Key Points
Question
What is the probability of skin disease remission over time for patients with dermatomyositis treated with standard-of-care therapies?
Findings
In this prospective cohort study of 74 adults with dermatomyositis, 28 patients (38%) achieved clinical skin remission during a 3-year follow-up period. Increasing age, having an associated malignancy, and treatment with mycophenolate mofetil were significantly associated with clinical remission of skin disease, while having anti–melanoma differentiation-associated gene 5 antibodies was significantly associated with worse outcomes.
Meaning
The majority of adult patients with dermatomyositis do not achieve satisfactory control of skin disease despite long-term aggressive therapy. Our data support the need for better therapies for dermatomyositis.
This cohort study examines factors associated with clinical remission of skin disease in dermatomyositis.
Abstract
Importance
Cutaneous disease represents a significant burden for patients with dermatomyositis. However, quantitative estimates of the probability of skin disease remission and clinical factors associated with skin outcomes are lacking.
Objective
To characterize cutaneous disease course in adult patients with dermatomyositis.
Design, Setting, and Participants
Prospective cohort study conducted at a dermatology clinic at a tertiary academic referral center. All adult patients with dermatomyositis (age >18 years) seen between May 15, 2007, and October 28, 2016, were eligible. Patients were included in the current analysis if they had a baseline Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) activity score of 12 or higher, and 2 or more CDASI scores separated by 3 months or more within their first 3 years of follow-up.
Main Outcomes and Measures
The percentage of patients who achieved clinical remission of their cutaneous disease as measured by the CDASI over a 3-year follow-up.
Results
A total of 74 patients met our inclusion criteria (mean [SD] age at initial CDASI scoring, 54 [13] years; 58 women [78%]), and 28 (38%) achieved clinical remission during our 3-year follow-up period. Increased age (odds ratio [OR], 1.07; 95% CI, 1.02-1.12; P = .01), a dermatomyositis-associated malignancy (OR, 14.46; 95% CI, 2.18-96.07; P = .01), and treatment with mycophenolate mofetil (OR, 6.00; 95% CI, 1.66-21.78; P = .01) were significantly associated with clinical remission of skin disease in multivariable analysis. Patients with anti–melanoma differentiation-associated protein 5 antibodies had a significantly lower probability of meeting outcome criteria in our time-to-event analysis. Baseline cutaneous disease activity, disease duration at baseline, and disease duration before first systemic therapy were not significantly associated with clinical remission of skin disease.
Conclusions and Relevance
Clinical remission was relatively uncommon in our population despite aggressive systemic therapy, and patients with anti–melanoma differentiation-associated protein 5 antibodies were even less likely to enter clinical remission during a 3-year follow-up period. Although mycophenolate mofetil compared favorably with other treatment options, our data provide evidence that a substantial population of patients with dermatomyositis have skin disease that is not adequately managed with standard-of-care therapies.
Introduction
Dermatomyositis (DM) is an idiopathic inflammatory myopathy that manifests as inflammation in the skin, muscles, joints, and lungs.1,2 Chronic activity and long-term damage in these organ systems are problems for patients. Cutaneous disease is an important component of DM disease burden, as it is present in patients with classic myositis, patients who have clinically amyopathic DM and never develop muscle disease, and patients with persistent skin inflammation despite adequate control of their myositis. This notion is supported by data3 from large cohorts showing that patients with juvenile DM often continue to experience chronic skin inflammation despite control of their myositis. This is especially problematic because skin disease is associated with a significant negative impact on quality of life.4
Despite our understanding that the course of cutaneous disease in juvenile DM can be chronic, quantitative estimates of the duration and severity of cutaneous disease in the adult DM population are lacking. The Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) is a validated instrument that quantifies cutaneous disease, both activity (CDASI-a) and damage (CDASI-d), and allows for objective assessments of disease severity.5,6,7,8 Our aims were to estimate the percentage of patients with DM with clinically significant skin inflammation who achieve clinical remission (CR) during a 3-year follow-up period and to examine if skin disease course is associated with selected clinical variables. We present data from our ongoing, prospective study of CDASI scores in a US cohort of adult patients with DM.
