 | 3 (1.0) | −1.0 (−2.1 to 0.1) | 1 (0.2) | 6 (2.0) | −1.8 (−3.5 to −0.2) |
| Hospitalization for valve-related symptoms or worsening congestive heart failure, No. (%) | 10 (1.7) | 9 (3.0) | −1.3 (−3.5 to 0.9) | 66 (11.2) | 41 (13.8) | −2.6 (−7.2 to 2.1) |
| Permanent pacemaker implantation | ||||||
| All patients, No. (%) | 175 (29.1) | 48 (15.8) | 13.3 (7.8 to 18.8) | 201 (34.2) | 55 (18.5) | 15.7 (9.9 to 21.6) |
| Pacemaker-naive patients, No./total (%) | 175/493 (35.5) | 48/245 (19.6) | 15.9 (9.4 to 22.4) | 201/485 (41.4) | 55/239 (23.0) | 18.4 (11.5 to 25.3) |
| New onset of atrial fibrillation, No. (%) | 35 (5.8) | 13 (4.3) | 1.5 (−1.4 to 4.5) | 39 (6.6) | 14 (4.7) | 1.9 (−1.2 to 5.1) |
| Prosthetic aortic valve malpositioning, No. (%)c | 0 | 8 (2.6) | −2.6 (−4.4 to −0.8) | 0 | 8 (2.7) | −2.7 (−4.5 to −0.9) |
| TAV-in-TAV deployment, No. (%)d | 0 | 9 (3.0) | −3.0 (−4.9 to −1.1) | 0 | 11 (3.7) | −3.7 (−5.9 to −1.6) |
| Prosthetic aortic valve thrombosis, No. (%) | 0 | 0 | 9 (1.5) | 0 | 1.5 (0.5 to 2.5) | |
| Prosthetic aortic valve endocarditis, No. (%) | 1 (0.2) | 0 | 0.2 (−0.2 to 0.5) | 4 (0.7) | 0 | 0.7 (0.0 to 1.3) |
| Effective orifice area, mean (SD), cm2 | 1.59 (0.45) (n=506) |
1.98 (0.51) (n=238) |
−0.39 (−0.47 to −0.32) | 1.49 (0.45) (n=420) |
1.69 (0.52) (n=199) |
−0.20 (−0.28 to −0.12) |
| Mean aortic valve gradient, mean (SD), mm Hg | 12.00 (6.08) (n=544) |
7.25 (3.44) (n=261) |
4.75 (3.95 to 5.54) | 12.29 (5.83) (n=462) |
7.88 (3.48) (n=219) |
4.40 (3.57 to 5.24) |
| Peak aortic valve gradient, mean (SD), mm Hg | 21.46 (10.27) (n=545) |
13.59 (6.21) (n=261) |
7.87 (6.52 to 9.22) | 22.74 (10.53) (n=462) |
15.22 (6.44) (n=219) |
7.52 (6.01 to 9.03) |
| Peak aortic velocity, mean (SD), m/s | 2.26 (0.46) (n=545) |
1.80 (0.40) (n=261) |
0.46 (0.39 to 0.52) | 2.33 (0.51) (n=462) |
1.91 (0.41) (n=219) |
0.42 (0.34 to 0.49) |
All percentages are binary rate estimates at 30 days or 1 year in the full analysis patient population with 30-day (>21 days) or 12-month (>335 days) follow-up or a Valve Academic Research Consortium event. Neurologic examinations were performed by a neurology specialist following any suspected stroke.
Stage 2/3 acute kidney injury based on the Acute Kidney Injury Network system.
Prosthetic aortic valve malpositioning included valve migration, valve embolization, and ectopic valve deployment.
An additional transcatheter aortic valve (TAV) prosthesis implanted within a previously implanted prosthesis.
Exploratory clinical outcomes at 30 days and 1 year are shown for the full analysis set in Table 3 (for the population receiving implants in eTable 9 in Supplement 1). There was no significant difference in the combined end point of all-cause mortality or disabling stroke (Table 3 and Figure 2). Disabling stroke as an individual end point occurred less frequently with the MEV. The need for a new permanent pacemaker within 1 year was more common with the MEV (Table 3), as was valve thrombosis. Repeat procedures for valve-related dysfunction, prosthetic valve malpositioning, and TAV-in-TAV deployment were less frequent in the MEV group. No differences in New York Heart Association class, National Institutes of Health Stroke Scale, or Modified Rankin Scale were observed (eTables 10 and 11 in Supplement 1).
