Key Points
Question
Is there an association between undergoing bariatric surgery with laparoscopic banding, Roux-en-Y gastric bypass, or laparoscopic sleeve gastrectomy vs nonsurgical usual care management and all-cause mortality among patients with obesity?
Findings
In this retrospective cohort study of 8385 patients who underwent bariatric surgery and 25 155 matched patients who received usual care, the mortality rate over approximately 4.5 years was 1.3% among surgical patients compared with 2.3% among nonsurgical patients, a significant difference.
Meaning
Bariatric surgery was associated with reduced all-cause mortality.
Abstract
Importance
Bariatric surgery is an effective and safe approach for weight loss and short-term improvement in metabolic disorders such as diabetes. However, studies have been limited in most settings by lack of a nonsurgical group, losses to follow-up, missing data, and small sample sizes in clinical trials and observational studies.
Objective
To assess the association of 3 common types of bariatric surgery compared with nonsurgical treatment with mortality and other clinical outcomes among obese patients.
Design, Setting, and Participants
Retrospective cohort study in a large Israeli integrated health fund covering 54% of Israeli citizens with less than 1% turnover of members annually. Obese adult patients who underwent bariatric surgery between January 1, 2005, and December 31, 2014, were selected and compared with obese nonsurgical patients matched on age, sex, body mass index (BMI), and diabetes, with a final follow-up date of December 31, 2015. A total of 33 540 patients were included in this study.
Exposures
Bariatric surgery (laparoscopic banding, Roux-en-Y gastric bypass, or laparoscopic sleeve gastrectomy) or usual care obesity management only (provided by a primary care physician and which may include dietary counseling and behavior modification).
Main Outcomes and Measures
The primary outcome, all-cause mortality, matched and adjusted for BMI prior to surgery, age, sex, socioeconomic status, diabetes, hyperlipidemia, hypertension, cardiovascular disease, and smoking.
Results
The study population included 8385 patients who underwent bariatric surgery (median age, 46 [IQR, 37-54] years; 5490 [65.5%] women; baseline median BMI, 40.6 [IQR, 38.5-43.7]; laparoscopic banding [n = 3635], gastric bypass [n = 1388], laparoscopic sleeve gastrectomy [n = 3362], and 25 155 nonsurgical matched patients (median age, 46 [IQR, 37-54] years; 16 470 [65.5%] women; baseline median BMI, 40.5 [IQR, 37.0-43.5]). The availability of follow-up data was 100% for all-cause mortality. There were 105 deaths (1.3%) among surgical patients during a median follow-up of 4.3 (IQR, 2.8-6.6) years (including 61 [1.7%] who underwent laparoscopic banding, 18 [1.3%] gastric bypass, and 26 [0.8%] sleeve gastrectomy), and 583 deaths (2.3%) among nonsurgical patients during a median follow-up of 4.0 (IQR, 2.6-6.2) years. The absolute difference was 2.51 (95% CI, 1.86-3.15) fewer deaths/1000 person-years in the surgical vs nonsurgical group. Adjusted hazard ratios (HRs) for mortality among nonsurgical vs surgical patients were 2.02 (95% CI, 1.63-2.52) for the entire study population; by surgical type, HRs were 2.01 (95% CI, 1.50-2.69) for laparoscopic banding, 2.65 (95% CI, 1.55-4.52) for gastric bypass, and 1.60 (95% CI, 1.02-2.51) for laparoscopic sleeve gastrectomy.
Conclusions and Relevance
Among obese patients in a large integrated health fund in Israel, bariatric surgery using laparoscopic banding, gastric bypass, or laparoscopic sleeve gastrectomy, compared with usual care nonsurgical obesity management, was associated with lower all-cause mortality over a median follow-up of approximately 4.5 years. The evidence of this association adds to the limited literature describing beneficial outcomes of these 3 types of bariatric surgery compared with usual care obesity management alone.
This cohort study uses Israeli electronic health record data to compare mortality of obese adult patients who underwent bariatric surgery (laparoscopic banding, Roux-en-Y gastric bypass, or laparoscopic sleeve gastrectomy) vs obese adults receiving nonsurgical usual care management.
Introduction
Although much is known about short-term outcomes after bariatric surgery, relatively little is known about the long-term effects of these operations. Several reviews have reported long-term results on weight loss and diabetes control, and a recent study demonstrated beneficial, long-term effects for gastric bypass but was limited by a fairly homogeneous population and operations that were performed by only a few surgeons. A large (2500 patients) retrospective cohort study from the US Veterans Affairs system of patients who underwent bariatric procedures (74% gastric bypass, 10% laparoscopic banding, and 15% laparoscopic sleeve gastrectomy) showed reduced mortality attributable to bariatric surgery. That study’s generalizability was also limited because the patients were mostly men; in the general population, this surgery is performed more often on women.
Obesity is a chronic disease and to fully understand the effects of its treatments, outcomes need to be assessed in the long term. Although there is a large body of bariatric surgical literature, the vast majority of studies report very short-term outcomes. Consequently, there is a need for more information about bariatric surgery outcomes. Additionally, most of the available long-term outcome data focuses on 1 of 2 outdated procedures: vertical band gastroplasty or laparoscopic banding, or gastric bypass. Recently, sleeve gastrectomy has become a very popular approach for surgically induced weight loss. Very little is known about the long-term outcomes for sleeve gastrectomy.
The main objective of the present study was to evaluate the association between 3 common bariatric operations and all-cause mortality as compared with matched obese patients who did not undergo surgery. A secondary objective was to evaluate long-term complications from these surgical procedures and also to observe the association of bariatric surgery on various metabolic conditions such as diabetes, hyperlipidemia, and hypertension.
Methods
Study Design Overview
This is a retrospective cohort study of patients from Clalit Health Service who underwent bariatric surgery between January 1, 2005, and December 31, 2014. The index date was defined as the date of first bariatric surgery. The surgical patients were matched with obese nonsurgical patients who received only usual care obesity management. Usual care obesity management was provided by primary care physicians and may have included dietary counseling and behavior modification. Baseline demographic and clinical characteristics were gathered for each surgical patient and each matched patient using extensive data from the Clalit Health Service electronic health record data system during the 3 years prior to the index date. The follow-up period was the time between the index date until occurrence of an event (all-cause mortality, occurrence of bariatric surgery among the matched comparison patients, or end of study follow-up period set as December 31, 2015) to allow at least a minimum follow-up of 1 year for all participants (eFigure in the Supplement).
This study was approved by the institutional review board of Clalit Health Service. Individual patient consent was not required because the study used only existing medical records data and individual patient identities were masked.
Source Population
In Israel, all citizens are entitled to free, basic health care from any of the 4 integrated payer-provider health funds. Clalit Health Service covers and provides care for more than half of the Israeli population (approximately 4.4 million insured patient members) who are older than 21 years of age. The ethnic composition of the membership includes approximately 20% Arab patients, with the remainder being the Jewish population with origins from Europe (approximately 60%) and North Africa or Asia (approximately 40%).
In contrast to health maintenance organizations and health insurance plans in the United States, Clalit Health Service and the other Israeli health funds are characterized by extremely low annual turnover of approximately 1%, facilitating nearly complete patient follow-up to study medium- to long-term outcomes for all 3 types of bariatric surgery.
Study Population
Members were considered potential bariatric surgery patients if they met all the following inclusion criteria: documentation of bariatric surgery during the study period, 24 years or older on the index date (to avoid absence of data during mandatory military service in Israel), and continuous membership in Clalit Health Service during the baseline period. Surgical and nonsurgical patients were excluded from the study if they met 1 or more of the following criteria: missing body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) measurement during the baseline period, BMI of equal to or less than 30 in all BMI measurements during the baseline period, pregnancy during 4 years prior to index date, or documentation of severe comorbidities during the baseline period (active cancer, Crohn disease, end-stage renal disease, or ascites). Additional information about the study population is in eMethods 1 in the Supplement.
