This cohort study addresses whether current skin surveillance intervals and threshold for biopsy of suspicious lesions are adequate for the increased tendency of solid organ transplant recipients to develop potentially aggressive cutaneous squamous cell carcinomas.
Key Points
Question
Do current dermatologic screening recommendations address the increased risk of potentially aggressive cutaneous squamous cell carcinomas in solid organ transplant recipients?
Findings
This cohort study compared 167 squamous cell carcinomas from 58 solid organ transplant recipients with 111 squamous cell carcinomas from 40 high-risk immunocompetent patients. Differences of tumor thickness and poorer outcomes between the 2 groups were not statistically significant.
Meaning
Increased risk for developing aggressive squamous cell carcinomas in solid organ transplant recipients may be successfully managed by close adherence to current skin surveillance recommendations and to a marginally lower threshold for biopsy of suspicious lesions.
Abstract
Importance
Solid organ transplant recipients (SOTRs) have a 100-fold increased risk of squamous cell carcinoma (SCC), and they may develop more aggressive SCCs compared with immunocompetent individuals.
Objective
To compare outcomes associated with aggressive behavior of SCC in SOTRs and high-risk immunocompetent patients.
Design, Setting, and Participants
A retrospective cohort study of 58 SOTRs and 40 immunocompetent patients evaluated at the Yale Transplant Dermatology Clinic in New Haven, Connecticut, who had at least 1 SCC confirmed histopathologically between January 1, 2008, and December 31, 2015. Cumulative follow-up time for this study was 369 patient-years.
Exposure
Immunosuppressive medication regimen for SOTRs.
Main Outcomes and Measures
The primary outcome measure was tumor depth of SCC. Secondary outcome measures that reflected tumor aggressiveness included perineural invasion, regional metastases, nodal metastases, disease-specific death, and overall death.
Results
Of the 58 SOTR study participants, 14 were women and 44 were men; the mean (SD) age was 61.3 (8.4) years. Of the 40 immunocompetent study participants, 16 were women and 24 were men; the mean (SD) age was 69.8 (10.9) years, resulting in a statistically significant difference from the SOTR group. The mean (SD) number of years that SOTRs were immunosuppressed was 14.6 (9.2) years (range, 2-37 years). The SOTR and immunocompetent groups were statistically comparable regarding race and sex, patient care, follow-up time, numbers of skin lesions, and field cancerization and chemopreventive therapies. The SOTR group had a significantly higher annual frequency of visits (mean [SD], 4 [2] vs 3 [2] office visits per patient per year, P = .02) and annual biopsy rates (mean [SD], 6 [4] vs 5 [3] biopsies per patient per year, P = .04). The SOTRs developed SCCs that did not appear to be significantly more aggressive than those found in the immunocompetent control group. These SOTRs also did not develop significantly thicker tumors than the immunocompetent control group (median [IQR] tumor depth, 1.30 [0.90-1.60] mm in 35 SOTRs vs 1.22 [1.10-1.60] mm in 20 immunocompetent patients).
Conclusions and Relevance
The increased risk and the potential for aggressive behavior of SCCs in SOTRs may be successfully managed at a level comparable to that in high-risk immunocompetent individuals through close adherence to current dermatologic surveillance recommendations and a marginally lower threshold for biopsy of suspicious lesions for SOTRs.
Introduction
The approximately 100-fold increased incidence of squamous cell carcinoma (SCC) in solid organ transplant recipients (SOTRs) compared with the general population is well documented in the literature. This risk increases for SOTRs with greater cumulative time spent receiving immunomodulatory therapy and with increasing intensity of the immunomodulatory regimen. Overall, the evidence appears to substantiate that a subset of SCCs in SOTRs can behave aggressively.
The consensus for clinical practice by transplant physicians is to manage all SOTR skin cancers as higher risk for local recurrence and metastasis. American and European transplantation organizations recommend skin cancer screening for renal transplant recipients every 6 to 12 months. Following diagnosis of SCC, total body skin examination is recommended for patients every 3 to 6 months for up to 5 years, since 95% of local recurrences and metastases are thought to occur within this time frame.
