Key Points
Question
Is weight loss at 5 years equivalent with laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass in patients with morbid obesity?
Findings
In this randomized clinical equivalence trial that included 240 adults with morbid obesity, the estimated mean percentage excess weight loss at 5 years was 49% after laparoscopic sleeve gastrectomy compared with 57% after laparoscopic Roux-en-Y gastric bypass, a difference that did not meet prespecified criteria for equivalence.
Meaning
This study did not demonstrate equivalence of percentage excess weight loss at 5 years between laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass.
Abstract
Importance
Laparoscopic sleeve gastrectomy for treatment of morbid obesity has increased substantially despite the lack of long-term results compared with laparoscopic Roux-en-Y gastric bypass.
Objective
To determine whether laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass are equivalent for weight loss at 5 years in patients with morbid obesity.
Design, Setting, and Participants
The Sleeve vs Bypass (SLEEVEPASS) multicenter, multisurgeon, open-label, randomized clinical equivalence trial was conducted from March 2008 until June 2010 in Finland. The trial enrolled 240 morbidly obese patients aged 18 to 60 years, who were randomly assigned to sleeve gastrectomy or gastric bypass with a 5-year follow-up period (last follow-up, October 14, 2015).
Interventions
Laparoscopic sleeve gastrectomy (n = 121) or laparoscopic Roux-en-Y gastric bypass (n = 119).
Main Outcomes and Measures
The primary end point was weight loss evaluated by percentage excess weight loss. Prespecified equivalence margins for the clinical significance of weight loss differences between gastric bypass and sleeve gastrectomy were −9% to +9% excess weight loss. Secondary end points included resolution of comorbidities, improvement of quality of life (QOL), all adverse events (overall morbidity), and mortality.
Results
Among 240 patients randomized (mean age, 48 [SD, 9] years; mean baseline body mass index, 45.9, [SD, 6.0]; 69.6% women), 80.4% completed the 5-year follow-up. At baseline, 42.1% had type 2 diabetes, 34.6% dyslipidemia, and 70.8% hypertension. The estimated mean percentage excess weight loss at 5 years was 49% (95% CI, 45%-52%) after sleeve gastrectomy and 57% (95% CI, 53%-61%) after gastric bypass (difference, 8.2 percentage units [95% CI, 3.2%-13.2%], higher in the gastric bypass group) and did not meet criteria for equivalence. Complete or partial remission of type 2 diabetes was seen in 37% (n = 15/41) after sleeve gastrectomy and in 45% (n = 18/40) after gastric bypass (P > .99). Medication for dyslipidemia was discontinued in 47% (n = 14/30) after sleeve gastrectomy and 60% (n = 24/40) after gastric bypass (P = .15) and for hypertension in 29% (n = 20/68) and 51% (n = 37/73) (P = .02), respectively. There was no statistically significant difference in QOL between groups (P = .85) and no treatment-related mortality. At 5 years the overall morbidity rate was 19% (n = 23) for sleeve gastrectomy and 26% (n = 31) for gastric bypass (P = .19).
Conclusions and Relevance
Among patients with morbid obesity, use of laparoscopic sleeve gastrectomy compared with use of laparoscopic Roux-en-Y gastric bypass did not meet criteria for equivalence in terms of percentage excess weight loss at 5 years. Although gastric bypass compared with sleeve gastrectomy was associated with greater percentage excess weight loss at 5 years, the difference was not statistically significant, based on the prespecified equivalence margins.
Trial Registration
clinicaltrials.gov Identifier: NCT00793143
This study reports 5-year weight loss outcomes for participants in the SLEEVEPASS equivalence trial with morbid obesity managed with laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass.
Introduction
Obesity is highly prevalent in the US population. Roughly one-third of US residents have a body mass index (BMI) exceeding 30 (calculated as weight in kilograms divided by height in meters squared), and 5% to 10% have BMIs of more than 40. Only bariatric surgery results in substantial and long-term weight loss for very obese patients. However, outcomes from bariatric surgery remain uncertain, because few bariatric surgery studies report long-term results with sufficient patient follow-up. The 2 most commonly performed operations are now the laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy. Sleeve gastrectomy is a simpler operation than the gastric bypass, and short-term results suggest that it very effectively treats morbid obesity and type 2 diabetes. Sleeve gastrectomy is technically easier to perform and has fewer early perioperative complications, is associated with less dumping syndrome, and does not involve the risk of internal hernias based on lack of small bowel rearrangement also providing access to an intact intestinal passage.
Although outcomes for gastric bypass are reasonably well described, long-term outcomes for sleeve gastrectomy are not well known, and it is also not known if outcomes from sleeve gastrectomy are similar to those for gastric bypass.
The Sleeve vs Bypass (SLEEVEPASS) trial was designed as a multicenter, multisurgeon, open-label randomized clinical trial to test the hypothesis that the long-term results of laparoscopic sleeve gastrectomy would be equivalent to those of laparoscopic Roux-en-Y gastric bypass with regard to weight loss and resolution of comorbidities. The 30-day and 6-month outcomes of this trial have been reported. This article reports the findings at 5-year follow-up.
Methods
Trial Design, Participants, and Interventions
The study design, rationale, and methods, including operative techniques, have been reported. The complete study protocol (Supplement 1) was approved by institutional ethics committees, and written informed consent was obtained from all patients. The trial was carried out at 3 hospitals in Finland (Turku, Vaasa, and Helsinki).
Briefly, the trial was a multicenter, multisurgeon, open-label, randomized clinical equivalence trial involving morbidly obese patients randomized to undergo either laparoscopic sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass from March 2008 to June 2010, with a final 5-year follow-up date of October 14, 2015. Eligibility criteria included age 18 to 60 years, BMI greater than 40 or greater than 35 with a significant obesity-associated comorbidity, and previous failed adequate conservative treatment. Exclusion criteria were BMI greater than 60, significant psychiatric or eating disorder, active alcohol or substance abuse, active gastric ulcer disease, severe gastroesophageal reflux with a large hiatal hernia, and previous bariatric surgery.
All of the participating surgeons were experienced laparoscopists. The standardized surgical technique for the gastric bypass entailed creating a small gastric pouch and constructing an antecolic end-to-side gastrojejunostomy, as either a circular or a linear anastomosis. The biliopancreatic jejunal limb was measured to 50 to 80 cm, the alimentary limb to 150 cm, and a side-to-side jejunojejunostomy was created. At the time of the study, mesenteric defects were not routinely closed. The sleeve gastrectomy was created narrow along a 33-Fr to 35-Fr calibration bougie. The resection was initiated 4 to 6 cm proximal to the pylorus, preserving the majority of the antrum.
Objective
The primary objective of the trial was to compare laparoscopic sleeve gastrectomy with laparoscopic Roux-en-Y gastric bypass in the treatment of morbid obesity, with a hypothesis that percentage excess weight loss after sleeve gastrectomy would be equivalent to that after gastric bypass. The secondary objective was to test the possible differences between the operations regarding resolution of comorbidities, quality of life (QOL), and overall morbidity and mortality.
Randomization
Patients were randomized by a closed-envelope method to undergo either laparoscopic sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass. Randomization was performed with a 1:1 equal allocation ratio. The opaque, sealed, and sequentially numbered randomization envelopes were shuffled and then distributed to each participating hospital. To randomize an eligible patient after the clinical decision of proceeding to bariatric surgery for treating morbid obesity, the surgeon opened a sealed envelope containing the information of the assigned randomization group. All of the treating surgeons were part of the study team.
Outcome Measures
The primary end point was weight loss defined by percentage excess weight loss, calculated as (initial weight − follow-up weight)/(initial weight − ideal weight for BMI 25) × 100%. Baseline weight was recorded at the start of the bariatric surgery evaluation process. At the time of study initiation, bariatric surgery in Finland was quite novel, and the primary end point was originally intended to be assessed at 1 year. Based on increasing understanding of the importance of long-term outcomes after bariatric surgery, the primary end point was revised to be assessed at 5-year follow-up, as this had no effect on the sample size calculation.
