Key Points
Question
Can treatment with an oral probiotic reduce the SCORAD index and the use of topical steroids in children with moderate atopic dermatitis?
Findings
This randomized clinical trial of 50 children treated with a mixture of probiotics or placebo for 12 weeks found that SCORAD and topical steroid use decreased significantly in the probiotic group compared with the placebo group.
Meaning
This probiotic is an effective and safe coadjuvant treatment to reduce the SCORAD index and topical steroid use in children with moderate atopic dermatitis.
This randomized clinical trial investigates whether a mixture of oral probiotics is safe and effective in the treatment of atopic dermatitis symptoms and evaluates its influence on the use of topical steroids in children.
Abstract
Importance
Oral intake of new probiotic formulations may improve the course of atopic dermatitis (AD) in a young population.
Objective
To determine whether a mixture of oral probiotics is safe and effective in the treatment of AD symptoms and to evaluate its influence on the use of topical steroids in a young population.
Design, Setting, and Participants
A 12-week randomized, double-blind, placebo-controlled intervention trial, from March to June 2016, at the outpatient hospital Centro Dermatológico Estético de Alicante, Alicante, Spain. Observers were blinded to patient groupings. Participants were children aged 4 to 17 years with moderate atopic dermatitis. The groups were stratified and block randomized according to sex, age, and age of onset. Patients were ineligible if they had used systemic immunosuppressive drugs in the previous 3 months or antibiotics in the previous 2 weeks or had a concomitant diagnosis of intestinal bowel disease or signs of bacterial infection.
Interventions
Twelve weeks with a daily capsule containing freeze-dried powder with 109 total colony-forming units of the probiotic strains Bifidobacterium lactis CECT 8145, B longum CECT 7347, and Lactobacillus casei CECT 9104 and maltodextrin as a carrier, or placebo (maltodextrin-only capsules).
Main Outcomes and Measures
SCORAD index score and days of topical steroid use were analyzed.
Results
Fifty children (26 [50%] female; mean [SD] age, 9.2 [3.7] years) participated. After 12 weeks of follow-up, the mean reduction in the SCORAD index in the probiotic group was 19.2 points greater than in the control group (mean difference, −19.2; 95% CI, −15.0 to −23.4). In relative terms, we observed a change of −83% (95% CI, −95% to −70%) in the probiotic group and −24% (95% CI, −36% to −11%) in the placebo group (P < .001). We found a significant reduction in the use of topical steroids to treat flares in the probiotic arm (161 of 2084 patient-days [7.7%]) compared with the control arm (220 of 2032 patient-days [10.8%]; odds ratio, 0.63; 95% CI, 0.51 to 0.78).
Conclusions and Relevance
The mixture of probiotics was effective in reducing SCORAD index and reducing the use of topical steroids in patients with moderate AD.
Trial Registration
clinicaltrials.gov Identifier: NCT02585986
Introduction
Atopic dermatitis (AD) is a chronic recurrent inflammatory skin disease characterized by intense pruritus, inflammation, and skin barrier disruption. The prevalence of AD is approximately 3% to 10% in adults and up to 20% in children worldwide. The first symptoms usually develop during childhood, and approximately 50% of cases are diagnosed in the first year of life. Atopic dermatitis significantly reduces the quality of life of patients and their families. Moreover, patients with AD have an increased risk of other atopic disorders, including asthma, allergic rhinitis, and chronic sinusitis.
As occurs in other atopic disorders, a predominance of T helper 2 cells rather than T helper 1 causes an imbalance that might also aggravate the pathogenesis of AD, increasing IgE and activating interleukins. Another point to consider is skin barrier integrity. Filaggrin interacts with intermediate filaments, particularly keratins, causing their aggregation into macrofibrils. Defects in filaggrin cause dysfunctions in the skin barrier, resulting in decreased protection from microbes and allergens.
Clinically, cutaneous manifestations related to AD include erythema, edema and/or papules, exudate, excoriation, and lichenification, as well as the resulting symptoms (pruritus and loss of sleep). Topical corticosteroids have been the keystone of pharmacological treatments for mild to moderate AD. Alternatively, cases of moderate-to-severe AD are treated with long-term applications of topical corticosteroids, with topical calcineurin inhibitors providing second-line effective agents. However, long-term data for these medications are lacking for pediatric patients. In cases of severe refractory disease, patients might benefit from a short course of systemic therapy with immunosuppressants such as corticosteroids, cyclosporine, and azathioprine, but these drugs have a potentially more severe adverse effect profile and risks of rebound after treatment discontinuation.
