Key Points
Question
Is bariatric surgery, as compared with specialized medical obesity treatment, associated with improvement and prevention of metabolic comorbidities but higher rates of complications?
Findings
In this cohort study of 1888 treatment-seeking adult patients with severe obesity who underwent either bariatric surgery or specialized medical treatment with a median 6.5 years of follow-up, bariatric surgery was associated with higher complication rates, including any gastrointestinal surgery (risk difference, 16%), gastroduodenal ulcers (risk difference, 4.7%), and iron deficiency (risk difference, 14%). Surgery was associated with better outcomes for hypertension, diabetes, and dyslipidemia.
Meaning
Although bariatric surgery has the potential to improve obesity-related comorbidities, clinically important rates of complication should be considered in the decision-making process.
Abstract
Importance
The association of bariatric surgery and specialized medical obesity treatment with beneficial and detrimental outcomes remains uncertain.
Objective
To compare changes in obesity-related comorbidities in patients with severe obesity (body mass index ≥40 or ≥35 and at least 1 comorbidity) undergoing bariatric surgery or specialized medical treatment.
Design, Setting, and Participants
Cohort study with baseline data of exposures from November 2005 through July 2010 and follow-up data from 2006 until death or through December 2015 at a tertiary care outpatient center, Vestfold Hospital Trust, Norway. Consecutive treatment-seeking adult patients (n = 2109) with severe obesity assessed (221 patients excluded and 1888 patients included).
Exposures
Bariatric surgery (n = 932, 92% gastric bypass) or specialized medical treatment (n = 956) including individual or group-based lifestyle intervention programs.
Main Outcomes and Measures
Primary outcomes included remission and new onset of hypertension based on drugs dispensed according to the Norwegian Prescription Database. Prespecified secondary outcomes included changes in comorbidities. Adverse events included complications retrieved from the Norwegian Patient Registry and a local laboratory database.
Results
Among 1888 patients included in the study, the mean (SD) age was 43.5 (12.3) years (1249 women [66%]; mean [SD] baseline BMI, 44.2 [6.1]; 100% completed follow-up at a median of 6.5 years [range, 0.2-10.1]). Surgically treated patients had a greater likelihood of remission and lesser likelihood for new onset of hypertension (remission: absolute risk [AR], 31.9% vs 12.4%); risk difference [RD], 19.5% [95% CI, 15.8%-23.2%], relative risk [RR], 2.1 [95% CI, 2.0-2.2]; new onset: AR, 3.5% vs 12.2%, RD, 8.7% [95% CI, 6.7%-10.7%], RR, 0.4 [95% CI, 0.3-0.5]; greater likelihood of diabetes remission: AR, 57.5% vs 14.8%; RD, 42.7% [95% CI, 35.8%-49.7%], RR, 3.9 [95% CI, 2.8-5.4]; greater risk of new-onset depression: AR, 8.9% vs 6.5%; RD, 2.4% [95% CI, 1.3%-3.5%], RR, 1.5 [95% CI, 1.4-1.7]; and treatment with opioids: AR, 19.4% vs 15.8%, RD, 3.6% [95% CI, 2.3%-4.9%], RR, 1.3 [95% CI, 1.2-1.4]). Surgical patients had a greater risk for undergoing at least 1 additional gastrointestinal surgical procedure (AR, 31.3% vs 15.5%; RD, 15.8% [95% CI, 13.1%-18.5%]; RR, 2.0 [95% CI, 1.7-2.4]). The proportion of patients with low ferritin levels was significantly greater in the surgical group (26% vs 12%, P < .001).
Results
Among 1888 patients included in the study, the mean (SD) age was 43.5 (12.3) years (1249 women [66%]; mean [SD] baseline body mass index, 44.2 [6.1]; 100% completed follow-up at a median of 6.5 years [range, 0.2-10.1]). Surgically treated patients had a greater likelihood of hypertension remission and lesser likelihood for new-onset hypertension. Surgically treated patients also had a greater likelihood of diabetes remission, risk of new-onset depression, treatment initiation with opioids, and greater risk of undergoing at least 1 additional gastrointestinal surgical procedure. The proportion of patients with low ferritin levels was significantly greater in the surgical group (26% vs 12%, P < .001).
| Absolute Risk, % (95% CI) | Risk Difference, % (95% CI) | Relative Risk (95% CI) | ||
|---|---|---|---|---|
| Surgical Group (n = 932) | Medical Group (n = 956) | |||
| Hypertension remission | 31.9 (28.4-35.4) | 12.4 (9.5-15.3) | 19.5 (15.8-23.2) | 2.1 (2.0-2.2) |
| Hypertension, new onset | 3.5 (2.2-4.9) | 12.2 (9.3-15.1) | 8.7 (6.7-10.7) | 0.4 (0.3-0.5) |
| Diabetes remission | 57.5 (53.8-61.2) | 14.8 (11.7-17.9) | 42.7 (35.8-49.7) | 3.9 (2.8-5.4) |
| Depression, new onset | 8.9 (6.8-11.0) | 6.5 (4.3-8.7) | 2.4 (1.3-3.5) | 1.5 (1.4-1.7) |
| Opioid use, new onset | 19.4 (16.5-22.3) | 15.8 (13.1-18.5) | 3.6 (2.3-4.9) | 1.3 (1.2-1.4) |
| Any gastrointestinal surgery | 31.3 (26.0-36.5) | 15.5 (12.8-18.2) | 15.8 (13.1-18.5) | 2.0 (1.7-2.4) |
Conclusions and Relevance
Among patients with severe obesity followed up for a median of 6.5 years, bariatric surgery compared with medical treatment was associated with a clinically important increased risk for complications, as well as lower risks of obesity-related comorbidities. The risk for complications should be considered in the decision-making process.
This cohort study uses Norwegian registry data to compare long-term changes in hypertension, diabetes, depression, and opioid use among patients with severe obesity treated with bariatric surgery or specialized medical treatment.