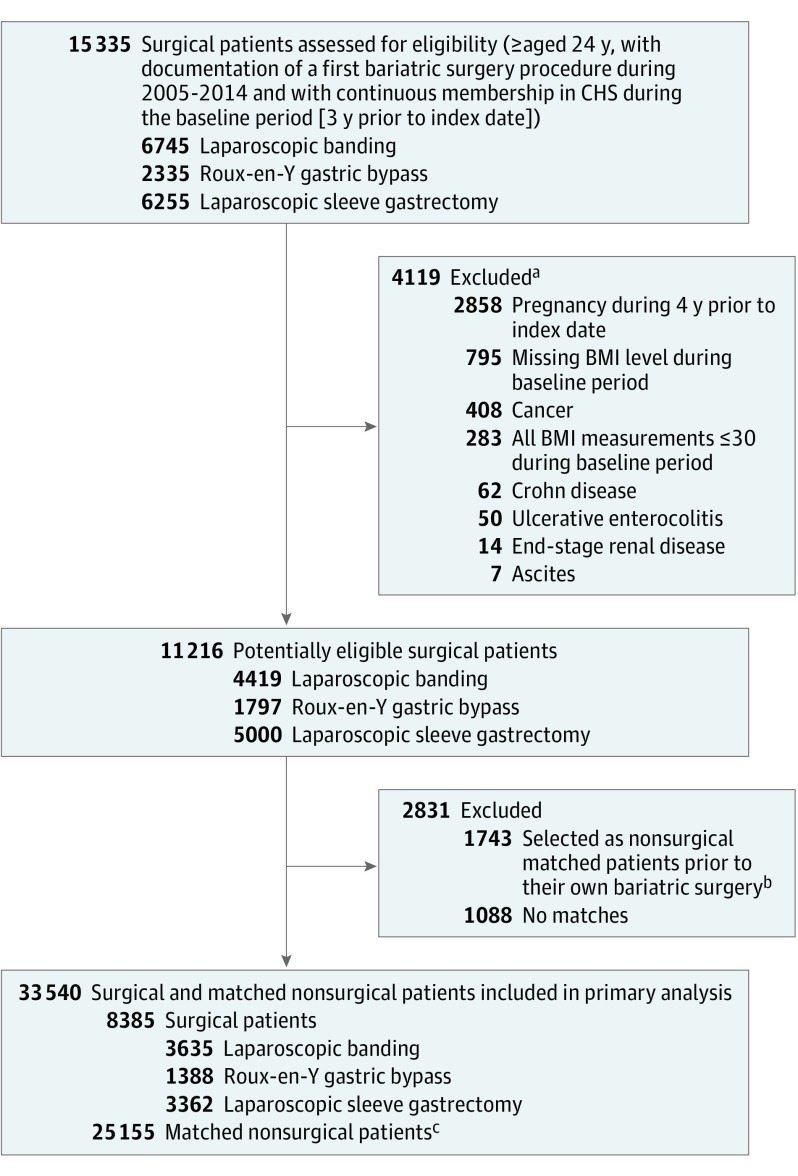
For each bariatric surgery patient, 3 matched nonsurgical patients were selected according to age group stratified by 5-year intervals, sex, BMI group stratified from greater than 30 to greater than 50 in 5-unit intervals, and diagnosis of diabetes. Because these characteristics as well as bariatric surgery status for the nonsurgical patients may change over time, a sequential/simultaneous (time-dependent) stratification matching, which preserves the time-dependent prospective structure, was used. Nonsurgical patients were considered as potential matched candidates at different points in time. Similar to a randomized clinical trial, potential matches were selected irrespective of their future bariatric surgery status; therefore, surgical patients were considered potential matches for those who underwent bariatric surgery prior to their own surgery. Nonsurgical matched patients at index date, who subsequently (after matching) underwent a bariatric surgical procedure, were censored at the time of their surgery from the nonsurgical group and were not added to the surgical group. Potential nonsurgical matches met the same inclusion and exclusion criteria as the surgical patients as of index date. Within each set of potential matches, 3 nonsurgical patients, who had not already been matched with a surgical patient, were selected randomly. Surgical patients for whom suitable matches were not found were excluded (Figure 1). Each patient who had repeat procedures was classified according to his or her initial surgery type.
Figure 1. Selection of Surgical and Nonsurgical Patients for the Study Population.
CHS indicates Clalit Health Services; BMI, body mass index.
aNot mutually exclusive.
bBy using a sequential stratification method, 1743 nonsurgical matched patients at index date, who subsequently (after matching) underwent a bariatric surgical procedure, were censored at the time of their surgery from the nonsurgical group and were not added to the surgical group.
cBy using a sequential stratification method, for each surgical patient 3 nonsurgical matches were selected (total nonsurgical patients, 25 155). Potential matches met all inclusion and exclusion criteria as the surgical patients, except for having bariatric surgery as of index date.
Baseline Measurements as of Index Date
Surgical data included the type and date of the procedure. Three types of bariatric procedures were identified—laparoscopic banding, gastric bypass, and sleeve gastrectomy—based on first indication of the relevant International Classification of Diseases, Ninth Revision (ICD-9) code in the Clalit Health Service data warehouse during 2005-2014. BMI value as of index date was determined based on the last documentation in the primary care clinics during the 3 years prior to index date. Socioeconomic variables measured at index date included age, biological sex, population sector (Jewish, non-Jewish), immigrant status (immigrated to Israel or born in Israel), and socioeconomic status (SES; low, medium, high). Population sector and SES are available and can be determined only at the clinic level in accordance with the designation of each member’s primary care clinic based on census designations from the Israeli Central Bureau of Statistics.
Comorbidity variables were evaluated as of the index date based on any documentation prior to index date and included diabetes (based on a reported algorithm that incorporates relevant ICD-9 diagnostic codes, HbA1c concentration, glucose levels, and diabetes medications), diagnosis of hyperlipidemia, diagnosis of hypertension, diagnosis of cardiovascular disease (myocardial infarction, unstable angina pectoris, angioplasty, coronary artery bypass graft, stable angina, and ischemic stroke), and diagnosis of lower leg amputation. Medical diagnoses as of index date were primarily defined based on ICD-9 codes extracted from hospital discharge records or ambulatory medical records. Ambulatory records missing ICD-9 codes were identified by analyzing available written text. ICD-9 codes of the medical diagnoses are described in eTable 1 in the Supplement. Smoking status (current, former, or nonsmoker as reported by the patient) and laboratory tests were evaluated based on last documentation in the outpatient setting during the baseline period. Laboratory tests included HbA1c concentration (%), blood glucose concentration (mg/dL), total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides. Medication use was assessed through Clalit Health Service records of prescriptions filled as coded in the Anatomical Therapeutic Chemical (ATC) classification system. Medication use was based on at least 1 purchase during the year before index date for blood glucose–lowering drugs and for cardiovascular system drugs (eTable 2 in the Supplement). Medications are significantly subsidized by the Clalit Health Service, therefore, these data are considered to be highly accurate.
Outcomes
All-cause mortality (yes or no) during the follow-up period was the primary outcome. Information regarding mortality events was obtained from Ministry of Interior data which includes current and complete information for the entire Israeli population. Cause of death was not available.
Secondary outcomes included additional clinical outcomes, and complications were reported for each individual through the end of the follow-up period (which varied in length across individuals) and included: change in BMI (based on last recorded BMI value during the follow-up period), new diagnosis of diabetes, remission of diabetes (last recorded HbA1c concentration equal to or less than 6% during the last year of follow-up and no diabetes medications were prescribed during the same time period), new diagnosis of hypertension, new diagnosis of hyperlipidemia, major adverse cardiovascular events ( defined as myocardial infarction, unstable angina pectoris, or coronary artery bypass graft), laboratory test values associated with nutritional status (hemoglobin and albumin concentrations [mmol/mol], both defined as the last measurements taken during the follow-up period), use of cardiometabolic medications (including β-blocking agents, calcium channel blockers, agents acting on the renin-angiotensin system, lipid-modifying agents, oral hypoglycemics, and insulin or glucagon-like peptide-1 receptor agonists drugs, each defined as a patient being prescribed ≥1 type of the medication in the last year of follow-up).