Post hoc analyses of the primary effectiveness end point, secondary end point, and PVL were performed to compare the SEV-E subgroup with the MEV group. The primary effectiveness end point with the SEV-E was 20.3% vs 15.4% with MEV (difference, −4.9%; 1-sided Farrington-Manning 97.5% CI, −∞ to 1.5%), suggesting that the MEV was still noninferior to the SEV despite the significantly reduced SEV sample size and correspondingly larger confidence interval for the difference in rates. Post hoc analysis also showed less PVL overall with the MEV than with the SEV-E; however, moderate or greater PVL was not statistically different between the SEV-E (2.9%) and the MEV (0.9%; difference, −2.0%; 95% CI, −5.3% to 1.3%) (eTables 12 and 13 in Supplement 1).
Discussion
In this clinical trial among high-risk patients with aortic stenosis undergoing TAVR, the use of the MEV, compared with the SEV, met criteria for noninferiority for the composite primary safety end point at 30 days and for the composite primary effectiveness end point at 1 year and also demonstrated lower rates for the secondary end point of moderate or severe PVL at 1 year. Additional differences between the valves included fewer disabling strokes and repeat procedures and less TAV-in-TAV deployment and valve malpositioning with the MEV compared with the SEV but more new pacemaker implantations and valve thrombosis and smaller valve areas with higher transvalvular gradients with the MEV.
Transcatheter aortic valve replacement has achieved a class I indication for extreme and high-risk patients and a class IIA indication for intermediate-risk patients in the current American Heart Association/American College of Cardiology guidelines. The MEV was randomized against a commercially available TAVR valve. Although comparison against a balloon-expandable valve might have been reasonable, the SEV was chosen because of its widespread use, lowest reported PVL of the available valves at the time of trial initiation, and fewer anticipated design changes during the trial.
Moderate or greater PVL has been associated with an increased risk of mortality, which is why PVL was included in the primary efficacy end point and as a secondary end point; the importance of mild PVL is less clear. Paravalvular leak can result from undersizing, malpositioning (in which the sealing zone is not properly aligned), or lack of a sealing zone due to calcification or irregularities from compression of the native valve. The MEV was designed to minimize the risk of PVL, and consistent with this, PVL was found to be significantly reduced with the MEV in this study Reports of newer iterations of SEVs and balloon-expandable valves have been notable for incremental improvement in PVL rates, with lower rates in intermediate- vs high-risk patients.
The frequency of overall stroke for the SEV was 9.4%, which was higher than reported in some high-risk studies (range, 6%-10%). Overall stroke with the MEV was 7.0%, which was comparable with the SEV rate in the CoreValve High Risk trial (8.8%) and rates in other MEV trials (between 4.9% and 9.2%). The rates of disabling stroke were lower with the MEV (21 events [3.6%] among 587 patients) than with the SEV (21 events [7.1%] among 297 patients).
The absence of valve malpositioning and TAV-in-TAV deployment with the MEV may reflect differences in implant mechanism or technique and the ability to reposition the MEV. The rate of TAV-in-TAV deployment with the SEV in this trial is consistent with rates seen in prior US pivotal SEV trials, which ranged from 3.5% to 7.0%.
The need for pacemaker implantation with the MEV was greater than with the SEV (among patients without a prior pacemaker: 35.5% vs 19.6% at 30 days). Pacemaker implantation is associated with increased costs, longer hospital stay, and perhaps patient morbidity but has not been associated with decreased survival. This study used the first-generation MEV, which has an implant mechanism involving significant interaction with the left ventricular outflow tract and conducting system. The MEV is being iterated to reduce this interaction, thereby potentially lowering the pacemaker implantation rate, but this will need to be tested in clinical trials.
Valve thrombosis was uncommon. There were more valve thromboses, defined by VARC-2 criteria, with the MEV (1.5% vs 0%). None of these patients died or had stroke. The findings of this study are consistent with a recent investigation that suggested that the supra-annular SEV may have a lower rate of valve thrombosis (MEV, 14%; SEV, 6%; balloon-expandable valve, 14%). Approaches to prevention, detection, and treatment of valve thrombosis are currently being studied.