Introduction
Obesity is associated with increased morbidity and mortality due to cardiometabolic disease, cancer, and mental disorders. Current treatment options for severe obesity encompass medical therapy and bariatric surgery. Intensive lifestyle intervention is associated with 5% to 8% weight loss and improvement of cardiovascular risk factors, while bariatric surgery is associated with larger weight loss and medium- to long-term (1- to 5-year) remission of hypertension, type 2 diabetes, and dyslipidemia. Bariatric surgery is associated with a number of somatic and mental complications, but few studies report long-term complication rates.
Pragmatic studies comparing clinical outcomes after surgical and medical treatment may complement randomized explanatory trials by providing data that may help clinicians and patients make shared decisions about treatment choice. To our knowledge, no large-scale clinical practice–based study has compared the long-term association of bariatric surgery and specialized medical obesity treatment with obesity-related somatic and mental comorbidities, nor their respective complication rates.
In a publicly funded tertiary care setting, this study aimed to compare the long-term outcomes of bariatric surgery and specialized medical obesity treatment. It was hypothesized that, as compared with medical therapy, bariatric surgery would be associated with higher rates of remission, and lower rates of new-onset drug-treated hypertension, diabetes, and dyslipidemia, at the expense of higher medical, mental, and surgical complication rates.
Methods
Study Design
This registry-based cohort study conducted at Vestfold Hospital Trust, Norway, was designed to compare changes in drug-treated obesity-related comorbidities among patients with severe obesity (body mass index [BMI, calculated as weight in kilograms divided by height in meters squared] ≥40 or BMI ≥35 and at least 1 obesity-related comorbidity) undergoing bariatric surgery or specialized medical obesity treatment. Baseline data were collected from the Registry- and Biobank (RB) study, which contains complete clinical and laboratory information from all included patients at their first consultation at the center. The RB study was approved by the Regional Committee for Medical and Health Research Ethics (reference No. S-05175), and the present study, including retrieval of data from the Norwegian Patient Registry, was approved by South East Regional Committee for Medical and Health Research Ethics (reference No. 2010/2329). Linkage to the Norwegian Prescription Database (NorPD) was approved by the Data Protection Authority (reference No. 16/00135), and the linkage of data was performed according to guidelines and legal requirements. Written informed consent was obtained from every participant.
Setting
The Morbid Obesity Center is a publicly funded tertiary care center, accredited by the European Association for the Study of Obesity as an EASO Collaborating Centre for Obesity Management, providing both specialized medical and surgical obesity treatment. In the present study, the physicians informed the patients about beneficial and detrimental effects of various treatment options and encouraged the patients to incorporate their own values and preferences in the decision-making process before the patient and physician agreed on the specific choice of therapy: medical therapy or bariatric surgery. Patients who opted for medical treatment could choose between individual- or group-based programs, either in the outpatient clinic or at a rehabilitation center. The rehabilitation centers offered multidisciplinary intensive lifestyle intervention programs as previously reported (eAppendix 1 in the Supplement). Patients were offered clinical follow-up at the tertiary clinic for at least 2 years after finishing the surgical or medical treatment programs.
Participants
Consecutive treatment-seeking patients referred to a tertiary care center were informed about the research question before giving their written informed consent and before collection of baseline and follow-up data. Those patients enrolled in the RB study from November 2005 until July 2010 were informed by letter about the present follow-up study, and they were given the choice of declining participation. Information on treatment choice was obtained from electronic patient records in July 2015, and information on drugs was obtained from the NorPD in June 2016. The NorPD contains information on all prescribed drugs dispensed from pharmacies to patients living outside institutions and data on all-cause mortality from the National Registry. Anonymous data on medical complications and surgical procedures were obtained from the Norwegian Patient Registry from 2008 to 2015. Laboratory follow-up data were obtained from the laboratory files of Vestfold Hospital Trust. The physician completed the standard registry questionnaire regarding race by fixed categories (white: yes or no). This variable was included to show the predominance of white patients, which reflects the Norwegian population (>90% white).
Definitions and Outcomes
Baseline, including start of medical treatment, was defined as the date of the first consultation and inclusion in the RB study, while start of surgical treatment was the operation date. Comorbidities were defined according to their respective Anatomical Therapeutic Chemical (ATC) codes and defined as present in patients who had dispensed at least 1 prescribed drug the year before treatment and within each year of follow-up (eTable 1 in the Supplement). Dispensing of specific drugs (yes/no) from the year before start of treatment was used to categorize each patient as an initial user or nonuser of a given drug. The prevalence of specific comorbidities was defined as the proportion of patients who were dispensed a drug from the defined ATC codes. Patients who no longer received drugs related to a specific comorbidity were categorized as having remission, while those who had a drug dispensed for the first time were categorized as having new-onset comorbidity. Medical complications and surgical procedures were defined according to International Statistical Classification of Diseases and Related Health Problems, Tenth Revision and NOMESCO Classification of Surgical Procedures (eTable 2 in the Supplement).
The proportion of patients with anemia, hypoproteinemia, and vitamin and mineral deficiencies more than 3 months after the start of treatment were assessed in a subgroup of patients with available laboratory results. Detailed methods for baseline measurements in the database are described elsewhere. The end of follow-up was December 31, 2015, or death, whichever came first.
Study Size
It was estimated that the proportions of patients with remission of hypertension at 5 years (primary outcome) would be 35% and 15% in the surgical and medical group, respectively, and the basis for these assumptions is found in the trial protocol (eAppendix 2 in the Supplement). To achieve power greater than 85%, 170 patients with baseline hypertension should be followed up for 5 years or more. However, the final sample size was much larger, resulting in a post-hoc power greater than 99% (Table 1).