Additional outcomes included hospital admissions at least 30 days after index date (in total and for hypoglycemia specifically), bariatric reoperations (performed ≥30 days after the first operation; taken from the hospital records and financial payment data), and nonbariatric reoperations (intestinal obstruction, hernia of abdominal cavity, gastric ulcer, and esophageal stricture recorded during the follow-up period and taken from the hospital discharge summaries). ICD-9 codes of the medical diagnoses are described in eTable 1 (in the Supplement). ATC classification codes are listed in eTable 2 (in the Supplement). Information regarding secondary outcomes was not available once patients left Clalit Health Service.
Statistical Analysis
The main characteristics of the total study population and nonmortality bariatric surgical outcomes, stratified by surgery type and the additional clinical outcomes, were described using proportions for categorical variables and means with standard deviations (SDs) or medians with interquartile ranges (IQRs) for continuous variables. Differences between surgical patients and their matched nonsurgical patients were evaluated using the unpaired t test and Mann-Whitney U test for continuous variables and the χ2 test for categorical variables. The statistically significant threshold of P value less than .05 and 2-sided tests were used throughout. A large number of additional clinical outcomes were described as exploratory results, without multivariable analysis or adjustments, in addition to the primary mortality analysis. Clinically improbable laboratory values were removed (eTable 3 in the Supplement).
We evaluated the association between bariatric surgery and all-cause mortality using a Kaplan-Meier nonparametric model comparing groups. The log-rank test (using R package survival) was used to determine differences in the survival distribution between surgical patients and nonsurgical patients (separately for the different types of bariatric surgery).
Stratified Cox proportional hazards regression using multiple matched pairs (3:1) was used to assess the hazard ratio (HR) and 95% CI for the association between exposure to bariatric surgery and all-cause mortality, using both unadjusted and adjusted models. In addition to the variables included in the matching (age group, sex, BMI group, and diagnosis of diabetes), the following potential confounders were included in the adjusted model: age (continuous), SES, population sector, immigrant status, diagnosis of hyperlipidemia, diagnosis of hypertension, cardiovascular disease, smoking status, level of BMI (continuous), total cholesterol, high-density lipoprotein cholesterol, and triglycerides.
Termination of follow-up was defined as death, end of study period, or undergoing bariatric surgery (among matches). Post hoc, to assess if mortality differed by surgery type, an interaction term (surgery type [laparoscopic banding, gastric bypass, and sleeve gastrectomy] × surgical status [y/n]) was incorporated into the adjusted stratified Cox regression model.
Missing data of patient characteristics were imputed using the R package MICE version 2.22 applying chained equations. Information about the multiple imputation procedures is located in eMethods 2 (in the Supplement).
For all stratified Cox proportional hazards regression calculations, model assumptions were tested by modeling residuals as well as by graphical observation of the dependence of coefficients on time. We used R version 3.4.0 64-bit for all analyses.
Results
During the study period, there were 15 335 members, aged 24 or older, with documentation of a first bariatric surgery procedure and with continuous membership in Clalit Health Service during the baseline period. Of these, 4119 (26.9%) were excluded due to uncertainty regarding BMI value or any documentation of cancer, Crohn’s disease, ulcerative enterocolitis, end-stage renal disease, or ascites during the baseline period, resulting in 11 216 surgical patients (4419 underwent laparoscopic banding, 1797 underwent gastric banding, and 5000 underwent sleeve gastrectomy) remaining. The attempt to match 3 nonsurgical patients for each bariatric surgery patient failed for 1088 patients due to absence of nonsurgical patients with congruent age, sex, BMI, or diagnosis of diabetes. These 1088 patients were younger, more obese, less likely to have type 2 diabetes and more likely to be women than the final surgical cohort. A comparison between surgical patients who were included in the study and those who were excluded due to failure in the matching process is described in eTable 4 (in the Supplement). In addition, 1743 patients who underwent bariatric surgery were initially selected as nonsurgical matches prior to their own surgery.
The final study cohort consisted of 33 540 patients of whom 8385 were surgical (laparoscopic banding [n=3635], gastric bypass [n=1388], sleeve gastrectomy [n=3362]), and 25 155 nonsurgical matching patients (Figure 1). Main characteristics of the total study cohort, stratified by surgery type, are described in Table 1. Missing values of baseline characteristics were imputed (16.8% patients had missing values). (eMethods 2 in the Supplement).
Table 1. Baseline Characteristics of the Bariatric Surgery Patients and Matched Nonsurgical Patients.
| Laparoscopic Banding | Gastric Bypass | Laparoscopic Sleeve Gastrectomy | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Surgical Patients (n = 3635) |
Nonsurgical Patients (n = 10 905) |
Surgical Patients (n = 1388) |
Nonsurgical Patients (n = 4164) |
Surgical Patients (n = 3362) |
Nonsurgical Patients (n = 10 086) |
Surgical Patients (n = 8385) |
Nonsurgical Patients (n = 25 155) |
|
| Duration of follow-up, median (IQR) [range], y | 6.2 (4.3-8.5)a
[0.6-11] |
5.7 (3.7-8.2)a
[0.1-11] |
5.5 (3.0-6.7)a
[0.1-10.7] |
4.8 (2.6-6.6)a
[0.1-10.7] |
3.2 (2.2-4.1)a
[0.1-5.6] |
3.0 (2.0-4.0)a
[0.1-5.6] |
4.3 (2.8-6.6) [0.1-11] |
4.0 (2.6-6.2) [0.1-11] |
| Age, median (IQR), y | 45 (35-53) | 45 (35-53) | 48 (39-55) | 48 (39-55) | 47 (37-55) | 47 (38-55) | 46 (37-54) | 46 (37-54) |
| Sex, No. (%) | ||||||||
| Men | 1197 (32.9) | 3591 (32.9) | 448 (32.3) | 1344 (32.3) | 1250 (37.2) | 3750 (37.2) | 2895 (34.5) | 8685 (34.5) |
| Women | 2438 (67.1) | 7314 (67.1) | 940 (67.7) | 2820 (67.7) | 2112 (62.8) | 6336 (62.8) | 5490 (65.5) | 16 470 (65.5) |
