Key Points
Question
Does age influence clinical outcomes and tolerance to immunotherapy in metastatic melanoma?
Finding
In this retrospective cohort study in a real-life setting, patients older than 65 years treated by immunotherapy for a metastatic melanoma had a better mean progression-free survival (4.8 vs 3.4 months) and overall survival (not reached vs 10.1 months) than younger patients. Common immune-related adverse effects were similar in both cohorts.
Meaning
Age might be associated with a better clinical outcome after treatment with immunotherapy in the real-life setting, and older patients did not have more immune-related adverse events.
Abstract
Importance
Melanoma treatment has been revolutionized with the development of immune-based therapies that offer durable clinical responses in a subset of patients. Clinical outcomes after treatment by immunotherapy can be influenced by the host’s immune system. The immune system is modified with age by age-related immune dysfunction.
Objective
To evaluate if age influences clinical outcome and immune adverse events in patients treated by immunotherapy for metastatic melanoma.
Design, Setting, and Participants
This was a single-center cohort analysis in patients treated with immunotherapy for metastatic melanoma between January 2007 and February 2016, in the Lyon Sud Hospital, France. A total of 92 patients with metastatic melanoma treated with ipilimumab, nivolumab, or pembrolizumab were retrospectively analyzed.
Main Outcomes and Measures
Overall survival, progression-free survival, and immune-related adverse events were evaluated for each treatment line according to the patients’ age.
Results
A total of 92 patients were eligible and included in this study for a total of 120 lines of treatment. Fifty-four patients were included in the cohort that was 65 years or younger (24 [44%] were female; mean [SD] age, 48.1 [12.5] years), and 38 patients were included in the cohort that was older than 65 years (12 [34%] were female; mean [SD] age, 74.8 [6.9] years). Mean follow-up duration starting at treatment initiation was 12.5 months. Patients older than 65 years treated with immunotherapy had a better mean progression-free survival (4.8 vs 3.4 months; P = .04) and overall survival (not reached vs 10.1 months; P = .009) than younger patients in univariate analysis, and after adjusting on prognosis covariates. This was particularly true with patients treated with anti–programmed cell death protein 1. Common immune-related adverse effects were similar in both cohorts.
Conclusions and Relevance
Age might be associated with a better clinical outcome after treatment with immunotherapy in the real-life setting. In our cohort, older patients did not have more immune-related adverse events. Further studies are warranted to confirm our results and describe the underlying mechanisms involved.
This cohort study evaluates if age influences clinical outcome and immune adverse events in patients treated by immunotherapy for `metastatic melanoma.
Introduction
Ipilimumab, nivolumab, and pembrolizumab, are immune checkpoint inhibitors that have recently been approved by the US Food and Drug Administration (FDA) for treatment of late-stage melanoma.
In the preclinical setting, age-related immune dysfunction has been shown to affect response to immune checkpoint inhibitors. In this study, we sought to evaluate if age affects response and tolerance to immunotherapy in patients treated for metastatic melanoma, in a real-life setting.
Methods
Setting and Participants
Inclusion criteria for this study were patients with unresectable or metastatic melanoma treated from January 2007 to February 2016 with ipilimumab, nivolumab, or pembrolizumab in the dermatology department of the Lyon Sud University Hospital in Pierre Bénite, France. Eligible patients were screened by interrogating 2 prospective databases: Melbase and PAIR (Programme d'Actions Intégrées de Recherche) Melanoma. Melbase is a French multi-institution database that follows patients with unresectable stage III and IV melanoma (NCT02828202), PAIR is a single-institution database that follows patients treated with nivolumab as a second-line treatment in the dermatology department of the Lyon Sud University Hospital (NCT02626065). All patients gave their written informed consent to be included in these databases; the study was authorized by the ethical review board of the Hospices Civils de Lyon. Patients were not compensated for their participation.
Data Collection
Data collection was performed retrospectively using electronic medical records by 2 dermatologists (A.B. and M.P.M.).