In this study, we aimed to assess if current care recommendations for SOTRs are addressing their increased risk of potentially aggressive SCCs by comparing differences in behavior and outcomes of SCCs between a group of iatrogenically immunosuppressed SOTRs and a control group of immunocompetent patients.
Methods
Participants
The Yale Dermatopathology database was searched for all carcinoma specimens biopsied at Yale Transplant Dermatology Clinic between January 1, 2008, and December 31, 2015. Two hundred eighty-nine patients had a biopsy performed with pathology report results containing the term carcinoma. Patients without a diagnosis of primary SCC (eg, basal cell carcinoma, SCC in situ, and recurrent SCC) were excluded. The electronic medical records of 105 patients with SCC confirmed histopathologically were reviewed to determine immune status. Seven patients, who were not SOTRs or who lost their transplant to rejection before the study period, were excluded. The remaining 98 patients who were either immunocompetent or SOTRs and had SCC confirmed histopathologically were included. Approval for the study was obtained from the Yale University institutional review board. Informed consent by the participants was waived by the Yale University institutional review board for this retrospective study.
All Yale Transplant Dermatology Clinic patients received standard sun protection instructions during each visit. Patients with extensive actinic damage, actinic keratoses, or multiple well-differentiated SCCs in situ were offered field cancerization treatment options, such as imiquimod, topical fluorouracil, or photodynamic therapy. The immunosuppressive regimens of SOTRs developing multiple SCCs may have been revised to substitute a calcineurin inhibitor with a mammalian target of rapamycin (mTOR) inhibitor such as sirolimus. Patients who developed more than 5 SCCs per year may also have initiated acitretin chemoprophylaxis therapy. Immunosuppression dosage reduction or discontinuation was considered for SOTRs who developed more than 10 SCCs per year. It was recommended to patients with confirmed nonmelanoma skin cancer within the past 2 to 3 years that skin screening be repeated at least every 6 months. A patient who developed multiple skin cancers and/or skin cancers with high-risk features, such as perineural invasion, was recommended to schedule follow-up visits as frequently as every 3 months.
Study Design
In this retrospective cohort study of patients with SCC confirmed histopathologically, 58 SOTRs were compared with 40 immunocompetent patients in the control group.
Demographic data collected included sex, race, date of birth, and immune status (SOTR or immunocompetent patient). Other variables collected for all patients included date of first transplant dermatology visit, total number of visits at transplant dermatology clinic within the study period, date of first biopsy confirmed as SCC histopathologically, and total number of biopsy procedures undergone by the patient within the study period.
Additional data collected on SOTRs included date of transplant, number of organs transplanted, number of transplant operations, and most recent confirmed immunosuppressive medications taken within the study period. Patients remained in the study until death, last visit with transplant dermatologist, last visit at the Yale–New Haven Transplantation Center, or study end date, whichever occurred first.
For each SCC tumor, the biopsy date, gross description, and presence of perineural invasion were documented from dermatopathology reports. Absence of perineural invasion was assumed unless otherwise noted. Skin biopsy margins were examined in a subset of 76 SCC biopsy specimens randomly selected for reevaluation and tumor depth measurement. Biopsy specimens were reevaluated by 2 board-certified dermatopathologists (two of us, C.J.K. and J.M.M.). Discordance from SCC diagnosis in the 76 randomly selected specimens was noted in 21 specimens, thus 55 specimens were ultimately included in the SCC tumor analysis (eTable in the Supplement). In cases in which the deep tumor margin was transected, the tumor was noted to have “at least” the depth of that measurement. A consensus diagnosis was reached after discussion in cases of disagreement.
Outcome Measures
The primary outcome measure was SCC tumor depth. Secondary outcomes were local recurrence, regional metastases, nodal metastases, disease-specific death, and overall death.
