Abstract
Necroptosis is a form of regulated necrosis and is dependent on a signaling pathway involving receptor interacting protein kinase-3 (RIPK3) and mixed lineage kinase domain-like protein (MLKL). Necroptosis is considered to have important functions in inflammation and, based on studies with animal disease models, is believed likely to be involved in the pathogenesis of many human inflammatory diseases. In neutrophils, necroptosis has recently been reported to be triggered by tumor necrosis factor (TNF) stimulation, ligation of adhesion receptors, exposure to monosodium urate (MSU) crystals, or phagocytosis of Staphylococcus aureus (S. aureus). Because neutrophils are involved in many kinds of tissue inflammation and disease, neutrophil necroptosis probably plays a vital role in such processes. Dissecting the signaling pathway of neutrophil necroptotic death may help to identify novel drug targets for inflammatory or autoimmune diseases. In this review, we discuss different mechanisms which regulate neutrophil necroptosis and are thus potentially important in neutrophil-associated disorders.
Facts
Necroptosis is one type of regulated necrosis and is dependent on RIPK3 and MLKL activities, showing morphologic features similar to necrosis.
Death receptors, Toll-like receptors (TLRs), the IFN-α receptor (IFNAR), adhesion receptors, and DNA-dependent activator of IFN (DAI) regulatory factors have been shown to trigger RIPK3-MLKL-dependent necroptosis.
Neutrophil necroptosis can be induced by TNF stimulation, ligation of adhesion receptors, exposure to monosodium urate (MSU) crystals, or phagocytosis of S. aureus.
Reactive oxygen species (ROS) are important contributors to neutrophil necroptosis induced by ligation of adhesion receptors or MSU stimulation.
Human neutrophils migrating to inflammatory sites can activate the RIPK3-MLKL pathway as seen in neutrophilic diseases such as cutaneous vasculitis, ulcerative colitis, and psoriasis.
Open questions
Is necroptosis involved in the pathogenesis of human inflammatory diseases?
How does neutrophil necroptosis impact on inflammatory and autoimmune diseases?
How can one distinguish between neutrophil extracellular trap (NET) formation and necroptosis-related, passive release of chromatin?
How is RIPK3 activated by adhesion receptors in neutrophil necroptosis?
How is p38 MAPK activated by the RIPK3-MLKL complex?
Are ROS initiators or executors in neutrophil necroptosis?
How can XIAP restrict the switch to TNF-induced necroptosis in mouse neutrophils? What is the role of XIAP in human neutrophils?
What is the executor of RIPK3-dependent regulated necrosis induced in human neutrophils by the phagocytosis of S. aureus?
Why do mouse neutrophils lacking MLKL clear S. aureus at the site of infection only poorly?
Introduction
Apoptosis was long thought to be the only form of programmed cell death during homeostasis, development and disease. The key regulators of apoptotic cell death are caspases and the characteristic morphological hallmarks, cell shrinkage, nuclear condensation, cell membrane blebbing, and formation of apoptotic bodies. In contrast, necrosis was long considered to be an unregulated and uncontrollable accidental cell death, for which the characteristic morphologic changes are cell swelling, plasma membrane rupture, and release of intracellular contents1,2. However, recent evidence has shown that necrosis can also occur in a regulated manner called necroptosis, now recognized as one type of regulated necrosis, dependent on RIPK33–5 and MLKL6–9 activities and exhibiting morphologic features similar to necrosis. This necrosis-like regulated cell death can be triggered when caspases are inhibited, as was already demonstrated in 199610. Since 2000, the important role of RIPK1 and of its kinase inhibitor, necrostatin-1, in this caspase-independent necrosis have been recognized11–13. The subsequent discovery of RIPK3 as the key protein in necroptosis3–5 and the identification of the pseudokinase MLKL as an effector protein downstream of RIPK36 were crucial for understanding the necroptosis pathway. (Fig. 1) illustrates the history of progress in defining the necroptosis pathway. Death receptors1,2, Toll-like receptors (TLRs)14–16, IFN-α receptor (IFNAR)16–18, adhesion receptors19,20 and DNA-dependent activator of IFN (DAI) regulatory factors21,22, have all been shown to be involved in RIPK3-MLKL-dependent necroptosis (Fig. 2).
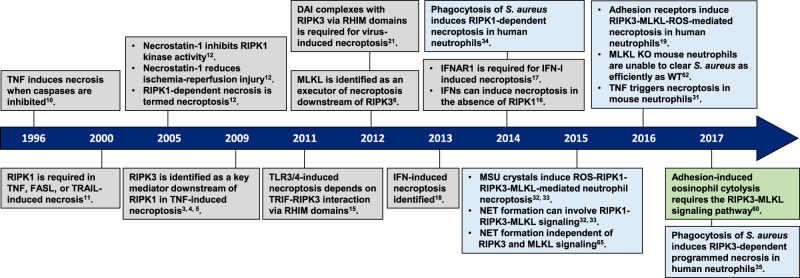
Fig. 1. Timeline for the discovery of key proteins in the molecular pathways of necroptosis.