Patient characteristics included age at treatment initiation, sex, presence or absence of autoimmune diseases, and cumulative illness rating score (CIRS). Eastern Cooperative Oncology Group performance status and body mass index were collected prior to treatment with immunotherapy and at first assessment of treatment response. Melanoma characteristics at diagnosis included Breslow index, genotype, results of sentinel lymph node biopsy, presence or absence of ulceration, and melanoma subtype.
Treatment characteristics included American Joint Committee on Cancer stage at treatment initiation, number of metastatic sites prior to treatment, presence or absence of brain metastasis, number and type of prior treatments, date of treatment initiation, treatment dosage, concomitant use of oral corticosteroids (defined as prescription of >20 mg of oral corticosteroids for more than 10 days) or immunosuppressants, date of clinician-based progression, and date of death or last control.
Biological blood parameters collected to prior treatment were lactate dehydrogenase, C-reactive protein, albumin, and creatinine levels and complete blood cell count.
Data collected to evaluate drug toxic effects included the presence or absence of diarrhea during treatment, cutaneous eruption, thyroid modifications, hepatitis, hypophysitis, vitiligo, and other, with patients’ date of diagnosis and their tumor grading from 1 to 4 according to National Cancer Institutes–Common Terminology Criteria for Adverse Events, version 3.0.
Clinical and serological follow-up was scheduled every 2 or 3 weeks during treatment. Radiological follow-up was scheduled every 3 months.
Statistical Analysis
The characteristics of patients in the 2 age groups were compared using 2-tailed univariate analysis. Fisher exact test was used to compare qualitative variables as appropriate. Mann-Whitney test was used to compare quantitative variables as appropriate.
Primary end points were overall survival (OS) and progression-free survival (PFS); OS was defined as the time from treatment initiation to death from any cause, and PFS was defined as the time from treatment initiation to progressive disease or death from any cause, whichever came first. Probabilities of survival were calculated using Kaplan-Meier estimates and compared using 2-tailed log-rank test. Covariates, including age, were considered statistically associated with PFS or OS if the associated P value was less than 0.05. All variables with a statistically significant impact on PFS or OS in univariate analysis were included in multivariate Cox proportional hazard models. Then variable selection was done using a stepwise backward procedure.
Secondary end points were percentage of immune-related adverse events and type of adverse events according to patient’s age. All analyses were performed using R statistical software (R Foundation for Statistical Computing). Database follow-up was closed in August 2016. Data were rarely missing, and no data imputation was performed through the analyses.
Results
Patients and Treatment Characteristics
Ninety-two patients were eligible and included in this study for a total of 120 lines of treatment. Fifty-four patients were included in the cohort 65 years or younger, and 38 patients were included in the cohort older than 65 years.
Patient characteristics, as seen in Table 1; and eTable 1 in the Supplement, were similar in both age groups except for CIRS comorbidity score (P < .001), the duration between initial diagnosis and first systemic treatment (P = .01), C-reactive protein dosage (P = .001), and neutrophil count (P = .01).
Table 1. Patient Characteristics in Each Group.