Table 1. Baseline Characteristics of Patients Who Underwent Either Surgical or Specialized Medical Treatment for Severe Obesity.
| Characteristic | Surgical Group (n = 932) |
Medical Group (n = 956) |
P Value |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD), y | 41.2 (10.5) | 45.7 (13.5) | <.001 |
| Women, No. (%) | 640 (68.7) | 609 (63.7) | .03 |
| White, No. (%) | 906 (97.4) | 930 (97.3) | .44 |
| Coronary heart disease, No. (%) | 33 (3.5) | 59 (6.2) | .01 |
| Family history of cardiovascular disease, No. (%) | 329 (35.3) | 353 (36.9) | .47 |
| Family history of diabetes, No. (%) | 318 (34.2) | 313 (32.7) | .53 |
| Duration of diabetes, median (range), y | 4 (1-38) | 5 (0-37) | .11 |
| Onset of obesity, No. (%) | |||
| <12 y | 326 (35.3) | 291 (30.4) | <.001 |
| 12-20 y | 262 (28.4) | 202 (21.1) | |
| >20 y | 335 (36.3) | 457 (47.8) | |
| General measures, mean (SD) | |||
| Height, cm | 171.4 (9.4) | 171.4 (9.1) | .99 |
| Weight, kg | 133.8 (22.9) | 126.5 (20.7) | <.001 |
| BMI | 45.4 (6.2) | 42.9 (5.7) | <.001 |
| Waist circumference, cm | 133.0 (14.7) | 128.4 (14.0) | <.001 |
| Systolic blood pressure, mm Hg | 132 (15.4) | 132 (16.4) | .58 |
| Diastolic blood pressure, mm Hg | 82 (9.6) | 81 (9.9) | .01 |
| Laboratory values | |||
| Hemoglobin A1c, mean (SD), % | 6.1 (1.2) | 6.1 (1.4) | .12 |
| Normal glucose tolerance (hemoglobin A1c <5.7%), No. (%)a | 421 (45.2) | 439 (45.9) | .61 |
| Prediabetes (hemoglobin A1c 5.7%-6.4%), No. (%)a | 249 (26.7) | 223 (23.3) | .04 |
| Fasting serum insulin, mean (SD), μU/mL | 18.6 (12.6) | 17.6 (20.0) | .20 |
| Median (Q1-Q3) | 15.7 (10.5-22.6) | 14.4 (9.5-21.9) | |
| Total cholesterol, mean (SD), mg/dL | 197 (38) | 195 (34) | .18 |
| HDL cholesterol, mean (SD), mg/dL | 46 (12) | 47 (13) | .17 |
| LDL cholesterol, mean (SD), mg/dL | 120 (34) | 116 (35) | .12 |
| LDL cholesterol ≥100 mg/dL, No. (%) | 673 (73.9) | 651 (68.1) | .04 |
| Triglycerides, mean (SD), mg/dL | 168 (141) | 169 (146) | .59 |
| C-reactive protein, median (Q1-Q3), mg/L | 1.4 (0.1-1.7) | 0.8 (0.1-1.5) | .01 |
| Hemoglobin, median (Q1-Q3), g/100 mL | 14.1 (13.2-14.9) | 14.1 (13.3-15.0) | .28 |
| Ferritin, median (Q1-Q3), μg/L | 77 (41-153) | 83 (40-153) | .63 |
| Vitamin D, median (Q1-Q3), ng/mL | 50 (38-64) | 50 (38-65) | .94 |
| Calcium, total, median (Q1-Q3), mg/dL | 9.3 (9.1-9.6) | 9.3 (9.1-9.6) | .95 |
| Medical conditions and use of drugs, No (%) | |||
| Anxiety or depression (self-reported) | 365 (39.2) | 398 (41.6) | .28 |
| Obstructive sleep apnea | 160 (17.2) | 200 (20.9) | .04 |
| Treatment with CPAP or BiPAP | 102 (10.9) | 147 (15.4) | .01 |
| Use of antihypertensivesb | 430 (47.8) | 467 (48.8) | .24 |
| Use of antidiabeticsb | 236 (25.3) | 255 (26.7) | .84 |
| Insulin treatmentb | 51 (5.5) | 54 (5.6) | .92 |
| Metformin treatment aloneb | 157 (16.8) | 161 (16.8) | .99 |
| Use of lipid-modifying agentsb | 198 (21.2) | 222 (20.9) | .30 |
| Use of antidepressantsb | 196 (21.0) | 192 (20.1) | .72 |
| Use of anxiolytics, hypnotics, and sedativesb | 242 (26.0) | 258 (27.0) | .51 |
| Use of opioidsb | 339 (36.4) | 270 (28.2) | <.01 |
| Smoking, No. (%) | |||
| Current | 258 (27.7) | 235 (24.6) | .31 |
| Former | 296 (31.8) | 309 (32.3) | |
| Never | 378 (40.6) | 409 (42.8) |
Abbreviations: BiPAP, bilevel positive airway pressure; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CPAP, continuous positive airway pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
SI conversion factors: To convert C-reactive protein to nmol/L, multiply by 9.524; serum insulin to pmol/L, multiply by 6.945; total, HDL, and LDL cholesterol to mmol/L, multiply by 0.0259; and triglycerides to mmol/L, multiply by 0.0113.
Patients using antidiabetics at start of treatment excluded.
Use of drugs at inclusion for patients in the medical group and at date of surgery for patients in the surgical group. All other measurements are at inclusion for both groups.
Statistical Analysis
Crude comparisons between groups were performed using independent-samples t test, Mann-Whitney Wilcoxon test, or χ2 test as appropriate. To assess possible differences between treatment groups when all measurements were considered, binary logistic regressions were applied using mixed models for repeated measures. Possible confounders (age, sex, and BMI at baseline) and an interaction term group by time were initially included into the models. However, the latter was not statistically significant so group by time was removed from the models to increase precision in the remaining estimates. As patients with higher BMI tend to opt for bariatric surgery, and to minimize selection bias, BMI was included in the multivariable models.