| SES, No. (%)b,c | ||||||||
| Low | 847 (23.4)a | 4283 (39.4)a | 376 (27.2)a | 1680 (40.5)a | 986 (29.4)a | 4128 (41.0)a | 2209 (26.4)a | 10 091 (40.2)a |
| Medium | 1705 (47.2)a | 4103 (37.7)a | 610 (44.1)a | 1538 (37.0)a | 1397 (41.6)a | 3759 (37.4)a | 3712 (44.4)a | 9400 (37.5)a |
| High | 1064 (29.4)a | 2484 (22.9)a | 397 (28.7)a | 934 (22.5)a | 972 (29.0)a | 2174 (21.6)a | 2433 (29.1)a | 5592 (22.3)a |
| Population sector, No. (%)b | ||||||||
| Jewish | 3166 (87.1)a | 7470 (68.5)a | 1092 (78.7)a | 2763 (66.4)a | 2521 (75.0)a | 6569 (65.1)a | 6779 (80.8) | 16 802 (66.8) |
| Non-Jewish | 469 (12.9) | 3435 (31.5) | 296 (21.3) | 1401 (33.6) | 841 (25.0)a | 3517 (34.9)a | 1606 (19.2) | 8353 (33.2) |
| Immigrant status, No. (%) | ||||||||
| Born in Israel | 2642 (72.7)a | 8275 (75.9)a | 1037 (74.7) | 3118 (74.9) | 2643 (78.6) | 7786 (77.2) | 6322 (75.4) | 19 179 (76.2) |
| Immigrant | 993 (27.3) | 2630 (24.1) | 351 (25.3) | 1046 (25.1) | 719 (21.4) | 2300 (22.8) | 2063 (24.6) | 5976 (23.8) |
| Diabetes, No. (%) | ||||||||
| Diagnosisd | 767 (21.1) | 2301 (21.1) | 524 (37.8) | 1572 (37.8) | 1100 (32.7) | 3300 (32.7) | 2391 (28.5) | 7173 (28.5) |
| Duration, median (IQR), y | 3.6 (1.3-6.4) | 4.0 (1.9-6.5) | 6.2 (2.7-9.0) | 5.0 (2.3-7.5) | 5.9 (1.9-9.5) | 5.4 (2.3-8.9) | 4.9 (1.8-8.5) | 4.7 (2.2-7.9) |
| Hyperlipidemia, No. (%)d | 1673 (46.0)a | 4669 (42.8)a | 843 (60.7)a | 2246 (53.9)a | 1916 (57.0)a | 5334 (52.9)a | 4432 (52.9)a | 12 249 (48.7)a |
| Hypertension, No. (%)d | 1400 (38.5)a | 4424 (40.6)a | 712 (51.3)a | 1960 (47.1)a | 1609 (47.9)a | 4280 (42.4)a | 3721 (44.4)a | 10 664 (42.4)a |
| CVD diagnosis, No. (%)d | 341 (9.4) | 966 (8.9) | 210 (15.1)a | 507 (12.2)a | 439 (13.1) | 1193 (11.8) | 990 (11.8)a | 2666 (10.6)a |
| Myocardial infarction | 115 (3.2) | 328 (3.0) | 68 (4.9) | 179 (4.3) | 135 (4.0) | 388 (3.8) | 318 (3.8) | 895 (3.6) |
| Unstable angina | 88 (2.4) | 219 (2.0) | 53 (3.8) | 128 (3.1) | 127 (3.8) | 287 (2.8) | 268 (3.2)a | 634 (2.5)a |
| Angioplasty | 97 (2.7) | 260 (2.4) | 64 (4.6) | 157 (3.8) | 160 (4.8) | 356 (3.5) | 321 (3.8)a | 773 (3.1)a |
| Coronary artery bypass graft | 28 (0.8) | 105 (1.0) | 15 (1.1) | 61 (1.5) | 35 (1.0) | 122 (1.2) | 78 (0.9) | 288 (1.1) |
| Stable angina | 102 (2.8) | 258 (2.4) | 68 (4.9)a | 149 (3.6)a | 119 (3.5) | 349 (3.5) | 289 (3.4) | 756 (3.0) |
| Ischemic stroke | 93 (2.6) | 281 (2.6) | 46 (3.3) | 134 (3.2) | 109 (3.2) | 346 (3.4) | 248 (3.0) | 761 (3.0) |
| Lower leg amputation, No. (%)sd | 14 (0.4) | 27 (0.2) | 6 (0.4) | 23 (0.6) | 17 (0.5) | 47 (0.5) | 37 (0.4) | 97 (0.4) |
| Body mass index, No. (%) | ||||||||
| >30-35 | 208 (5.7) | 624 (5.7) | 102 (7.3) | 306 (7.3) | 153 (4.6) | 459 (4.6) | 463 (5.5) | 1389 (5.5) |
| >35-40 | 1179 (32.4) | 3537 (32.4) | 474 (34.1) | 1422 (34.1) | 1289 (38.3) | 3867 (38.3) | 2942 (35.1) | 8826 (35.1) |
| >40-45 | 1555 (42.8) | 4665 (42.8) | 529 (38.1) | 1587 (38.1) | 1334 (39.7) | 4002 (39.7) | 3418 (40.8) | 10 254 (40.8) |
| >45-50 | 470 (12.9) | 1410 (12.9) | 190 (13.7) | 570 (13.7) | 408 (12.1) | 1224 (12.1) | 1068 (12.7) | 3204 (12.7) |
| >50 | 223 (6.1) | 669 (6.1) | 93 (6.7) | 279 (6.7) | 178 (5.3) | 534 (5.3) | 494 (5.9) | 1482 (5.9) |
| Median (IQR) | 40.6 (38.8-43.7) |
40.8 (37.1-43.8) |
40.6 (38.4-44.0) |
40.5 (37.0-43.7) |
40.5 (38.3-43.5) |
40.4 (36.8-43.2) |
40.6 (38.5-43.7) |
40.5 (37.0-43.5) |
| Smoking status, No. (%)e | ||||||||
| Nonsmoker | 1755 (58.0)a | 6067 (68.4)a | 807 (59.3)a | 2849 (70.3)a | 1967 (58.5)a | 6652 (66.1)a | 4529 (58.5)a | 15 568 (67.7)a |
| Former | 470 (15.5)a | 926 (10.4)a | 281 (20.6)a | 505 (12.5)a | 702 (20.9)a | 1434 (14.2)a | 1453 (18.8)a | 2865 (12.5)a |
| Current | 800 (26.4)a | 1881 (21.2)a | 274 (20.1)a | 700 (17.3)a | 691 (20.6)a | 1981 (19.7)a | 1765 (22.8)a | 4562 (19.8)a |
| HbA1c, median (IQR), %f | 6.2 (5.7-7.3)a | 6.2 (5.7-7.3)a | 6.4 (5.8-7.9) | 6.7 (5.9-7.9) | 6.2 (5.7-7.2)a | 6.4 (5.8-7.5)a | 6.2 (5.7-7.4)a | 6.4 (5.8-7.5)a |
| Glucose, median (IQR), mg/dLg | 98.0 (88.0-116.0) |
97.0 (88.0-113.0) |
105.0 (91.0-136.0)a |
103.0 (91.0-131.0)a |
102.0 (92.0-126.0)a |
101.0 (90.0-121.0)a |
101.0 (90.0-122.0)a |
99.0 (89.0-119.0)a |
| Total cholesterol, mean (SD), mg/dLh | 194.0 (37.1)a | 190.4 (36.4)a | 189.8 (38.4)a | 187.1 (37.5)a | 186.7 (37.6)a | 184.7 (36.8)a | 190.4 (37.7)a | 187.5 (36.8)a |
| HDL-C, mean (SD), mg/dLi | 45.9 (11.8)a | 45.1 (11.2)a | 46.2 (12.1)a | 45.2 (11.1)a | 44.3 (11.1) | 44.6 (11.0) | 45.3 (11.6)a | 44.9 (11.1)a |
| LDL-C, mean (SD), mg/dLj | 115.0 (32.0)a | 113.0 (30.9)a | 112.0 (33.0)a | 109.0 (31.0)a | 109.0 (32.0) | 109.0 (32.0) | 112.0 (32.0)a | 110.6 (31.4)a |
| Triglycerides, median (IQR), mg/dLk | 148.0 (107.0-207.0)a |
141.0 (104.0-195.0)a |
149.0 (106.0-205.0) |
145.0 (106.0-199.0) |
151.0 (109.0-210.0)a |
144.0 (106.0-196.0)a |
149.0 (108.0-208.0)a |
143.0 (105.0-196.0)a |
| Pharmaceutical treatment, No. (%) | ||||||||
| Oral hypoglycemics | 571 (15.7) | 1762 (16.2) | 422 (30.4) | 1188 (28.5) | 879 (26.1) | 2480 (24.6) | 1872 (22.3) | 5430 (21.6) |
| Injectable therapy for blood glucose loweringl | 163 (4.5) | 467 (4.3) | 229 (16.5)a | 338 (8.1)a | 389 (11.6)a | 845 (8.4)a | 781 (9.3)a | 1650 (6.6)a |
| β-Blocking agents | 614 (16.9) | 1921 (17.6) | 286 (20.6) | 871 (20.9) | 679 (20.2)a | 1807 (17.9)a | 1579 (18.8) | 4599 (18.3) |
| Calcium channel blockers | 412 (11.3) | 1220 (11.2) | 233 (16.8)a | 580 (13.9)a | 482 (14.3)a | 1219 (12.1)a | 1127 (13.4)a | 3019 (12.0)a |
| Agents acting on the renin-angiotensin system | 920 (25.3) | 2858 (26.2) | 517 (37.2) | 1464 (35.2) | 1174 (34.9)a | 3216 (31.9)a | 2611 (31.1)a | 7538 (30.0)a |
| Lipid-modifying agents | 1094 (30.1)a | 3093 (28.4)a | 605 (43.6)a | 1654 (39.7)a | 1337 (39.8)a | 3794 (37.6)a | 3036 (36.2)a | 8541 (34.0)a |
Abbreviations: HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; SES, socioeconomic status.
SI conversion factors: To convert glucose to mmol/L, multiply values by 0.0555, to convert cholesterol to mmol/L, multiply values by 0.0259, to convert triglyceride values to mmol/L, multiply by 0.0113.
Indicates a significant P value of <.05.
Variable was determined at the clinic level in accordance with the designation of each individual’s primary care clinic based on census designations from the Israel Bureau of Statistics.