| Characteristic | Age Group, No. (%) | Fisher Test (P Value) | |
|---|---|---|---|
| ≤65 y (n = 54) |
>65 y (n = 38) |
||
| Demographic Characteristics of 92 Patients | |||
| Age, mean (SD), y | 48.1 (12.5) | 74.8 (6.9) | |
| Sex | .50 | ||
| Male | 30 (56) | 24 (63) | |
| Female | 24 (44) | 14 (34) | |
| Relevant Disease-Associated Characteristics | |||
| AJCC stage at treatment | .06 | ||
| IV M1a | 10 (18) | 5 (13) | |
| IV M1b | 7 (13) | 13 (34) | |
| IV M1c | 37 (69) | 20 (53) | |
| Genotype | .77 | ||
| BRAF | 20 (37) | 11 (29) | |
| NRAS | 13 (24) | 12 (32) | |
| Other (GNAQ, KIT) | 2 (4) | 2 (5) | |
| Wild type | 19 (35) | 13 (34) | |
| Duration between initial diagnosis and first systemic treatment, mo | .01 | ||
| <12 | 30 (83) | 23 (61) | |
| ≥12 | 24 (17) | 15 (39) | |
| BMI | .87 | ||
| Mean | 25.7 | 25.4 | |
| Median (q1, q3) [range] | 24.9 (22.0, 28.6) [17.6-35.5] | 25.5 (21.7, 28.4) [15-36.9] | |
| History of autoimmune disease | .68 | ||
| No | 51 (95) | 35 (92) | |
| Yes | 3 (5) | 3 (8) | |
| Comorbidity score CIRS | <.001 | ||
| Mean | 2.46 | 5.0 | |
| Median (q1, q3) [range] | 2.0 (0, 4) [0-13] | 5.0 (3, 7) [0-10] | |
| Treatment Lines (n = 120) | |||
| ECOG prior to treatment (n = 120/120)a | .07 | ||
| 0-1 | 54 (81) | 49 (92) | |
| ≥2 | 13 (19) | 4 (8) | |
| Neutrophil count prior to treatment (n = 112)a | .01 | ||
| ≤3500/μL | 21 (42) | 44 (66) | |
| >3500/μL | 29 (58) | 23 (34) | |
| CRP level prior to treatment, mg/L (n = 64)a | .001 | ||
| ≤5 | 11 (35) | 17 (71) | |
| >5 | 29 (65) | 7 (29) | |
| Immune-related adverse events (n = 120)a | .19 | ||
| No | 31 (46) | 18 (34) | |
| Yes | 36 (54) | 35 (66) | |
| Maximum grade of adverse events (n = 120)a | .63 | ||
| 0 | 20 (36) | 13 (27) | |
| I-II | 23 (41) | 23 (48) | |
| III-IV | 13 (23) | 12 (25) | |
Abbreviations: AJCC, American Joint Committee on Cancer; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CIRS, cumulative illness rating score; CRP, C-reactive protein; ECOG, Eastern Cooperative Oncology Group; q, quartile.
SI conversion factors: To convert CRP to nanomoles per liter, multiply by 9.524; to convert neutrophil count to 109/L, multiply by 0.001.
A total of 92 patients received 120 lines of treatments. Characteristics of the patients or of the tumor that are unlikely to vary according to treatment line are reported by patient (n = 92). Characteristics that may vary according to treatment line are reported by treatment line (n = 120).
Mean follow-up duration starting at treatment initiation was 12.5 months.
Patient Outcomes
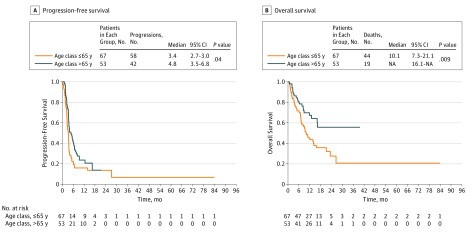
Patients older than 65 years treated with immunotherapy (ipilimumab and anti–programmed cell death protein 1 [PD-1]) had a better PFS (4.8 vs 3.4 months; P = .04) and OS (10.1 months vs not reached; P = .009) than patients 65 years or younger (Figure). These results were confirmed in subgroup analysis selecting patients treated with either ipilimumab or anti-PD1 (eFigure 1 and 2 in the Supplement). Multivariate analysis showed that age was independently associated with a better OS and PFS (eTables 2 and 3 in the Supplement).
Figure. Progression-Free Survival and Overall Survival in Function of Age at Treatment Initiation.
Kaplan-Meier progression-free survival and overall survival curves of all patients included in the study in function of their age at treatment initiation. NA indicates not applicable.