Two models for each selected drug code modeling remission and new onset were fitted separately. Odds ratios were transformed to relative risk (RRs) with 95% CIs, as suggested by Zhang and Yu, and comparative absolute rates and weighted risk differences are presented for most outcomes. Overall incidence was estimated as a weighted average of all the yearly incidences. The weights used to transform odds ratios to RRs were constructed as proportions of individuals followed up at a given point. Sensitivity analyses were performed, excluding either those who did not undergo gastric bypass (n = 77), the outpatient group (n = 492), or the rehabilitation center group (n = 464). The analyses were not adjusted for multiple testing, but P values less than .01 were considered statistically significant. Therefore, except for the primary outcomes, the study findings should be considered exploratory. All tests were 2-sided. All analyses were performed using SPSS version 22 (SPSS Inc) and Stata version 9 (StataCorp).
Results
Participant Characteristics
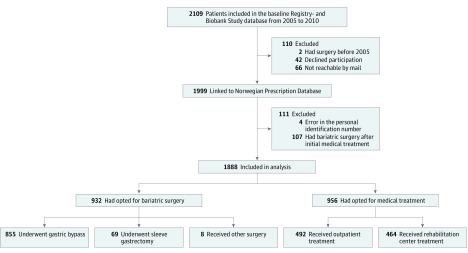
A total of 2109 consecutive patients with severe obesity were assessed for eligibility. After the exclusion of 221 patients, including 107 medically treated patients who later underwent bariatric surgery, complete data from 1888 patients were included in the prespecified analyses (Figure 1). A total of 956 patients (51%) voluntarily opted for specialized medical treatment, while 932 patients (49%) voluntarily opted for bariatric surgery, mostly gastric bypass (92%) or sleeve gastrectomy (7%).
Figure 1. Flowchart of Patients With Severe Obesity Treated With Either Bariatric Surgery or Specialized Medical Treatment.
At baseline, the patients had a mean (SD) age of 43.5 (12.3) years and BMI of 44.2 (6.1) (66% were women). As compared with medical patients, surgically treated patients were significantly younger with a higher BMI and earlier debut of obesity (Table 1). The patients were followed up for a median of 6.5 years (range, 0.2-10.1 years) after the start of treatment, and the mean (SD) time from the first consultation to surgery was 23 (12) months. The total median follow-up durations in the medical and surgical treatment groups were 7.1 years (range, 0.2-10.1 years) and 7.8 years (range, 2.5-10.0 years), respectively, and the median follow-up after the bariatric surgical procedure was 5.8 years (range, 0.6-9.2 years).
Prevalence of Obesity-Related Comorbidities
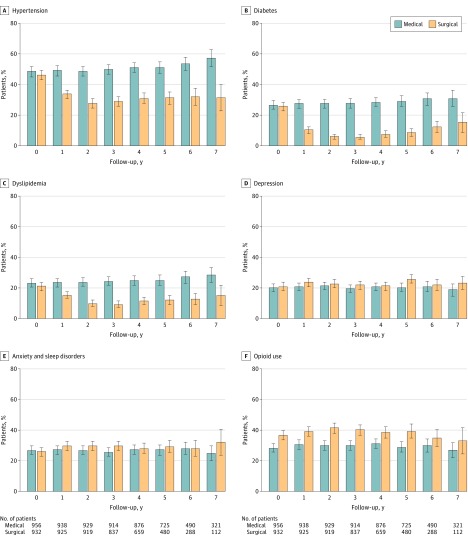
At the start of treatment, the proportions of patients treated with drugs for hypertension (48%), diabetes (26%), dyslipidemia (21%), depression (21%), or anxiety and sleep disorders (26%) did not differ significantly between treatment groups, while the proportion of opioid users was significantly higher in the surgical group (Table 1).
The prevalences of hypertension, diabetes, and dyslipidemia after treatment start were significantly lower in the surgical group than in the medical group (all P < .001; Figure 2). By contrast, the prevalences of depression and opioid use were significantly higher in the surgical group (P = .004 and P < .001, respectively) while the prevalence of anxiety and sleep disorders did not differ significantly between groups (P = .02; Figure 2).
Figure 2. Yearly Prevalence of Obesity-Related Comorbidities and Opioid Use.
Time 0 (start of treatment) was at inclusion for medically treated patients and time of surgery for patients undergoing bariatric surgery. The condition was defined as present if a patient collected at least 1 prescription for the given disease each year after the start of treatment. The error bars indicate 95% CIs.
Primary Outcomes
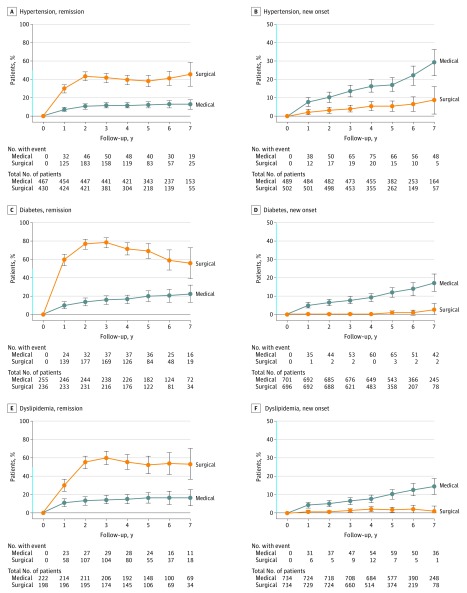
Surgically treated patients had a higher likelihood for hypertension remission (absolute risk [AR], 31.9% vs 12.4%; risk difference [RD], 19.5% [95% CI, 15.8%-23.2%]; adjusted relative risk [RR], 2.1 [95% CI, 2.0-2.2]) and lower risk for developing new-onset hypertension (AR, 3.5% vs 12.2%; RD, 8.7% [95% CI, 6.7%-10.7%]; RR, 0.4 [95% CI, 0.3-0.5]) than their medically treated counterparts (Figure 3, Table 2).
Figure 3. Yearly Remission and New-Onset Rates of Hypertension, Diabetes, and Dyslipidemia.
Time 0 (start of treatment) was inclusion for medically treated patients and time of surgery for patients undergoing bariatric surgery. The condition was defined as present if a patient collected at least 1 prescription for the given disease each year after start of treatment. Y-axis shown in blue indicates range from 0% to 50%. The error bars indicate 95% CIs.
