This study compares functional speech, swallowing, and quality-of-life outcomes between patients with oropharangeal cancer who underwent transoral robotic surgery onlyand those who underwent transoral robotic surgery and received adjuvant radiotherapy or chemoradiotherapy.
Key Points
Question
How does adjuvant radiotherapy with and without concomitant chemotherapy affect functional speech and swallowing outcomes and quality-of-life metrics in patients with squamous cell carcinoma of the oropharynx who have undergone transoral robotic surgery?
Findings
This longitudinal cohort study of 74 patients undergoing transoral robotic surgery for the initial treatment of oropharyngeal squamous cell carcinoma found that immediately after surgery, there was a significant reduction in quality-of-life and functional speech and swallowing measures, with the transoral robotic surgery–only group having the greatest recovery with the least weight loss and best swallowing-related outcomes at long-term follow-up.
Meaning
Patients who undergo adjuvant treatment after transoral robotic surgery may have poorer long-term outcomes, with continued dysphagia more than 1 year after surgery.
Abstract
Importance
In recent years, transoral robotic surgery (TORS) has emerged as a useful treatment for oropharyngeal squamous cell carcinoma (OPSCC). In appropriately selected patients, the use of TORS may allow avoidance of adjuvant chemotherapy and/or radiotherapy, thereby avoiding the long-term adverse effects of these therapies.
Objective
To compare functional speech, swallowing, and quality-of-life outcomes longitudinally between those undergoing TORS only and those undergoing TORS and adjuvant radiotherapy (TORS+RT) or TORS and chemoradiotherapy (TORS+CRT).
Design, Setting, and Participants
This prospective, longitudinal cohort study performed from June 1, 2013, through November 31, 2015, included 74 patients undergoing TORS for initial treatment of OPSCC at a single tertiary academic hospital.
Main Outcomes and Measures
Data were collected at baseline, postoperatively (7-21 days), at short-term follow-up (6-12 months), and at long-term follow-up (>12 months). The quality-of-life metrics included the 10-item Eating Assessment Tool and the University of Michigan Head and Neck Quality of Life instrument. Data were also collected on tumor staging, surgical and adjuvant therapy details, patient comorbidities, tracheostomy and feeding tube use, and functional speech and swallowing status using the Performance Status Scale for Head and Neck Cancer Patients.
Results
Seventy-four patients were enrolled in the study (mean [SD] age, 61.39 [7.99] years; 68 [92%] male). Median long-term follow-up was 21 months (range, 12-36 months). The response rates were 86% (n = 64) postoperatively, 88% (n = 65) at short-term follow-up, and 86% (n = 64) at long-term follow-up. In all 3 groups, there was a significant worsening in pain and all swallowing-related measures postoperatively. There was subsequent improvement over time, with different trajectories observed across the 3 intervention groups. Postoperative dysphagia improved significantly more quickly in the TORS-only group. At long-term follow-up, weight loss differed between the TORS-only and TORS+RT groups (mean difference, −16.1; 97.5% CI, −29.8 to −2.4) and the TORS-only and TORS+CRT groups (mean difference, −14.6; 97.5% CI, −29.2 to 0) in a clinically meaningful way. In addition, the TORS-only group had significantly better scores than the TORS+CRT group on the Performance Status Scale–Eating in Public scale (mean difference, 21.8; 97.5% CI, 4.3-39.2) and Head and Neck Quality of Life–Eating scale (mean difference, 21.2; 97.5% CI, 4.0-38.3).
Conclusions and Relevance
Patients who underwent TORS+CRT demonstrated poorer long-term outcomes, with continued dysphagia more than 1 year after surgery. These findings support the investigation of adjuvant de-escalation therapies to reduce the long-term adverse effects of treatment.
Introduction
The incidence of human papillomavirus (HPV)–associated oropharyngeal squamous cell carcinoma (OPSCC) is increasing and accounts for most new oropharyngeal cancer cases. Historically, oropharyngeal cancer was treated with highly invasive surgery through a variety of open approaches followed by adjuvant radiotherapy (RT); this regimen was associated with significant complications and postoperative comorbidities. More than 20 years ago, OPSCC treatment shifted to primary chemoradiotherapy (CRT) after several studies demonstrated comparable oncologic outcomes and lower morbidity. However, long-term adverse effects of CRT, such as xerostomia and dysphagia, can be severe. After US Food and Drug Administration approval in 2009, transoral robotic surgery (TORS) emerged as an innovative surgical technique, allowing access to the oropharynx without the need for mandibulotomy. Studies have found oncologic outcomes comparable to those of primary CRT and surgery using a mandibulotomy approach. The use of TORS may allow for de-escalation of RT and/or chemotherapy, thereby potentially improving long-term functional outcomes. As more centers are offering TORS as a primary surgical modality, reports have emerged of long-term functional outcomes after TORS. However, these series are somewhat small, and there is a lack of standardized outcome metrics beyond long-term tracheostomy and gastrostomy dependence. We report the long-term, prospective functional outcomes and quality-of-life (QOL) outcomes after TORS using several instruments validated in the population with head and neck cancer.