Toxic Effects in Patients
No difference in frequency or grade of immune adverse events (IAEs) was observed between younger and older patients (Table 2), even though older patients had more comorbidities (Table 1).
Table 2. Immune Adverse Events in Both Cohorts.
| Adverse Event (n = 120) | Age Group, No. (%) | χ2 Test (P Value) | |
|---|---|---|---|
| ≤65 y (n = 67) |
>65 y (n = 53) |
||
| Immune-related adverse events | .24 | ||
| No | 31 (46) | 18 (34) | |
| Yes | 36 (54) | 38 (66) | |
| Adverse event | |||
| ≥1 (no other) | 33 (49) | 31 (58) | .41 |
| ≥1 (with other) | 37 (56) | 34 (64) | .42 |
| Time to first adverse event | |||
| Missing | 31 | 17 | |
| Mean | 7.8 | 6.19 | |
| Median (q1, q3) [range] | 7.0 (4.8, 10.0) [3-19] | 5.5 (4.0, 8.0) [3-14] | |
| Diarrhea enterocolitisa | .19 | ||
| No | 55 (83) | 38 (72) | |
| Yes | 11 (17) | 15 (28) | |
| Cutaneous eruption | >.99 | ||
| No | 54 (80) | 42 (79) | |
| Yes | 13 (20) | 11 (21) | |
| Thyroid | .81 | ||
| No | 59 (88) | 45 (75) | |
| Yes | 8 (12) | 8 (15) | |
| Hypophysis | .93 | ||
| No | 66 (99) | 51 (96) | |
| Yes | 1 (1) | 2 (4) | |
| Hepatitis | .84 | ||
| No | 54 (80) | 41 (77) | |
| Yes | 13 (20) | 12 (23) | |
| Vitiligo | >.99 | ||
| No | 60 (89) | 47 (89) | |
| Yes | 7 (11) | 6 (11) | |
| Other toxic effects | .04 | ||
| No | 58 (86) | 37 (70) | |
| Yes | 9 (14) | 16 (30) | |
Abbreviation: q, quartile.
n = 119.
The frequencies of most common IAEs were similar among younger and older patients but “other” IAEs were more frequent among older patients as older patients more frequently developed meningitis (3 of 38 [7.8%] vs 0 of 54 [0%]) and immunologic nephritis (3 of 38 [7.8] vs 0 of 54 [0%]) than younger patients. Younger patients tended to more frequently develop immunogenic interstitial pneumopathy (3 of 54 [5.5%] vs 1 of 38 [2.6%]) compared with older patients.
Discussion
In our study, older patients treated by immunotherapy had the same incidence of common IAEs as younger patients. The expansion of protective regulatory mechanisms, such as a higher production of peripheral T-regulatory cells, could contribute to limit IAEs in older patients. In our single-institution, retrospective study, patients treated for metastatic melanoma who were older than 65 years had better OS (not reached vs 10.1 months; P = .009) and PFS (4.8 vs 3.4 months; P = .04) compared with patients 65 years or younger. This association between older age and a better prognosis was independent of other prognosis covariates, and was stronger for patients treated with anti–PD-1 compared with patients treated with ipilimumab.
In our study, the biological mechanisms that might contribute to better responses in older patients were not evaluated. A high level of Il-2 soluble receptor at baseline, high levels of myeloid derived suppressor cells, a low neutrophil count, absence of ICOS+ CD4-positive T lymphocyte induction, high PD-L1 expression by the tumor, high PD1 expression on CD8-positive T cells, and CD8-positive T-cell tumor infiltration, have been shown to influence clinical responses to immunotherapy, and have been observed in the presence of ARID.
In addition, gut microbiota is known to influence responses to immunotherapy and could account for age-related differences in response to treatment, as it can be modified by age. Finally, differences in pharmacokinetic parameters, like drug clearance and drug exposure, that can be modified by sarcopenia, renal dysfunction, and polymedication could also explain differences in immunotherapy efficiency.