During recent years, many authors have suggested an association between a disruption in intestinal barrier function and the origin of AD, mediated by immunological activation leading to a type 2 dominant inflammation. In this respect, gut microbiota may play an important immunomodulatory role in the development of normal immune tolerance. Recently an analysis of the gut microbiota of patients with AD has shown an intraspecies compositional change in Faecalibacterium prausnitzii that reduces the number of high butyrate and propionate producers. Butyrate and propionate are microbial-produced short-chain fatty acids with an anti-inflammatory role. Moreover, butyrate has been shown to be a key player in maintaining gut barrier integrity. Therefore, reduced levels in the microbiota of both butyrate and propionate producers may result in a pro-inflammatory state in the gut and a loss of barrier integrity. All these data indicate the potential role of probiotics as microbiota recovery players, and consequently as potential nutritional supplements in AD treatment.
The primary goal of the present study was to determine the efficacy of a mixture of probiotics in improving the SCORAD (Scoring Atopic Dermatitis) index and in reducing the percentage of days with topical steroid treatment during flares in patients with moderate AD.
Methods
Study Design
The study design was a double-blind, 2-arm placebo-controlled trial with stratified randomization by baseline variables (ratio, 1:1). The study received approval from the Ethics Committee for Clinical Research of the Hospital General Universitario de Alicante, and the Spanish Medicines Agency. There was no change to the trial protocol (Supplement 1) after it commenced.
Study Population
Inclusion criteria of the protocol were as follows: children between 4 and 17 years old, with a diagnosis of AD that meets the Hanifin and Rajka criteria (eTable 1 in Supplement 2) and moderate SCORAD index (from 20 to 40) who had been prescribed topical steroids for the treatment of AD. Participants were also required to be currently consuming a high-quality Mediterranean diet with a Mediterranean Diet Quality Index (KIDMED) score more than 7. Written informed consent was obtained from parents or a legal representative (and the child if >12 years).
Among the exclusion criteria, patients were ineligible for the study if they had used systemic corticosteroids, methotrexate, cyclosporine, or anti–tumor necrosis factor drugs in the previous 3 months, antibiotics in the previous 2 weeks, or had a concomitant diagnosis of intolerance to gluten and/or lactose or signs of bacterial infection (the Trial Protocol in Supplement 1 provides a complete list of exclusion criteria).
Recruitment and Randomization of Participants
Children aged 4 to 17 years were recruited between March and June 2016 from a single outpatient dermatological clinic where 3 dermatologists (A.R.-B. and 2 others) with expertise in pediatric dermatology evaluated their AD and SCORAD index. After informed consent was obtained from their legal representatives, participants completed the dietary screener KIDMED short questionnaire for quality of the Mediterranean diet (eTable 2 in Supplement 2).
Each child was classified into 1 of the 8 possible strata combining 3 binary variables at baseline: sex, age (4-12 vs >12 years), age of onset (0-4 vs >4 years). Patients were then assigned to 1 of the 2 trial arms (probiotics or control) following a computerized randomization list that been previously prepared for each stratum by the principal investigator of the study (V.N.-L.).
Interventions
All patients received treatment during the 12-week study period with topical methylprednisolone aceponate, moisturizer, and 1 oral antihistamine, according to the guidelines for the management of AD. Participants in the probiotic group received daily a pill containing 109 colony-forming units (CFUs) of a mixture of the 3 probiotic strains in a 1:1:1 ratio, freeze-dried powder with maltodextrin as a carrier, and participants in the placebo group received a pill containing only maltodextrin. The probiotic and placebo pills were matched for size, shape, and volume of contents (gelatin capsules of 9.85 × 16.4 mm) and were dispensed by the pharmacy department staff.
The product was designed not as a single strain but as a bacteria mixture with lactobacilli and bifidobacteria because previous AD clinical trials with probiotics have obtained better results with mixed products. The mixture was composed of Bifidobacterium lactis CECT 8145, B longum CECT 7347, and Lactobacillus casei CECT 9104. The blend was selected on the basis of previous data: oxidative stress has been associated with AD, and strain B lactis CECT 8145 has previously shown antioxidant properties in the Caenorhabditis elegans model. Strain B longum CECT 7347 has an anti-inflammatory profile, previously demonstrated in both preclinical and clinical studies, and it modulates the gut microbiota. Finally, strain L casei CECT 9104 has in vitro activity against certain gut pathogens (E.C.-C., S.G.-M., D.R.-V., unpublished data, June 2009).
Outcome Measurements
Two primary outcomes were compared between the trial arms to assess the treatment effect: (1) change in SCORAD index between baseline and 12 weeks of follow-up and (2) the proportion of days of topical steroid use during flares within the 12 weeks of follow-up. The SCORAD index was measured at the time of inclusion and every 4 weeks until the end of the 12-week follow-up period. The number of days of topical steroid use during flares was recorded biweekly until week 12. A flare was defined as a worsening of the disease leading to use of topical corticosteroids for at least 3 consecutive days (so steroid use on only 1 or 2 isolated days was not considered a flare).