Necroptosis (Grey box); neutrophil necroptosis (Blue box); eosinophil necroptosis (Green box). TNF tumor necrosis factor, FASL FAS ligand, TRAIL TNF-related apoptosis-inducing ligand, RIPK1 receptor interacting protein kinase-1, RIPK3 receptor interacting protein kinase-3, DAI DNA-dependent activator of IFN regulatory factors, RHIM RIP homotypic interaction motif, MLKL mixed lineage kinase domain-like protein, TLR Toll-like receptor, TRIF Toll/IL-1 receptor domain-containing adaptor protein-inducing interferon-β, S. aureus Staphylococcus aureus, IFNs interferons, IFN-I type I interferons IFNAR1 IFN-α receptor type I, MSU monosodium urate, PMA phorbol 12-myristate 13-acetate, ROS reactive oxygen species, NET neutrophil extracellular trap, KO knockout, WT wild-type
Fig. 2. RIPK3-MLKL-mediated necroptosis induced by receptors or DAI.
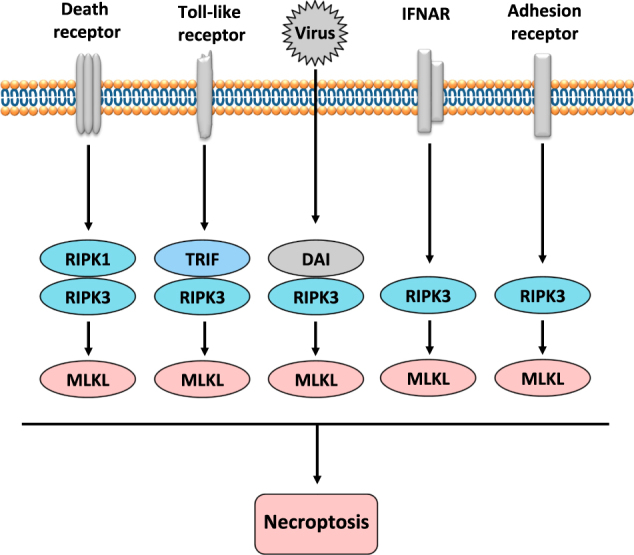
Death receptors can trigger a RIPK1-RIPK3-MLKL-mediated necroptosis and TLRs are able to induce a TRIF-RIPK3-MLKL-mediated necroptosis. Viruses, too, can trigger a DAI-RIPK3-MLKL-mediated necroptosis as can adhesion receptors or IFN-I signaling (IFNAR), inducing a RIPK3-MLKL-mediated necroptosis. RIPK3 can be engaged by RIPK1, TRIF, or DAI through their respective RHIM domains and, furthermore, MLKL can be recruited and phosphorylated by RIPK3 to trigger necroptosis
In contrast to apoptosis, which can limit inflammation, necroptotic cells can release massive amounts of damage-associated molecular patterns (DAMPs) from disintegrating membranes, depending on the cellular environment; thus, necroptosis is generally considered to be a contributor to inflammation1,23. On the other hand, necroptosis may also reduce the production of pro-inflammatory cytokines by shortening the cell lifespan, decreasing the overall inflammatory response induced by tumor necrosis factor (TNF) or lipopolysaccharide (LPS), thus suppressing the host immune response and benefiting intracellular pathogens24. Moreover, necroptosis is also considered to be involved in normal development2,25.
Neutrophils are important effector cells in innate immunity, being the most abundant population of leukocytes in the circulation26. They are produced in the bone marrow, where they mature before being released into the bloodstream. Neutrophils are essential for the innate immune system and rapidly migrate to inflamed tissues in response to pathogens or sterile harmful stimuli27,28. At the inflammatory sites, neutrophils can fight infection with an array of strategies, including phagocytosis, generation of reactive oxygen species (ROS), degranulation, and release of neutrophil extracellular traps (NETs)29. These functions are responsible for the vital contribution of neutrophils in fighting pathogens and in preventing spread of infection; however, when they overreact, they also damage host tissues27.
Apoptosis is the most common physiological and pathological cell death in neutrophils28. In the resolution of inflammation, neutrophils accumulated locally rapidly undergo apoptosis and are removed by other phagocytic cells. Therefore, neutrophil apoptosis is considered to limit tissue damage by preventing the release of histotoxic contents from dying cells28. In contrast, neutrophil necrosis is considered highly detrimental to the resolution of inflammation owing to the release of toxic contents and potential escape of pathogens into the surroundings30. Recently, several groups have reported that necroptosis can also occur in neutrophils. For instance, TNF receptor 1 (TNFR1) stimulation31, ligation of adhesion receptors19,20, activation by MSU crystals32,33, or phagocytosis of S. aureus34,35 can all trigger neutrophil necroptosis (Fig. 3). Neutrophils are involved in many kinds of tissue inflammation and disease;36 unlike apoptosis, necroptotic death of neutrophils may induce tissue injury and inflammation similar to necrosis and is thus likely to be important for the pathogenesis of infectious or autoimmune diseases. In this article, we discuss the different mechanisms regulating neutrophil necroptosis and their potential for causing neutrophil-associated disorders.