Methods
Patient Population
The Stanford institutional review board approved the collection of plasma and clinical information used in this study, and all participants provided informed consent. The study population consists of adults (age >18 years) with DM seen at the Stanford University outpatient dermatology clinic between May 15, 2007, and October 28, 2016. Dermatomyositis was diagnosed using Bohan and Peter criteria,9,10 or, for patients with clinically amyopathic DM, Sontheimer criteria.11 Patients were considered to have clinically amyopathic DM if they had cutaneous disease for longer than 6 months with no evidence of muscle weakness or elevation of muscle enzymes beyond 20% of the upper limit of normal. Dermatomyositis-associated malignancy was defined as any malignancy diagnosed within 5 years of DM onset.
Patients were considered for this analysis if their baseline CDASI-a score in our clinic was 12 or higher. This number includes all patients with moderate-to-severe cutaneous disease5 and represents a biologic disease threshold as the minimum cutaneous disease activity score associated with increased interferon signaling in the blood.12 In addition, patients were required to have 2 or more CDASI scores separated by 3 months or more within their first 3 years of follow-up. There were 161 patients with DM in the enrolled population; 55 patients were excluded because they only had 1 CDASI score, 7 patients were excluded because they had less than 3 or more than 36 months between their first and last CDASI scores, and 25 patients were excluded because they had a baseline CDASI-a of less than 12. This left a total of 74 patients for analysis.
Because our clinic is a tertiary referral center, the majority of our patients (57 of 74 [77%]) were previously treated, and had variable disease duration at baseline. All patients were treated with standard-of-care therapy at the discretion of the investigators. In our study, the term systemic therapy specifically refers to antimalarials, mycophenolate mofetil, methotrexate, intravenous immunoglobulin, and oral or intravenous corticosteroids.
Assessment of Skin Activity and Definition of Clinical Remission
The extent of each patient’s skin disease was assessed by the same board-certified dermatologist using the CDASI. We used an exploratory definition for CR of skin disease based on the total CDASI-a score. The CDASI-a captures mild erythema that can be a normal variant in some areas. Consequently, there is a range of low scores that all represent functional remission—we defined this as a CDASI-a of 5 or less. We excluded the score for periungual changes from our CR definition for 2 reasons: first, we were interested in defining an end point that would represent clinically important skin activity to the patient; second, in our experience nailfold telangiectasias are often slow to improve, and we were concerned that including this score would prevent too many patients whose skin was otherwise in remission from reaching CR. Finally, we reasoned that any patient with significant erythema at a given location (score >1) or ulcerations would not meet our criteria for CR, regardless of the total CDASI-a score.
Antibody Detection
Plasma was typically collected at the first visit to our clinic. Antibodies against transcriptional intermediary factor 1γ (TIF-1γ), chromodomain-helicase-DNA-binding protein Mi-2 (Mi-2), nuclear matrix protein 2 (NXP2), small ubiquitin-like modifier activating enzyme 1 (SAE1), melanoma differentiation-associated gene 5 (MDA5), and E3 ubiquitin-protein ligase TRIM21 (Ro52) were assayed as previously described.13
Statistical Analysis
Wilcoxon rank sum tests or Welch t tests were used to compare continuous variables, and Fisher exact tests were used to compare categorical variables. P values less than .05 were considered statistically significant. Univariable and multivariable logistic regression analysis was performed using age, race, sex, amyopathic status, DM-associated malignancy, baseline skin disease activity, disease duration from first symptom to enrollment into the database and from first symptom to first systemic therapy, DM-autoantibody phenotype, and medication as covariates. Patients were required to have received 3 or more consecutive months of a medication for it to be considered a treatment. Variables with P values less than .10 in the univariable analysis were retained in the multivariable model. For selected variables and the outcome of CR, Kaplan-Meier plots were created and log-rank analysis used to compare curves. Analyses were conducted using SAS version 9.4 (SAS Institute Inc).