Limitations
Our study is limited by its monocentric and retrospective nature and its small sample size. However, compared with previous clinical trials and meta-analysis, it might be more reflective of current practice, as our patients were unselected and therefore tended to have more polymedication, more prior treatments, and more severe diseases than in clinical trials.
Conclusions
In this real-life, single-institution retrospective study, an age greater than 65 years was not associated with more IAEs in patients treated by immunotherapy for metastatic melanoma, confirming that age should not be a limiting factor for immunotherapy.
eFigure 1. Overall survival in function of age at treatment initiation in patients treated by ipilimumab
eFigure 2. Overall survival in function of age at treatment initiation in patients treated by anti-PD1
eTable 1. Patient characteristics in each group by treatment line
eTable 2. Univariate analysis of variables that impact PFS
eTable 3. Univariate analysis of variables that impact Overall Survival
References
- 1.Robert C, Thomas L, Bondarenko I, et al. . Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517-2526. [DOI] [PubMed] [Google Scholar]
- 2.Robert C, Long GV, Brady B, et al. . Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320-330. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Schachter J, Long GV, et al. ; KEYNOTE-006 investigators . Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521-2532. [DOI] [PubMed] [Google Scholar]
- 4.Hurez V, Padrón ÁS, Svatek RS, Curiel TJ. Considerations for successful cancer immunotherapy in aged hosts. Clin Exp Immunol. 2017;187(1):53-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vadasz Z, Haj T, Kessel A, Toubi E. Age-related autoimmunity. BMC Med. 2013;11:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sade-Feldman M, Kanterman J, Klieger Y, et al. . Clinical significance of circulating CD33+CD11b+HLA-DR- myeloid cells in patients with stage iv melanoma treated with ipilimumab. Clin Cancer Res. 2016;22(23):5661-5672. [DOI] [PubMed] [Google Scholar]
- 7.Tietze JK, Angelova D, Heppt MV, Ruzicka T, Berking C. Low baseline levels of NK cells may predict a positive response to ipilimumab in melanoma therapy. Exp Dermatol. 2017;26(7):622-629. [DOI] [PubMed] [Google Scholar]
- 8.Zhang T, Xie J, Arai S, et al. . The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory cancers: a meta-analysis. Oncotarget. 2016;7(45):73068-73079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daillère R, Vétizou M, Waldschmitt N, et al. . Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity. 2016;45(4):931-943. [DOI] [PubMed] [Google Scholar]
- 10.Longoria TC, Tewari KS. Evaluation of the pharmacokinetics and metabolism of pembrolizumab in the treatment of melanoma. Expert Opin Drug Metab Toxicol. 2016;12(10):1247-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong AC, Ma B. An update on the pharmacodynamics, pharmacokinetics, safety and clinical efficacy of nivolumab in the treatment of solid cancers. Expert Opin Drug Metab Toxicol. 2016;12(10):1255-1261. [DOI] [PubMed] [Google Scholar]
- 12.Escudier B, Sharma P, McDermott DF, et al. ; CheckMate 025 Investigators . CheckMate 025 randomized phase 3 study: outcomes by key baseline factors and prior therapy for nivolumab versus everolimus in advanced renal cell carcinoma. Eur Urol. 2017;S0302-2838(17)30099-4. [DOI] [PubMed] [Google Scholar]
- 13.Borghaei H, Paz-Ares L, Horn L, et al. . Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. [DOI] [PubMed] [Google Scholar]
- 15.Nishijima TF, Muss HB, Shachar SS, Moschos SJ. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: a systematic review and meta-analysis. Cancer Treat Rev. 2016;45:30-37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Overall survival in function of age at treatment initiation in patients treated by ipilimumab
eFigure 2. Overall survival in function of age at treatment initiation in patients treated by anti-PD1
eTable 1. Patient characteristics in each group by treatment line
eTable 2. Univariate analysis of variables that impact PFS
eTable 3. Univariate analysis of variables that impact Overall Survival