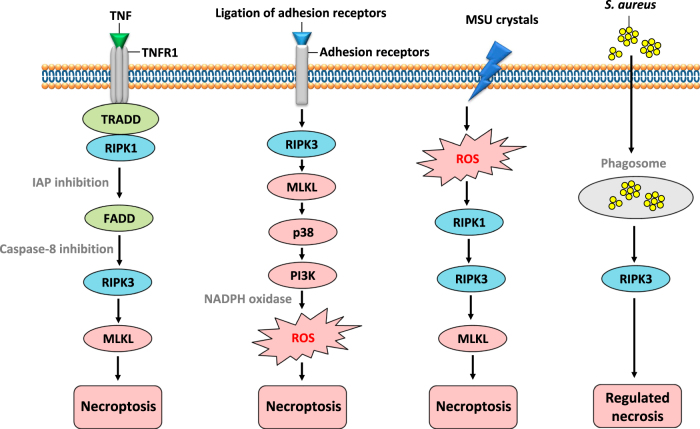
Fig. 3. The signaling pathway of neutrophil necroptosis.
Neutrophil necroptosis triggered by various stimuli, including TNF, adhesion receptors, MSU crystals or phagocytosis of S. aureus. TNF-induced mouse neutrophil necroptosis is dependent on a RIPK1-RIPK3-MLKL signaling pathway. Ligation of adhesion receptors, including CD44, CD11b, CD18, and CD15, can induce a human neutrophil necroptosis involving the RIPK3 – MLKL - p38 MAPK - PI3K axis, in which all of these molecular components are prerequisite for generation of ROS induced by NADPH oxidase, and subsequent necroptosis. However, how the RIPK3-MLKL complex activates p38 MAPK requires further investigation and confirmation in other cell types. MSU crystal-induced neutrophil necroptosis is triggered by ROS production which further activates RIPK1-RIPK3-MLKL and subsequent cell death. The mechanism of ROS production by MSU exposure remains to be investigated. Phagocytosis of S. aureus can also trigger RIPK3-dependent regulated necrosis in human neutrophils
The signaling pathway of TNF-induced necroptosis
TNFR1 is one of the death receptors in the TNF superfamily and much of our knowledge of necroptosis comes from studies of the TNFR1 signaling pathway (Fig. 4)1. TNFR1 stimulation induces an early complex formation comprising TNFR1-associated death domain protein (TRADD) and RIPK1. Cellular inhibitors of apoptosis (cIAPs), including cIAP1 and cIAP2, and the linear ubiquitin chain assembly complex (LUBAC) are recruited to the initial complex, inducing Lys63-linked or linear ubiquitylation of RIPK1, respectively2. In consequence of ubiquitylation, the initial complex is stabilized, going on to activate Nuclear Factor-κB (NF-κB) transcriptional activity, which contributes to cell survival, proliferation, and the production of pro-inflammatory cytokines (Fig. 4)1,2. Conversely, inhibiting the ubiquitylation of RIPK1 through blocking of cIAPs (Smac mimetics or genetic deletion of IAPs)37–40 facilitates the formation of a different complex composed of TRADD, RIPK1, and oligomerized FAS-associated death domain protein (FADD)2. This second complex then recruits and activates caspase-8, finally inducing an RIPK1 activity-dependent apoptosis (Fig. 4). Moreover, LUBAC inhibition2,41, transforming growth factor-β-activated kinase 1 inhibition42, NF-κB essential modulator silencing43, or Pellino silencing44 also favor the formation of a FADD-containing complex and thus promote apoptosis1.
Fig. 4. Necroptosis signaling pathway induced by TNFR1 stimulation via TNF.
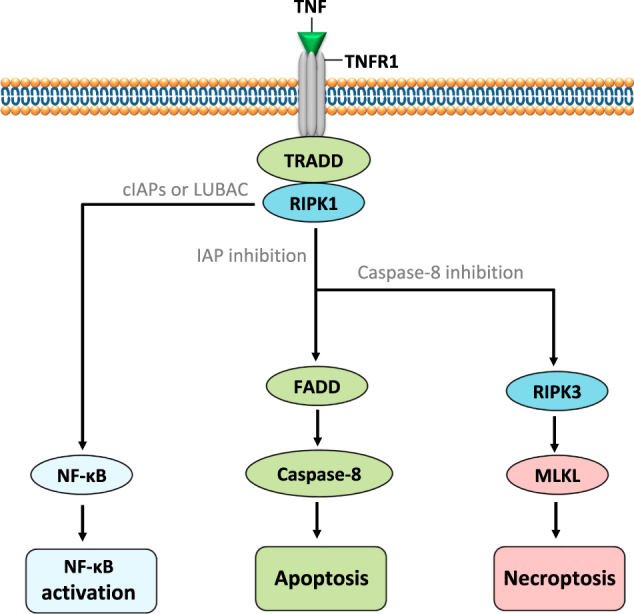
TNF is a pleiotropic cytokine playing a key role in infection and tissue injury. TNF has a role not only in inflammation, since, under some conditions, as a consequence of TNFR1 ligation, it can also trigger regulated cell death pathways such as apoptosis or necroptosis
Following the stimulation of apoptosis, if caspase-8 activity is blocked, RIPK1 recruits RIPK3 through interactions between the RIP homotypic interaction motif (RHIM) domains. This RIPK3-containing complex is termed the necrosome. The RIPK3 can subsequently recruit and phosphorylate MLKL to trigger necroptosis (Fig. 4)6,9. MLKL, a downstream target of RIPK3, is considered as the effector of necroptosis6,9. RIPK3 can activate MLKL and induce its oligomerization, which facilitates oligomerized MLKL translocation to the plasma membrane and triggers cell permeabilization and necroptosis45–48.