Secondary outcomes were laboratory values. Peripheral blood samples were collected at baseline and after 12 weeks of treatment and analyzed for routine biochemical laboratory values and for interleukin 4 (IL-4), IL-5, IL-10, and IL-13 levels.
Sample Size
According to the trial protocol, 25 patients per group would be needed to detect a difference of clinical improvement as evaluated by the SCORAD index, assuming 5% loss to follow-up, type I error of .05, and 80% power to detect a difference of 4.13 units on the SCORAD index with a standard deviation of 5 units between the 2 groups on a 2-sided t test.
Statistical Analysis
Data were analyzed as intention to treat, and the last available value was not carried forward for missing observations. Quantitative variables were summarized as means and standard deviations or median and interquartile range, and categorical variables were summarized with proportions.
We used linear mixed-effects models with a group-by-week interaction term to compare the mean SCORAD score between groups at weeks 4, 8, and 12. Participant-level random intercepts accounted for the correlation due to repeated measurements. The model was adjusted for variables used to stratify randomization (age at recruitment, sex, and age at onset). Residuals were examined visually and tested for normality with the Kolmogorov-Smirnov test. To analyze the proportion of days of topical steroid use, we used logistic regression to model the odds per individual with treatment group as the main explanatory variable and adjusted for the variables used for stratified randomization.
Laboratory determinations from blood samples were log-transformed to achieve more normally distributed variables and avoid extreme values. They were then analyzed with linear mixed-effects models (as for the SCORAD although with only 2 time observations, baseline and end of study).
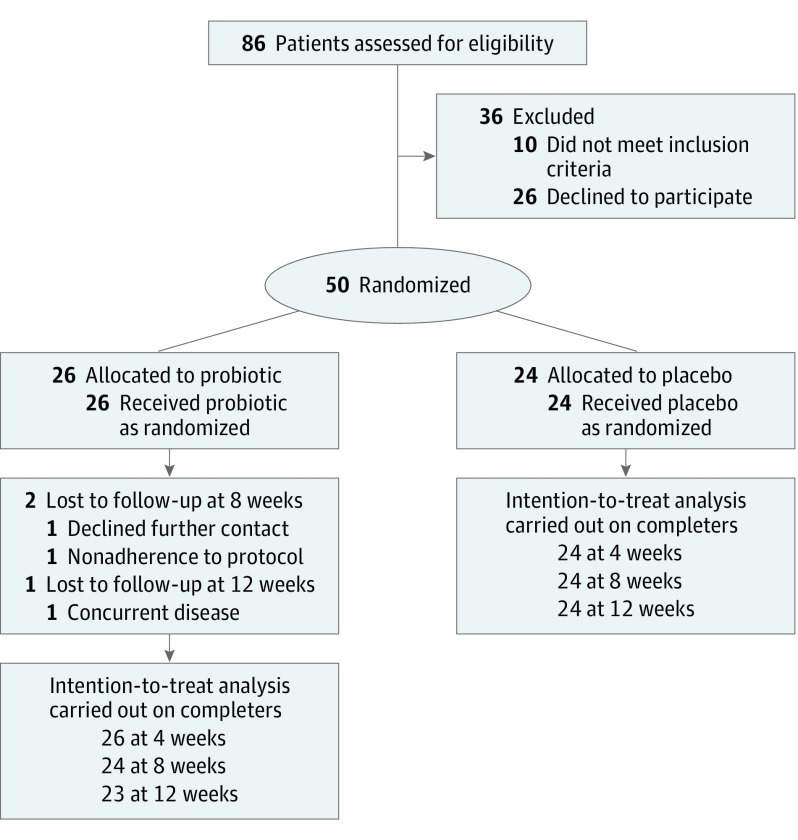
We followed CONSORT recommendations for reporting of randomized clinical trials (Figure 1). All statistical analyses were performed using IBM SPSS statistics, version 22 (SPSS Inc), and R, version 3.2.3.
Figure 1. CONSORT Diagram.
Results
From March to June 2016, 50 children with a diagnosis of AD were enrolled in the study. All met the criteria for a high-quality Mediterranean diet (KIDMED score >7). There were no significant differences in the baseline characteristics between the 2 groups (Table 1). Three of the patients in the probiotic group failed to provide data in some of the visits. Two of them declined contact in the second and third visit due to a work-related problem of the parents. Another patient had a concurrent disease unrelated to the treatment and could not provide data on the third visit. All reasons for missing data were unrelated to the treatment or the outcome, so we do not expect effect bias. Because missing data were only 2.5% of follow-up measurements, we decided not to use it and we used only observed data. Patients completed the KIDMED test at inclusion and repeated the test at the end of the intervention. The data show no relevant differences in the KIDMED score between the trial arms at baseline (Table 1) or end of the study (mean [SD] score, 8.7 [0.63] vs 8.6 [0.66] for the placebo and treatment groups, respectively).