Data on socioeconomic status, representing tertiles of Clalit Health Service patients, were missing for 31 of the surgical patients and for 72 of the nonsurgical patients (103 in total).
Any time before index date.
Data on smoking status were missing for 638 of the surgical patients and for 2160 of the nonsurgical patients (2798 in total).
Data on HbA1c level were missing for 63 of the surgical patients with diabetes at index date and for 364 of the nonsurgical patients with diabetes at index date (427 in total).
Data on glucose level were missing for 73 of the surgical patients and for 2053 of the nonsurgical patients (2126 in total).
Data on total-C were missing for 128 of the surgical patients and for 2148 of the nonsurgical patients (2276 in total).
Data on HDL-C were missing for 164 of the surgical patients and for 2348 of the nonsurgical patients (2512 in total).
Data on LDL-C were missing for 325 of the surgical patients and for 2806 of the nonsurgical patients (3131 in total).
Data on triglycerides were missing for 144 of the surgical patients and for 2219 of the nonsurgical patients (2363 in total).
Insulins and noninsulin.
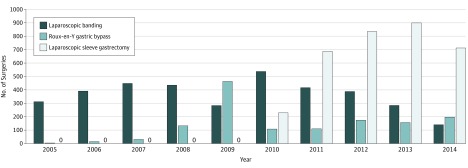
Trends in type of surgery performed changed over time. A preponderance of laparoscopic banding procedures was seen early in the study period, while sleeve gastrectomy procedures were introduced in 2010. Beginning in 2011, the majority of surgeries were for sleeve gastrectomy (Figure 2).
Figure 2. Number of Laparoscopic Banding, Gastric Bypass, and Laparoscopic Sleeve Gastrectomy Procedures, 2005-2014.
Of the total study cohort, 30 759 (91.7%) individuals completed their respective follow-up period. Of the remainder, 688 (2.1%) died and 2093 (6.2%) nonsurgical patients, who underwent bariatric surgery during follow-up (of whom 1743 up to 2014), were censored before December 31, 2015. For the main outcome, receipt of information from the Ministry of Interior ensured 100% follow-up. For secondary outcomes, 1089 patients left Clalit Health Service, yielding an overall retention rate of 96.8%. Median follow-up was 4.3 years (IQR, 2.8-6.6) for surgical patients and 4.0 years (IQR, 2.6-6.2) for nonsurgical patients as described in Table 1. Detailed description of duration of follow-up by surgery type is provided in Table 1. Given the later introduction of the sleeve gastrectomy procedure in this cohort, median follow-up is shorter in this group compared with gastric bypass and laparoscopic banding.
Primary Outcome: Association Between Bariatric Surgery and Mortality
Table 2 shows that during the follow-up period, there were 688 (2.1%) deaths among the entire cohort, with 105 (1.3%) among surgical patients (61 [1.7%] laparoscopic banding, 18 [1.3%] gastric bypass, and 26 [0.8%] sleeve gastrectomy) and 583 (2.3%) among the nonsurgical patients. The median time to mortality was 3.7 years among patients who died. The absolute mortality rate difference per 1000 person-years, comparing overall surgical vs medical group, was 2.51 (95% CI, 1.86-3.15) for the entire study population (for laparoscopic banding, 2.6 [95% CI, 1.7-3.5]; for gastric bypass, 3.4 [95% CI, 1.7-5.0]; and for sleeve gastrectomy, 1.8 [95% CI, 0.6-3.0]).
Table 2. Association Between Bariatric Surgery and Mortality.
| Laparoscopic Banding | Gastric Bypass | Laparoscopic Sleeve Gastrectomy | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Surgical Patients (n = 3635) |
Nonsurgical Patients (n = 10 905) |
Surgical Patients (n = 1388) |
Nonsurgical Patients (n = 4164) |
Surgical Patients (n = 3362) |
Nonsurgical Patients (n = 10 086) |
Surgical Patients (n = 8385) |
Nonsurgical Patients (n = 25 155) |
|
| Follow-up, median (IQR), y | 6.2 (4.3-8.5)a | 5.7 (3.7-8.2)a | 5.5 (3.0-6.7)a | 4.8 (2.6-6.6)a | 3.2 (2.2-4.1)a | 3.0 (2.0-4.0)a | 4.3 (2.8-6.6)a | 4.0 (2.6-6.2)a |
| Total deaths, No. (%) | 61 (1.7)a | 338 (3.1)a | 18 (1.3)a | 116 (2.8)a | 26 (0.8)a | 129 (1.3)a | 105 (1.3)a | 583 (2.3)a |
| Mortality/1000 person-years (95% CI) | 2.6 (2.0-3.4) | 5.3 (4.7-5.8) | 2.6 (1.6-4.2) | 6.0 (5.0-7.2) | 2.4 (1.6-3.6) | 4.2 (3.5-5.0) | 2.6 (2.1-3.1) | 5.1 (4.7-5.5) |
| Mortality rate difference/1000 person-years, mean (95% CI) | [Reference] | 2.6 (1.7-3.5) | [Reference] | 3.4 (1.7- 5.0) | [Reference] | 1.8 (0.6-3.0) | [Reference] | 2.51 (1.86-3.15) |
| Nonsurgical patients vs surgical, hazard ratio (95% CI) for mortality | ||||||||
| Unadjusted | 1 [Reference] | 2.00 (1.52-2.63) | 1 [Reference] | 2.29 (1.39-3.76) | 1 [Reference] | 1.66 (1.09-2.54) | 1 [Reference] | 1.97 (1.59-2.42) |
| Adjusted, before multiple imputationb,c | 1 [Reference] | 2.13 (1.47-3.09) | 1 [Reference] | 2.46 (1.43-4.24) | 1 [Reference] | 1.59 (1.00-2.53) | 1 [Reference] | 2.03 (1.58-2.61) |
| Adjusted, after multiple imputationc | 1 [Reference] | 2.01 (1.50-2.69) | 1 [Reference] | 2.65 (1.55-4.52) | 1 [Reference] | 1.60 (1.02-2.51) | 1 [Reference] | 2.02 (1.63-2.52) |
Indicates a significant P value of <.05.
5060 patients with missing values were eliminated, leaving 28 480 patients in the model (number of events = 557).
Variables included: all-cause mortality (y/n) (dependent variable); age introduced as continuous variable (years); SES introduced as dummy variable: (low-reference group, medium, high); population sector (Jewish, non-Jewish), immigrant status (immigrated to Israel/born in Israel); diagnosis of hyperlipidemia (y/n); diagnosis of hypertension (y/n); diagnosis of CVD (y/n); smoking status introduced as an ordinal variable (nonsmoker, former, current); BMI introduced as continuous variable; total cholesterol level introduced as continuous variable (mg/dL); HDL cholesterol level and triglycerides introduced as the log transformed of the continuous level. No collinearity between variables was observed (max VIF <2.1).