The predefined secondary end points included resolution of obesity-related comorbidities, improvement of disease-specific QOL measured by the Moorehead-Ardelt QOL score (range, −3 to +3, with higher score indicating better QOL), and overall morbidity and mortality. Postoperatively, comorbidities were assessed as persisting (same medications as before surgery), improved (reduction in medications), or resolved (no medications needed). These definitions were used for all of the recorded comorbidities (type 2 diabetes, dyslipidemia, and hypertension). In addition, at 5-year follow-up the remission of diabetes was defined and analyzed according to American Diabetes Association criteria (complete remission defined as glycated hemoglobin [HbA1c] value less than 6.0% (42 mmol/mol) and fasting glucose level less than 100 mg/dL [<5.6 mmol/L]; partial remission defined as HbA1c value less than 6.5% (48 mmol/mol) and a fasting glucose level of 100 to 125 mg/dL (5.6-6.9 mmol/L), both for at least 1 year’s duration in the absence of active pharmacologic therapy or ongoing procedures. For dyslipidemia, the whole study group was also evaluated for possible lipid disturbances (changes in levels of total cholesterol, high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], and triglycerides) at all time points. The decision to discontinue dyslipidemia medication was based on the treating physician’s decision using European Society of Cardiology/European Atherosclerosis Society guidelines. At 5-year follow-up, true remission of dyslipidemia as defined by these guidelines (LDL-C level less than 115.8 mg/dL [<3.0 mmol/L] and no dyslipidemia medications) was performed for all patients with baseline dyslipidemia.
Postoperative complications were classified as major or minor. Morbidity resulting in death, reoperation, hospital stay exceeding 7 days, or need for blood transfusions of 4 or more units constituted a major complication adapted from a classification scheme for complications of endoscopic sphincterotomy. All other postoperative adverse events were classified as minor complications. For the 5-year follow-up, all late complications recorded between 30 days and 5 years after surgery were also retrospectively classified according to Clavien-Dindo classification (I: any deviation in postoperative recovery; II: requiring pharmacological treatment, blood transfusions, or parenteral nutrition; III: requiring intervention [a: no general anesthesia, b: under general anesthesia]; IV: life-threatening complication requiring intensive care unit; V: death of patient).
Follow-Up
After the preliminary and 6-month early follow-up, patient outcomes were assessed at 1, 2, 3, and 5 years, with a follow-up plan extending up to 20 years (7, 10, 15, and 20 years). Patients were evaluated at outpatient control visits, and all prespecified data were thoroughly recorded. Patients lost to follow-up were contacted multiple times by mail or telephone.
Statistical Analysis
The statistical analysis plan is available in Supplement 2. Sample size calculations were performed for percentage excess weight loss using an equivalence design. Calculations were based on a test of mean difference between gastric bypass and sleeve gastrectomy, assuming the mean of 60 and standard deviation of 20 in the gastric bypass group. An α level of .05 and power of 90% were used in calculations. The prespecified equivalence margins for the clinical significance of weight loss differences between gastric bypass and sleeve gastrectomy were −9% to +9% excess weight loss; the aim was to evaluate the margins based on minimal clinically important difference. Based on these calculations, 108 patients per group were needed, and taking into account 10% dropout rate, a total of 240 study patients were enrolled in the study.
Continuous variables were characterized using means and standard deviations except for micronutrient levels, for which medians and ranges were used. Categorical variables were characterized using frequencies and percentages.
Equivalence of percentage excess weight loss between the operations at different time points was evaluated using repeated-measurements analysis of variance (ANOVA). The model included operation, time, center, and diabetes status as independent variables, excess weight at the beginning of the study as a covariate, and interaction of operation and time. Confidence intervals (95%) for the difference between the study groups were calculated at every time point, and equivalence was evaluated using the predefined margins of equivalence (−9 to 9). If the 95% CI of difference is within equivalence margins, the groups are equivalent.
Repeated-measurements ANOVA was used to analyze the dependent variables, ie, fasting plasma glucose levels and HbA1c values for patients with diabetes and levels of total cholesterol, LDL-C, HDL-C, and triglycerides for all patients. All of the models included operation, time, and center as independent variables and also included interaction of operation and time. In the analyses of fasting plasma glucose and HbA1cvalues, preoperative use of insulin was also included in the model as an independent variable. In the analyses of lipid values, diabetes status was also included in the model as an independent variable. Repeated-measurements ANOVA tests for general differences across time points and, with the test of interaction of operation and time, tests whether the difference between the operations have any differences between the time points. According to the idea of repeated-measurements ANOVA, the difference between the study groups was evaluated separately at 4 points (0.5, 1, 3, and 5 years) only when the interaction of operation and time was statistically significant. If the interaction was not statistically significant, the results are presented by main-effects operation and time, meaning that mean estimates for operations are calculated across time points and mean estimates for time points are calculated for the whole dataset, not separately for operations. The QOL score was also analyzed using repeated-measurements ANOVA but including only baseline and 5 years in the analysis.
Normality of the residuals of the models was evaluated visually and using the Kolmogorov-Smirnov test. For skewed variables (HbA1C, fasting plasma glucose, HDL-C, and triglycerides), logarithmic transformation was used to achieve normality. The analysis results were quantified using least-squares mean (95% CI) estimates and difference (95% CI) between operations. When logarithmic transformation was used for analyses, estimates were transformed to the original scale, but for those variables differences are not presented, because back-transformed estimates for difference represent the ratio of group means, not the difference. For categorical variables, differences between study groups were studied using Pearson χ2 or Fisher exact test. Post hoc analyses included BMI for the whole study group and percentage excess weight loss and BMI in patients with diabetes. All post hoc analyses were performed using repeated-measurements ANOVA as described above. Differences between groups at the 5-year point regarding vitamin deficiencies in the whole study group were evaluated using the Mann-Whitney U-test.
P values for multiple comparisons were adjusted using the step-down Bonferroni method of Holm. Analyses were performed according to the intention-to-treat population, ie, all patients were analyzed in their original intervention group, and missing data were excluded from the analyses. Because of missing values at at least 1 time point (60/240 patients [25%]), a sensitivity analysis using multiple imputation was performed for the primary outcome (percentage excess weight loss). Multivariate imputation by fully conditional specification method was performed. The predictive mean matching method was used to construct 10 imputed datasets, and repeated-measurements ANOVA was performed for each. The results of these sensitivity analyses were compared with the original analysis of percentage excess weight loss.
Two-sided P values less than .05 were considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc), and all figures were drawn with R version 3.2.0 (R Foundation for Statistical Computing).