Protection of Patient Confidentiality
Data were stored in a password-protected spreadsheet (Excel; Microsoft) on a secure network server specifically encrypted for storage of electronic protected health information.
Statistical Analysis
Demographic data were summarized using mean (SD, range) or median (interquartile range [IQR]) for continuous variables and frequency (percentage) for categorical variables. Group comparisons were made using the χ2 test or Fisher exact test for categorical variables and the unpaired 2-sided t test or Wilcoxon rank sum test for continuous variables. Age- and sex-adjusted negative binomial regression with robust sandwich estimator was used to account for overdispersion of tumor count data. Follow-up time was used as an offset variable to estimate incidence rate. Two-sided P < .05 was considered significant. Data summarization and group comparisons were performed using Stata/SE, version 13.1 (StataCorp LP). Negative binomial regression and Poisson regression were performed using SAS, version 9.4 (SAS Institute Inc).
Results
Patient Demographics
Demographic data for the 98 patients are shown in Table 1. Of the 58 SOTR study participants, 14 (24.1%) were women and 44 (75.9%) were men; the mean (SD) age was 61.3 (8.4) years. Of the 40 immunocompetent study participants, 16 (40%) were women and 24 (60%) were men; the mean (SD) age was 69.8 (10.9) years. There was no significant difference in sex or race distribution between groups. The immunocompetent group was significantly older than the SOTR group (69.5 vs 62.3 years, P < .001).
Table 1. Characteristics of the Study Population.
| Characteristic | SOTR Group (n = 58) |
Immunocompetent Group (n = 40) |
P Value | ||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| Demographic | |||||
| Age, y | 61.3 (8.4) | 62.3 (56-66) | 69.8 (10.9) | 69.5 (63.5-80.0) | <.001a |
| Sex, No. (%) | |||||
| Male | 44 (75.9) | NA | 24 (60) | NA | .09 |
| Female | 14 (24.1) | NA | 16 (40) | NA | |
| Race/ethnicity, No. (%) | |||||
| White | 56 (96.5) | NA | 40 (100) | NA | .24 |
| Hispanic or Latino | 2 (3.5) | NA | 0 | NA | |
| Transplant Data | |||||
| No. of transplants, No. (%) | |||||
| 1 | 46 (79.3) | NA | NA | NA | |
| >1 | 12 (20.7) | NA | NA | NA | |
| Years of immunosuppression | 14.6 (9.2) | 12 (7-20.5) | NA | NA | |
| Patient care receivedb | |||||
| Follow-up time, y | 3.8 (2.2) | 4 (1.5-6) | 3.7 (2.4) | 3.8 (1-6) | .82 |
| No. of office visits | 11 (8) | 11 (5-16) | 9 (9) | 7 (3-15) | .24 |
| Visit frequency | 4 (2) | 3 (2-4) | 3 (2) | 2 (2-3) | .02a |
| No. of skin biopsies | 21 (17) | 14 (8-34) | 17 (17) | 11 (4-24) | .25 |
| Annual biopsy rate | 6 (4) | 6 (3-8) | 5 (3) | 4 (2-7) | .04a |
Abbreviations: IQR, interquartile range; NA, not applicable; SOTR, solid organ transplant recipient.
Statistically significant association. Visit frequency and annual biopsy rate were calculated using variable follow-up time for each patient.
Total number of office visits was 1026; total number of biopsies for suspicious skin lesions for 98 patients, 1866.
SOTR Characteristics
Some transplant data for the SOTR group are shown in Table 1. Of 46 patients who underwent a single transplant operation, 26 were kidney recipients, 9 were heart recipients, 4 were lung recipients, 3 were liver recipients, and 4 were combined kidney/pancreas recipients. The remaining 12 patients underwent from 2 to 4 different transplant operations involving some combination of previously listed transplanted organs.
This study reported a wide range of years of cumulative immunosuppression therapy for SOTRs. The mean (SD) number of years that SOTRs were immunosuppressed was 14.6 (9.2) years, (range, 2-37 years). At the end of the study period, the most represented immunosuppressive regimen consisted of some combination of mycophenolate mofetil, prednisone, and tacrolimus and was observed in 10 of 58 patients (17.2%).