Results
Patient Characteristics
Our study group comprised 74 patients with moderate-to-severe cutaneous disease (CDASI-a ≥ 12) at their baseline visit. The median (interquartile range [IQR]) duration of follow-up was 17.5 (11-28.3) months, with a median (IQR) interval between CDASI scores of 4 (3-6) months. Our inclusion group was representative of our overall population with 2 differences (Table 1): the median CDASI scores were significantly higher in the inclusion vs exclusion group (25 vs 12 for CDASI-a; P < .001 and 4 vs 2 for CDASI-d; P < .001); and the inclusion group had a greater percentage of patients with anti–TIF-1γ antibodies (50% vs 22%; P < .001). We expected this because the inclusion group was designed to capture patients with more severe disease, and data suggest that patients with anti–TIF-1γ antibodies have more severe cutaneous disease.14
Table 1. Baseline Characteristics of the Inclusion and Exclusion Groups.
| Characteristic | Inclusion
Group (n = 74) |
Exclusion
Group (n = 87) |
P Value |
|---|---|---|---|
| Female sex, No. (%) | 58 (78) | 57 (66) | .08 |
| Age at initial CDASI, mean (SD), y | 54 (13) | 54 (15) | .82 |
| Race/ethnicity, No. (%) | |||
| White | 42 (57) | 43 (49) | .43 |
| African American | 2 (3) | 2 (2) | >.99 |
| Asian | 8 (11) | 9 (10) | >.99 |
| Hispanic/Latino | 13 (18) | 11 (13) | .51 |
| Mixed | 3 (4) | 1 (1) | .33 |
| Disease duration at baseline, median (IQR), mo | 18 (8-41) | 19 (9-69) | .29 |
| Disease duration before first systemic therapy, median (IQR) moa | 3 (1-8) | 4.5 (1-8) | .76 |
| Baseline CDASI-a score, median (IQR) | 25 (19-30) | 12 (7-22) | <.001b |
| Baseline CDASI-d score, median (IQR) | 4 (2-7) | 2 (1-5) | <.001b |
| Clinically amyopathic disease, No. (%) | 14 (19) | 19 (22) | .70 |
| DM-associated malignancy, No. (%) | 9 (12) | 10 (11) | >.99 |
| Autoantibody positivity, No. (%) | |||
| Mi-2 | 2 (3) | 5 (6) | .45 |
| NXP2 | 5 (7) | 4 (5) | .73 |
| MDA5 | 10 (14) | 13 (15) | .83 |
| SAE1 | 8 (11) | 6 (7) | .41 |
| TIF-1γ | 37 (50) | 19 (22) | <.001b |
| Ro52 | 18 (24) | 20 (23) | .85 |
| Mi-2/TIF-1γ | 4 (5) | 5 (6) | >.99 |
| Antibody overlap besides Mi-2/TIF-1γ | 0 | 5 (6) | .06 |
| None | 7 (10) | 14 (16) | .35 |
| Prior medication exposure, No. (%)c | |||
| Intravenous immunoglobulin | 13 (18) | 18 (21) | .69 |
| Mycophenolate mofetil | 20 (27) | 28 (32) | .49 |
| Methotrexate | 36 (49) | 38 (44) | .63 |
| Antimalarials | 36 (49) | 32 (37) | .15 |
| >10 mg prednisone | 56 (76) | 72 (83) | .33 |
| No systemic oral medications or intravenous immunoglobulin | 17 (23) | 24 (28) | .59 |
Abbreviations: CDASI-a, Cutaneous Dermatomyositis Disease Area and Severity Index Activity Score; CDASI-d, Cutaneous Dermatomyositis Disease Area and Severity Index Damage Score; DM, dermatomyositis; IQR, interquartile range; MDA5, melanoma differentiation-associated gene 5; Mi-2, chromodomain-helicase-DNA-binding protein Mi-2; NXP2, nuclear matrix protein 2; Ro52, E3 ubiquitin-protein ligase TRIM21; SAE1, small ubiquitin-like modifier activating enzyme 1; TIF-1γ, transcriptional intermediary factor 1γ.
Systemic therapy includes oral or intravenous corticosteroids, mycophenolate mofetil, methotrexate, antimalarials, and intravenous immunoglobulin.
Statistically significant.
Positive exposure counts as any duration of treatment prior to baseline CDASI score.