Results
Trial Patients
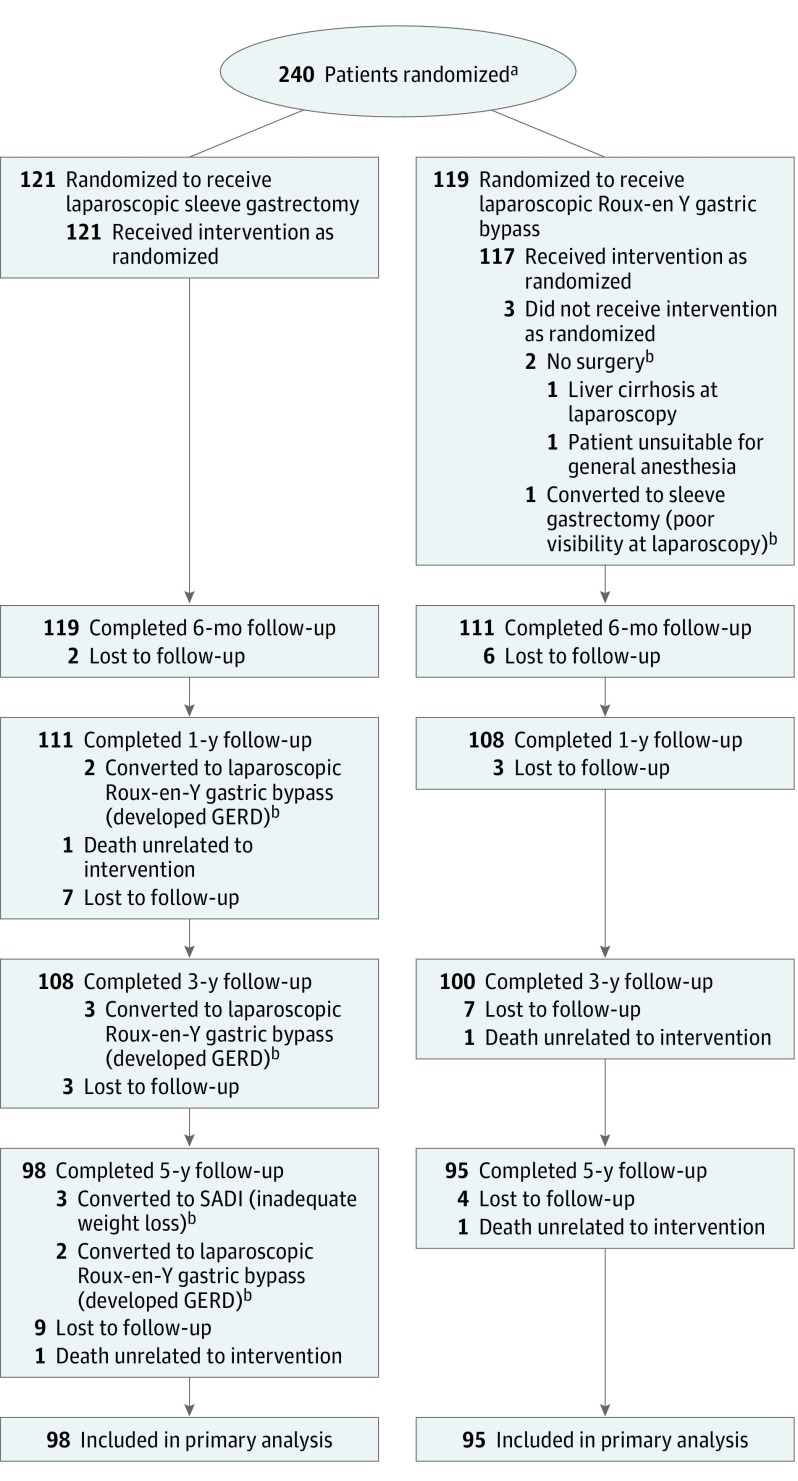
Among 240 patients randomized (mean age, 48 [SD, 9] years; mean baseline BMI, 45.9 [SD, 6.0]; 69.6% women), 80.4% completed the 5-year follow-up. Of the randomized patients, 2 in the group undergoing laparoscopic Roux-en-Y gastric bypass did not undergo bariatric surgery, resulting in a total of 238 operated patients. Baseline characteristics are presented in Table 1; there were no differences in demographic characteristics between the study groups regarding patient age, sex, BMI, and obesity-associated comorbidities. After 5 years, 24 patients in the gastric bypass group and 23 patients in the laparoscopic sleeve gastrectomy group were lost to follow-up; the remaining 193 patients (80.4%) were evaluated at 5-year follow-up. Figure 1 shows the flow of participants through the trial.
Table 1. Baseline Characteristics.
| Characteristic | Laparoscopic Sleeve Gastrectomy (n = 121) |
Laparoscopic Roux-en-Y Gastric Bypass (n = 119) |
|---|---|---|
| Sex, No. (%) | ||
| Women | 87 (71.9) | 80 (67.2) |
| Men | 34 (28.1) | 39 (32.8) |
| Age, mean (SD), y | 48.5 (9.6) | 48.4 (9.3) |
| Weight, mean (SD), kg | 130.1 (21.5) | 134.9 (22.5) |
| BMI, mean (SD)a | 45.5 (6.2) | 46.4 (5.9) |
| Type 2 diabetes, No. (%) | 52 (43.0) | 49 (41.2) |
| Hypertension, No. (%) | 83 (68.6) | 87 (73.1) |
| Dyslipidemia, No. (%) | 39 (32.2) | 45 (37.8) |
| Moorehead-Ardelt QOL total score, mean (SD)b | 0.10 (0.94) | 0.12 (1.12) |
| Hospitals participating in the study, No. | ||
| Turku | 40 | 40 |
| Vaasa | 40 | 40 |
| Helsinki | 41 | 39 |
Abbreviations: BMI, body mass index; QOL, quality of life.
Calculated as weight in kilograms divided by height in meters squared.
Score range −3 to +3, with higher score indicating better QOL.
Figure 1. Flow of Participants Through the SLEEVEPASS Trial of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass.
GERD indicates gastroesophageal reflux disease; SADI, single duodenoileal bypass.
aThe number of patients assessed for eligibility was not recorded.
bAnalyzed according to intention-to-treat.
Primary End Point
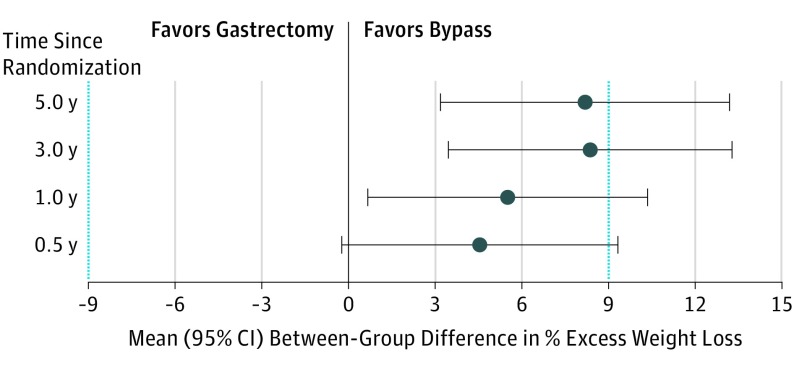
The estimated mean percentage excess weight loss at 5 years was 49% (95% CI, 45%-52%) after sleeve gastrectomy and 57% (95% CI, 53%-61%) after gastric bypass; percentage excess weight loss data are presented in detail in Table 2, Figure 2, Figure 3, and Figure 4. At 5 years, the model-based estimate of mean percentage excess weight loss was 8.2 percentage units (95% CI, 3.2%-13.2%) higher in the gastric bypass group than in sleeve gastrectomy group. Predefined margins of equivalence were −9 to 9; based on those limits, the groups are not equivalent, because the whole confidence interval is not within the margins. The difference in mean percentage excess weight loss between the sleeve gastrectomy and gastric bypass groups did not meet the criteria for equivalence at any of the time points (6 months and 1, 3, and 5 years). Gastric bypass resulted in statistically greater weight loss than sleeve gastrectomy at 5 years, but the difference was not clinically significant, as the minimal clinically important difference of 9 is within the confidence interval. The results were very similar in sensitivity analyses in which multiple imputation was used to fill in the missing values.