Table 1. Baseline Clinical Characteristics of Study Participants in the Placebo and Probiotic Groups.
| Characteristic | Probiotic Group (n = 26) |
Placebo Group (n = 24) |
|---|---|---|
| Female sex, No. (%) | 13 (50) | 13 (54) |
| Age, mean (SD), y | 9.35 (3.58) | 8.96 (3.94) |
| Race/ethnicity, No. (%) | ||
| White | 25 (96) | 24 (100) |
| Black, No. (%) | 1 (4) | 0 |
| Hispanic, No. (%) | 26 (100) | 23 (96) |
| Weight, mean (SD), kg | 34.80 (14.77) | 38.33 (14.54) |
| Height, mean (SD), cm | 136.31 (19.69) | 137.58 (22.72) |
| KIDMED index, mean (SD) | 8.6 (0.66) | 8.7 (0.63) |
| SCORAD, mean SD | ||
| Total | 33.58 (3.38) | 31.64 (5.05) |
| Extension | 15.58 (5.65) | 14.25 (5.49) |
| Intensity | 24.5 (2.62) | 22.75 (3.50) |
| Subjective symptoms | 5.96 (2.34) | 6.04 (2.22) |
| Investigator Global Assessment index, median (IQR) | 3 (2-3) | 3 (2-3) |
| Eosinophil count, mean (SD), /μL | 300 (545) | 390 (465) |
| Serum IgE, mean (SD), μg/L | 989 (1714) | 773 (1528) |
| Lactate dehydrogenase, mean (SD), U/L | 537.42 (123.56) | 544.91 (140.34) |
| C-reactive protein, median (IQR), mg/L | 0.25 (0.25-0.25) | 0.25 (0.25-0.25) |
| Mean (SD), pg/mL | ||
| IL-4 | 32.19 (8.35) | 30.11 (4.56) |
| IL-5 | 4.35 (0.68) | 4.34 (0.45) |
| IL-10 | 13.68 (5.08) | 12.31 (3.41) |
| IL-13 | 85.52 (12.48) | 81.82 (13.24) |
Abbreviations: IL, interleukin; IQR, interquartile range; SCORAD, Scoring Atopic Dermatitis.
SI conversion factors: To convert C-reactive protein to nanomoles per liter, multiply by 9.524; to convert eosinophil count to billions per liter, multiply by 0.001; to convert IgE to milligrams per liter, multiply by 0.001; to convert lactate dehydrogenase to microkatals per liter, multiply by 0.0167.
Effects on Disease Severity
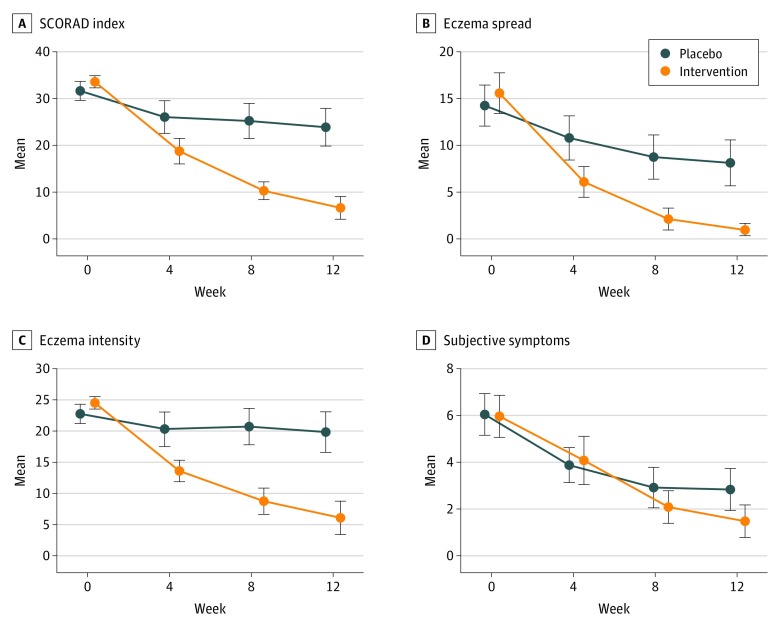
Twenty-two of 23 (96%) patients in the probiotic group and 11 of 24 (46%) in the placebo group improved in the SCORAD index. The mean reduction and 95% confidence interval for the SCORAD index and its components (eczema extension, eczema intensity, and subjective symptoms) for different weeks are shown in Figure 2, whereas the estimated changes in each trial arm and the differences between the 2 arms (intervention effect) are presented in Table 2 for the total SCORAD index, eTable 4 in Supplement 2 for the subcomponents, and eFigure 1 in Supplement 2 for all. These eTables also give the changes as percentages over baseline values of the variable. At baseline, there were no large differences between arms in any of the variables. As time passed after starting treatment, the differences between arms increased, with better improvements in the probiotic group for total SCORAD, eczema intensity, and eczema spread (with CI excluding the null) but not for subjective symptoms. After 12 weeks of follow-up, the mean change in the SCORAD index (main outcome) was, in relative terms, −83% (95% CI, −95% to −70%) in the probiotic group and −24% (95% CI, −36% to −11%) in the placebo group. This is a difference in effectiveness of −19.2 (95% CI, −23.4 to −15.0) SCORAD points, or −59% (95% CI, −72% to −46%; P < .001), in favor of the probiotic. Patients in both probiotic and placebo groups showed improvement in atopic dermatitis disease activity as measured with Investigator Global Assessment (IGA) over the 12-week study period. Comparison between groups showed significant differences in the number of patients with response to treatment: 21 patients (91%) in the probiotic group achieved IGA scores of 0 or 1 (95% CI, 72%-99%) and 5 patients (21%; 95% CI, 7%-42%) achieved these scores in the placebo group. The percentage of patients with IGA score less than 2, measured at each checkup in weeks 4, 8, and 12, is included in eFigure 2 in Supplement 2.