Table 2. Relative and Absolute Risks for Remission and New Onset of Comorbidities and Complications in Patients With Severe Obesity Undergoing Surgical or Specialized Medical Treatment.
| Condition | % (95% CI) | Relative Risk (95% CI) |
||
|---|---|---|---|---|
| Absolute Risk Surgical Group (n = 932) |
Absolute Risk Medical Group (n = 956) |
Risk Difference | ||
| Hypertensiona | ||||
| Remission | 31.9 (28.4-35.4) | 12.4 (9.5-15.3) | 19.5 (15.8-23.2) | 2.1 (2.0-2.2) |
| New onset | 3.5 (2.2-4.9) | 12.2 (9.3-15.1) | 8.7 (6.7-10.7) | 0.4 (0.3-0.5) |
| Diabetesa | ||||
| Remission | 57.5 (53.8-61.2) | 14.8 (11.7-17.9) | 42.7 (35.8-49.7) | 3.9 (2.8-5.4) |
| New onset | 0.3 (0-0.7) | 7.5 (5.2-9.8) | 7.2 (5.3-9.1) | 0.07 (0.03-0.11) |
| Dyslipidemiaa | ||||
| Remission | 43.0 (39.3-46.7) | 13.2 (10.2-16.2) | 29.8 (23.4-36.2) | 2.6 (2.4-2.8) |
| New onset | 1.1 (0.3-1.9) | 6.4 (4.3-8.5) | 5.3 (3.7-6.9) | 0.3 (0.2-0.4) |
| Depressiona | ||||
| Remission | 26.7 (23.4-30.0) | 27.4 (23.5-31.3) | 1.1 (0.0-2.3) | 1.2 (1.0-1.4) |
| New onset | 8.9 (6.8-11.0) | 6.5 (4.3-8.7) | 2.4 (1.3-3.5) | 1.5 (1.4-1.7) |
| Anxiety and sleep disordersa | ||||
| Remission | 25.0 (21.8-28.2) | 28.1 (24.2-32.0) | 3.1 (0.7-5.5) | 0.9 (0.8-1.1) |
| New onset | 12.2 (9.8-14.6) | 9.4 (6.8-11.9) | 2.8 (1.6-4.0) | 1.3 (1.2-1.5) |
| Opioid usea | ||||
| Remission | 34.2 (28.8-39.6) | 41.4 (35.8-46.9) | 7.2 (3.6-10.8) | 0.8 (0.7-0.9) |
| New onset | 19.4 (16.5-22.3) | 15.8 (13.1-18.5) | 3.6 (2.3-4.9) | 1.3 (1.2-1.4) |
| Complications | ||||
| Abdominal pain | 26.1 (21.1-31.1) | 13.5 (9.6-17.4) | 12.6 (8.8-16.3) | 1.9 (1.6-2.3) |
| Gastroduodenal ulcers | 6.7 (3.9-9.5) | 2.0 (1.0-3.0) | 4.7 (3.1-6.3) | 3.4 (2.0-5.6) |
| Iron deficiency anemia | 6.8 (4.9-8.7) | 2.8 (0.9-4.7) | 4.0 (1.8-6.2) | 2.4 (1.6-3.8) |
| Vitamin and mineral deficiency | 1.7 (0.2-3.2) | 1.6 (0.7-2.5) | 0.2 (0-0.5) | 1.1 (0.5-2.2) |
| Protein-energy malnutrition | 2.3 (0.6-4.0) | 1.9 (0.4-3.4) | 0.4 (0.0-1.1) | 1.2 (0.6-2.3) |
| Hypoglycemia | 1.3 (0.5-2.1) | 0.3 (0.0-0.7) | 1.0 (0.3-1.7) | 4.1 (1.2-14.6) |
| Nausea and vomiting | 0.9 (0.0-2.1) | 1.4 (0.0-2.7) | 0.5 (0.0-1.3) | 0.6 (0.3-1.5) |
| Surgical procedures | ||||
| Any gastrointestinal surgery | 31.3 (26.0-36.5) | 15.5 (12.8-18.2) | 15.8 (13.1-18.5) | 2.0 (1.7-2.4) |
| Operations for intestinal obstruction | 8.7 (6.6-10.8) | 0.8 (0.0-1.8) | 7.9 (4.8-10.9) | 10.5 (5.1-21.5) |
| Operations for abdominal pain | 5.4 (2.8-8.0) | 0.4 (0.0-0.9) | 5.0 (3.4-6.6) | 12.9 (4.7-35.7) |
| Operations on gallbladder | 8.1 (5.0-11.2) | 2.4 (0.7-4.1) | 5.7 (3.1-8.3) | 3.4 (2.1-5.3) |
| Operation of incisional hernia | 2.8 (1.6-4.0) | 1.3 (0.5-2.1) | 1.4 (0.5-2.3) | 2.1 (1.1-4.0) |
Relative risk ratios were converted from adjusted odds ratios derived from multiple logistic regression models for repeated measurements. The analyses were adjusted for sex, age, and body mass index at baseline. The absolute risk for each condition was calculated as a weighted average of incidences of all the yearly incidences available. The weights were constructed as proportions of individuals followed up at a given point. Risk differences were calculated as the difference between weighted incidences using data from the whole follow-up.
Prespecified Secondary Outcomes
Surgically treated patients had a higher likelihood for remission of diabetes and dyslipidemia (diabetes remission: AR, 57.5% vs 14.8%; RD, 42.7% [95% CI, 35.8%-49.7%]; RR, 3.9 [95% CI, 2.8-5.4]; and dyslipidemia remission: AR, 43.0% vs 13.2%; RD, 29.8% [95% CI, 23.4%-36.2%]; RR, 2.6 [95% CI, 2.4-2.8]) (Figure 3, Table 2). Odds ratios are presented in eTable 3 in the Supplement.