Table 2. Excess Weight Loss Mean Differences and Body Mass Index Model-Based Means for the Whole Study Group and for Patients With Diabetes After Laparoscopic Sleeve Gastrectomy and Laparoscopic Roux-en-Y Gastric Bypass at Baseline, 6 Months, and 1, 3, and 5 Yearsa.
| Baseline | 6 mo | 1 y | 3 y | 5 y | P Value for Effects in ANOVA | |
|---|---|---|---|---|---|---|
| Excess Weight Loss, Mean Difference Between Procedures, % (95% CI)b,c,d | ||||||
| All patients | 4.5 (−0.2 to 9.3) | 5.5 (0.7 to 10.3) | 8.4 (3.5 to 13.3) | 8.2 (3.2 to 13.2) | ||
| Patients with diabetes | 6.0 (−1.7 to 13.8) | 8.3 (0.5 to 16.1) | 7.4 (−0.6 to 15.3) | 11.7 (3.7 to 19.7) | ||
| Body Mass Index | ||||||
| All patients | <.001 for operation × time interaction | |||||
| Sleeve gastrectomy | ||||||
| No. | 121 | 119 | 111 | 108 | 98 | |
| Model-based mean (95% CI)c,e | 47.3 (46.2 to 48.3) | 35.8 (34.7 to 36.8) | 34.4 (33.3 to 35.5) | 35.2 (34.1 to 36.3) | 36.5 (35.4 to 37.6) | |
| Gastric bypass | ||||||
| No. | 119 | 111 | 108 | 100 | 95 | |
| Model-based mean (95% CI)c,e | 48.4 (47.3 to 49.5) | 35.3 (34.2 to 36.4) | 33.6 (32.5 to 34.7) | 34.0 (32.9 to 35.1) | 35.4 (34.3 to 36.5) | |
| Difference | 0.4 (−1.1 to 2.0) | 0.8 (−0.8 to 2.3) | 1.2 (−0.3 to 2.8) | 1.1 (−0.5 to 2.6) | ||
| P value (corrected with step-down Bonferroni) | .65 | .65 | .47 | .54 | ||
| Patients with diabetes | <.001 for operation × time interaction | |||||
| Sleeve gastrectomy | ||||||
| No. | 52 | 50 | 50 | 45 | 41 | |
| Model-based mean (95% CI)c,e | 46.3 (44.7 to 47.9) | 35.2 (33.6 to 36.7) | 34.3 (32.7 to 35.8) | 35.2 (33.6 to 36.8) | 36.6 (35.0 to 38.3) | |
| Gastric bypass | ||||||
| No. | 49 | 44 | 43 | 42 | 41 | |
| Model-based mean (95% CI)c,e | 47.4 (45.8 to 49.0) | 34.4 (32.7 to 36.0) | 32.9 (31.2 to 34.5) | 33.9 (32.2 to 35.5) | 34.5 (32.8 to 36.1) | |
| Difference | 0.8 (−1.5 to 3.1) | 1.4 (−0.9 to 3.7) | 1.3 (−1.0 to 3.6) | 2.1 (−0.2 to 4.5) | ||
| P value (corrected with step-down Bonferroni) | .70 | .70 | .70 | .29 | ||
Abbreviation: ANOVA, analysis of variance.
All results adjusted for center and diabetes status.
Equivalence design was used in the analyses, and equivalence margins were set from −9 to +9.
Repeated-measurements ANOVA.
Percentage excess weight loss calculated as (initial weight − follow-up weight)/(initial weight − ideal weight for body mass index 25).
Superiority design was used in the analysis.
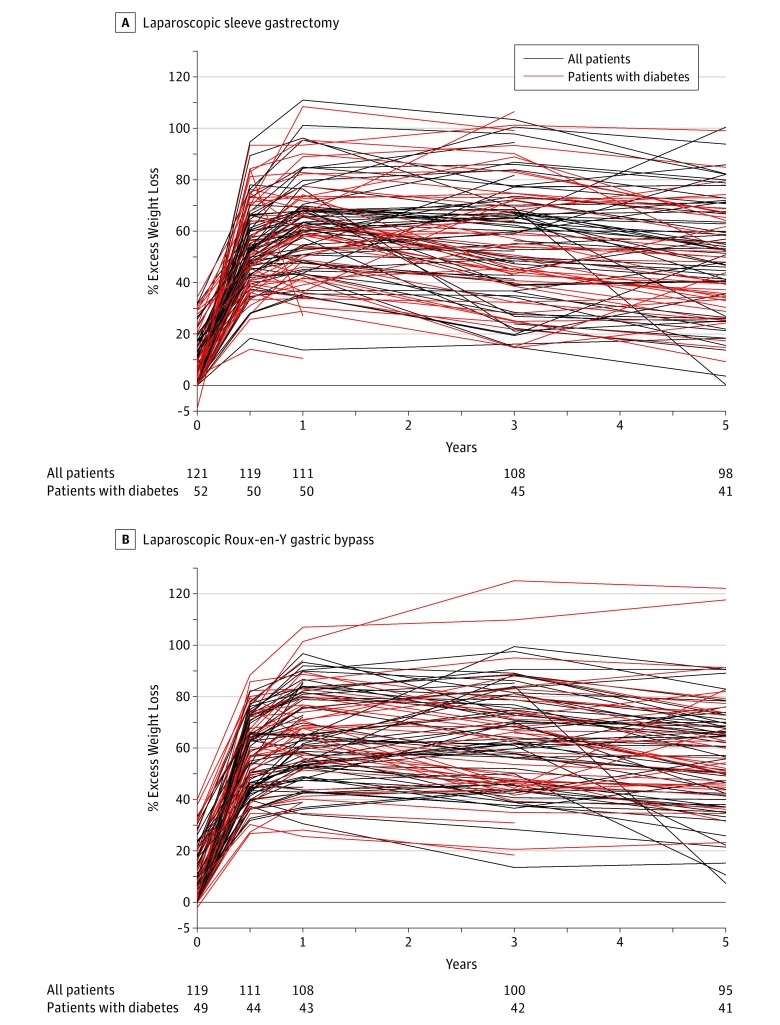
Figure 2. Percentage Excess Weight Loss Over 5-Year Follow-up for Individual Patients After Laparoscopic Sleeve Gastrectomy (n = 121) and Laparoscopic Roux-en-Y Gastric Bypass (n = 119).
Percentage excess weight loss at time 0 represents preoperative weight loss between day of randomization and day of surgery.
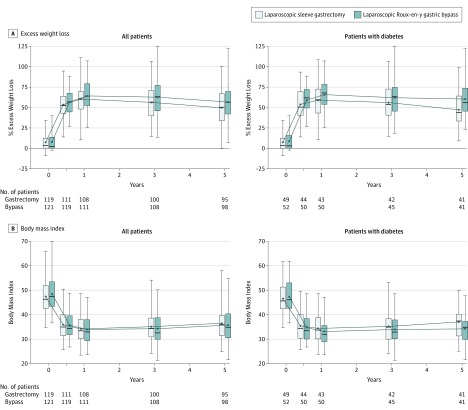
Figure 3. Percentage Excess Weight Loss and Body Mass Index for the Whole Study Group and by Procedure Over 5-Year Follow-up.
Percentage excess weight loss and body mass index for the whole study group and for patients with diabetes after laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass at baseline, 6 months, and 1, 3, and 5 years. Lower and upper borders of boxes indicate 25th and 75th quartiles, respectively; lower and upper ends of error bars indicate minimum and maximum values, respectively; horizontal lines in boxes indicate median values; dots indicate mean values. Percentage excess weight loss at time 0 represents preoperative weight loss between day of randomization and day of surgery.
Figure 4. Differences in Estimates of Mean Percentage Excess Weight Loss Between Laparoscopic Sleeve Gastrectomy and Laparoscopic Roux-en-Y Gastric Bypass Over 5-Year Follow-up.
Prespecified equivalence margins (blue dotted lines) for the clinical significance of weight loss differences between gastric bypass and sleeve gastrectomy were −9% to +9% excess weight loss. Error bars indicate 95% confidence intervals.
Secondary End Points
Type 2 Diabetes
At baseline, 101 patients (42%) had type 2 diabetes (52 sleeve gastrectomy, 49 gastric bypass), and 29 were using insulin (16 sleeve gastrectomy, 13 gastric bypass). After 5 years there was no significant difference between the study groups in diabetes remission (P > .99). Complete remission was seen in 5 of 41 patients (12%) in the sleeve gastrectomy group and in 10 of 40 (25%) in the gastric bypass group. Improved glycemic control was seen at 3 and 5 years after surgery in both study groups compared with baseline. After 5 years there was no statistically significant difference between the study groups in mean estimated fasting plasma glucose level: 135.1 (95% CI, 124.3-147.8) mg/dL (7.5 [95% CI, 6.9-8.2] mmol/L) in the sleeve gastrectomy group compared with 120.7 (95% CI, 109.9-131.56.7) mg/dL (6.7 [95% CI, 6.1-7.3] mmol/L) in the gastric bypass group, P = .052). There was no difference between the study groups regarding glycated hemoglobin; the mean estimated HbA1c value across the follow-up time was 6.6% (95% CI, 6.4%-6.8%) in the sleeve gastrectomy group and 6.6% (95% CI, 6.4%-6.8%) in the gastric bypass group (P = .93) (Table 3).