Patient Care Metrics
This study included a total follow-up of 369 patient-years for both SOTRs and immunocompetent patients. The 98 patients were seen at 1026 office visits and underwent 1866 biopsies of suspicious skin lesions. Data on patient care received are compared in Table 1. There were no significant differences in mean follow-up time (mean [SD] years, 3.8 [2.2] vs 3.7 [2.4] years; P = .82), mean number of office visits per patient (mean [SD], 11 [8] vs 9 [9]; P = .24), or mean number of skin biopsies per patient (mean [SD], 21 [17] vs 17 [17]; P = .25). Patient visit frequency and annual biopsy rate were calculated using variable follow-up time per patient. As a result, patient visit frequency (number of office visits per patient per year) was significantly higher (mean [SD], 4 [2] vs 3 [2]; P = .02) and annual biopsy rate (number of skin biopsies per patient per year) was also significantly greater (mean [SD], 6 [4] vs 5 [3]; P = .04) in the SOTR group compared with the immunocompetent group.
Tumor Counts
Of 1866 skin biopsies performed on the 98 patients, 756 were diagnosed as nonmelanoma skin cancers, 115 were basal cell carcinomas, 278 were SCC, 1 was an atypical fibroxanthoma, and the remainder were SCC in situ.
Data on the number of skin lesions diagnosed in each group are shown in Table 2. The SOTRs were diagnosed with a greater median number of actinic keratoses [median (IQR): 4.5 (1.0-10.0) vs 2.5 (0.5-5.0)], nonmelanoma skin cancers [median (IQR): 5.5 (3.0-11.0) vs 2.0 (2.0-13.0)], and SCCs [median (IQR): 2.0 (1.0-4.0) vs 1.0 (1.0-3.0)] than the immunocompetent group. However, no significant differences between the incidence rates (with or without age and sex adjustment) of actinic keratoses, SCCs, or nonmelanoma skin cancers were observed between the 2 groups.
Table 2. Comparison of Skin Tumor Incidence After Adjusting for Age and Sex.
| Tumor Incidence | SOTR Group (n = 58) |
Immunocompetent Group (n = 40) |
Unadjusted IRR (95% CI) | Adjusted IRR (95% CI) | P Value | |
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | |||||
| No. of AKs | 409 | 231 | ||||
| Mean (SD) | 7 (8) | 6 (9) | 1.4 (0.9-2.2) | 1.4 (0.8-2.3) | .11 | .19 |
| Median (IQR) | 4.5 (1-10) | 2.5 (0.5-5) | ||||
| Unadjusted IR (95% CI) | 2.0 (1.6-2.6) | 1.4 (1.0-2.0) | ||||
| Adjusted IR (95% CI) | 1.8 (1.4-2.5) | 1.3 (0.9-1.9) | ||||
| No. NMSCs | 480 | 276 | ||||
| Mean (SD) | 8 (8) | 7 (8) | 1.2 (0.8-1.8) | 1.2 (0.8-1.7) | .36 | .31 |
| Median (IQR) | 5.5 (3-11) | 2 (2-13) | ||||
| Unadjusted IR (95% CI) | 2.5 (2.0-3.1) | 2.1 (1.5-2.8) | ||||
| Adjusted IR (95% CI) | 2.6 (2.0-3.2) | 2.2 (1.6-3.1) | ||||
| No. of SCCs | 167 | 111 | ||||
| Mean (SD) | 3 (3) | 3 (4) | 1.1 (0.7-1.7) | 1.4 (0.9-2.0) | .83 | .14 |
| Median (IQR) | 2 (1-4) | 1 (1-3) | ||||
| Unadjusted IR (95% CI) | 0.9 (0.7-1.3) | 0.9 (0.6-1.3) | ||||
| Adjusted IR (95% CI) | 1.0 (0.7-1.4) | 0.9 (0.6-1.2) | ||||
Abbreviations: AKs, actinic keratoses; IQR, interquartile range; IR, incidence rate; IRR, incidence rate ratio; NMSCs, nonmelanoma skin cancers; SCCs, squamous cell carcinomas; SOTR, solid organ transplant recipient.