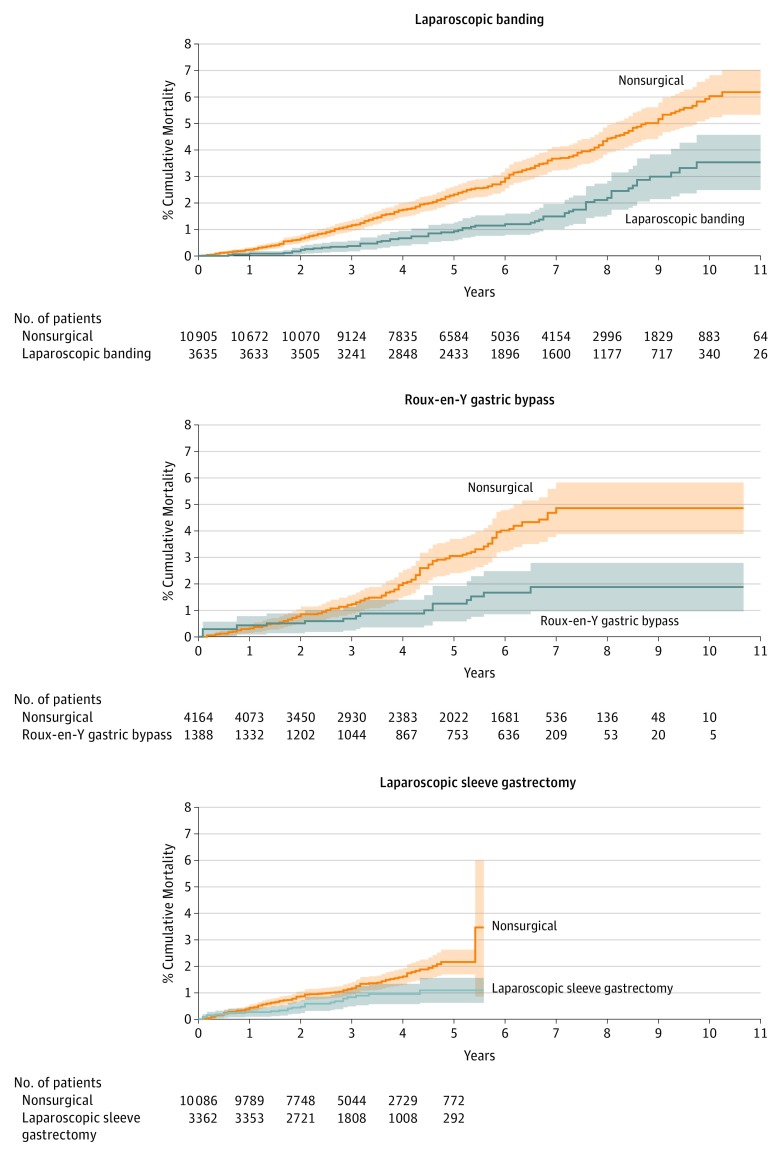
Kaplan-Meier nonparametric models comparing groups demonstrated significant differences in the survival distribution between nonsurgical patients and surgical patients, with a higher mortality rate among nonsurgical patients (Figure 3).
Figure 3. Kaplan-Meier Estimated Mortality Curves for 3 Types of Surgical Patients and Matched Nonsurgical Obese Patients.
Cumulative mortality for matched bariatric surgical and nonsurgical patients, by surgical procedure, with 95% CIs. For laparoscopic banding, the median (IQR) time of follow-up was 6.2 (4.3-8.5) years for surgical patients and 5.7 (3.7-8.2) years for nonsurgical (P<.001 by log-rank test); for gastric bypass, 5.5 (3.0-6.7) years vs 4.8 (2.6-6.6) years (P<.001) and laparoscopic sleeve gastrectomy, 3.2 (2.2-4.1) years vs 3.0 (2.0-4.0) years (P = .006). Overall (not shown), median (IQR) follow-up time was 4.3 years (IQR, 2.8-6.6) for surgical patients and 4.0 years (IQR, 2.6-6.2) for nonsurgical patients (P<.001).
After adjustment for patient characteristics, comorbidities, laboratory tests, and use of medications, stratified Cox proportional hazards models over the full follow-up period demonstrated significant associations between bariatric surgery status and all-cause mortality, with higher risk among nonsurgical patients vs those who underwent bariatric surgery for all 3 types (overall HR, 2.02 [95% CI, 1.63-2.52]; laparoscopic banding HR, 2.01 [95% CI, 1.50-2.69]; gastric bypass HR, 2.65 [95% CI, 1.55-4.52]; and sleeve gastrectomy HR, 1.60, [95% CI, 1.02-2.51]). These results were consistent with the unadjusted and the nonimputed models (Table 2).
Introduction of multiplicative interaction terms of surgical status × surgery type were not statistically significant for either the preimputed model (P = .52) or the postimputed model (P = .45). Because all the Cox models described previously met the proportional hazards assumption, these interaction P values suggest that the survival association of surgery was not statistically significantly different across the 3 surgery types.
Secondary Outcomes: Additional Clinical Outcomes and Complications
Table 3 shows secondary analyses comparing surgical patients with nonsurgical patients on additional clinical outcomes at last follow-up included greater BMI unit reduction in surgical patients (mean [SD], 9.3 [5.8] compared with 1.2 [6.1]), higher proportion of individuals with 20% or more reduction in BMI (59.3% compared with 7.6%), lower rates of incident diabetes (0.2% compared with 2.1%), higher rate of diabetes remission (23.6% compared with 5.1%), and lower rates of new hypertension diagnoses (3.2% compared with 8.1%). Incident rates for major adverse cardiovascular events, hemoglobin or albumin concentrations, total hospital admissions, and specific admissions for hypoglycemia were comparable in the surgical and nonsurgical groups. Reoperations occurred in 8.0% of the laparoscopic band patients, 1.6% of gastric bypass patients, and 1.6% of sleeve gastrectomy patients. In total, surgical patients had more nonbariatric reoperations compared with nonsurgical patients. A detailed breakdown by specific surgical procedures is given in Table 3.
Table 3. Description of Additional Outcomes.
| Laparoscopic Banding | Gastric Bypass | Sleeve Gastrectomy | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Surgical Patients | Nonsurgical Patients | Surgical Patients | Nonsurgical Patients | Surgical Patients | Nonsurgical Patients | Surgical Patients | Nonsurgical Patients | |
| No. at risk | 3635 | 10 905 | 1388 | 4164 | 3362 | 10 086 | 8385 | 25 155 |
| Follow-up, median (IQR), y | 6.2 (4.3-8.5) | 5.7 (3.7-8.2) | 5.5 (3.0-6.7) | 4.8 (2.6-6.6) | 3.2 (2.2-4.1) | 3.0 (2.0-4.0) | 4.3 (2.8-6.6) | 4.0 (2.6-6.2) |
| BMI | ||||||||
| Last measurement, median (IQR)a | 32.5 (28.3-37.2)b | 39.8 (35.6-44.1)b | 30.9 (27.3-35.5)b | 39.3 (35.4-43.4)b | 29.8 (26.6-33.3)b | 39.3 (35.6-43.1)b | 31.0 (27.3-35.4)b | 39.6 (35.6-43.6)b |
| Reduction from baseline, mean (SD) | 8.1 (6.3)b | 1.2 (6.3)b | 9.4 (5.6)b | 1.4 (6.3)b | 10.6 (4.9)b | 1.3 (5.8)b | 9.3 (5.8)b | 1.2 (6.1)b |
| Individuals with ≥20% reduction, No. (%) | 1702 (48.2)b | 854 (8.2)b | 829 (60.9)b | 287 (7.3)b | 2278 (70.8)b | 645 (7.1)b | 4809 (59.3)b | 1786 (7.6)b |
| Diabetes | ||||||||
| New diagnosis, No. (%)c | 11 (0.3)b | 236 (2.2)b | 2 (0.1)b | 89 (2.1)b | 6 (0.2)b | 196 (1.9)b | 19 (0.2)b | 521 (2.1)b |
| Control, median (IQR), HbA1c %d | 5.7 (5.4-6.1)b | 6.1 (5.6-7.0)b | 5.8 (5.4-6.4)b | 6.3 (5.7-7.6)b | 5.6 (5.3-6.0)b | 6.3 (5.7-7.4)b | 5.7 (5.3-6.1)b | 6.2 (5.7-7.3)b |
| Remission, No. (%)e | 89 (14.7)b | 70 (3.6)b | 84 (19.3)b | 64 (4.8)b | 280 (31.9)b | 179 (6.4)b | 453 (23.6)b | 313 (5.1)b |
| Hypertension | ||||||||
| New diagnosis, No. (%)f | 199 (5.5)b | 1163 (10.7)b | 34 (2.4)b | 344 (8.3)b | 32 (1.0)b | 531 (5.3)b | 265 (3.2)b | 2038 (8.1)b |
| SBP, mean (SD), mm Hgg | 121.7 (14.3)b | 126.4 (14.0)b | 121.2 (14.4)b | 126.8 (13.7)b | 120.5 (13.5)b | 126.6 (13.7)b | 121.2 (14.0)b | 126.5 (13.8)b |
| DBP, mean (SD), mm Hgh | 74.2 (9.4)b | 76.3 (9.3)b | 73.6 (9.3)b | 76.0 (9.1)b | 73.7 (9.1)b | 76.5 (9.2)b | 73.9 (9.3)b | 76.3 (9.2)b |
| SBP/DBP ≤130/80 mm Hg, No. (%)i | 2521 (69.4)b | 6502 (59.6)b | 987 (71.1)b | 2456 (59.0)b | 2354 (70.0)b | 5675 (56.3)b | 5862 (69.9)b | 14 633 (58.2)b |
| Hyperlipidemia | ||||||||
| New diagnosis, No. (%)j | 412 (11.3)b | 1940 (17.8)b | 63 (4.5)b | 482 (11.6)b | 95 (2.8)b | 678 (6.7)b | 570 (6.8)b | 3100 (12.3)b |
| Total cholesterol, median (IQR), mg/dLk | 192 (168-217)b |
188 (166-213)b |
187 (163-212)b |
185 (161-209)b |
184 (161-209)b |
182 (159-207)b |
188 (164-213)b |
185 (162-210)b |