Table 3. Improvement of Glycemic Control in Patients With Diabetes After Laparoscopic Sleeve Gastrectomy and Laparoscopic Roux-en-Y Gastric Bypass at Baseline, 6 Months, and 1, 3, and 5 Years.
| Model-Based Mean (95% CI) in Operations | Baseline | 0.5 y | 1 y | 3 y | 5 y | P Value for Effects in ANOVA | |
|---|---|---|---|---|---|---|---|
| Fasting glucose, mmol/La | .03 for operation × time interaction | ||||||
| Sleeve gastrectomy | |||||||
| No. | 52 | 50 | 50 | 46 | 41 | ||
| Model-based mean (95% CI) | 7.5 (6.9 to 8.1) | 6.2 (5.9 to 6.5) | 6.4 (6.0 to 6.7) | 6.6 (6.2 to 7.0) | 7.5 (6.9 to 8.2) | ||
| Gastric bypass | |||||||
| No. | 49 | 43 | 43 | 42 | 40 | ||
| Model-based mean (95% CI) | 7.8 (7.2 to 8.5) | 6.1 (5.7 to 6.4) | 5.9 (5.6 to 6.3) | 6.7 (6.2 to 7.2) | 6.7 (6.1 to 7.3) | ||
| P value (corrected with step-down Bonferroni) | >.99 | .30 | >.99 | .21 | |||
| Glycated hemoglobin, %a | .05 for operation × time interaction | ||||||
| Sleeve gastrectomy | 6.6 (6.4 to 6.8) | .93 for main effect of operation | |||||
| Gastric bypass | 6.6 (6.4 to 6.8) | ||||||
| Model-based means (95% CI) in time points | 7.2 (7.0 to 7.5) | 6.6 (6.2 to 6.5) | 6.2 (6.1 to 6.4) | 6.4 (6.2 to 6.6) | 6.7 (6.5 to 6.9) | <.001 for main effect of time | |
| Glycemic status, No./total (%) | |||||||
| Complete remissionb | |||||||
| Sleeve gastrectomy | 9/50 (18.0) | 13/50 (26.0) | 7/46 (15.2) | 5/41 (12.2) | |||
| Gastric bypass | 10/43 (23.3) | 13/43 (30.2) | 11/42 (26.2) | 10/40 (25.0) | |||
| Partial remissionc | |||||||
| Sleeve gastrectomy | 8/50 (16.0) | 7/50 (14.0) | 14/46 (30.4) | 10/41 (24.4) | |||
| Gastric bypass | 11/43 (25.6) | 11/43 (25.6) | 10/42 (23.8) | 8/40 (20.0) | |||
| Reduction in medication | |||||||
| Sleeve gastrectomy | 25/50 (50.0) | 28/50 (56.0) | 22/46 (47.8) | 21/41 (51.2) | |||
| Gastric bypass | 19/43 (44.2) | 16/43 (37.2) | 19/42 (45.2) | 20/40 (50.0) | |||
| No change in medication (no deterioration) | |||||||
| Sleeve gastrectomy | 8/50 (16.0) | 2/50 (4.0) | 3/46 (6.5) | 5/41 (12.2) | |||
| Gastric bypass | 3/43 (7.0) | 3/43 (7.0) | 2/42 (4.8) | 2/40 (5.0) | |||
| P value (corrected with step-down Bonferroni)d | >.99 | >.99 | >.99 | >.99 |
SI conversion factor: To convert glucose values to mg/dL, divide by 0.0555.
Repeated-measurements ANOVA; logarithmic transformation was used in the analyses, and results are transformed back to original scale. Results are adjusted for center and preoperative use of insulin.
Glycated hemoglobin value less than 6.0% 42 mmol/mol) and fasting plasma glucose level less than 100 mg/dL (5.6 mmol/L) for at least 1 year’s duration in the absence of active pharmacologic therapy or ongoing procedures.
Glycated hemoglobin value less than 6.5% (48 mmol/mol) and fasting plasma glucose level 100 to 125 mg/dL (5.6-6.9 mmol/L) for at least 1 year’s duration in the absence of active pharmacologic therapy or ongoing procedures.
χ2 test performed 1 year at a time.
Dyslipidemia
At baseline, 83 patients (35%) had dyslipidemia. At 5-year follow-up, 14 of 30 patients (47%) in the sleeve gastrectomy group and 24 of 40 (60%) in the gastric bypass group had discontinued dyslipidemia medications; 6 of 30 patients (20%) in the sleeve gastrectomy group and 2 of 40 (5%) in the gastric bypass group needed less medications; and no improvement in dyslipidemia medication use was detected in 10 of 30 patients (33%) in the sleeve gastrectomy group and 14 of 40 (35%) in the gastric bypass group (P = .15).
All lipid values at all time points are reported in Table 4. For the whole study group, there was no statistically significant difference (P = .053) in total cholesterol values after 5 years between the groups: 189.2 (95% CI, 181.5-193.1) mg/dL (4.9 [95% CI, 4.7-5.0] mmol/L) for the sleeve gastrectomy group and 177.6 (95% CI, 173.8-185.3) mg/dL (4.6 [95% CI, 4.5-4.8] mmol/L) for the gastric bypass group. LDL-C values were significantly lower (P = .02) in the gastric bypass group at 5-year follow-up compared with the sleeve gastrectomy group: 96.5 (95% CI, 88.0-100.4) mg/dL (2.5 [95% CI, 2.3-2.6] mmol/L) and 104.3 (95% CI, 100.4-112.0) mg/dL (2.7 [95% CI, 2.6-2.9] mmol/L), respectively. The mean estimates of triglyceride values across the time were 109.7 (95% CI, 102.7-116.8] mg/dL (1.2 [95% CI, 1.2-1.3] mmol/L) for the sleeve gastrectomy group and 102.7 (95% CI, 96.5-109.7) mg/dL (1.2 [95% CI, 1.1-1.2] mmol/L) for the gastric bypass group (P = .18). Mean estimates of HDL-C values across time were 53.3 (95% CI, 51.4-55.6) mg/dL (1.4 [95% CI, 1.3-1.4] mmol/L) and 53.7 (95% CI, 51.7-56.0) mg/dL (1.4 [95% CI, 1.3-1.5] mmol/L), respectively (P = .79), with no statistically significant differences between the study groups. Of the 38 patients in the whole study group who discontinued dyslipidemia medication, 22 had true dyslipidemia remission (LDL-C level <115.8 mg/dL [3.0 mmol/L] and no dyslipidemia medications) at 5-year follow-up; the remission rate was 20% (6/30) in the sleeve gastrectomy group and 40% (n = 16/40) in the gastric bypass group.