Methods
From June 1, 2013, through November 31, 2015, a total of 74 consecutive patients diagnosed with OPSCC with planned TORS resection were prospectively enrolled in the study. Patients with known distant metastatic disease, prior CRT, and preexisting speech, swallowing, or cognitive deficits (eg, attributable to neurologic disease) were excluded. All cases were formally discussed at a head and neck cancer multidisciplinary tumor board meeting before finalizing treatment plans and were treated according to our standard of care. The study was approved by the institutional review board at Oregon Health and Science University (OHSU), Portland, and written informed consent was obtained. Data were deidentified after all data were collected.
Treatment
Patients underwent TORS partial pharyngectomy or hemiglossectomy with concomitant neck dissection. The da Vinci SI Surgical System (Intuitive Surgical Inc) was used for all procedures. Indications for adjuvant C RT included positive margins and extracapsular extension in metastatic lymph nodes in accordance with current National Comprehensive Cancer Network guidelines. Indications for adjuvant RT without concomitant chemotherapy included perineural invasion, lymphovascular invasion, or pathologic N2a or greater disease. Adjuvant RT was delivered using intensity-modulated RT in 2-Gy increments for a total dose of 60 to 66 Gy. Patients who were treated with adjuvant CRT received cisplatin therapy weekly or triweekly during the adjuvant RT.
Data Collection
Data collected preoperatively included age at surgery, sex, smoking history, Charlson Comorbidity Index, weight, and weight loss. Surgical information included extent of surgery and associated procedures, length of hospitalization, and complications. Pathologic data included tumor stage, nodal status, p16 positivity, and details of adjuvant therapy. At follow-up appointments, data were recorded on patient status, weight, recurrence, and tracheostomy and gastrostomy tube (G tube) use. Functional and QOL outcomes were measured at baseline (preoperatively), postoperatively (7-21 days after surgery), at short-term follow-up (6-12 months postoperatively), and at long-term follow-up (12-36 months postoperatively).
Functional Outcome Measures
The Performance Status Scale for Head and Neck Cancer Patients (PSS-HN) is a validated, clinician-administered instrument designed to quantify the functional status of patients with head and neck cancer and has been used in some prospective TORS studies. The measure contains 3 domains: normalcy of diet, speech, and the ability to eat in public. Each scale is rated from 0 to 100, with higher scores indicating better function.
QOL Outcomes
Two QOL instruments were used. The Eating Assessment Tool (EAT-10) is a 10-item general measure of swallowing difficulty that was previously validated in the population with head and neck cancer. Scores range from 0 to 40, with higher scores indicating more severe dysphagia. The Head and Neck Quality of Life (HNQOL) instrument was developed at the University of Michigan specifically for patients with head and neck cancer. It is a multidimensional tool that assesses eating, emotion, pain, and communication. The best possible score in each category is 100, indicating superior QOL.
Statistical Analysis
Statistical analysis was conducted using SPSS, version 21 (IBM Inc). Patients were divided into 3 groups based on the adjuvant therapy received postoperatively. Because of concerns about differences in the duration of long-term follow-up (12-36 months), the data were analyzed to determine whether long-term data should be analyzed as 1 or 2 time points. First, a Pearson correlation was performed to determine whether there was a significant association between length of time at long-term follow-up and any of the outcome measures of interest. No significant associations were found. Second, a split half analysis was performed for the long-term outcomes based on the median value of 21 months. No significant differences were found for any of the outcomes of interest; therefore, the long-term follow-up data were pooled as a single time point. The normality assumption was tested using the Shapiro-Wilk test. Of the variables examined, only age and weight loss met the normality assumption. Within-group comparisons were made using the Wilcoxon signed rank test to compare baseline and postoperative values. Between-group comparisons were made using analysis of variance for age and weight loss, the Kruskal-Wallis test for all other continuous variables, and χ2 tests for categorical variables. Effect size was calculated using the F test for means and φ and Cramer V for categorical variables. Pearson correlations were used to assess the association among measures. All tests were 2-tailed with an α level of .05. Because within- and between-groups analyses were performed for the long-term data, a Bonferroni correction was used (α = .05/2 = .025) to avoid the risk of a type I error.