| HDL-C, mean (SD), mg/dLl | 45.9 (11.8)b | 45.1 (11.2)b | 46.2 (12.1)b | 45.2 (11.1)b | 44.3 (11.1) | 44.6 (11.0) | 45.3 (11.6)b | 44.9 (11.1)b |
| LDL-C, mean (SD), mg/dLm | 115.1 (31.8)* | 113.2 (30.9)b | 111.5 (33.3)b | 109.0 (31.3)b | 108.8 (32.4) | 108.5 (31.8) | 111.9 (32.4)b | 110.6 (31.4)b |
| Triglycerides, median (IQR), mg/dLn | 147.5 (107.0-207.0)b |
141.0 (104.0-195.0)b |
149.0 (106.0-205.0) |
145.0 (106.0-199.0) |
151.0 (109.0-210.0)b |
144.0 (106.0-196.0)b |
149.0 (108.0-208.0)b |
143.0 (105.0-196.0)b |
| MACE, No. (%)o | 132 (3.6) | 408 (3.7) | 55 (4.0) | 163 (3.9) | 96 (2.9) | 268 (2.7) | 283 (3.4) | 839 (3.3) |
| Laboratory tests suggestive of nutritional status | ||||||||
| Hemoglobin, mean (SD), g/Lp | 13.5 (1.4)b | 13.4 (1.5)b | 13.4 (1.4)b | 13.3 (1.5)b | 13.5 (1.4)b | 13.4 (1.5)b | 13.5 (1.4)b | 13.4 (1.5)b |
| Albumin, median (IQR), g/dLq | 4.1 (3.9-4.3) | 4.1 (3.9-4.3) | 4.1 (3.9-4.3) | 4.1 (3.9-4.3) | 4.2 (4.0-4.4) | 4.2 (4.0-4.3) | 4.1 (3.9-4.3) | 4.2 (3.9-4.3) |
| Pharmaceutical treatment, No. (%)r | ||||||||
| Oral hypoglycemics | 713 (19.6)b | 3367 (30.9)b | 378 (27.2)b | 1628 (39.1)b | 628 (18.7)b | 3138 (31.1)b | 1719 (20.5)b | 8133 (32.3)b |
| Insulin or GLP-1 | 197 (5.4)b | 1023 (9.4)b | 167 (12.0) | 502 (12.1) | 249 (7.4)b | 975 (9.7)b | 613 (7.3)b | 2500 (9.9)b |
| β-Blocking agents | 761 (20.9)b | 2828 (25.9)b | 319 (23.0)b | 1109 (26.6)b | 602 (17.9)b | 1981 (19.6)b | 1682 (20.1)b | 5918 (23.5)b |
| Calcium channel blockers | 548 (15.1)b | 2049 (18.8)b | 228 (16.4)b | 782 (18.8)b | 430 (12.8)b | 1409 (14.0)b | 1206 (14.4)b | 4240 (16.9)b |
| Agents acting on the renin-angiotensin system | 1169 (32.2)b | 4546 (41.7)b | 527 (38.0)b | 1870 (44.9)b | 1052 (31.3)b | 3732 (37.0)b | 2748 (32.8)b | 10 148 (40.3)b |
| Lipid-modifying agent | 1457 (40.1)b | 4910 (45.0)b | 557 (40.1)b | 2048 (49.2)b | 1058 (31.5)b | 3943 (39.1)b | 3072 (36.6)b | 10 901 (43.3)b |
| Hospital admissionss | ||||||||
| No. (%) hospitalized for any reason | 1890 (52.0)b | 4720 (43.3)b | 692 (49.9)b | 1675 (40.2)b | 1041 (31.0) | 2948 (29.2) | 3623 (43.2)b | 9343 (37.1)b |
| No. of total hospitalizations, median (IQR) | 2 (1-3) | 2 (1-3) | 2 (1-3) | 1 (1-3) | 1 (1-2) | 1 (1-2) | 1 (1-2)b | 1 (1-3)b |
| No. (%) hospitalized for hypoglycemia | 9 (0.2) | 48 (0.4) | 11 (0.8)b | 17 (0.4)b | 13 (0.4) | 27 (0.3) | 33 (0.4) | 92 (0.4) |
| Nonbariatric reoperations, No. (%)t | ||||||||
| Intestinal obstruction (without mention of hernia) | 41 (1.1)b | 55 (0.5)b | 32 (2.3)b | 13 (0.3)b | 13 (0.4)b | 17 (0.2)b | 86 (1.0)b | 85 (0.3)b |
| Hernia of abdominal cavity | 285 (7.8)b | 368 (3.4)b | 159 (11.5)b | 123 (3.0)b | 265 (7.9)b | 176 (1.7)b | 709 (8.5)b | 667 (2.7)b |
| Gastric ulcer | 33 (0.9)b | 35 (0.3)b | 10 (0.7)b | 12 (0.3)b | 9 (0.3)b | 6 (0.1)b | 52 (0.6)b | 53 (0.2)b |
| Esophageal stricture | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; GLP-1, glucagon-like peptide 1; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LB, laparoscopic banding; LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiac event; SBP, systolic blood pressure.
The last BMI taken during the follow-up period. Data were missing for 275 surgical patients and 1799 nonsurgical patients.
Indicates a significant P value of <.05.
A new diagnosis of diabetes recorded during the follow-up period among patients with no history of diabetes (y/n), based on an algorithm which incorporates relevant ICD-9 diagnostic codes, HbA1c levels, glucose levels, and diabetes medications.
The last HbA1c level taken during the follow-up period (%). Data were missing for 83 surgical patients with diabetes and 279 nonsurgical patients with diabetes.
Last HbA1c level taken in the last year of the follow-up period that was equal to or less than 6.0%, and no diabetes medication was prescribed in the last year of the follow-up period (y/n). HbA1c measurement in the last year of follow-up was missing for 473 surgical patients with diabetes and for 1090 nonsurgical patients with diabetes.
New diagnosis of hypertension recorded during the follow-up period among patients with no history of hypertension (y/n), based on any documentation in the hospital or community setting.
The last SBP level recorded during the follow-up period. Data were missing for 50 surgical patients with hypertension and 253 nonsurgical patients with hypertension.
The last DBP level recorded during the follow-up period. Data were missing for 50 surgical patients with hypertension and 253 for nonsurgical patients with hypertension.
The last blood pressure measurement recorded in the follow-up period. Data were missing for 50 surgical patients with hypertension and 253 nonsurgical patients with hypertension.
A new diagnosis of hyperlipidermia during follow-up period among patients with no history of hyperlipidemia (y/n), based on any documentation in the hospital or community setting.
Last total-C level taken during the follow-up period. Data were missing for 17 surgical patients with hyperlipidemia and 244 nonsurgical patients with hyperlipidemia.
Last HDL-C level taken during the follow-up period. Data were missing for 19 surgical patients with hyperlipidemia and 268 nonsurgical patients with hyperlipidemia.
Last LDL-C level taken during the follow-up period. Data were missing for 138 surgical patients with hyperlipidemia and 637 nonsurgical patients with hyperlipidemia.
Last triglycerides level taken during the follow-up period. Data were missing for 18 surgical patients with hyperlipidemia and 257 nonsurgical patients with hyperlipidemia.
At least 1 episode of MACE (myocardial infarction, unstable angina, angioplasty, or coronary artery bypass graft) in hospital records during the follow-up period (y/n).