Table 4. Lipid Profiles for the Whole Study Group After Laparoscopic Sleeve Gastrectomy and Laparoscopic Roux-en-Y Gastric Bypass at Baseline, 6 Months, and 1, 3, and 5 Yearsa.
| Model-Based Mean (95% CI) in Operations | Baseline | 0.5 y | 1 y | 3 y | 5 y | P Value for Effects in ANOVA | |
|---|---|---|---|---|---|---|---|
| Total cholesterol, mmol/La | .02 for operation × time interaction | ||||||
| Sleeve gastrectomy | |||||||
| No. | 106 | 115 | 110 | 108 | 97 | ||
| Model-based mean (95% CI) | 4.5 (4.4 to 4.7) | 4.5 (4.3 to 4.7) | 4.7 (4.5 to 4.8) | 5.0 (4.8 to 5.1) | 4.9 (4.7 to 5.0) | ||
| Gastric bypass | |||||||
| No. | 106 | 107 | 105 | 100 | 91 | ||
| Model-based mean (95% CI) | 4.6 (4.4 to 4.8) | 4.1 (4.0 to 4.3) | 4.3 (4.1 to 4.5) | 4.5 (4.4 to 4.7) | 4.6 (4.5 to 4.8) | ||
| Difference | 0.4 (0.1 to 0.6) | 0.4 (0.1 to 0.6) | 0.4 (0.2 to 0.7) | 0.2 (−0.003 to 0.5) | |||
| P value (corrected with step-down Bonferroni) | .008 | .006 | <.001 | .053 | |||
| LDL-C, mmol/Lb | .04 for operation × time interaction | ||||||
| Sleeve gastrectomy | |||||||
| No. | 101 | 111 | 109 | 107 | 95 | ||
| Model-based mean (95% CI) | 2.6 (2.4 to 2.8) | 2.6 (2.5 to 2.8) | 2.6 (2.5 to 2.8) | 2.8 (2.7 to 2.9) | 2.7 (2.6 to 2.9) | ||
| Gastric bypass | |||||||
| No. | 103 | 107 | 104 | 100 | 91 | ||
| Model-based mean (95% CI) | 2.6 (2.4 to 2.7) | 2.3 (2.2 to 2.4) | 2.3 (2.2 to 2.4) | 2.4 (2.3 to 2.5) | 2.5 (2.3 to 2.6) | ||
| Difference | 0.3 (0.1 to 0.5) | 0.3 (0.2 to 0.5) | 0.4 (0.2 to 0.6) | 0.2 (0.04 to 0.5) | |||
| P value (corrected with step-down Bonferroni) | .006 | .003 | <.001 | .02 | |||
| HDL-C, mmol/Lb,c | .39 for operation × time interaction | ||||||
| Sleeve gastrectomy | 1.4 (1.3 to 1.4) | n = 105 | n = 114 | n = 109 | n = 108 | n = 97 | .79 for main effect of operation |
| Gastric bypass | 1.4 (1.3 to 1.5) | n = 106 | n = 107 | n = 105 | n = 100 | n = 91 | |
| Model-based means (95% CI) in time points | 1.2 (1.1 to 1.2) | 1.3 (1.2 to 1.3) | 1.5 (1.4 to 1.5) | 1.5 (1.5 to 1.6) | 1.5 (1.5 to 1.6) | <.01 for main effect of time | |
| Triglycerides, mmol/La,b | .14 for operation × time interaction | ||||||
| Sleeve gastrectomy | 1.2 (1.2 to 1.3) | n = 103 | n = 112 | n = 109 | n = 108 | n = 97 | .18 for main effect of operation |
| Gastric bypass | 1.2 (1.1 to 1.2) | n = 105 | n = 107 | n = 105 | n = 100 | n = 91 | |
| Model-based means (95% CI) in time points | 1.5 (1.4 to 1.6) | 1.2 (1.1 to 1.2) | 1.1 (1.0 to 1.1) | 1.1 (1.0 to 1.2) | 1.2 (1.1 to 1.3) | <.01 for main effect of time |
Abbreviations: ANOVA, analysis of variance; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
SI conversion factors: To convert total cholesterol, LDL-C, and HDL-C values to mg/dL, divide by 0.0259; triglyceride values to mg/dL, divide by 0.0113.
All results adjusted for center and diabetes status.
Repeated-measurements ANOVA.
Logarithmic transformation was used in the analyses, and results are transformed back to original scale.
Hypertension
At baseline, 170 patients (71%) were using medication for hypertension. After 5 years, 20 of 68 patients (29%) in the sleeve gastrectomy group and 37 of 73 (51%) in the gastric bypass group had discontinued hypertension medications; 24 of 68 patients (35%) in the sleeve gastrectomy group and 22 of 73 (30%) in the gastric bypass group needed less hypertension medications compared with baseline; and no improvement in hypertension medication use was detected in 24 of 68 patients (35%) in the sleeve gastrectomy group and 14 of 73 (19%) in the gastric bypass group (P = .02).
Quality of Life
At baseline, the mean QOL total score (Moorehead-Ardelt QOL) was 0.10 (SD, 0.94) in the sleeve gastrectomy group and 0.12 (SD, 1.12) in the gastric bypass group; mean QOL total scores were 0.85 (SD, 1.08) at 5 years after sleeve gastrectomy (n = 90) and 0.76 (SD, 1.01) after gastric bypass (n = 86). The change in QOL did not differ significantly between the study groups (P = .70 for operation × time interaction). There was no statistically significant difference in QOL between groups (P = .85), but total QOL score increased statistically significantly by 0.7 (95% CI, 0.6-0.9) units from baseline to the 5-year follow-up point (P < .001).
Morbidity and Mortality
The 30-day and 6-month complications have been reported, and detailed early complications (<30 days) and late complications (>30 days) are reported in Table 5. For this study, the late complications were evaluated from 30 days after surgery until the 5-year follow-up, with an overall morbidity rate of 19% (n = 23) for sleeve gastrectomy and 26% (n = 31) for gastric bypass (P = .19). The late minor complication rate (Clavien-Dindo I-IIIa) was 10.7% (n = 13) in the sleeve gastrectomy group and 10.9% (n = 13) in the gastric bypass group (P = .96). The late major complications in both study groups were all reoperations; the late major complication rate (Clavien-Dindo IIIb) was 8.3% (n = 10) after sleeve gastrectomy and 15.1% (n = 18) after gastric bypass (P = .10). Seven of the 10 reoperations after sleeve gastrectomy were performed for severe reflux, with patients undergoing conversion to gastric bypass at a median of 14 months (range, 6-59 months). In the gastric bypass group, 17 patients underwent reoperation for suspected internal herniation; all of these patients had closure of the mesenteric defect at repeat laparoscopy. There was no treatment-related mortality during the 5-year follow-up.
Table 5. Complications Reported for Laparoscopic Sleeve Gastrectomy and Laparoscopic Roux-en-Y Gastric Bypass.
| Complication Category and Type | Sleeve Gastrectomy (n = 121) | Gastric Bypass (n = 119) | P Value |
|---|---|---|---|
| Minor Early (<30 d) Complications, No. (%) | |||
| Bleeding | 3 (2.5) | 2 (1.7) | |
| Intra-abdominal infection/infection of unknown origin | 2 (1.7) | 8 (6.8) | |
| Pneumonia | 1 (0.8) | 6 (5.1) | |
| Superficial wound infection | 2 (1.7) | 3 (2.6) | |
| Troacar site pain | 1 (0.8) | ||
| Dehydration | 1 (0.9) | ||
| Total | 9 (7.4) | 20 (17.1) | .02 |
| Major Early (<30 d) Complications, No. (%) | |||
| Bleeding | 3 (2.5) | 7 (6.0) | |
| Intra-abdominal infection/infection of unknown origin | 1 (0.8) | 3 (2.6) | |
| Pneumonia | 1 (0.8) | ||
| Bowel perforation | 1 (0.8) | ||
| Torsion of the enteroanastomosis | 1 (0.9) | ||
| Outlet obstruction | 1 (0.8) | ||
| Total | 7 (5.8) | 11 (9.4) | .29 |
| Minor Late (>30 d) Complications, No. (%) | |||
| Vomiting/dehydration | 3 (2.5) | ||
| Gastroesophageal reflux | 11 (9.1) | ||
| Ulcer/stricture at gastrojejunal anastomosis | 2 (1.7) | 6 (5.0) | |
| Dumping | 3 (2.5) | ||
| Nonspecific abdominal pain | 1 (0.8) | ||
| Total | 13 (10.7) | 13 (10.9) | .96 |
| Major Late (>30 d) Complications, No. (%) | |||
| Gastroesophageal reflux | 7(5.8) | ||
| Internal herniation | 17 (14.3) | ||
| Incisional hernia | 3 (2.5) | 1 (0.8) | |
| Total | 10 (8.3) | 18 (15.1) | .10 |
Post Hoc Outcomes
The change in BMI differed significantly between the study groups (P < .001 for operation × time interaction). At 5-year follow-up, the mean estimate of BMI was 1.1 (95% CI, −0.5 to 2.6) units higher after sleeve gastrectomy, without statistically significant difference between the operations (P = .54). For patients with diabetes, BMI results at 5 years were similar to the results in the whole study group; the mean estimate of BMI was 2.1 (95% CI, −0.2 to 4.5) units higher after sleeve gastrectomy compared with gastric bypass (P = .29). For patients with diabetes, study groups were not equivalent with regard to percentage excess weight loss at any of the points: at 5-year follow-up, the estimate of mean percent excess weight loss was 11.7% (95% CI, 3.7%-19.7%) higher in patients undergoing gastric bypass than in those undergoing sleeve gastrectomy. These results are presented in detail in Table 2 and Figure 3.