Characteristics of Patients Achieving Clinical Remission
Of the 74 patients in our study, 28 (38%) met criteria for CR within 3 years (Table 2). Patients achieving CR were significantly older at baseline and more likely to have a DM-associated malignancy compared with those with persistent skin disease. Of 9 total patients with a DM-associated malignancy, 7 achieved CR (Table 2). Six of these 7 patients were in clinical remission for their cancer. In contrast, the 2 patients with a DM-associated malignancy who did not enter CR had persistently active malignancies. Patients with anti-MDA5 antibodies were significantly less likely to be in the CR group compared with the nonremission group (0 vs 10; P = .01). The remaining characteristics were not significantly different between the CR and nonremission group, including baseline CDASI scores, disease duration at baseline, and disease duration before first systemic therapy.
Table 2. Comparison of Groups Achieving and Not Achieving Clinical Remission.
| Characteristic | Inclusion
Group (n = 74) |
P Value | |
|---|---|---|---|
| Clinical
Remission (n = 28) |
No Clinical
Remission (n = 46) |
||
| Female sex, No. (%) | 24 (86) | 34 (74) | .26 |
| Age at initial CDASI, mean (SD), y | 59 (12) | 50 (12) | .01a |
| Race/ethnicity, No. (%) | |||
| White | 16 (57) | 26 (57) | >.99 |
| African American | 1 (4) | 1 (2) | >.99 |
| Asian | 2 (7) | 6 (13) | .70 |
| Hispanic/Latino | 4 (14) | 9 (20) | .76 |
| Mixed | 2 (7) | 1 (2) | .55 |
| Disease duration at baseline, median (IQR), mo | 16 (8-58) | 20 (9-41) | .82 |
| Disease duration before first systemic therapy, median (IQR), mob | 3 (1-8) | 3 (2-8) | .52 |
| Baseline CDASI-a score, median (IQR) | 25 (20-28) | 25 (19-30) | .55 |
| Baseline CDASI-d score, median (IQR) | 4 (2-6) | 5 (2-9) | .25 |
| Clinically amyopathic disease, No. (%) | 6 (21) | 8 (17) | .76 |
| DM-associated malignancy, No. (%) | 7 (25) | 2 (4) | .02a |
| Autoantibody positivity, No. (%) | |||
| Mi-2 | 1 (4) | 1 (2) | >.99 |
| NXP2 | 3 (11) | 2 (4) | .36 |
| MDA5 | 0 (0) | 10 (22) | .01a |
| SAE1 | 3 (11) | 5 (11) | >.99 |
| TIF-1γ | 14 (50) | 23 (50) | >.99 |
| Ro52 | 6 (21) | 12 (26) | .78 |
| Mi-2/TIF-1γ | 2 (7) | 2 (4) | >.99 |
| None | 5 (18) | 2 (4) | .10 |
| Treatment, No. (%)c | |||
| Mycophenolate mofetil | 14 (50) | 13 (28) | .08 |
| Antimalarials | 13 (46) | 15 (33) | .32 |
| Methotrexate | 10 (36) | 19 (41) | .81 |
| Intravenous immunoglobulin | 8 (29) | 10 (22) | .58 |
| No systemic oral medications or intravenous immunoglobulin | 1 (4) | 7 (15) | .25 |
Abbreviations: CDASI-a, Cutaneous Dermatomyositis Disease Area and Severity Index Activity Score; CDASI-d, Cutaneous Dermatomyositis Disease Area and Severity Index Damage Score; DM, dermatomyositis; IQR, interquartile range; MDA5, melanoma differentiation-associated gene 5; Mi-2, chromodomain-helicase-DNA-binding protein Mi-2; NXP2, nuclear matrix protein 2; Ro52, E3 ubiquitin-protein ligase TRIM21; SAE1, small ubiquitin-like modifier activating enzyme 1; TIF-1γ, transcriptional intermediary factor 1γ.
Statistically significant.
Systemic therapy includes oral or intravenous corticosteroids, mycophenolate mofetil, methotrexate, antimalarials, and intravenous immunoglobulin.
Positive treatment requires 3 or more continuous months of treatment between time of baseline and final CDASI.