Aortic valve area was increased and transvalvular pressure gradient was decreased with both devices. The improved mean gradient was stable for both devices up to 1 year. Valve area was larger with the SEV because the leaflets reside in a less constrained supra-annular location. Hemodynamics observed with the MEV in this study were similar to what is reported in commercially available balloon-expandable valves and similar to or better than reported for many surgical valves. The long-term effect of hemodynamic differences between the MEV and the SEV observed in this study is unknown; longer-term follow-up will help elucidate this.
This study has several limitations. First, the noninferiority margins used were based on available data at the time of protocol development. The margins were considered clinically reasonable and were similar on a relative basis to margins used in other major TAVR trials. However, the observed primary end-point event rates were lower than the expected rates used to develop these margins, perhaps because of rapid improvements in the TAVR procedure over time. In assessing the safety profile of the MEV, it is therefore important to consider the confidence intervals around the differences in primary end-point rates in addition to the prespecified statistical testing. The Kaplan-Meier time-to-event curves for the primary safety end point for the MEV and the SEV are very similar, providing additional clinical evidence that supports the statistical conclusion of noninferiority. Second, composite end points inherently include events of differing severities; assessment of all components individually (Table 3) is necessary for a more complete understanding of harms and benefits. Third, the MEV was compared in part against an early-generation SEV TAVR prosthesis, which has been largely replaced by the newer-generation SEV-E, which may have less PVL. Fourth, this study does not provide comparative data between MEVs and balloon-expandable valves. Fifth, only transfemoral access was used in this study, and because the MEV arterial sheath size was larger, there were more instances of the MEV not being implanted (Figure 1). Sixth, balloon aortic valvuloplasty before dilatation was required per protocol for all patients, which is no longer routine clinical practice. Seventh, all patients met defined high-risk criteria based on STS risk assessment or other factors; however, the mean STS score was less than 8, similar to other contemporary high-risk trial populations. Eighth, long-term assessment of durability of all bioprosthetic TAVR devices remains a limitation in this field.
Conclusions
Among high-risk patients with aortic stenosis, use of the MEV compared with use of the SEV did not result in inferior outcomes for the primary safety end point at 30 days or for the primary effectiveness end point at 1 year. These findings suggest that the MEV may be a useful addition for the treatment of high-risk patients with severe aortic stenosis undergoing TAVR.
Abbreviations List
- MEV
mechanically expanded valve
- PVL
paravalvular leak
- SEV
self-expanding valve
- TAVR
transcatheter aortic valve replacement
eAppendix. Supplemental Methods and Results
eTable 1. REPRISE III Investigators and Study Support by Site Name
eTable 2. REPRISE III Committees, and Core Laboratories
eTable 3. REPRISE III Inclusion Criteria
eTable 4. REPRISE III Exclusion Criteria
eTable 5. Additional Measurements
eTable 6. Antiplatelet/Anticoagulant Medications
eTable 7. Hierarchical Testing of the Prespecified Safety and Effectiveness Endpoints in Alternative Patient Populations
eTable 8. Grades of Aortic Regurgitation or Paravalvular Leak Presented Over Time
eTable 9. Clinical Outcomes at 30 Days and 1 Year Post Procedure in the Implanted Patient Population
eTable 10. Functional Status Over Time
eTable 11. Neurological Assessment From Baseline Through 1 Year Post-Procedure
eTable 12. Effectiveness Endpoints in SEV-E vs MEV Patients
eTable 13. Paravalvular Leak in SEV-E vs MEV Patients
eFigure 1. Missing Data Sensitivity Analysis for the 1-Year Primary Effectiveness Endpoint for Inferiority Testing
eFigure 2. Missing Data Sensitivity Analysis for the 1-Year Primary Effectiveness Endpoint for Superiority Testing
eFigure 3. Patient Flow for the Secondary Endpoint