Figure 2. Change in SCORAD Index and Its Components.
Error bars indicate 95% confidence interval.
Table 2. Estimation of the Effect of Intervention on the Scoring Atopic Dermatitis (SCORAD) Scalea.
| Parameter | Baseline | Change From Baseline | ||||||
|---|---|---|---|---|---|---|---|---|
| Week 4 | Week 8 | Week 12 | ||||||
| Placebo (n = 24) |
Probiotic (n = 26) |
Placebo (n = 24) |
Probiotic (n = 26) |
Placebo (n = 24) |
Probiotic (n = 24) |
Placebo (n = 24) |
Probiotic (n = 23) |
|
| SCORAD score | 32.2 (27.4 to 37.0) |
33.8 (29.0 to 38.5) |
−5.6 (−9.8 to −1.4) |
−14.8 (−18.8 to −10.8) |
−6.4 (−10.6 to −2.3) |
−23.2 (−27.3 to −19.1) |
−7.8 (−11.9 to −3.6) |
−27.0 (−31.1 to −22.8) |
| Proportional effect, %b | N/A | N/A | −17 (−30 to −4) |
−45 (−58 to −33) |
−20 (−32 to −7) |
−71 (−84 to −59) |
−24 (−36 to −11) |
−83 (−95 to −70) |
| Difference | ||||||||
| SCORAD score | 1.5 (−2.3 to 5.3) | −9.2 (−13.4 to −5.1) | −16.7 (−20.9 to −12.6) | −19.2 (−23.4 to −15.0) | ||||
| Proportional effect, %b | N/A | −28 (−41 to −16) | −51 (−64 to −39) | −59 (−72 to −46) | ||||
Abbreviation: N/A, not applicable.
Effects are estimated using linear mixed-effects models with a group-by-week interaction, participant-level random intercepts, and adjusted for variables used to stratify randomization.
These rows are the effects on the rows above divided by baseline SCORAD mean of all participants (32.6) and multiplied by 100. This yields the proportional effect, or percentage effectiveness.
Effect on Topical Use of Steroids
At the end of follow-up, there were 2032 patient-days of observation in the placebo group and 2084 patient-days in the probiotic group. Steroids were used to treat flares on 220 (10.8%) and 161 (7.7%) patient-days, respectively. The distribution of number of days per individual did not seem to follow a Poisson distribution (eFigure 3 in Supplement 2). Considering that follow-up was approximately 84 days, some individuals used steroids for a large proportion of them. The logistic regression adjusted for baseline stratification variables estimated an odds ratio of 0.63 (95% CI, 0.51-0.78; P < .001) for the effect of the treatment on the reduction of use of corticosteroids. We also did a sensitivity analysis comparing the total number of days of steroid use (including nonflare use). The placebo arm had 336 patient-days of use (16.5%) whereas the probiotic arm had 291 patient-days of use (14.0%). The adjusted logistic regression model produced an odds ratio of 0.77 (95% CI, 0.65-0.91; P < .003), a statistically significant finding of less corticosteroid use in the probiotic group.
Comparison of Blood Marker Levels
Baseline blood marker levels of patients in the probiotic and placebo groups are given in Table 1. During the 12-week intervention period, no significant differences were observed between the 2 groups in the changes in blood levels of IL-4, IL-5, IL-10, IL-13, eosinophils, IgE, and lactate dehydrogenase (eTable 3 in Supplement 2).
Adverse Events
No relevant adverse events were associated with drug or placebo intake.
Discussion
Several important studies have explored the efficacy of certain probiotics in the prevention and treatment of AD. Overall, the current evidence suggests that probiotics could be an option to improve moderate and severe AD recovery rates in children and adults; however, to date, there is no strong experimental evidence supporting their effectiveness and safety in clinical practice. Importantly, evidence and clinical trials demonstrating strain-specific effects are lacking.