Some additional proteins have been reported to be involved in the function of the necrosome. The FADD-like interleukin (IL)-1β-converting enzyme (FLICE)-inhibitory protein (FLIP) plays an important role in the control of necroptosis induced by TNF2,38,49, since caspase-8—FLIP heterodimers are believed to inhibit necroptosis by disrupting RIPK1-RIPK3 necrosome formation2,49. Cylindromatosis protein (CYLD) is an ubiquitin-editing enzyme which can remove ubiquitin chains from RIPK1 to facilitate the induction of necroptosis50,51. Upon TNF stimulation, CYLD can trigger necroptosis by promoting necrosome formation, phosphorylation and activation of RIPK351, but caspase-8 can cleave CYLD to suppress necroptosis50. Moreover, heat shock protein 90 and its co-chaperone CDC37 complex are required for RIPK3 activation during necroptosis52. In contrast, both the phosphatase PPM1B53 and the ubiquitin-modifying enzyme A2054 can negatively regulate necroptosis through suppressing RIPK3 activity.
TNF-induced necroptosis in neutrophils
A TNF-induced neutrophil necroptosis was reported recently which is dependent on a RIPK1- RIPK3-MLKL signaling31. In that work, the authors showed that in mouse neutrophils responding to high concentrations of TNF, X-linked IAP (XIAP) plays a crucial role in determining the type of neutrophil death, i.e. either apoptosis or necroptosis31. XIAP and cIAPs are all members of the IAP family. Like the cIAPs, XIAP was also reported to ubiquitylate RIPK1 and trigger NF-κB activation2. Thus, blocking XIAP could also facilitate the formation of the FADD-containing complex to induce either apoptosis or necroptosis in response to TNF (Fig. 4)2,37. In other cell types, however, evidence shows that the role of XIAP in restricting TNF-induced death is dispensable, because loss of XIAP fails to trigger TNF-induced apoptosis in mouse embryonic fibroblasts39 and cIAP1 inhibition alone is a sufficient induction for either apoptosis or necroptosis upon TNF stimulation37,39.
Moreover, in mouse neutrophils, the inhibition of XIAP results also in increased ubiquitylation of RIPK1 instead of the reduced ubiquitylation observed when cIAPs are blocked31. In line with these findings in mouse neutrophils, XIAP has also been reported to restrict TNF and RIPK3-dependent cell death in dendritic cells55 and to limit TLR and TNFR1-induced necroptosis in macrophages56. Similarly, loss of XIAP can also lead to elevated ubiquitylation of RIPK155. Taken together, XIAP appears to be able to function independently of cIAPs in regulating the neutrophil death pathways and the complicated XIAP functions in different kinds of cells may well imply that the role of XIAP in regulating necroptosis is cell type dependent.
LPS can activate TLR4 to induce macrophage necroptosis when caspases are inhibited; here, autocrine TNF plays only a minor role15. However, in mouse neutrophils, it has been reported that only when cIAPs and XIAP are both blocked, can LPS trigger RIPK1-dependent apoptosis, which is then almost completely dependent on autocrine TNF31. Thus, XIAP can maintain neutrophil viability when cIAPs are blocked in response to LPS. RIPK1-dependent apoptosis initiated by LPS-induced autocrine TNF can then switch to an RIPK3 and MLKL-dependent necroptosis when caspases are inhibited31. However, these authors also show that low concentrations of TNF can also induce neutrophil cell death when cIAPs alone, or both cIAPs and XIAP, are blocked by Smac mimetics31. Accordingly, this cell death is probably an RIPK1-dependent apoptosis. Thus, it seems reasonable that low concentrations of TNF should also be able to induce neutrophil necroptosis in the presence of Smac mimetics when caspases are also inhibited. This, however, needs to be confirmed. Furthermore, we cannot exclude a role for XIAP in maintaining neutrophil viability by blocking LPS-induced TNF production when cIAPs are functionally absent. This hypothesis is also suggested by XIAP restriction of granulocyte/macrophage colony-stimulating factor (GM-CSF)-primed neutrophil death in response to TNF and increased XIAP expression upon GM-CSF stimulation31. Finally, the execution of necroptosis in mouse neutrophils appears to be dependent on MLKL translocation to the plasma membrane31. However, the role of ROS in TNF-induced necroptosis in mouse neutrophils has so far not been well investigated. Moreover, it should be noted, that TNF-induced necroptosis in human neutrophils has not been demonstrated so far.