Chemoprevention and Field Cancerization
Twenty-three of 40 high-risk immunocompetent patients (58%) and 26 of 58 SOTRs (45%) received a field cancerization or chemopreventive therapy within the study period. Field cancerization is the presence of numerous actinic keratoses to their confluence over a large cutaneous area, typically in sun-exposed areas of the body. There was no significant difference in the proportion of patients receiving these therapies (P = .30).
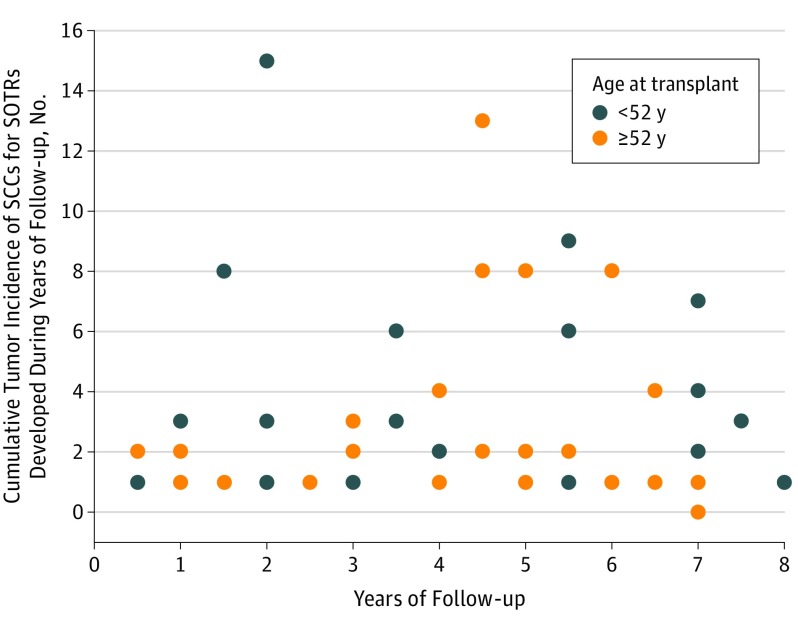
Cumulative Tumor Incidence Among SOTRs
The cumulative tumor incidence of SCC developed during years of follow-up for SOTRs is shown in the Figure, as stratified by median age at transplant (52 years). Regarding years of follow-up and cumulative incidence of SCC, there was no significant correlation in the entire cohort or in each age stratum (entire cohort: r = 0.11, P = .43; younger group [<52 years old]: r = 0.14, P = .48; older group [≥52 years old] r = 0.09, P = .65). However, in a multivariate logistic regression model examining the odds of having a cumulative incidence of 2 or more SCCs, age at transplant was an independent factor associated with a cumulative incidence of 2 or more SCCs after controlling for years of follow-up and years of immunosuppression (Table 3). The adjusted odds of cumulative incidence of 2 or more SCCs decreased by 20% per every 5-year increase in age at transplant (adjusted OR per 5-year age increase, 0.80; 95% CI, 0.63-0.99; P = .04). The association for total years of immunosuppression therapy was trivial (adjusted OR per additional 5 years of therapy, 0.99; 95% CI, 0.99-1.00; P = .10).
Figure. Cumulative Incidence Rate of Squamous Cell Carcinoma (SCC) During the Study Period vs Years of Follow-up for 58 Solid Organ Transplant Recipients (SOTRs) Stratified by Median Age at Transplant.