The clinical trial reported herein explores the role of a mixture of probiotics administered to patients with moderate AD. Several variables, such as antibiotic use, diet, and other concomitant allergenic diseases were controlled for, and, to avoid bias, all cases included in the study were matched for these variables. Our results suggest that administration of this mixture of probiotics, as adjuvant treatment, may be effective in reducing the SCORAD index and, subsequently, decreasing the use of steroids during AD flares. The response rate was significant when the reduction in baseline SCORAD index was compared between groups (Table 2). The clinical response documented in the probiotic group was greater than that obtained with other probiotics tested in a previous placebo-controlled clinical trial. This study showed better response in SCORAD (83% relative reduction) than previously communicated results by Farid et al (68% of response), Iemoli et al (63%), or Yeşilova et al (64%). Several factors may influence the response to probiotic treatment in AD, and they were taken into account when the protocol of this clinical study was designed: treatment longer than 8 weeks might condition the positive effect of probiotic use, patients older than 1 year have a greater response to probiotics, patients with moderate to severe AD have a better response, and a mixture of probiotics has better beneficial effects than a single probiotic, especially when lactobacilli and bifidobacteria are included in the mixture. The final blend used in the study was selected on the basis of published results and internal unpublished data, as described in the Methods.
Two of 3 subcomponents of the SCORAD index (eczema spread and intensity) showed a clear improvement in favor of the probiotic group compared with the placebo group (Figure 2, B and C). In contrast, there was no statistically significant difference in subjective symptoms (eTable 4 in Supplement 2 and Figure 2D). This finding was not due to a lack of effect in the probiotic group, which showed a clinically relevant proportional reduction of 77%, but rather because there was also a reduction in the placebo group of 53%. A possible explanation is that the placebo group was able to reduce symptoms such as pruritus by using more corticosteroids.
After 12 weeks, we saw a slightly greater reduction of IL-4, IL-5, and IL-13 in the probiotic arm compared with placebo (eTable 3 in Supplement 2, last column). This may suggest decreased activity of the T helper 2 cells in the probiotic arm. We did not find statistical significance in any of these differences, but lack of statistical significance does not mean evidence of no difference, especially when the trial was not powered to test differences in cytokines. An investigation of the biological mechanism will require a specifically designed study.
Limitations
The limitations of the study should be considered and clarified through further research. These limitations include the applicability of our results to patients consuming a different diet in different geographic areas, and whether the results can be extended to other population groups such as newborns (<1 year) or adults older than 17 years. In addition, the short follow-up of 12 weeks, the fact that topical corticosteroid dose was not recorded, and the inclusion of patients from a single center should also be considered limitations of this clinical trial. Finally, questions should be answered about adequate dosage, the duration of probiotic administration, and at what age the use of probiotics would be most efficacious. Future trials will be necessary and should consider all these questions to assess the probiotic mixture used in this clinical trial and other specific probiotic strains.
Conclusions
The results of our study indicate a strong positive effect in reducing the SCORAD index and use of topical corticosteroids in the group treated with the probiotic mixture. This evidence supports the efficacy of administering this probiotic mixture to patients with moderate AD and suggests that it could be used more extensively in clinical practice.
Trial Protocol
eTable 1. Major and minor criteria in the study participants within placebo and probiotic groups
eTable 2. KIDMED test to assess the Mediterranean Diet
eTable 3. Estimation of the effect of the intervention in laboratory parameters
eTable 4. Estimation of the effect of the intervention in SCORAD components
eFigure 1. Estimated differences between the two trial arms
eFigure 2. Percentage of patients with IGA score under 2, measured at each checkup in weeks 4, 8, and 12
eFigure 3. Distribution of number of days using steroids to treat flares
References
- 1.Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97(4):267-271. [PubMed] [Google Scholar]