The mechanism of neutrophil necroptosis induced by adhesion receptors
Ligation of adhesion receptors, including CD44, CD11b, CD18, and CD15, can induce necroptosis in human neutrophils if the cells have been exposed to GM-CSF (Fig. 3)19. This process is characterized by cytoplasmic vacuolization, a phenomenon seen both in vitro and in vivo under inflammatory conditions19,57. This necroptosis pathway involves a RIPK3–MLKL—p38 MAPK–PI3K axis, for which all these molecular components are required to generate ROS via NADPH oxidase and subsequent necrosis. It is unknown how RIPK3 is activated by adhesion receptors. TLR stimulation can induce necroptosis by the TRIF-RIPK3 interaction through their RHIM domains (Fig. 2)14,15, for which TRIF may also play a role by activating RIPK3 in the neutrophil necroptosis induced by adhesion receptors. Clearly, this pathway needs confirmation. While the essential roles of p38 and PI3K in the activation of the NADPH oxidase within neutrophil cell death pathways had been reported earlier57–59, it remains unclear how the RIPK3-MLKL complex is able to bring about p38 MAPK activation.
Increased ROS production is indispensable for neutrophil necroptosis, because neutrophils from patients with chronic granulomatous disease (CGD) exhibiting a genetic defect in NADPH oxidase were unable to undergo necroptosis induced by adhesion receptors19. The strong increase in ROS production in human neutrophils may result in irreversible damage to biomolecules and is therefore severely deleterious for the cell. However, a direct MLKL-mediated plasma membrane disruption does not seem to occur. Instead, MLKL, perhaps together with RIPK3, was required to activate p38 MAPK, PI3K and NADPH oxidase, which finally triggered ROS production and neutrophil death. Therefore, MLKL should be seen not only as a necroptosis executor protein, but also as an adaptor protein for necrosis signaling.
In line with this idea, it has been shown that MLKL is able to activate p38 MAPK and NADPH oxidase, leading to regulated necrosis induced by adhesion in human eosinophils60. The execution of both neutrophil and eosinophil necroptosis appears to involve an ROS-dependent permeabilization of vacuole membranes, intracellular structures containing toxic granule proteins20,57,60. Moreover, MLKL can contribute to inflammasome activation56. In addition, RIPK3 fails to trigger phosphorylation on the mitochondrial protein phosphatase PGMA5S in the absence of MLKL61 and overexpression of MLKL was reported to activate JNK9. Interestingly, at the site of infection, wild-type mouse neutrophils are able to clear S. aureus while neutrophils lacking MLKL, like human neutrophils from CGD patients, kill microbes poorly62,63. Furthermore, this cell death pathway appears to be independent of autocrine TNF and can be induced without blocking IAP or caspase activities. Thus, the mechanism of necroptosis induced by adhesion receptors is quite different from TNF-induced necroptosis, though this cell death pathway does appear to be a close analogy to the necroptosis observed under physiological conditions. Clearly, the mechanism of RIPK3 activation by adhesion receptors in cytokine-primed neutrophils should be further investigated.
The role of MSU crystals in neutrophil necroptosis
Neutrophil necroptosis has also been shown to be induced by phorbol 12-myristate 13-acetate (PMA) treatment or exposure to MSU crystals32,33. This cell death, measured 2 h after stimulation, is triggered by ROS production which further activates RIPK1, RIPK3, and MLKL. Thus, ROS are upstream of RIPK1-RIPK3-MLKL signaling and are believed to be initiators of the necroptosis induced by PMA or MSU crystals (Fig. 3). This cell death, whether induced by PMA or MSU crystals, can be inhibited by necrostatin-1 (a RIPK1 inhibitor) or necrosulfonamide (NSA; a MLKL inhibitor)6,13. However, these findings have been contradicted in a publication arguing that NSA was unable to block human neutrophil death in response to PMA under the same stimulation conditions64.
On the other hand, neutrophil necroptosis induced by MSU crystals was shown to be reduced by either necrostatin-1 treatment or by silencing the Ripk3 gene in experimental mouse gouty arthritis models32,65. Furthermore, MSU crystals have also been shown to induce necroptosis in other cell types66. Clearly, more work needs to be done to confirm the role of ROS as initiators of neutrophil necroptosis (Fig. 3). This is also true, because it has also been shown that MLKL can be proximal to the generation of ROS in TNF-induced necroptosis9 and RIPK3 triggered ROS production plays an important role in myocardial necroptosis67.
NETs were first interpreted as a mechanism for fighting microbial infection68,69. Subsequently, it was found that NETs are generated in the course of neutrophil necroptosis induced by PMA or MSU crystals32,33. In contrast, another report described NET formation induced in live neutrophils independent of RIPK3 and MLKL64. One explanation may be that the so-called NET release during necroptosis is in fact a necrosis-related, passive release of chromatin32,33. So interpreted, NET formation would be independent of necroptosis64. In fact, it is unlikely that necroptosis would be beneficial for the host under conditions where NET formation would seem to be an appropriate anti-microbial response70.