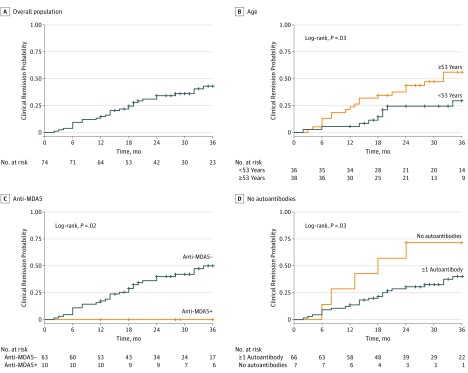
Timing of Clinical Remission of Skin Disease
The overall probability of achieving CR increased from 0.25 after 18 months to 0.43 after 36 months (Figure, A). When categorizing patients as older or younger than 53 years (median age of cohort), the time-to-remission curve was statistically favorable for the older population (Figure, B). Patients without anti-MDA5 antibodies also fared better (Figure, C), as did patients who were seronegative for any of the tested DM-specific antibodies (Figure, D).
Figure. Clinical Remission Probabilities.
A, Overall probability of achieving clinical remission (CR); B, Probability of achieving CR based on age; C, Probability of patients with anti-MDA5 antibodies achieving CR; D, Probability of patients without DM-specific autoantibodies achieving CR. MDA5 indicates melanoma differentiation-associated gene 5.
Despite not achieving CR, the 46 patients with persistent skin disease experienced a significant improvement in cutaneous disease activity. Their median change in CDASI-a was −9.5 at 3 years, and 31 (67%) had a decrease in CDASI-a of 5 or greater, the minimal clinically important difference for improvement.5
Variables Affecting Skin Disease Remission
All demographic and disease characteristics listed in Table 2 were also tested in a univariable and multivariable logistic regression using CR as the outcome. In the multivariable model, age at baseline CDASI (odds ratio [OR], 1.07; 95% CI, 1.02-1.12; P = .01), DM-associated malignancy (OR, 14.46; 95% CI, 2.18-96.07; P = .01), and treatment with mycophenolate mofetil (OR, 6.00; 95% CI, 1.66-21.78; P = .01) were all significantly associated with achieving CR (Table 3).
Table 3. Univariable and Multivariable Analysis Using Clinical Remission as End Point.
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Univariable analysis | ||
| Age at baseline CDASI | 1.06 (1.01-1.10) | .01 |
| No DM-specific autoantibodies | 4.67 (0.84-26) | .08 |
| Treatment with mycophenolate mofetil | 2.54 (0.95-6.76) | .06 |
| DM-associated malignancy | 7.33 (1.40-38.38) | .02 |
| Length of time between baseline and final CDASI | 0.96 (0.01-1.01) | .09 |
| Multivariable analysis | ||
| Age at baseline CDASI | 1.07 (1.02-1.12) | .01 |
| Treatment with mycophenolate mofetil | 6.00 (1.66-21.78) | .01 |
| DM-associated malignancy | 14.46 (2.18-96.07) | .01 |
| Length of time between baseline and final CDASI | 0.94 (0.88-1.00) | .06 |
Abbreviations: CDASI, Cutaneous Dermatomyositis Disease Area and Severity Index; DM, dermatomyositis; OR, odds ratio.
We further investigated mycophenolate mofetil response in our cohort. Higher doses of mycophenolate mofetil were associated with efficacy, as 64% of those who achieved CR were treated with 3 g/d, compared with only 15% of those who did not achieve CR (P = .02). Patients treated with mycophenolate mofetil who met CR criteria were also more likely to be on mycophenolate mofetil monotherapy at the visit where they entered CR compared with the final visit for those who did not enter CR (64% vs 8%; P = .004). We next tested if a higher tolerability for mycophenolate mofetil allowed for relatively longer treatment times, and thus a greater chance of reaching CR. However, the majority of patients treated with intravenous immunoglobulin (16 of 18 [89%]), mycophenolate mofetil (21 of 27 [78%]), methotrexate (22 of 29 [76%]), or antimalarials (20 of 28 [74%]) remained on therapy at the end of follow-up.