Last hemoglobin level taken during the follow-up period (mmol/mol). Data were missing for 973 surgical patients and 5753 nonsurgical patients.
Last albumin level taken during the follow-up period (mmol/mol). Data were missing for 65 surgical patients and 2008 nonsurgical patients.
At least 1 prescription of medication type recorded in last year of follow-up (y/n).
Number of patients hospitalized at least once after 30 d from index date up to end of the follow-up period.
Nonbariatric reoperations that occurred during the follow-up period, taken from hospital records.
Discussion
In this retrospective cohort study in a large integrated health system, patients who underwent any of the 3 common types of bariatric surgery experienced statistically significantly lower rates of all-cause mortality during up to 11 years of follow-up compared with nonsurgical patients. Duration of follow-up for sleeve gastrectomy was shorter than for the other 2 types; however, the study included large numbers of sleeve gastrectomy patients and there was lower mortality for these patients as well. Secondary analyses suggest favorable patterns for several additional outcomes including achieved reduction in BMI, remission of diabetes, and reduction of incident hypertension in surgical patients vs matched nonsurgical patients.
A major limitation of the previous literature on bariatric surgery is loss to follow-up in both clinical trials and observational studies. A meta-analysis by Zhou et al included 7 randomized studies, of which no definitive conclusions could be made regarding a mortality benefit from bariatric surgery due to short follow-up times and low numbers of events. Pooled unadjusted estimates, in the same meta-analysis, from 19 nonrandomized studies of all-cause mortality showed statistically lower all-cause mortality among surgical patients (4.4%) compared with nonsurgical patients (8.5%). Based on the follow-up period and number of events reported in that meta-analysis, one can estimate a mean follow up among the 19 nonrandomized studies of 3.6 years and a mortality rate of 6.4 per 1000 person-years among surgical patients compared with 22.2 per 1000 person-years among nonsurgical patients. Unfortunately, most of these studies lacked methodological rigor and often could not account for relatively high loss to follow-up. Uncertainty regarding patients lost to follow-up tempers any conclusions from studies with poor follow-up because it is conceivable that these patients had poor outcomes, biasing reports from these cohorts toward more favorable results.
In a major study from the VA system, most of the patients underwent gastric bypass (74%) with relatively few patients having laparoscopic banding (10%) or sleeve gastrectomy procedures (15%). Their study is limited by 75% follow-up rate at 4 years and a predominance of men (75%).
The current study addresses some of the previous limitations of other studies and thus provides additional data regarding the beneficial effects of bariatric surgery on all-cause mortality for the 3 types of bariatric surgery widely used in the United States, Israel, and world-wide. The study includes a large number of men and women, large numbers of the 3 main types of surgery, and nearly complete follow-up for total mortality. The base study population includes more than 50% of the entire Israeli citizenry, and thus, the results are expected to be highly representative of the experience of the entire country.
Several additional clinical outcomes in surgical and matched nonsurgical patients were also assessed to provide a more complete overview of the benefits and risks of different approaches to obesity treatment in this population. These were considered secondary analyses, are exploratory, and were not subjected to any analysis. The results are suggestive of benefit associated with surgery for these additional clinical outcomes (Table 3).
Some important additional observations are that hemoglobin levels were not affected, and there was little evidence of malnutrition or greater incidence of admissions for hypoglycemia in the gastric bypass group. This operation is commonly associated with anemia, malnutrition, and hypoglycemia, but little is known about how often this becomes a clinically important phenomenon. These observations are important because of the paucity of data regarding these long-term complications for the gastric bypass procedure and will have to be further assessed.
Specific mention should be made regarding bariatric and nonbariatric reoperations. Most of the bariatric reoperations occurred in patients who had laparoscopic banding as their primary operation, and these high reoperation rates have resulted in fewer laparoscopic banding procedures being performed. As expected, there were fewer procedures for repair of abdominal hernias in the nonsurgical group.
This study has several strengths, including large numbers of both surgical and nonsurgical patients, high availability of follow-up data within the health care system, and the extensive and robust nature of the available data.
This study also has several limitations. The first is the observational nature of the study and the need to retrospectively match surgical patients with similar nonsurgical patients as opposed to random allocation. Matching was performed with respect to age, sex, BMI, and diagnosis of diabetes. Although matching by additional characteristics or possible use of propensity score matching could have reduced group imbalance, it would have caused considerable loss of unmatched cases. There was a higher proportion of low SES among nonsurgical patients after matching. Given the higher mortality among low SES patients in general, SES could have been a confounder. This and other potential confounding characteristics were adjusted for in the models.
Second, in an observational study, values may be entered in a less than rigorous fashion. Although this limitation is acknowledged, the observational nature of the study allows us to report medium- to long-term follow-up and outcomes as applied to a real-world setting. Third, many surgical patients were excluded from the study due to limited baseline BMI or matching failure, with differences between the matched and the unmatched patients.
Fourth, because the different procedures were performed at varying points in time, the present study compared their respective mortality rates but should not be interpreted as a head-to-head comparison of the 3 methodologies.
Conclusions
Among obese patients in a large integrated health fund in Israel, bariatric surgery using laparoscopic banding, gastric bypass, or sleeve gastrectomy, vs usual care nonsurgical obesity management, was associated with lower all-cause mortality over a median follow-up of approximately 4.5 years. The evidence of this association adds to the limited literature describing beneficial outcomes of these 3 types of bariatric surgery compared with usual care obesity management.
eFigure 1. Study design
eMethods 1. Clalit Health Services (CHS) population
eTable 1. ICD-9 codes of medical diagnoses
eTable 2. ATC codes
eTable 3. Valid range of values for lab tests
eMethods 2. Multiple imputation process and results for imputing missing data
eTable 4. A comparison between final surgical cohort and those surgical who were excluded
References
- 1.Pontiroli AE, Zakaria AS, Mantegazza E, et al. . Long-term mortality and incidence of cardiovascular diseases and type 2 diabetes in diabetic and nondiabetic obese patients undergoing gastric banding. Cardiovasc Diabetol. 2016;15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J, Eisenberg D, Azagury D, et al. . American Society for Metabolic and Bariatric Surgery position statement on long-term survival benefit after metabolic and bariatric surgery. Surg Obes Relat Dis. 2016;12(3):453-459. [DOI] [PubMed] [Google Scholar]
- 3.Puzziferri N, Roshek TB III, Mayo HG, et al. . Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ. 2014;349:g3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dicker D, Yahalom R, Comaneshter DS, Vinker S. Long-term outcomes of three types of bariatric surgery on obesity and type 2 diabetes control and remission. Obes Surg. 2016;26(8):1814-1820. [DOI] [PubMed] [Google Scholar]
- 6.Adams TD, Davidson LE, Litwin SE, et al. . Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377(12):1143-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arterburn DE, Olsen MK, Smith VA, et al. . Association between bariatric surgery and long-term survival. JAMA. 2015;313(1):62-70. [DOI] [PubMed] [Google Scholar]
- 8.Torgersen Z, Osmolak A, Forse RA. Sleeve gastrectomy and Roux-en-Y gastric bypass. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):352-357. [DOI] [PubMed] [Google Scholar]
- 9.Schaubel DE, Wolfe RA, Port FK. A sequential stratification method for estimating the effect of a time-dependent experimental treatment in observational studies. Biometrics. 2006;62(3):910-917. [DOI] [PubMed] [Google Scholar]
- 10.Karpati T, Cohen-Stavi CJ, Leibowitz M, et al. . Towards a subsiding diabetes epidemic: trends from a large population-based study in Israel. Popul Health Metr. 2014;12(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/. Accessed April 25, 2017.
- 12.Zhou X, Yu J, Li L, et al. . Effects of bariatric surgery on mortality, cardiovascular events, and cancer outcomes in obese patients. Obes Surg. 2016;26(11):2590-2601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study design
eMethods 1. Clalit Health Services (CHS) population
eTable 1. ICD-9 codes of medical diagnoses
eTable 2. ATC codes
eTable 3. Valid range of values for lab tests
eMethods 2. Multiple imputation process and results for imputing missing data
eTable 4. A comparison between final surgical cohort and those surgical who were excluded