Table 3. Association With Cumulative Incidence of 2 or More SCCs Among SOTRs.
| Characteristic | Unadjusted OR (95% CI)a | Unadjusted P Value | Adjusted OR (95% CI)b | Adjusted P Value |
|---|---|---|---|---|
| Age at transplant | 0.82 (0.67-1.01) | .06 | 0.80 (0.63-0.99) | .04 |
| Years of follow-up | 1.23 (1.01-1.50) | .04 | 1.30 (0.98-1.73) | .07 |
| Years of immunosuppression therapy | 0.99 (0.99-1.00) | .12 | 0.99 (0.99-1.00) | .10 |
Abbreviations: OR, odds ratio; SCCs, squamous cell carcinomas; SOTRs, solid organ transplant recipients.
The OR corresponds to 5-year change in odds of having cumulative incidence of 2 or more SCCs.
Adjusted for age and sex.
Outcomes
Patient outcomes were examined, including local recurrence, regional metastases, nodal metastases, disease-specific death, and overall death. No local recurrences of SCC were observed among the 98 patients within the follow-up time period. There were 2 SOTRs diagnosed with regional metastases. A man in his 70s, the recipient of a lung transplant, was diagnosed with regional metastases about 8 months after the initial diagnosis of SCC. A man in his 80s, the recipient of a kidney transplant, was also diagnosed with regional metastases of a scalp SCC. Nine months after diagnosis of an SCC, nodal metastases that were likely from SCC were noted in a woman in her 70s who had a kidney transplant. No disease-specific death was observed in the study population. Nine patients (22.5%) in the immunocompetent group and 8 SOTRs (13.8%) died of non-SCC–related causes during the study period. The difference in mortality rates was not statistically significant (P = .26).
SCC Tumor Characteristics
The 98 patients in this study developed 278 histologically confirmed SCCs within the study period. Of these 278 SCCs, 111 (40%) came from the 40 immunocompetent patients, while 167 (60%) came from the 58 SOTRs.
Perineural Invasion
One SCC biopsy result (0.4%) demonstrated evidence of perineural invasion. The histologic biopsy specimen was reported to be acantholytic, and the SCC was located on the scalp of a male recipient of a heart transplant (1 of 167 [0.6%]).
Tumor Base Transection
Tumor base transection was noted on the pathology report in 53 of the 278 SCCs (19%) biopsied, whereas tumor base was not transected in 225 of the 278 SCCs (81%) biopsied in the study population (Table 4). There was no significant age- and sex-adjusted difference in the risk of transecting tumor base on biopsy between the SOTR and immunocompetent groups (P = .24).
Table 4. Comparison of Other SCC Tumor Characteristics Adjusted for Age and Sex.
| Tumor Characteristic | SOTR Group (n = 58) |
Immunocompetent Group (n = 40) |
P Value |
|---|---|---|---|
| Tumor base transection, No. of tumors/patientsa | |||
| Base transected | 36/5 | 17/15 | |
| Not transected | 131/12 | 94/32 | |
| Unadjusted | .27 | ||
| Adjusted | .24 | ||
| Tumor depth in subset of SCCs (n = 55) | (n = 35) | (n = 20) | |
| Mean (SD) depth, mm | 1.42 (0.82) | 1.31 (0.36) | .53 |
| Median (IQR) depth, mm | 1.30 (0.90-1.60) | 1.22 (1.10-1.60) | |
| No. of SCCs of “at least” measured depth, No. (%) | 19 (54) | 8 (40) | .31 |
Abbreviations: IQR, interquartile range; SCCs, squamous cell carcinomas; SOTR, solid organ transplant recipient.
For the immunocompetent group vs the SOTR group, unadjusted relative risk (95% CI) was 1.4 (0.8-2.5); after adjustment for age and sex, 1.4 (0.8-2.6).
Tumor Depth
Tumor depth measured in 55 randomly selected specimens independently confirmed SCC according to 2 board-certified dermatopathologists.