Results
Patient, Tumor, and Treatment Characteristics
Seventy-four patients were enrolled in the study (mean [SD] age, 61.39 [7.99] years; 68 [92%] male). Baseline patient demographic data and tumor staging are provided in Table 1. One patient underwent tonsillectomy and TORS base-of-tongue resection for an unknown primary squamous cell carcinoma, although a primary lesion was not identified. Weight loss was reported in 5 patients (6.5%), with a mean (SD) weight loss of 10.5 (3.0) lb (4.8 [1.4] kg). Characteristics of the 3 treatment groups are given in Table 1. A robust effect size between treatment groups was noted for clinical nodal stage, pathologic nodal stage, and the presence of angiolymphatic invasion and extranodal extension. Across the 3 groups, pathologic N2 nodal stage prevalence was 20% (TORS only), 62% (TORS+RT), and 87% (TORS+CRT); angiolymphatic invasion prevalence was 20% (TORS only), 65% (TORS+RT), and 65% (TORS+CRT); and extranodal extension rates were 5% (TORS only), 13% (TORS+RT), and 74% (TORS+CRT). Pathologic staging of lymph nodes resulted in upstaging in 21 patients (28%) and downstaging in 8 patients (11%).
Table 1. Patient Demographics and Tumor Characteristics by Groupa.
| Characteristic | All (N = 74) |
TORS Only (n = 20) |
TORS + RT (n = 31) |
TORS + CRT (n = 23) |
Effect Size (95% CI)b |
|---|---|---|---|---|---|
| Age, mean (SD), y | 61.39 (7.99) | 62.10 (9.09) | 61.84 (7.95) | 60.17 (7.22) | −0.28 (−0.80 to 0.23) |
| CCI | 0.36 (−0.11 to 0.82) | ||||
| 0 | 50 (68) | 12 (60) | 21 (68) | 17 (74) | |
| 1 | 16 (22) | 5 (25) | 7 (23) | 4 (17) | |
| 2 | 5 (7) | 1 (5) | 2 (5) | 2 (9) | |
| 3 | 2 (2) | 1 (5) | 1 (3) | 0 | |
| 4 | 1 (1) | 1 (5) | 0 | 0 | |
| Male | 68 (92) | 18 (90) | 28 (90) | 22 (96) | 0.19 (−0.27 to −0.64) |
| Smoking status | 0.33(−0.13 to 0.79) | ||||
| Never | 31 (42) | 10 (50) | 13 (42) | 8 (35) | |
| Former (quit <6 mo ago) | 34 (46) | 8 (40) | 12 (39) | 14 (61) | |
| Current | 9 (12) | 2 (10) | 6 (19) | 1 (4) | |
| Tumor subsite | 0.36 (−0.11 to 0.81) | ||||
| Tonsil | 42 (57) | 13 (65) | 18 (58) | 11 (48) | |
| Base of tongue | 31 (42) | 6 (30) | 13 (42) | 12 (52) | |
| Unknown | 1 (1) | 1 (5) | 0 | 0 | |
| Clinical T stagec | 0.62 (0.15 to 1.10) | ||||
| T1 | 43 (58) | 14 (70) | 13 (42) | 16 (70) | |
| T2 | 30 (41) | 5 (25) | 18 (58) | 7 (30) | |
| Unknown | 1 (1) | 1 (5) | 0 | 0 | |
| Clinical N stagec | 0.81 (0.32 to 1.30) | ||||
| N0 | 13 (18) | 7 (35) | 6 (19) | 0 | |
| N1 | 24 (32) | 5 (25) | 12 (39) | 7 (30) | |
| N2a | 9 (12) | 5 (25) | 3 (10) | 1 (4) | |
| N2b | 27 (37) | 3 (15) | 10 (32) | 14 (62) | |
| N2c | 1 (1) | 0 | 0 | 1 (4) | |
| N3 | 0 | 0 | 0 | 0 | |
| Pathologic T stagec | 0.42 (−0.05 to 0.88) | ||||
| T1 | 39 (53) | 13 (65) | 13 (42) | 13 (57) | |
| T2 | 34 (46) | 6 (30) | 18 (58) | 10 (43) | |
| Tx | 1 (1) | 1 (5) | 0 (0) | 0 | |
| Pathologic N stagec | 1.03 (0.52 to 1.55) | ||||
| N0 | 10 (14) | 7 (35) | 3 (10) | 0 | |
| N1 | 18 (24) | 9 (45) | 7 (23) | 2 (9) | |
| N2a | 11 (15) | 1 (5) | 7 (23) | 3 (13) | |
| N2b | 30 (41) | 3 (15) | 12 (39) | 15 (65) | |
| N2c | 2 (3) | 0 | 0 | 2 (9) | |
| N3 | 3 (4) | 0 | 2 (6) | 1 (4) | |
| Pathologic features | |||||
| p16+ | 70 (95) | 19 (95) | 30 (97) | 21 (92) | 0.24 (−0.22 to 0.70) |
| Positive margin | 17 (23) | 2 (10) | 6 (19) | 9 (39) | 0.57 (0.09 to 1.04) |
| Perineural spread | 13 (18) | 3 (15) | 5 (16) | 5 (22) | 0.15 (−0.31 to 0.61) |
| Angiolymphatic invasion | 39 (53) | 4 (20) | 20 (65) | 15 (65) | 0.87 (0.37 to 1.36) |
| Extranodal extension | 22 (30) | 1 (5) | 4 (13) | 17 (74) | 1.72 (1.12 to 2.33) |
Abbreviations: CCI, Charlson Comorbidity Index; CRT, chemoradiation; RT, radiation; TORS, transoral robotic surgery.