As intravenous immunoglobulin has been associated with significant improvement in DM skin disease,15,16 we examined why our data did not reflect a significant association between its use and CR. We hypothesized that patients with more severe and/or recalcitrant skin disease were channeled into receiving intravenous immunoglobulin therapy due to its perceived efficacy. However, there was no significant difference in median baseline CDASI-a scores between the 18 patients treated with intravenous immunoglobulin and the 56 not receiving intravenous immunoglobulin (26 vs 23; P = .23). Similarly, there was no difference in median CDASI-a score prior to initiation of intravenous immunoglobulin therapy (27 vs 27; P = .52) for those who did and did not meet CR criteria. Finally, there was no significant difference in median number of failed systemic oral medications in those who did and did not receive intravenous immunoglobulin (2.5 vs 2.0, respectively; P = .19).
We also investigated whether patients treated with intravenous immunoglobulin experienced significant improvement that fell short of our definition of CR. Thus, we performed a similar regression analysis using mean absolute change in CDASI-a as the outcome. In the unadjusted model, treatment with intravenous immunoglobulin was not statistically associated with CDASI-a change. In the multivariable model, the association between intravenous immunoglobulin and change in CDADI-a remained insignificant after adjusting for other variables (β, 2.12; 95% CI, −3.03 to 7.26; P = .41).
It is somewhat surprising that baseline CDASI-a score does not appear to affect the risk of CR. One explanation for this is that patients with comparatively mild disease were treated less aggressively. To investigate this, patients were divided into quartiles based on their baseline CDASI-a score. We found no significant difference in the median number of systemic medications used for treatment; the frequency of treatment with intravenous immunoglobulin, mycophenolate mofetil, methotrexate, or antimalarials; or the number of patients treated with 2 or more systemic therapies simultaneously among the quartiles.
As our definitions of CR and medication exposure are exploratory, we conducted several sensitivity analyses. We first set a more rigorous definition of remission, changing the outcome criteria to a CDASI-a score of 2 or less. Treatment with mycophenolate mofetil and age both increased the odds of meeting outcome criteria in the multivariable model, although malignancy was no longer significant. We also changed the definition of medication treatment to 6 or more consecutive months of therapy. Treatment with mycophenolate mofetil was again significant in the multivariable, and, as in our original analysis, no other medications were significantly associated with CR.
Discussion
Despite evidence that cutaneous disease in DM is highly impactful and often chronic, there are no robust and quantitative studies examining cutaneous disease course. In this study we used the CDASI-a score to calculate the probability of achieving skin disease remission and measure the impact of modifiable and fixed covariates. The strengths of our study include that these data are quantitative, are collected prospectively, use a validated skin disease outcome instrument, and use evaluations performed by a single investigator.
One important finding is that remission of skin activity is uncommon. Despite aggressive use of systemic therapies, only 28 of 74 patients (38%) in our inclusion group achieved CR within 3 years. This result is consistent with the data in the juvenile DM population, which show that many patients have persistent skin disease and require treatment for more than 3 years following diagnosis.3
Increased age was consistently associated with increased probability of achieving CR in our study (Tables 2 and 3; Figure). This result might be explained by unmeasured confounders, such as increased medication adherence or likelihood of coming to visits despite remission of skin disease in the older population. We considered the latter possibility, but found that the older (>53 years) and younger (<53 years) patients had a similar mean duration of follow-up (17 vs 19 months, respectively). Our results may be rooted in biology, as aging is associated with dysregulation of the innate immune system, simultaneously leading to a proinflammatory environment17 and impairment of the type I interferon pathway as regulated by plasmacytoid dendritic cells.18 Research has shown an up-regulation of type 1 interferon-inducible genes within the skin of patients with DM,19 and an abundance of plasmacytoid dendritic cells localized to DM skin lesions.20 Thus, the natural impairment of the type 1 interferon pathway in older individuals may explain their improved outcomes in our study.
Dermatomyositis-associated malignancies were associated with increased probability of achieving CR (Tables 2 and 3). All of the patients who achieved clinical remission of their malignancy also entered CR for their skin disease. This observed association may reflect the paraneoplastic nature of DM in a subset of patients, as rapid remission of skin disease following definitive cancer treatment has been previously described in some patients.21,22
With respect to treatment, the use of mycophenolate mofetil was associated with increased odds of CR (Tables 2 and 3; Figure). We consider mycophenolate mofetil to be an aggressive therapy for severe skin disease, and we did not find evidence that it was preferentially used for patients with comparatively mild disease. Interestingly, the majority of patients who achieved CR with mycophenolate mofetil were treated with 3 g/d and were on mycophenolate mofetil monotherapy at the time of CR. This supports prolonged and high dosing of mycophenolate mofetil for skin disease in patients with DM, and suggests that the efficacy of mycophenolate mofetil is attributable to the drug alone and not due to combination therapy.