The overall mean (SD) tumor depth measured was 1.37 (0.69) mm (range, 0.32-5.1 mm), and the median tumor depth measured was 1.23 mm (IQR, 1.00-1.60) in all specimens. The median tumor depth measured in specimens from 35 SOTR patients was 1.30 mm (IQR, 0.90-1.60), whereas the median tumor depth from 20 immunocompetent patients was 1.22 mm (IQR, 1.10-1.60) (Table 4). There was no significant difference in tumor depth noted between the 2 groups in this study population (P = .53). Nineteen of the 35 tumors (54%) from SOTRs and 8 of the 20 tumors (40%) from immunocompetent patients were noted to be “at least” the measured depth. There was no significant difference between the proportions of tumors that were at least the noted measurement between the 2 groups (P = .31).
Discussion
The SCCs developed by the SOTR cohort did not appear to behave more aggressively than those developed by the immunocompetent control population. Of 55 randomly selected, histologically confirmed SCC specimens, 4 SCCs in the overall cohort were more than 2.0 mm thick; the maximum thickness of the SCCs was 5.1 mm, and all SCCs thicker than 2.0 mm occurred in SOTRs. However, we did not find a significant difference in tumor depth between the 2 groups in the study population. A secondary measure of aggressive behavior of SCCs was poor outcomes at the patient level. We described poor outcomes in patients with SCC in this study because of their rarity and general difficulty to compare statistically. All poor outcomes in this study occurred in the SOTR group, and there were too few to determine whether the differences were statistically significant.
The approximately 100-fold increased incidence of SCC in the SOTR population compared with the general population is well documented in the literature. Various retrospective studies have found that SCCs in SOTRs behave more aggressively than those in the general population. Solid organ transplant recipients have been reported to develop thicker, more infiltrative SCCs. Brantsch et al assessed 615 immunocompetent white patients over a median follow-up period of 43 months and found that SCCs greater than 2.0 mm thick showed a significant tendency to metastasize. Tumors greater than 6.0 mm thick were associated with a high risk of metastasis and local recurrence.
It was important to ensure that the immunocompetent control group was comparable to the SOTR group in as many clinically meaningful ways as possible, aside from the SOTRs’ iatrogenic immunosuppression. The SOTR group was demographically comparable to the immunocompetent control group regarding sex and race distribution. The SOTR group was younger compared with the immunocompetent group, and this is consistent with the well-documented earlier onset of SCCs in SOTRs. Multivariate analysis was adjusted for age to control for the association of age difference with outcomes. The SOTR and immunocompetent groups were of comparable health status because there was no significant difference in the frequency of death between groups. An important factor that could limit a study’s ability to detect the true differences between groups is the amount of medical care received by patients. The proportion of SOTRs receiving field cancerization or chemopreventive therapies was also comparable to that in immunocompetent patients. After adjusting for variable follow-up time per patient, the SOTR group did have significantly greater patient visit frequency (4 vs 3 office visits per patient per year, P = .02) and significantly greater annual biopsy rate (6 vs 5 skin biopsies per patient per year, P = .04) compared with the immunocompetent group. The greater annual visit frequency and biopsy rate reflect strict adherence to current dermatologic surveillance recommendations for SOTRs and greater clinical suspicion about the clinical course of the lesions that develop.
The SOTRs in the study had a wide range of years of cumulative immunosuppression therapy. Numerous studies have shown that the heightened risk of skin cancers increases with greater cumulative time receiving immunomodulatory therapy and with increasing intensity of the regimen. We did not observe a monotonic increase in incidence of SCCs. In logistic regression, we did not find a significant association between the cumulative incidence of more than 2 SCCs during the study period and years of immunosuppression at the end of the study. Age at transplantation was a more important factor associated with having 2 or more SCCs cumulatively.