At 5-year follow-up, the median micronutrient levels, regardless of possible vitamin supplementation, after sleeve gastrectomy and gastric bypass were 33 (range, 7-61) ng/mL (83 [range, 17-153] nmol/L) and 31 (range, 12-64) ng/mL (77 [range, 30-160] nmol/L) (P = .24), respectively, for vitamin D, 54 (range, 79-1999) pg/mL (409 [range, 58-1475] pmol/L) and 484 pg/mL (range, 153-2006) pg/mL (357 [range, 113-1480] pmol/L) (P = .07) for vitamin B12, 37 (range, 30-45) g/L and (range, 31-71) g/L (P = .28) for albumin, and 328 (range, 117-1277) ng/mL (743 [range, 265-2893] nmol/L) and 384 (range, 98-840) ng/mL (801 [range, 221-1904] nmol/L) (P = .78) for folate.
Discussion
Among patients with morbid obesity, use of laparoscopic sleeve gastrectomy compared with use of laparoscopic Roux-en-Y gastric bypass did not meet criteria for equivalence in terms of percentage excess weight loss at 5 years. Although gastric bypass compared with sleeve gastrectomy was associated with greater percentage excess weight loss at 5 years, the confidence interval for the difference extended both above and below the prespecified equivalence margin, and therefore no conclusion can be drawn about whether gastric bypass is clinically superior to sleeve gastrectomy. However, both procedures resulted in sustained weight loss, with a mean excess weight loss of 49% in the sleeve gastrectomy group and 57% in the gastric bypass group. There were no statistically significant differences between sleeve gastrectomy and gastric bypass for the secondary outcomes of type 2 diabetes remission, dyslipidemia resolution, QOL improvement, and late morbidity. Compared with sleeve gastrectomy, gastric bypass resulted in better resolution of hypertension, based on antihypertensive medication use.
The effectiveness of bariatric procedures should be assessed from long-term outcomes information, since obesity is a chronic disease, as are its comorbidities. With 240 patients, the SLEEVEPASS trial is, to our knowledge, the largest randomized clinical trial comparing laparoscopic sleeve gastrectomy with laparoscopic Roux-en-Y gastric bypass. The Swiss Multicenter Bypass or Sleeve Study (SM-BOSS) had a protocol very similar to that of the SLEEVEPASS trial, included 217 patients, and had findings similar to those from our study. Over long-term follow-up, gastric bypass showed significantly better weight loss than sleeve gastrectomy at each time point, evaluated by percentage excess weight loss (Figure 4). These findings are consistent with most previously published series and meta-analyses, taking into account the variation in definitions for reporting weight loss outcomes after bariatric surgery. The difference in weight loss between the gastric bypass and sleeve gastrectomy groups increased with time. Longer-term follow-up may help determine if these differences result from loss of effect of sleeve gastrectomy or from greater weight loss after gastric bypass.
In general, bariatric surgery has better outcomes than does intensive medical therapy for treating type 2 diabetes. Using the American Diabetes Association triple end point of HbA1c value, LDL-C level, and systolic blood pressure as the primary outcome, the Diabetes Surgery Study found that laparoscopic Roux-en-Y gastric bypass resulted in better outcomes than did intensive medical management using the Look AHEAD protocol. The Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) trial also showed greater efficacy for both laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy than medical therapy alone in decreasing or resolving hyperglycemia at 5-year follow-up. At 5 years, there were no significant differences in diabetes control between the 2 surgical procedures, but the single-surgeon, single-institution STAMPEDE study was not powered for detecting differences in this outcome.
In this study, the diabetes remission rate did not differ between the procedures at 5 years, but the diabetes remission rate was lower than that in the SM-BOSS trial. This may be attributable to possible differences in preoperative diabetes duration, because shorter diabetes duration at baseline is associated with more favorable short-term remission rates after bariatric surgery. Neither the current trial nor SM-BOSS were powered to detect differences for diabetes remission between sleeve gastrectomy and gastric bypass. However, 101 patients (42%) in this trial had baseline diabetes, a rate that was higher compared with other randomized clinical trials comparing sleeve gastrectomy and gastric bypass. As was found in other studies, in a post hoc analysis, the estimated mean percentage excess weight loss difference between the procedures was higher for patients with diabetes than for the whole study group, which also included patients without diabetes.
Although the dyslipidemia remission rate was not significantly different between the gastric bypass and sleeve gastrectomy groups, LDL-C levels were significantly lower in patients in the gastric bypass group than in those in the sleeve gastrectomy group—findings consistent with similar observations made in other studies. Hypertension remission rates were significantly better after gastric bypass, based on patients using fewer antihypertension medications. This observation is tempered by reliance on medication use as an indicator for hypertension, since medication adherence may not be optimal. This may explain the discrepancies observed in this study between dyslipidemia remission rates estimated from medication cessation relative to those estimated from measured LDL-C values.
One potential drawback of sleeve gastrectomy is the exacerbation or new onset of gastroesophageal reflux and high incidence reported for Barrett esophagus after this procedure, although there are discrepancies in rates for Barrett esophagus between the various studies. Nevertheless, some authors have suggested that the presence of severe reflux and Barrett esophagus are contraindications for sleeve gastrectomy. In the current study, 7 patients (6%) in the sleeve gastrectomy group underwent conversion from sleeve gastrectomy to gastric bypass for severe reflux, and 11 (9%) required daily proton pump inhibitors. All of the patients in this study underwent preoperative upper gastrointestinal endoscopy and, if severe gastroesophageal reflux disease with large hiatal hernia was found, those patients were excluded from the study; however, there was no standardized evaluation of hiatal hernia size or use of a validated symptom questionnaire. In addition, 3 sleeve gastrectomy procedures were converted into single-anastomosis duodeno-ileal bypass procedures without any trial protocol consensus; these 3 cases were considered protocol violations. In the gastric bypass group, almost all of the late reoperations were for suspected internal hernias. These conceivably could have been prevented by closure of mesenteric defects during gastric bypass, since these defects were not routinely closed in the operations performed in this study.
When this trial was designed in 2007, it was common to assess bariatric surgical success by reporting excess weight loss or excess BMI loss. Since the time the study was designed, outcome reporting standards were adopted, with percentage weight loss relative to baseline weight now being the standard. The findings for percentage total weight loss are presented in the eTable in Supplement 3 to facilitate comparison of these findings with those from other studies.
This study has several strengths. Major advantages include the large number of patients enrolled, the long-term follow-up of 5 years with a high degree of follow-up (>80%), and the randomized, multicenter, multisurgeon trial design, making the study results more generalizable to routine surgical practice.