- 2.Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33(3):281-288. [DOI] [PubMed] [Google Scholar]
- 3.Celakovská J, Bukač J. The severity of atopic dermatitis evaluated with the SCORAD index and the occurrence of bronchial asthma and rhinitis, and the duration of atopic dermatitis. Allergy Rhinol (Providence). 2016;7(1):8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nowicki R, Trzeciak M, Wilkowska A, et al. Atopic dermatitis: current treatment guidelines: statement of the experts of the Dermatological Section, Polish Society of Allergology, and the Allergology Section, Polish Society of Dermatology. Postepy Dermatol Alergol. 2015;32(4):239-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buzney CD, Gottlieb AB, Rosmarin D. Asthma and atopic dermatitis: a review of targeted inhibition of interleukin-4 and interleukin-13 as therapy for atopic disease. J Drugs Dermatol. 2016;15(2):165-171. [PubMed] [Google Scholar]
- 6.Hamilton JD, Ungar B, Guttman-Yassky E. Drug evaluation review: dupilumab in atopic dermatitis. Immunotherapy. 2015;7(10):1043-1058. [DOI] [PubMed] [Google Scholar]
- 7.D’Auria E, Banderali G, Barberi S, et al. Atopic dermatitis: recent insight on pathogenesis and novel therapeutic target. Asian Pac J Allergy Immunol. 2016;34(2):98-108. [DOI] [PubMed] [Google Scholar]
- 8.Stalder JF, Dutartre H, Laruche G, Litoux P. Photoprotection in children [in French]. Ann Dermatol Venereol. 1993;120(6-7):485-488. [PubMed] [Google Scholar]
- 9.Saeki H, Furue M, Furukawa F, et al. ; Committee for Guidelines for the Management of Atopic Dermatitis of the Japanese Dermatological Association . Guidelines for management of atopic dermatitis. J Dermatol. 2009;36(10):563-577. [DOI] [PubMed] [Google Scholar]
- 10.Luger T, Boguniewicz M, Carr W, et al. Pimecrolimus in atopic dermatitis: consensus on safety and the need to allow use in infants. Pediatr Allergy Immunol. 2015;26(4):306-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roekevisch E, Spuls PI, Kuester D, Limpens J, Schmitt J. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133(2):429-438. [DOI] [PubMed] [Google Scholar]
- 12.Kutlubay Z, Erdogan BÇ, Engin B, Serdaroglu S. Cyclosporine in dermatology. Skinmed. 2016;14(2):105-109. [PubMed] [Google Scholar]
- 13.Czarnowicki T, Gonzalez J, Shemer A, et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J Allergy Clin Immunol. 2015;136(1):104-115.e7. [DOI] [PubMed] [Google Scholar]
- 14.Lu CY, Ni YH. Gut microbiota and the development of pediatric diseases. J Gastroenterol. 2015;50(7):720-726. [DOI] [PubMed] [Google Scholar]
- 15.Lammers KM, Brigidi P, Vitali B, et al. Immunomodulatory effects of probiotic bacteria DNA: IL-1 and IL-10 response in human peripheral blood mononuclear cells. FEMS Immunol Med Microbiol. 2003;38(2):165-172. [DOI] [PubMed] [Google Scholar]
- 16.Song H, Yoo Y, Hwang J, Na YC, Kim HS. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016;137(3):852-860. [DOI] [PubMed] [Google Scholar]
- 17.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3(10):858-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plöger S, Stumpff F, Penner GB, et al. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci. 2012;1258:52-59. [DOI] [PubMed] [Google Scholar]
- 19.Weston S, Halbert A, Richmond P, Prescott SL. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90(9):892-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SO, Ah YM, Yu YM, Choi KH, Shin WG, Lee JY. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2014;113(2):217-226. [DOI] [PubMed] [Google Scholar]
- 21.Rather IA, Bajpai VK, Kumar S, Lim J, Paek WK, Park YH. Probiotics and atopic dermatitis: an overview. Front Microbiol. 2016;7:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serra-Majem L, Ribas L, García A, Pérez-Rodrigo C, Aranceta J. Nutrient adequacy and Mediterranean diet in Spanish school children and adolescents. Eur J Clin Nutr. 2003;57(suppl 1):S35-S39. [DOI] [PubMed] [Google Scholar]
- 24.Chang YS, Trivedi MK, Jha A, Lin YF, Dimaano L, García-Romero MT. Synbiotics for prevention and treatment of atopic dermatitis: a meta-analysis of randomized clinical trials. JAMA Pediatr. 2016;170(3):236-242. [DOI] [PubMed] [Google Scholar]
- 25.Ji H, Li XK. Oxidative stress in atopic dermatitis. Oxid Med Cell Longev. 2016;2016:2721469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martorell P, Llopis S, González N, et al. Probiotic strain Bifidobacterium animalis subsp lactis CECT 8145 reduces fat content and modulates lipid metabolism and antioxidant response in Caenorhabditis elegans. J Agric Food Chem. 2016;64(17):3462-3472. [DOI] [PubMed] [Google Scholar]