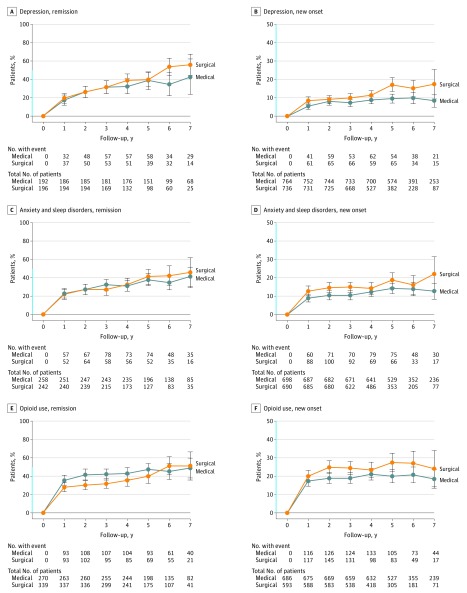
The surgical patients had a lower overall risk for new-onset diabetes (AR, 0.3% vs 7.5%; RD, 7.2% [95% CI, 5.3-9.1]; RR, 0.07 [95% CI, 0.03-0.11]) and dyslipidemia (AR, 1.1% vs 6.4%; RD, 5.3% [95% CI, 3.7%-6.9%]; RR, 0.3 [95% CI, 0.2-0.4]), but a higher risk of new-onset depression (AR, 8.9% vs 6.5%; RD 2.4% [95% CI, 1.3%-3.5%]; RR, 1.5 [95% CI, 1.4-1.7]), anxiety and sleep disorders (AR, 12.2% vs 9.4%; RD, 2.8% [95% CI, 1.6%-4.0%]; RR, 1.3 [95% CI, 1.2-1.5]), and treatment with opioids (AR, 19.4% vs 15.8%; RD, 3.6% [95% CI, 2.3%-4.9%], RR, 1.3 [95% CI, 1.2-1.4]) (Figure 3 and Figure 4, Table 2).
Figure 4. Yearly Remission and New-Onset Rates of Depression, Anxiety and Sleep Disorders, and Opioid Use.
Time 0 (start of treatment) was inclusion for medically treated patients and time of surgery for patients undergoing bariatric surgery. The condition was defined as present if a patient collected at least 1 prescription for the given disease each year after start of treatment. Y-axis shown in blue indicates range from 0% to 50%. The error bars indicate 95% CIs.
Adverse Events
The surgical patients had higher risk of several complications and surgical procedures: for example, at least 1 additional gastrointestinal surgical procedure (AR, 31.3% vs 15.5%; RD, 15.8% [95% CI, 13.1%-18.5%]; RR, 2.0 [95% CI, 1.7-2.4]), operation for intestinal obstruction (AR, 8.7% vs 0.8%; RD, 7.9% [95% CI, 4.8%-10.9%]; RR, 10.5 [95% CI, 5.1-21.5]), and abdominal pain (AR, 26.1% vs 13.5%; RD, 12.6% [95% CI, 8.8%-16.3%]; RR, 1.9 [95% CI, 1.6-2.3]) (Table 2). Relatively few patients had vitamin and mineral deficiencies, protein malnutrition, nausea/vomiting, and hypoglycemia.
Blood test results more than 3 months after treatment start demonstrated an increasing proportion of patients with anemia, hypocalcemia, and hypoalbuminemia, but with no significant differences between groups. However, the proportion of patients with low ferritin levels was significantly higher in the surgical group (26% vs 12%, P < .001) (eTable 4 in the Supplement).
A total of 26 (2.8%) and 65 (6.8%) patients in the surgical and medical groups died (eFigure in the Supplement), with no significant (age, sex, and BMI-adjusted) differences in overall mortality risk (hazard ratio, 0.74; 95% CI, 0.45-1.19). The 30-day mortality after surgery was zero.
Sensitivity Analysis
A sensitivity analysis of data from NorPD, excluding either those who did not undergo gastric bypass (n = 77), the outpatient group (n = 492), or the rehabilitation center group (n = 464), did not substantially change the main findings (eTable 5 in the Supplement). However, the association of bariatric surgery with remission of hypertension decreased from an RR of 2.1 (95% CI, 2.0-2.2) to 1.4 (95% CI, 1.1-1.7) after exclusion of the outpatient group.
Discussion
Among patients with severe obesity followed up for a median of 6.5 years, bariatric surgery compared with medical treatment was associated with a clinically important increased risk for complications, as well as lower risks of obesity-related metabolic comorbidities. Bariatric surgery was associated with higher risk of remission of hypertension (RD, 19.5%) and lower risk of new-onset hypertension (RD, 8.7%). Surgically treated patients also had significantly higher risk of remission of diabetes (RD, 42.7%), lower risk for new-onset diabetes (RD, 7.2%), but higher risk of new-onset depression (RD, 2.4%), anxiety and sleep disorders (RD, 2.8%), and treatment with opioids (RD, 3.6%). Surgical patients had higher complication rates, including abdominal pain (RD, 12.6%) and any gastrointestinal surgery (RD, 15.8%) (Table 2). The risk for complications should be considered in the decision-making process.
The beneficial effects of bariatric surgery are well known, but the present study adds knowledge about the totality of short- to long-term medical outcomes from a large number of patients with morbid obesity who underwent specialized treatment at a tertiary care center. The findings cannot, however, be directly compared with previous studies lacking control groups or that included control groups receiving routine clinical care or no active treatment at all.
The associations between bariatric surgery and remission and prevention of hypertension are consistent with the results from 2 nonrandomized clinical studies and some observational studies. Similarly, the strong association between bariatric surgery and the remission and prevention of diabetes confirms and extends findings from randomized and observational studies. Although few studies have specifically compared changes in dyslipidemia as judged by statin therapy, the beneficial association between surgical treatment and dyslipidemia is consistent with results from previous studies (Figure 3).