It was somewhat surprising that we did not identify intravenous immunoglobulin as a medication associated with CR or increased absolute reduction in CDASI-a score, because data suggest that it is very effective for skin disease.15,16 One explanation for this would be that intravenous immunoglobulin is reserved for patients in our clinic who have more recalcitrant and severe disease. However, we did not find evidence that it was preferentially used for patients with more severe or recalcitrant disease. We also wondered if patients could not tolerate intravenous immunoglobulin for long enough to enter CR, but this did not appear to be the case, as almost 90% of patients treated with intravenous immunoglobulin remained on therapy at their last assessment. Larger, prospective, randomized studies will be needed to examine comparative efficacy of intravenous immunoglobulin in the long-term treatment of DM skin disease.
Patients with anti-MDA5 antibodies had a lower chance of entering CR. As the presence of any ulcer precludes CR, and anti-MDA5 antibodies are associated with mucocutaneous ulcers,23 we wondered if this result reflects a higher ulcer burden in patients with anti-MDA5. However, persistent erythema and scale, not ulcers, prevented 9 of 10 patients with anti-MDA5 from entering CR at their final visit. Our results suggest that even the clinically nonvasculopathic skin disease in patients with anti-MDA5 may be biologically different from that seen in other patients with DM.
Studies in patients with juvenile DM have demonstrated that a shorter duration of untreated disease or less severe cutaneous disease at onset is associated with a shorter cutaneous disease course.24 It has also been shown that early treatment positively affects outcomes in juvenile patients with DM.25,26 Interestingly, we did not find an association between duration of untreated disease or baseline CDASI scores and CR. This could be due to methodological differences between our study and those for juvenile DM (eg, our cohort is not an inception cohort), or this could reflect inherent differences between adult and juvenile patients with DM.
Limitations
We recognize that there are unavoidable biases in this study. This is not an inception cohort, and the majority of patients (n = 57 [77%]) were exposed to a systemic therapy prior to the baseline visit. Nonetheless, our results provide insight into the average length of time before CR is reached for a patient with moderate-to-severe skin disease beginning aggressive treatment in a specialty clinic. As patients of a referral center, our patient population may have more recalcitrant skin disease than the general DM population, and therefore we may be underestimating the number of patients who achieve CR.
Because our patients are not randomized to therapy, any data regarding medication use and outcomes are subject to confounding by indication. Although we were unable to detect this using baseline CDASI-a as a proxy for skin disease that is difficult to manage, it is possible that other unmeasured factors influenced therapeutic decision making. Finally, it is important to note that our definition of CR is not validated. The purpose of this study is not necessarily to identify the best definition for clinical remission of skin disease but instead to compare outcomes among patients using a standardized end point. Importantly, our sensitivity analyses using an altered definition of CR did not appreciably change our results.
Conclusions
Clinical remission was relatively uncommon in our overall population and even less common in patients with anti-MDA5, despite the use of aggressive systemic therapy. Although increased age and mycophenolate mofetil were both associated with positive cutaneous outcomes, our overall findings highlight the need for new therapies to more effectively treat skin disease in DM.