The mechanism of RIPK3-dependent regulated necrosis induced by phagocytosis of S. aureus1 in neutrophils
Human neutrophils can phagocytose community-associated methicillin-resistant S. aureus (CA-MRSA) strain USA300 to fight infection. Some ingested S. aureus may survive within the neutrophils phagosome, however, preventing apoptosis and resulting in a necrosis-like cell death. Such human neutrophil death is considered to be an RIPK1-dependent necroptosis based on the inhibition of lysis by Nec-134. However, later, the same authors found that cell lysis inhibited by Nec-1 is owing to its off-target effects and that this necrosis-like death in neutrophils partially requires RIPK3 activity, though independent of RIPK1 and MLKL35. These authors did not identify the executioner protein in this RIPK3-dependent necrosis and they considered this regulated cell death pathway to be distinct from necroptosis. However, ischemia- and oxidative stress-induced myocardial necroptosis is also independent of RIPK1 and MLKL, acting through the RIPK3-Ca2+-calmodulin–dependent protein kinase (CaMKII) pathway67. Thus, lysis of neutrophils by CA-MRSA may also be interpreted as one type of RIPK3-dependent necroptosis (Fig. 3). This RIPK3-dependent regulated necrosis induced by phagocytosis of S. aureus in human neutrophils is believed to facilitate the release of DAMPs and to allow the escape of viable bacteria leading to an amplification of local tissue damage and persisting infection34.
The role of necroptosis in neutrophil-associated disorders
It has been predicted that necroptosis is involved in disease pathogenesis since studies in animal disease models provided strong evidence for this hypothesis. However, the role of necroptosis in human pathologies remains to be further identified1,2. Neutrophils are involved in many kinds of diseases; unlike apoptosis, necrosis of neutrophils can be very harmful during inflammation due to the liberation of their toxic contents and ROS production, which induce further tissue damage and an amplified inflammatory response. Furthermore, neutrophils undergoing necrosis may allow pathogens to escape from dead cells, inducing further infections30. Thus, an understanding of neutrophil necroptosis undoubtedly promises an approach for preventing neutrophil-associated excessive tissue injury or inflammatory disease by targeting key proteins in the necroptosis pathway.
The induction of necroptosis occurring under in vivo inflammatory conditions has been explored and the migration of human neutrophils to inflammatory sites was found to activate the RIPK3-MLKL pathway in tissue samples from patients with neutrophilic diseases including cutaneous vasculitis, ulcerative colitis, and psoriasis19. These diseases are characterized not only by neutrophilic tissues, but also by strong triggers of inflammation, which involve autoimmune mechanisms71–73. Although tissue samples from patients with neutrophilic diseases are without any experimental stimulation, adhesion receptors19,57, including CD44, CD11b, CD18, CD15, and Siglec-9 may be the triggers for neutrophil necroptosis, because the cytoplasmic vacuolization of neutrophils can also be observed in inflamed tissue samples of these patients57. Moreover, it has been previously demonstrated that hyaluronan, a natural ligand of CD44, is able to trigger the necroptotic pathway in neutrophils57. However, exactly when necroptosis triggering occurs is not clear, since it could be during migration, but could also be later at the site of inflammation. Thus, adhesion receptors, RIPK3 and MLKL are all potential therapy targets.
Neutrophil necroptosis seems also to be involved in the pathology of gout, because, in the mouse, gouty arthritis models based on the injection of MSU crystals into subcutaneous air pouches exhibit the gout-like tophus formation induced by neutrophil necrosis which can be reduced both by necrostatin-1 treatment or by silencing of the Ripk3 gene32. Thus, RIPK1, RIPK3, and MLKL might serve as molecular targets for gout therapy. Furthermore, in patients diagnosed with X-linked lymphoproliferative syndrome type 2 (deficient in XIAP), neutrophil necroptosis may play a role in disease progression31, and TNF, XIAP, RIPK1, RIPK3, and MLKL promise to be therapy targets. However, the role of TNF, adhesion receptors, MSU crystals, and phagocytosis-induced neutrophil necroptosis in human diseases remains to be further investigated.
Conclusions
The identification of the necroptosis death pathway helps us to better understand overall necrotic cell death, which is not always an accidental or uncontrolled process, and which appears to play an important role in inflammation and disease pathogenesis. The characterization of the neutrophil necroptosis signaling pathway may facilitate a better control of tissue damage or excessive inflammation induced by neutrophil dysfunction, helping us to identify appropriate drug targets in neutrophil-associated disorders such as cutaneous vasculitis, ulcerative colitis, and psoriasis. Although necroptosis has been extensively studied, neutrophil necroptosis has so far been little investigated. Given the important role of neutrophils in the immune system and in different pathologies, it is worthwhile to explore the mechanisms, as well as the triggers and key proteins of neutrophil necroptosis. A better understanding of this signaling pathway will hopefully benefit the treatment of inflammatory or autoimmune diseases in the future.
Acknowledgements
The work in the laboratories of the authors is supported by the Swiss National Science Foundation (number 31003A_173215 to S.Y. and number 310030_166473 to H.U.S).