Strengths and Limitations
The results of this study should be interpreted carefully in the context of its strengths and limitations. The detection of rare aggressive outcomes in SCC was limited by the study sample size. A considerable 369 patient-years of follow-up were included in this 8-year retrospective study, but larger, multicenter studies would be needed to compare rare poor outcomes of SCCs among SOTRs. We were limited in describing the poor outcomes that occurred in this cohort. This study was conducted at a single institution, which limits generalizability to populations dissimilar from those served by the Yale–New Haven Transplantation Center. However, this feature may strengthen the study because it allows control for hospital-related cofounders and for variations in biopsy technique, which we showed to be comparable between the 2 groups by examining the rate of margin positivity. This nonrandomized, retrospective, observational cohort study was conducted in a high-risk referral population with timely access to specialized transplant care, which unfortunately limits generalizability to the typical SOTR population. The immunocompetent control group was also composed of patients with high-risk skin cancer who were referred to a tertiary medical center. This may introduce referral bias, but it actually strengthens comparisons between the SOTR and immunocompetent patient groups since the immunocompetent group stringently matched the SOTR group regarding possible confounders, such as demographic composition, patient care received, and general tumor outcomes.
Conclusions
The findings suggest that the greatly increased risk of SCCs in SOTRs and the potentially more aggressive clinical course of SCCs may be successfully managed at a level comparable to high-risk immunocompetent individuals by adhering to current dermatologic surveillance recommendations for SOTRs and by allowing a marginally lower threshold for biopsy of suspicious lesions.
eTable. Diagnoses Upon Secondary Histopathologic Examination of Randomly Selected Specimens Initially Read as Squamous Cell Carcinoma
References
- 1.Berg D, Otley CC. Skin cancer in organ transplant recipients: epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47(1):1-17. [DOI] [PubMed] [Google Scholar]
- 2.Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management, part I: epidemiology of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65(2):253-261. [DOI] [PubMed] [Google Scholar]
- 3.Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management, part II: management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65(2):263-279. [DOI] [PubMed] [Google Scholar]
- 4.Moloney FJ, Comber H, O’Lorcain P, O’Kelly P, Conlon PJ, Murphy GM. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br J Dermatol. 2006;154(3):498-504. [DOI] [PubMed] [Google Scholar]
- 5.Weaver JL. Establishing the carcinogenic risk of immunomodulatory drugs. Toxicol Pathol. 2012;40(2):267-271. [DOI] [PubMed] [Google Scholar]
- 6.Winkelhorst JT, Brokelman WJ, Tiggeler RG, Wobbes T. Incidence and clinical course of de-novo malignancies in renal allograft recipients. Eur J Surg Oncol. 2001;27(4):409-413. [DOI] [PubMed] [Google Scholar]
- 7.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348(17):1681-1691. [DOI] [PubMed] [Google Scholar]
- 8.Lott DG, Manz R, Koch C, Lorenz RR. Aggressive behavior of nonmelanotic skin cancers in solid organ transplant recipients. Transplantation. 2010;90(6):683-687. [DOI] [PubMed] [Google Scholar]
- 9.Wong G, Chapman JR, Craig JC. Cancer screening in renal transplant recipients: what is the evidence? Clin J Am Soc Nephrol. 2008;3(suppl 2):S87-S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip: implications for treatment modality selection. J Am Acad Dermatol. 1992;26(6):976-990. [DOI] [PubMed] [Google Scholar]
- 11.Smith KJ, Hamza S, Skelton H. Histologic features in primary cutaneous squamous cell carcinomas in immunocompromised patients focusing on organ transplant patients. Dermatol Surg. 2004;30(4, pt 2):634-641. [DOI] [PubMed] [Google Scholar]
- 12.Schmults CD. High-risk cutaneous squamous cell carcinoma: identification and management. Adv Dermatol. 2005;21:133-152. [DOI] [PubMed] [Google Scholar]
- 13.Brantsch KD, Meisner C, Schönfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9(8):713-720. [DOI] [PubMed] [Google Scholar]
- 14.Lewis KG, Weinstock MA. Nonmelanoma skin cancer mortality (1988-2000): the Rhode Island follow-back study. Arch Dermatol. 2004;140(7):837-842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Diagnoses Upon Secondary Histopathologic Examination of Randomly Selected Specimens Initially Read as Squamous Cell Carcinoma