References
- 1.Callen JP. Dermatomyositis. Lancet. 2000;355(9197):53-57. [DOI] [PubMed] [Google Scholar]
- 2.Callen JP, Wortmann RL. Dermatomyositis. Clin Dermatol. 2006;24(5):363-373. [DOI] [PubMed] [Google Scholar]
- 3.Huber AM, Lang B, LeBlanc CM, et al. Medium- and long-term functional outcomes in a multicenter cohort of children with juvenile dermatomyositis. Arthritis Rheum. 2000;43(3):541-549. [DOI] [PubMed] [Google Scholar]
- 4.Goreshi R, Chock M, Foering K, et al. Quality of life in dermatomyositis. J Am Acad Dermatol. 2011;65(6):1107-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anyanwu CO, Fiorentino DF, Chung L, et al. Validation of the cutaneous dermatomyositis disease area and severity index: characterizing disease severity and assessing responsiveness to clinical change. Br J Dermatol. 2015;173(4):969-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yassaee M, Fiorentino D, Okawa J, et al. Modification of the cutaneous dermatomyositis disease area and severity index, an outcome instrument. Br J Dermatol. 2010;162(3):669-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein RQ, Bangert CA, Costner M, et al. Comparison of the reliability and validity of outcome instruments for cutaneous dermatomyositis. Br J Dermatol. 2008;159(4):887-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goreshi R, Okawa J, Rose M, et al. Evaluation of reliability, validity, and responsiveness of the CDASI and the CAT-BM. J Invest Dermatol. 2012;132(4):1117-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292(7):344-347. [DOI] [PubMed] [Google Scholar]
- 10.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292(8):403-407. [DOI] [PubMed] [Google Scholar]
- 11.Sontheimer RD. Dermatomyositis: an overview of recent progress with emphasis on dermatologic aspects. Dermatol Clin. 2002;20(3):387-408. [DOI] [PubMed] [Google Scholar]
- 12.Huard C, Gullà SV, Bennett DV, Coyle AJ, Vleugels RA, Greenberg SA. Correlation of cutaneous disease activity with type 1 interferon gene signature and interferon β in dermatomyositis. Br J Dermatol. 2017;176(5):1224-1230. [DOI] [PubMed] [Google Scholar]
- 13.Fiorentino DF, Chung LS, Christopher-Stine L, et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1γ. Arthritis Rheum. 2013;65(11):2954-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorentino DF, Kuo K, Chung L, Zaba L, Li S, Casciola-Rosen L. Distinctive cutaneous and systemic features associated with antitranscriptional intermediary factor-1γ antibodies in adults with dermatomyositis. J Am Acad Dermatol. 2015;72(3):449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalakas MC, Illa I, Dambrosia JM, et al. . A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med. 1993;329(27):1993-2000. [DOI] [PubMed] [Google Scholar]
- 16.Femia AN, Eastham AB, Lam C, Merola JF, Qureshi AA, Vleugels RA. Intravenous immunoglobulin for refractory cutaneous dermatomyositis: a retrospective analysis from an academic medical center. J Am Acad Dermatol. 2013;69(4):654-657. [DOI] [PubMed] [Google Scholar]
- 17.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol. 2010;22(4):507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stout-Delgado HW, Yang X, Walker WE, Tesar BM, Goldstein DR. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J Immunol. 2008;181(10):6747-6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong D, Kea B, Pesich R, et al. Interferon and biologic signatures in dermatomyositis skin: specificity and heterogeneity across diseases. PLoS One. 2012;7(1):e29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wenzel J, Schmidt R, Proelss J, Zahn S, Bieber T, Tüting T. Type I interferon-associated skin recruitment of CXCR3+ lymphocytes in dermatomyositis. Clin Exp Dermatol. 2006;31(4):576-582. [DOI] [PubMed] [Google Scholar]
- 21.Masuda H, Urushibara M, Kihara K. Successful treatment of dermatomyositis associated with adenocarcinoma of the prostate after radical prostatectomy. J Urol. 2003;169(3):1084. [DOI] [PubMed] [Google Scholar]
- 22.Luu X, Leonard S, Joseph KA. Dermatomyositis presenting as a paraneoplastic syndrome with resolution of symptoms following surgical management of underlying breast malignancy. J Surg Case Rep. 2015;2015(7):rjv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65(1):25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christen-Zaech S, Seshadri R, Sundberg J, Paller AS, Pachman LM. Persistent association of nailfold capillaroscopy changes and skin involvement over thirty-six months with duration of untreated disease in patients with juvenile dermatomyositis. Arthritis Rheum. 2008;58(2):571-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rider LG, Katz JD, Jones OY. Developments in the classification and treatment of the juvenile idiopathic inflammatory myopathies. Rheum Dis Clin North Am. 2013;39(4):877-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S, El-Hallak M, Dedeoglu F, Zurakowski D, Fuhlbrigge RC, Sundel RP. Complete and sustained remission of juvenile dermatomyositis resulting from aggressive treatment. Arthritis Rheum. 2009;60(6):1825-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]