- 27.Medina M, De Palma G, Ribes-Koninckx C, Calabuig M, Sanz Y. Bifidobacterium strains suppress in vitro the pro-inflammatory milieu triggered by the large intestinal microbiota of coeliac patients. J Inflamm (Lond). 2008;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivares M, Castillejo G, Varea V, Sanz Y. Double-blind, randomised, placebo-controlled intervention trial to evaluate the effects of Bifidobacterium longum CECT 7347 in children with newly diagnosed coeliac disease. Br J Nutr. 2014;112(1):30-40. [DOI] [PubMed] [Google Scholar]
- 29.Woo SI, Kim JY, Lee YJ, Kim NS, Hahn YS. Effect of Lactobacillus sakei supplementation in children with atopic eczema-dermatitis syndrome. Ann Allergy Asthma Immunol. 2010;104(4):343-348. [DOI] [PubMed] [Google Scholar]
- 30.Park CW, Youn M, Jung YM, et al. New functional probiotic Lactobacillus sakei probio 65 alleviates atopic symptoms in the mouse. J Med Food. 2008;11(3):405-412. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto M, Aranami A, Ishige A, Watanabe K, Benno Y. LKM512 yogurt consumption improves the intestinal environment and induces the T-helper type 1 cytokine in adult patients with intractable atopic dermatitis. Clin Exp Allergy. 2007;37(3):358-370. [DOI] [PubMed] [Google Scholar]
- 32.Chapman CM, Gibson GR, Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr. 2011;50(1):1-17. [DOI] [PubMed] [Google Scholar]
- 33.Wickens K, Black P, Stanley TV, et al. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin Exp Allergy. 2012;42(7):1071-1079. [DOI] [PubMed] [Google Scholar]
- 34.Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirjavainen PV, Salminen SJ, Isolauri E. Probiotic bacteria in the management of atopic disease: underscoring the importance of viability. J Pediatr Gastroenterol Nutr. 2003;36(2):223-227. [DOI] [PubMed] [Google Scholar]
- 36.Viljanen M, Savilahti E, Haahtela T, et al. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy. 2005;60(4):494-500. [DOI] [PubMed] [Google Scholar]
- 37.Passeron T, Lacour JP, Fontas E, Ortonne JP. Prebiotics and synbiotics: two promising approaches for the treatment of atopic dermatitis in children above 2 years. Allergy. 2006;61(4):431-437. [DOI] [PubMed] [Google Scholar]
- 38.Brouwer ML, Wolt-Plompen SA, Dubois AE, et al. No effects of probiotics on atopic dermatitis in infancy: a randomized placebo-controlled trial. Clin Exp Allergy. 2006;36(7):899-906. [DOI] [PubMed] [Google Scholar]
- 39.Grüber C, Wendt M, Sulser C, et al. Randomized, placebo-controlled trial of Lactobacillus rhamnosus GG as treatment of atopic dermatitis in infancy. Allergy. 2007;62(11):1270-1276. [DOI] [PubMed] [Google Scholar]
- 40.Roessler A, Friedrich U, Vogelsang H, et al. The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clin Exp Allergy. 2008;38(1):93-102. [DOI] [PubMed] [Google Scholar]
- 41.Chernyshov PV. Randomized, placebo-controlled trial on clinical and immunologic effects of probiotic containing Lactobacillus rhamnosus R0011 and L helveticus R0052 in infants with atopic dermatitis. Microb Ecol Health Dis. 2009;21(3-4):228-232. [Google Scholar]
- 42.Niccoli AA, Artesi AL, Candio F, et al. Preliminary results on clinical effects of probiotic Lactobacillus salivarius LS01 in children affected by atopic dermatitis. J Clin Gastroenterol. 2014;48(suppl 1):S34-S36. [DOI] [PubMed] [Google Scholar]
- 43.Gobel R, Larsen N, Mølgaard C, Jakobsen M, Michaelsen KF. Probiotics to young children with atopic dermatitis: a randomized placebo-controlled trial. Int J Probio Prebio. 2010;5(2):53-59. [Google Scholar]
- 44.Farid R, Ahanchian H, Jabbari F, Moghiman T. Effect of a new synbiotic mixture on atopic dermatitis in children: a randomized-controlled trial. Iran J Pediatr. 2011;21(2):225-230. [PMC free article] [PubMed] [Google Scholar]
- 45.Iemoli E, Trabattoni D, Parisotto S, et al. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J Clin Gastroenterol. 2012;46(suppl):S33-S40. [DOI] [PubMed] [Google Scholar]
- 46.Yeşilova Y, Çalka Ö, Akdeniz N, Berktaş M. Effect of probiotics on the treatment of children with atopic dermatitis. Ann Dermatol. 2012;24(2):189-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drago L, Iemoli E, Rodighiero V, Nicola L, De Vecchi E, Piconi S. Effects of Lactobacillus salivarius LS01 (DSM 22775) treatment on adult atopic dermatitis: a randomized placebo-controlled study. Int J Immunopathol Pharmacol. 2011;24(4):1037-1048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Major and minor criteria in the study participants within placebo and probiotic groups
eTable 2. KIDMED test to assess the Mediterranean Diet
eTable 3. Estimation of the effect of the intervention in laboratory parameters
eTable 4. Estimation of the effect of the intervention in SCORAD components
eFigure 1. Estimated differences between the two trial arms
eFigure 2. Percentage of patients with IGA score under 2, measured at each checkup in weeks 4, 8, and 12
eFigure 3. Distribution of number of days using steroids to treat flares