This study also has several limitations. First, only a small number (n = 430) of bariatric procedures were performed in Finland (5.5 million inhabitants) at trial initiation in 2008. A learning curve effect could have accounted for some of the technical complications observed earlier in the trial, resulting in a higher reoperation rate for gastric bypass. Second, the study had a higher reoperation rate for sleeve gastrectomy than reported in other studies, which also may be based on learning curve effect. Third, insufficient information was available for patients excluded from the study. Nevertheless, the trial population can be considered representative of the average bariatric surgery population, because the study group included most patients undergoing bariatric surgery at the study hospitals during the study enrollment period. Fourth, approximately 20% of the patient population was lost to follow-up, precluding a strict intention-to-treat analysis. However, the drop-out rates were similar in both groups. Multiple-imputation analysis suggested that there was little risk for bias when percentage excess weight loss was compared between the 2 procedures. Fifth, reliable information regarding diabetes duration at baseline was lacking, which constitutes a limitation when evaluating the remission rate for type 2 diabetes, because diabetes duration has been shown to be associated with predicted long-term remission.
Future studies should focus on assessing all factors affecting the sustainable long-term results of these 2 bariatric procedures to enhance optimal preoperative selection of the best possible procedure for each patient.
Conclusions
Among patients with morbid obesity, use of laparoscopic sleeve gastrectomy compared with use of laparoscopic Roux-en-Y gastric bypass did not meet criteria for equivalence in terms of percentage excess weight loss at 5 years. Although gastric bypass compared with sleeve gastrectomy was associated with greater percentage excess weight loss at 5 years, the difference was not statistically significant, based on the prespecified equivalence margins.
Study Protocol
Statistical Analysis Plan
eTable. Percentage Total Weight Loss and Weight, Model-Based Means After Laparoscopic Sleeve Gastrectomy and Laparoscopic Roux-en-Y Gastric Bypass at Baseline, 6 months, and 1, 3, and 5 Years
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puzziferri N, Roshek TB III, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helmiö M, Victorzon M, Ovaska J, et al. SLEEVEPASS: a randomized prospective multicenter study comparing laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: preliminary results. Surg Endosc. 2012;26(9):2521-2526. [DOI] [PubMed] [Google Scholar]
- 4.Helmiö M, Victorzon M, Ovaska J, et al. Comparison of short-term outcome of laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: a prospective randomized controlled multicenter SLEEVEPASS study with 6-month follow-up. Scand J Surg. 2014;103(3):175-181. [DOI] [PubMed] [Google Scholar]
- 5.Brethauer SA, Kim J, El Chaar M, et al. ; ASMBS Clinical Issues Committee . Standardized outcomes reporting in metabolic and bariatric surgery. Obes Surg. 2015;25(4):587-606. [DOI] [PubMed] [Google Scholar]
- 6.Moorehead MK, Ardelt-Gattinger E, Lechner H, Oria HE. The validation of the Moorehead-Ardelt Quality of Life Questionnaire II. Obes Surg. 2003;13(5):684-692. [DOI] [PubMed] [Google Scholar]
- 7.Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32(11):2133-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catapano AL, Reiner Z, De Backer G, et al. ; European Society of Cardiology (ESC); European Atherosclerosis Society (EAS) . ESC/EAS guidelines for the management of dyslipidaemias :the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis. 2011;217(1):3-46. [DOI] [PubMed] [Google Scholar]
- 9.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37(3):383-393. [DOI] [PubMed] [Google Scholar]
- 10.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeMaria EJ, Sugerman HJ, Kellum JM, Meador JG, Wolfe LG. Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg. 2002;235(5):640-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higa KD, Ho T, Boone KB. Laparoscopic Roux-en-Y gastric bypass: technique and 3-year follow-up. J Laparoendosc Adv Surg Tech A. 2001;11(6):377-382. [DOI] [PubMed] [Google Scholar]
- 13.Himpens J, Dapri G, Cadiere GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16(11):1450-1456. [DOI] [PubMed] [Google Scholar]
- 14.Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. doi: 10.1001/jama.2017.20897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401-407. [DOI] [PubMed] [Google Scholar]
- 16.Kehagias I, Karamanakos SN, Argentou M, Kalfarentzos F. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI <50 kg/m2. Obes Surg. 2011;21(11):1650-1656. [DOI] [PubMed] [Google Scholar]
- 17.Schauer PR, Bhatt DL, Kirwan JP, et al. ; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhao H, Cao Z, et al. A randomized clinical trial of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy for the treatment of morbid obesity in China: a 5-year outcome. Obes Surg. 2014;24(10):1617-1624. [DOI] [PubMed] [Google Scholar]
- 19.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149(3):275-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Lai D, Wu D. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy to treat morbid obesity-related comorbidities: a systematic review and meta-analysis. Obes Surg. 2016;26(2):429-442. [DOI] [PubMed] [Google Scholar]
- 21.Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg. 2015;150(10):931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014;149(7):716-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309(21):2240-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterli R, Wölnerhanssen BK, Vetter D, et al. Laparoscopic sleeve gastrectomy versus Roux-Y-gastric bypass for morbid obesity—3-year outcomes of the prospective randomized Swiss Multicenter Bypass Or Sleeve Study (SM-BOSS). Ann Surg. 2017;265(3):466-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon JB, Chuang LM, Chong K, et al. Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care. 2013;36(1):20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297-2304. [DOI] [PubMed] [Google Scholar]
- 27.Keidar A, Hershkop KJ, Marko L, et al. Roux-en-Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trial. Diabetologia. 2013;56(9):1914-1918. [DOI] [PubMed] [Google Scholar]
- 28.Lauffenburger JC, Shrank WH, Bitton A, et al. Association between patient-centered medical homes and adherence to chronic disease medications: a cohort study. Ann Intern Med. 2017;166(2):81-88. [DOI] [PubMed] [Google Scholar]
- 29.Arman GA, Himpens J, Dhaenens J, Ballet T, Vilallonga R, Leman G. Long-term (11+ years) outcomes in weight, patient satisfaction, comorbidities, and gastroesophageal reflux treatment after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2016;12(10):1778-1786. [DOI] [PubMed] [Google Scholar]
- 30.DuPree CE, Blair K, Steele SR, Martin MJ. Laparoscopic sleeve gastrectomy in patients with preexisting gastroesophageal reflux disease: a national analysis. JAMA Surg. 2014;149(4):328-334. [DOI] [PubMed] [Google Scholar]
- 31.Felsenreich DM, Kefurt R, Schermann M, et al. Reflux, sleeve dilation, and Barrett’s esophagus after laparoscopic sleeve gastrectomy: long-term follow-up. Obes Surg. 2017;27(12):3092-3101. [DOI] [PubMed] [Google Scholar]
- 32.Oor JE, Roks DJ, Ünlü Ç, Hazebroek EJ. Laparoscopic sleeve gastrectomy and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Surg. 2016;211(1):250-267. [DOI] [PubMed] [Google Scholar]
- 33.Melissas J, Braghetto I, Molina JC, et al. Gastroesophageal reflux disease and sleeve gastrectomy. Obes Surg. 2015;25(12):2430-2435. [DOI] [PubMed] [Google Scholar]
- 34.Stenberg E, Szabo E, Ågren G, et al. Closure of mesenteric defects in laparoscopic gastric bypass: a multicentre, randomised, parallel, open-label trial. Lancet. 2016;387(10026):1397-1404. [DOI] [PubMed] [Google Scholar]
- 35.Stenberg E, Szabo E, Ottosson J, Näslund I. Outcomes of laparoscopic gastric bypass in a randomized clinical trial compared with a concurrent national database. Br J Surg. 2017;104(5):562-569. [DOI] [PubMed] [Google Scholar]
- 36.Brethauer SA, Aminian A, Romero-Talamás H, et al. Can diabetes be surgically cured? long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258(4):628-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiménez A, Casamitjana R, Flores L, et al. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256(6):1023-1029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
Statistical Analysis Plan
eTable. Percentage Total Weight Loss and Weight, Model-Based Means After Laparoscopic Sleeve Gastrectomy and Laparoscopic Roux-en-Y Gastric Bypass at Baseline, 6 months, and 1, 3, and 5 Years