eReferences
Trial Protocol
References
- 1.Smith CR, Leon MB, Mack MJ, et al. ; PARTNER Trial Investigators . Transcatheter vs surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187-2198. [DOI] [PubMed] [Google Scholar]
- 2.Leon MB, Smith CR, Mack M, et al. ; PARTNER Trial Investigators . Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597-1607. [DOI] [PubMed] [Google Scholar]
- 3.Adams DH, Popma JJ, Reardon MJ, et al. ; US CoreValve Clinical Investigators . Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370(19):1790-1798. [DOI] [PubMed] [Google Scholar]
- 4.Reardon MJ, Van Mieghem NM, Popma JJ, et al. ; SURTAVI Investigators . Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376(14):1321-1331. [DOI] [PubMed] [Google Scholar]
- 5.Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement vs surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387(10034):2218-2225. [DOI] [PubMed] [Google Scholar]
- 6.Popma JJ, Reardon MJ, Khabbaz K, et al. Early clinical outcomes after transcatheter aortic valve replacement using a novel self-expanding bioprosthesis in patients with severe aortic stenosis who are suboptimal for surgery: results of the Evolut R US study. JACC Cardiovasc Interv. 2017;10(3):268-275. [DOI] [PubMed] [Google Scholar]
- 7.Toggweiler S, Wood DA, Rodés-Cabau J, et al. Transcatheter valve-in-valve implantation for failed balloon-expandable transcatheter aortic valves. JACC Cardiovasc Interv. 2012;5(5):571-577. [DOI] [PubMed] [Google Scholar]
- 8.Witkowski A, Jastrzebski J, Dabrowski M, Chmielak Z. Second transcatheter aortic valve implantation for treatment of suboptimal function of previously implanted prosthesis: review of the literature. J Interv Cardiol. 2014;27(3):300-307. [DOI] [PubMed] [Google Scholar]
- 9.Buellesfeld L, Stortecky S, Heg D, et al. Impact of permanent pacemaker implantation on clinical outcome among patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;60(6):493-501. [DOI] [PubMed] [Google Scholar]
- 10.Urena M, Webb JG, Tamburino C, et al. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation. 2014;129(11):1233-1243. [DOI] [PubMed] [Google Scholar]
- 11.Biner S, Michowitz Y, Leshem-Rubinow E, et al. Hemodynamic impact and outcome of permanent pacemaker implantation following transcatheter aortic valve implantation. Am J Cardiol. 2014;113(1):132-137. [DOI] [PubMed] [Google Scholar]
- 12.Escárcega RO, Magalhaes MA, Lipinski MJ, et al. Mortality in patients requiring pacemaker implantation following transcatheter aortic valve replacement: insights from a systematic review and meta-analysis. Int J Cardiol. 2014;174(1):207-208. [DOI] [PubMed] [Google Scholar]
- 13.Abdelghani M, Soliman OII, Schultz C, Vahanian A, Serruys PW. Adjudicating paravalvular leaks of transcatheter aortic valves: a critical appraisal. Eur Heart J. 2016;37(34):2627-2644. [DOI] [PubMed] [Google Scholar]
- 14.Meredith IT, Walters DL, Dumonteil N, et al. 1-year outcomes with the fully repositionable and retrievable lotus transcatheter aortic replacement valve in 120 high-risk surgical patients with severe aortic stenosis: results of the REPRISE II study. JACC. Cardiovasc Interv. 2016;9(4):376-384. [DOI] [PubMed] [Google Scholar]
- 15.Meredith IT, Hood KL, Haratani N, Allocco DJ, Dawkins KD. Boston Scientific Lotus valve. EuroIntervention. 2012;8(suppl Q):Q70-Q74. [DOI] [PubMed] [Google Scholar]
- 16.Meredith Am IT, Walters DL, Dumonteil N, et al. Transcatheter aortic valve replacement for severe symptomatic aortic stenosis using a repositionable valve system: 30-day primary endpoint results from the REPRISE II study. J Am Coll Cardiol. 2014;64(13):1339-1348. [DOI] [PubMed] [Google Scholar]
- 17.Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57(3):253-269. [DOI] [PubMed] [Google Scholar]
- 18.Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60(15):1438-1454. [DOI] [PubMed] [Google Scholar]
- 19.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people—results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53(10):1675-1680. [DOI] [PubMed] [Google Scholar]
- 20.Popma JJ, Adams DH, Reardon MJ, et al. ; CoreValve US Clinical Investigators . Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63(19):1972-1981. [DOI] [PubMed] [Google Scholar]