Authors' contributions
XW and HUS conceived the review. XW collected, analyzed literatures and wrote the draft. SY helped writing the draft. HUS corrected and finalized the manuscript. All authors have read and approved the final manuscript.
Competing interest
The authors declare that they have no competing financial interests.
Footnotes
Edited by B. Zhivotovsky
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 2.Weinlich R, Oberst A, Beere HM, Green DR. Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell. Biol. 2017;18:127–136. doi: 10.1038/nrm.2016.149. [DOI] [PubMed] [Google Scholar]
- 3.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 4.He S, Wang L, Miao L, Wang T, Du F, Zhao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 7.Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Zhou Z, Li L, Zhong CQ, Zheng X, Wu X, et al. Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. J. Biol. Chem. 2013;288:16247–16261. doi: 10.1074/jbc.M112.435545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci USA. 2012;109:5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray CA, Pickup DJ. The mode of death of pig kidney cells infected with cowpox virus is governed by the expression of the crmA gene. Virology. 1996;217:384–391. doi: 10.1006/viro.1996.0128. [DOI] [PubMed] [Google Scholar]
- 11.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 12.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 13.Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci USA. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McComb S, Cessford E, Alturki NA, Joseph J, Shutinoski B, Startek JB, et al. Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. Proc Natl Acad Sci USA. 2014;111:E3206–3213. doi: 10.1073/pnas.1407068111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci USA. 2013;110:E3109–3118. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, He Z, Liu H, Yousefi S, Simon HU. Neutrophil necroptosis is triggered by ligation of adhesion molecules following GM-CSF priming. J. Immunol. 2016;197:4090–4100. doi: 10.4049/jimmunol.1600051. [DOI] [PubMed] [Google Scholar]
- 20.Benarafa C, Simon HU. Role of granule proteases in the life and death of neutrophils. Biochem. Biophys. Res. Commun. 2017;482:473–481. doi: 10.1016/j.bbrc.2016.11.086. [DOI] [PubMed] [Google Scholar]
- 21.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell. Host. Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thapa RJ, Ingram JP, Ragan KB, Nogusa S, Boyd DF, Benitez AA, et al. DAI senses influenza A virus genomic RNA and activates RIPK3-dependent cell death. Cell. Host. Microbe. 2016;20:674–681. doi: 10.1016/j.chom.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Kearney CJ, Cullen SP, Tynan GA, Henry CM, Clancy D, Lavelle EC, et al. Necroptosis suppresses inflammation via termination of TNF- or LPS-induced cytokine and chemokine production. Cell. Death. Differ. 2015;22:1313–1327. doi: 10.1038/cdd.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linkermann A, Green DR. Necroptosis. N. Engl. J. Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nauseef WM, Borregaard N. Neutrophils at work. Nat. Immunol. 2014;15:602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 27.Nicolas-Avila JA, Adrover JM, Hidalgo A. Neutrophils in homeostasis, immunity, and cancer. Immunity. 2017;46:15–28. doi: 10.1016/j.immuni.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Simon HU. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol. Rev. 2003;193:101–110. doi: 10.1034/j.1600-065X.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 29.Hampson P, Hazeldine J, Lord JM. Neutrophil apoptosis and its induction as a potential treatment for chronic inflammatory disease. Curr. Opin. Hematol. 2013;20:10–15. doi: 10.1097/MOH.0b013e32835b06be. [DOI] [PubMed] [Google Scholar]
- 30.Geering B, Stoeckle C, Conus S, Simon HU. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends. Immunol. 2013;34:398–409. doi: 10.1016/j.it.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Wicki S, Gurzeler U, Wei-Lynn Wong W, Jost PJ, Bachmann D, Kaufmann T. Loss of XIAP facilitates switch to TNFalpha-induced necroptosis in mouse neutrophils. Cell Death Dis. 2016;7:e2422. doi: 10.1038/cddis.2016.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desai J, Kumar SV, Mulay SR, Konrad L, Romoli S, Schauer C, et al. PMA and crystal-induced neutrophil extracellular trap formation involves RIPK1-RIPK3-MLKL signaling. Eur. J. Immunol. 2016;46:223–229. doi: 10.1002/eji.201545605. [DOI] [PubMed] [Google Scholar]
- 33.Desai J, Mulay SR, Nakazawa D, Anders HJ. Matters of life and death. How neutrophils die or survive along NET release and is “NETosis” = necroptosis? Cell. Mol. Life. Sci. 2016;73:2211–2219. doi: 10.1007/s00018-016-2195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenlee-Wacker MC, Rigby KM, Kobayashi SD, Porter AR, DeLeo FR, Nauseef WM. Phagocytosis of Staphylococcus aureus by human neutrophils prevents macrophage efferocytosis and induces programmed necrosis. J. Immunol. 2014;192:4709–4717. doi: 10.4049/jimmunol.1302692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenlee-Wacker MC, Kremserova S, Nauseef WM. Lysis of human neutrophils by community-associated methicillin-resistant Staphylococcus aureus. Blood. 2017;129:3237–3244. doi: 10.1182/blood-2017-02-766253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soehnlein O, Steffens S, Hidalgo A, Weber C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017;17:248–261. doi: 10.1038/nri.2017.10. [DOI] [PubMed] [Google Scholar]