A recent meta-analysis showed that bariatric surgery was associated with postoperative decreases in the prevalence of depression, while limited evidence suggested that this improvement may not be maintained beyond the first years after surgery. However, the present study did not show any significant decline in the prevalence of drug-treated depression after treatment start in either group, while patients undergoing surgery had significantly higher risk of new-onset depression (RD, 2.4%) (Figure 2 and Figure 4).
A large US multicenter cohort study recently reported a significant increase in the prevalence of opioid users after bariatric surgery (14.7% to 20.3%). In contrast, the present study showed a higher prevalence of opioid users before surgery (36.4%), while the prevalence did not increase significantly after surgery (Figure 2). However, surgical patients had a slightly higher risk of new-onset opioid prescriptions after surgery (RD, 3.6%). The high prevalence of opioid prescriptions in the nonsurgical group indicates that obesity itself may be a risk factor for opioid use.
Recent evidence indicates that abdominal pain is common after gastric bypass, with a prevalence between 22% and 34% for 3 to 5 years after bariatric surgery. The present study supports and extends these findings, as 26.1% and 5.4% of patients in the surgical group were medically or surgically treated for abdominal pain, respectively, as compared with 13.5% and 0.4% of patients in the group who underwent specialized medical treatment.
A substantial number of patients underwent any gastrointestinal surgery, both after surgical and medical treatment (31.3% vs 15.5%), the former in line with a recent report from the Scandinavian Obesity Surgery Registry showing a rate of gastrointestinal surgery of 24.4% (median follow-up, 3.6 years) after gastric bypass. Most surgical complications were caused by intestinal obstruction (8.7%), gallbladder disease (8.1%), and abdominal pain (5.4%). However, the relatively high prevalence of gastrointestinal surgery in the medically treated group probably reflects that obesity by itself may be associated with gastrointestinal diseases and symptoms.
Strengths
Strengths of this study include a nearly 100% follow-up rate on both serious complications and dispensed drugs for comorbidities, and high external validity and generalizability to similar treatment programs in publicly funded health care systems.
Limitations
This study has several limitations. First, age and baseline BMI, both probably associated with the primary outcomes, differed significantly between treatment groups, and patients with higher BMI levels and younger age were more likely to undergo bariatric surgery. Accordingly, selection bias and confounding by indication might have reduced the internal validity of the study. These differences were, however, minimized by adjustments for age and BMI in the multivariable analyses. In addition, unknown differences between groups might have biased the results. Second, the use of dispensed drugs as proxy outcomes for the various comorbidities might have underestimated the proportions of patients with comorbidities. However, this limitation may not have altered the absolute differences between the groups. Third, this study did not address other outcomes, such as weight loss, quality of life, or cardiovascular events, and it had insufficient power to compare mortality. The lack of weight loss data is a major limitation, as differences in weight loss might have mediated the associations between treatment and outcomes. Fourth, 107 patients who initially opted for medical treatment were excluded owing to later bariatric surgery. Fifth, medical treatment does not primarily intend to resolve comorbidities, but rather to improve somatic and mental health and/or relieve symptoms, and a number of drugs have shown beneficial effects on cardiovascular morbidity and mortality. Nevertheless, most bariatric surgery candidates expect improvement and prevention of obesity-related comorbidities after bariatric surgery. Sixth, only a minority (7%) of the surgical patients underwent sleeve gastrectomy, and the results might not be generalizable to patients undergoing this procedure. Seventh, laboratory data were exploratory and incomplete. Eighth, this study included treatment-seeking white patients with severe obesity, limiting the generalizability of the results to populations with other races/ethnicities, less severe obesity, or people not seeking treatment.
Conclusions
Among patients with severe obesity followed up for a median of 6.5 years, bariatric surgery compared with medical treatment was associated with a clinically important increased risk for complications, as well as lower risks of obesity-related comorbidities. The risk for complications should be considered in the decision-making process.
eTable 1. Definition of Drugs for Each Comorbid Disease.
eTable 2. Definitions of Complications and Surgical Procedures According to ICD-10 (International Statistical Classification of Diseases and Related Health Problems 10th Revision) and NCSP (NOMESCO Classification of Surgical Procedures).
eTable 3. Odds Ratios and 95 % CIs for remission and New-Onset of Hypertension, Diabetes, Dyslipidemia, Depression Anxiety and Sleep Disorders, and Use of Opioids in Patients Receiving Surgical Compared With Specialized Medical Treatment.
eTable 4. Laboratory Values.
eTable 5. Sensitivity Analysis.
eFigure. Overall Mortality of Patients Undergoing Medical or Surgical Treatment for Severe Obesity.
eAppendix 1. Supplementary Information on the Different Treatment Options in Specialized Medical Treatment.
eAppendix 2. Research Protocol.
References
- 1.World Health Organization Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed June 25, 2017.
- 2.Kalarchian MA, Marcus MD, Levine MD, et al. Psychiatric disorders among bariatric surgery candidates: relationship to obesity and functional health status. Am J Psychiatry. 2007;164(2):328-334. [DOI] [PubMed] [Google Scholar]
- 3.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376(3):254-266. [DOI] [PubMed] [Google Scholar]
- 4.American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel, 2013 Executive summary: guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines: based on a systematic review from the Obesity Expert Panel, 2013. Obesity (Silver Spring). 2014;22(suppl 2):S5-S39. [DOI] [PubMed] [Google Scholar]
- 5.Look AHEAD Research Group Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring). 2014;22(1):5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puzziferri N, Roshek TB III, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schauer PR, Bhatt DL, Kirwan JP, et al. ; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med. 2017;376(7):641-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964-973. [DOI] [PubMed] [Google Scholar]
- 9.Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309(21):2240-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA. 2016;315(2):150-163. [DOI] [PubMed] [Google Scholar]
- 11.Sox HC, Lewis RJ. Pragmatic trials: practical answers to “real world” questions. JAMA. 2016;316(11):1205-1206. [DOI] [PubMed] [Google Scholar]
- 12.Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valderhaug TG, Aasheim ET, Sandbu R, et al. The association between severity of King’s Obesity Staging Criteria scores and treatment choice in patients with morbid obesity: a retrospective cohort study. BMC Obes. 2016;3(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Association for the Study of Obesity EASO Collaborating Centres for Obesity Management (COMs). http://easo.org/coms/. Accessed June 27, 2017.