- 21.Surgery and portal hypertension In: Child CG, Turcotte JG. The Liver and Portal Hypertension. Philadelphia, PA: Saunders; 1964:50-64. [Google Scholar]
- 22.Pugh RNH, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646-649. [DOI] [PubMed] [Google Scholar]
- 23.Dolgin M, Association NYH, Fox AC, Gorlin R, Levin RI. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed Boston, MA: Lippincott Williams and Wilkins; 1994. [Google Scholar]
- 24.Zoghbi WA. Echocardiography at the point of care: an ultra sound future. J Am Soc Echocardiogr. 2011;24(2):132-134. [DOI] [PubMed] [Google Scholar]
- 25.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup . Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204-R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta RL, Kellum JA, Shah SV, et al. ; Acute Kidney Injury Network . Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura RA, Otto CM, Bonow RO, et al. AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159-e1195. [DOI] [PubMed] [Google Scholar]
- 28.Kodali S, Pibarot P, Douglas PS, et al. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards sapien valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J. 2015;36(7):449-456. [DOI] [PubMed] [Google Scholar]
- 29.Van Belle E, Juthier F, Susen S, et al. ; FRANCE 2 Investigators . Postprocedural aortic regurgitation in balloon-expandable and self-expandable transcatheter aortic valve replacement procedures: analysis of predictors and impact on long-term mortality: insights from the FRANCE2 Registry. Circulation. 2014;129(13):1415-1427. [DOI] [PubMed] [Google Scholar]
- 30.Hayashida K, Lefèvre T, Chevalier B, et al. Impact of post-procedural aortic regurgitation on mortality after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5(12):1247-1256. [DOI] [PubMed] [Google Scholar]
- 31.Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol. 2013;61(15):1585-1595. [DOI] [PubMed] [Google Scholar]
- 32.Falk V, Wöhrle J, Hildick-Smith D, et al. Safety and efficacy of a repositionable and fully retrievable aortic valve used in routine clinical practice: the RESPOND study. Eur Heart J. 2017. [DOI] [PubMed] [Google Scholar]
- 33.Dumonteil N, Meredith IT, Blackman DJ, et al. Insights into the need for permanent pacemaker following implantation of the repositionable LOTUS valve for transcatheter aortic valve replacement in 250 patients: results from the REPRISE II trial with extended cohort. EuroIntervention. 2017;13(7):796-803. [DOI] [PubMed] [Google Scholar]
- 34.Chakravarty T, Søndergaard L, Friedman J, et al. ; RESOLVE; SAVORY Investigators . Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. 2017;389(10087):2383-2392. [DOI] [PubMed] [Google Scholar]
- 35.Windecker S, Tijssen J, Giustino G, et al. Trial design: rivaroxaban for the prevention of major cardiovascular events after transcatheter aortic valve replacement: rationale and design of the GALILEO study. Am Heart J. 2017;184:81-87. [DOI] [PubMed] [Google Scholar]
- 36.Grover FL, Vemulapalli S, Carroll JD, et al. ; STS/ACC TVT Registry . 2016 annual report of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol. 2017;69(10):1215-1230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplemental Methods and Results
eTable 1. REPRISE III Investigators and Study Support by Site Name
eTable 2. REPRISE III Committees, and Core Laboratories
eTable 3. REPRISE III Inclusion Criteria
eTable 4. REPRISE III Exclusion Criteria
eTable 5. Additional Measurements
eTable 6. Antiplatelet/Anticoagulant Medications
eTable 7. Hierarchical Testing of the Prespecified Safety and Effectiveness Endpoints in Alternative Patient Populations
eTable 8. Grades of Aortic Regurgitation or Paravalvular Leak Presented Over Time
eTable 9. Clinical Outcomes at 30 Days and 1 Year Post Procedure in the Implanted Patient Population
eTable 10. Functional Status Over Time
eTable 11. Neurological Assessment From Baseline Through 1 Year Post-Procedure
eTable 12. Effectiveness Endpoints in SEV-E vs MEV Patients
eTable 13. Paravalvular Leak in SEV-E vs MEV Patients
eFigure 1. Missing Data Sensitivity Analysis for the 1-Year Primary Effectiveness Endpoint for Inferiority Testing
eFigure 2. Missing Data Sensitivity Analysis for the 1-Year Primary Effectiveness Endpoint for Superiority Testing
eFigure 3. Patient Flow for the Secondary Endpoint
eReferences
Trial Protocol