- 37.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moulin M, Anderton H, Voss AK, Thomas T, Wong WW, Bankovacki A, et al. IAPs limit activation of RIP kinases by TNF receptor 1 during development. EMBO. J. 2012;31:1679–1691. doi: 10.1038/emboj.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 41.Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 42.Dondelinger Y, Aguileta MA, Goossens V, Dubuisson C, Grootjans S, Dejardin E, et al. RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell. Death. Differ. 2013;20:1381–1392. doi: 10.1038/cdd.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Legarda-Addison D, Hase H, O’Donnell MA, Ting AT. NEMO/IKKgamma regulates an early NF-kappaB-independent cell-death checkpoint during TNF signaling. Cell. Death. Differ. 2009;16:1279–1288. doi: 10.1038/cdd.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang S, Wang B, Tang LS, Siednienko J, Callanan JJ, Moynagh PN. Pellino3 targets RIP1 and regulates the pro-apoptotic effects of TNF-alpha. Nat Commun. 2013;4:2583. doi: 10.1038/ncomms3583. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell. Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Li W, Ren J, Huang D, He WT, Song Y, et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell. Res. 2014;24:105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–981. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 49.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat. Cell. Biol. 2011;13:1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFalpha-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS. ONE. 2013;8:e76841. doi: 10.1371/journal.pone.0076841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li D, Xu T, Cao Y, Wang H, Li L, Chen S, et al. A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc Natl Acad Sci USA. 2015;112:5017–5022. doi: 10.1073/pnas.1505244112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen W, Wu J, Li L, Zhang Z, Ren J, Liang Y, et al. Ppm1b negatively regulates necroptosis through dephosphorylating Rip3. Nat. Cell. Biol. 2015;17:434–444. doi: 10.1038/ncb3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Onizawa M, Oshima S, Schulze-Topphoff U, Oses-Prieto JA, Lu T, Tavares R, et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat. Immunol. 2015;16:618–627. doi: 10.1038/ni.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yabal M, Muller N, Adler H, Knies N, Gross CJ, Damgaard RB, et al. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. 2014;7:1796–1808. doi: 10.1016/j.celrep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D’Cruz AA, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mihalache CC, Yousefi S, Conus S, Villiger PM, Schneider EM, Simon HU. Inflammation-associated autophagy-related programmed necrotic death of human neutrophils characterized by organelle fusion events. J. Immunol. 2011;186:6532–6542. doi: 10.4049/jimmunol.1004055. [DOI] [PubMed] [Google Scholar]
- 58.Geering B, Gurzeler U, Federzoni E, Kaufmann T, Simon HU. A novel TNFR1-triggered apoptosis pathway mediated by class IA PI3Ks in neutrophils. Blood. 2011;117:5953–5962. doi: 10.1182/blood-2010-11-322206. [DOI] [PubMed] [Google Scholar]
- 59.Geering B, Simon HU. Peculiarities of cell death mechanisms in neutrophils. Cell. Death. Differ. 2011;18:1457–1469. doi: 10.1038/cdd.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radonjic-Hoesli S, Wang X, de Graauw E, Stoeckle C, Styp-Rekowska B, Hlushchuk R, et al. Adhesion-induced eosinophil cytolysis requires the RIPK3-MLKL signaling pathway which is counter-regulated by autophagy. J. Allergy Clin. Immunol. 2017;140:1632–1642. doi: 10.1016/j.jaci.2017.01.044. [DOI] [PubMed] [Google Scholar]
- 61.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 62.Kitur K, Wachtel S, Brown A, Wickersham M, Paulino F, Penaloza HF, et al. Necroptosis promotes Staphylococcus aureus clearance by inhibiting excessive inflammatory signaling. Cell Rep. 2016;16:2219–2230. doi: 10.1016/j.celrep.2016.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 64.Amini P, Stojkov D, Wang X, Wicki S, Kaufmann T, Wong WW, et al. NET formation can occur independently of RIPK3 and MLKL signaling. Eur. J. Immunol. 2016;46:178–184. doi: 10.1002/eji.201545615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014;20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 66.Mulay SR, Desai J, Kumar SV, Eberhard JN, Thomasova D, Romoli S, et al. Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis. Nat Commun. 2016;7:10274. doi: 10.1038/ncomms10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat. Med. 2016;22:175–182. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- 68.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 69.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell. Death. Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 70.Yousefi S, Simon HU. NETosis – Does it really represent nature’s “suicide bomber. Front. Immunol. 2016;7:328. doi: 10.3389/fimmu.2016.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Micheletti RG, Werth VP. Small vessel vasculitis of the skin. Rheum. Dis. Clin. North. Am. 2015;41:21–32. doi: 10.1016/j.rdc.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 72.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 73.Sticherling M. Psoriasis and autoimmunity. Autoimmune Rev. 2016;15:1167–1170. doi: 10.1016/j.autrev.2016.09.004. [DOI] [PubMed] [Google Scholar]