- 15.Jakobsen GS, Hofsø D, Røislien J, Sandbu R, Hjelmesaeth J. Morbidly obese patients: who undergoes bariatric surgery? Obes Surg. 2010;20(8):1142-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofsø D, Nordstrand N, Johnson LK, et al. Obesity-related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol. 2010;163(5):735-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gjevestad E, Hjelmesaeth J, Sandbu R, Nordstrand N. Effects of intensive lifestyle intervention and gastric bypass on aortic stiffness: a 1-year nonrandomized clinical study. Obesity (Silver Spring). 2015;23(1):37-45. [DOI] [PubMed] [Google Scholar]
- 18.Furu K, Wettermark B, Andersen M, Martikainen JE, Almarsdottir AB, Sørensen HT. The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol. 2010;106(2):86-94. [DOI] [PubMed] [Google Scholar]
- 19.Helsedirektoratet Norwegian Patient Registry. https://helsedirektoratet.no/english/norwegian-patient-registry. Accessed October 22, 2017.
- 20.Zhang J, Yu KF. What’s the relative risk? a method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690-1691. [DOI] [PubMed] [Google Scholar]
- 21.Øvrebø B, Strømmen M, Kulseng B, Martins C. Bariatric surgery versus lifestyle interventions for severe obesity: 5-year changes in body weight, risk factors and comorbidities. Clin Obes. 2017;7(3):183-190. [DOI] [PubMed] [Google Scholar]
- 22.Adams TD, Davidson LE, Litwin SE, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377(12):1143-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308(11):1122-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douglas IJ, Bhaskaran K, Batterham RL, Smeeth L. Bariatric surgery in the United Kingdom: a cohort study of weight loss and clinical outcomes in routine clinical care. PLoS Med. 2015;12(12):e1001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlsson LMS, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367(8):695-704. [DOI] [PubMed] [Google Scholar]
- 26.Booth H, Khan O, Prevost T, et al. Incidence of type 2 diabetes after bariatric surgery: population-based matched cohort study. Lancet Diabetes Endocrinol. 2014;2(12):963-968. [DOI] [PubMed] [Google Scholar]
- 27.Risstad H, Søvik TT, Engström M, et al. Five-year outcomes after laparoscopic gastric bypass and laparoscopic duodenal switch in patients with body mass index of 50 to 60: a randomized clinical trial. JAMA Surg. 2015;150(4):352-361. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell JE, King WC, Chen JY, et al. Course of depressive symptoms and treatment in the Longitudinal Assessment of Bariatric Surgery (LABS-2) study. Obesity (Silver Spring). 2014;22(8):1799-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Booth H, Khan O, Prevost AT, Reddy M, Charlton J, Gulliford MC; King׳s Bariatric Surgery Study Group . Impact of bariatric surgery on clinical depression: interrupted time series study with matched controls. J Affect Disord. 2015;174(suppl C):644-649. [DOI] [PubMed] [Google Scholar]
- 30.King WC, Chen JY, Belle SH, et al. Use of prescribed opioids before and after bariatric surgery: prospective evidence from a US multicenter cohort study. Surg Obes Relat Dis. 2017;13(8):1337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierik AS, Coblijn UK, de Raaff CAL, van Veen RN, van Tets WF, van Wagensveld BA. Unexplained abdominal pain in morbidly obese patients after bariatric surgery. Surg Obes Relat Dis. 2017;13(10):1743-1751. [DOI] [PubMed] [Google Scholar]
- 32.Høgestøl IK, Chahal-Kummen M, Eribe I, et al. Chronic abdominal pain and symptoms 5 years after gastric bypass for morbid obesity. Obes Surg. 2017;27(6):1438-1445. [DOI] [PubMed] [Google Scholar]
- 33.Gribsholt SB, Pedersen AM, Svensson E, Thomsen RW, Richelsen B. Prevalence of self-reported symptoms after gastric bypass surgery for obesity. JAMA Surg. 2016;151(6):504-511. [DOI] [PubMed] [Google Scholar]
- 34.Bruze G, Ottosson J, Neovius M, Näslund I, Marsk R. Hospital admission after gastric bypass: a nationwide cohort study with up to 6 years follow-up. Surg Obes Relat Dis. 2017;13(6):962-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stenberg E, Szabo E, Ågren G, et al. Closure of mesenteric defects in laparoscopic gastric bypass: a multicentre, randomised, parallel, open-label trial. Lancet. 2016;387(10026):1397-1404. [DOI] [PubMed] [Google Scholar]
- 36.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957-967. [DOI] [PubMed] [Google Scholar]
- 37.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marso SP, Daniels GH, Brown-Frandsen K, et al. ; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. [DOI] [PubMed] [Google Scholar]
- 40.Fischer L, Nickel F, Sander J, et al. Patient expectations of bariatric surgery are gender specific: a prospective, multicenter cohort study. Surg Obes Relat Dis. 2014;10(3):516-523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Definition of Drugs for Each Comorbid Disease.
eTable 2. Definitions of Complications and Surgical Procedures According to ICD-10 (International Statistical Classification of Diseases and Related Health Problems 10th Revision) and NCSP (NOMESCO Classification of Surgical Procedures).
eTable 3. Odds Ratios and 95 % CIs for remission and New-Onset of Hypertension, Diabetes, Dyslipidemia, Depression Anxiety and Sleep Disorders, and Use of Opioids in Patients Receiving Surgical Compared With Specialized Medical Treatment.
eTable 4. Laboratory Values.
eTable 5. Sensitivity Analysis.
eFigure. Overall Mortality of Patients Undergoing Medical or Surgical Treatment for Severe Obesity.
eAppendix 1. Supplementary Information on the Different Treatment Options in Specialized Medical Treatment.
eAppendix 2. Research Protocol.