Data are presented as number (percentage) of patients unless otherwise indicated.
Statistical analysis was performed comparing the 3 groups using analysis of variance for age and χ2 test for all other categorical variables. An F test was used to determine effect size for means, and φ and Cramer V were used for categorical variables
Tumor staging according to the American Joint Committee on Cancer, seventh edition.
Functional and QOL Outcomes
The EAT-10, PSS, and HNQOL scores were obtained in 64 patients (86%) postoperatively. Nine patients elected to follow up with the referring surgeon because of their geographic distance from OHSU. One patient was readmitted with a hematoma during that time; therefore, data were not collected for this interval. At short-term follow-up, the same metrics were recorded in 65 patients (88%) because 9 patients (12%) were unavailable for follow-up despite multiple attempts to contact them. Data were obtained at long-term follow-up in 64 patients (86%). There were no confirmed deaths at the time of follow-up more than 1 year after surgery.
Only 1 patient underwent tracheostomy. Tracheostomy occurred on postoperative day 13 during readmission for neck hematoma evacuation, and the patient underwent decannulation 1 month later (6 weeks postoperatively). All patients underwent nasogastric feeding tube (NG tube) placement at the time of surgery except 2 patients who underwent planned G tube placement because of expected adjuvant treatment and baseline dysphagia. Of the 72 patients who underwent NG tube placement, NG tube removal occurred before hospital discharge in 35 patients (49%) and at the first postoperative outpatient visit in 26 patients (36%). The mean (SD) length of hospital stay was 4.85 (1.93) days (range, 2-11 days). Of the remaining patients, 6 (8%) had their NG tube removed subsequently and 5 (7%) subsequently underwent G tube placement because of persistent dysphagia and the need for adjuvant treatment. The mean (SD) duration of NG tube placement was 9.84 (8.16) days (range, 1-36 days). Overall, 7 of 74 patients (9%) received a G tube. Two patients underwent planned G tube placement because of expected adjuvant treatment and baseline dysphagia. Three patients completed adjuvant treatment at our institution, whereas 4 patients completed adjuvant treatment at another medical center. Five patients (7%) were still using a G tube at short-term follow-up, all of whom received adjuvant CRT. Two of these patients were treated at our institution, and the other 3 patients were treated at another facility. At long-term follow-up, only 1 patient (1%) continued to require a G tube. This individual had declined to participate in dysphagia rehabilitation postoperatively and during CRT and remained G tube dependent at last follow-up.
Overall, 54 patients received adjuvant therapy in addition to TORS: 31 patients underwent adjuvant RT and 23 patients underwent adjuvant CRT. Radiotherapy was completed at OHSU in 16 of 54 patients (30%), with a mean (SD) radiation dose of 63 (4) Gy (range, 50-70 Gy). Radiotherapy was completed at another medical center in 38 of 54 patients (70%), with a mean (SD) radiation dose of 60 (7) Gy (range, 20-66 Gy).
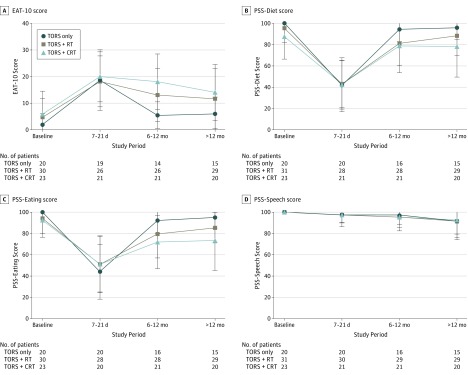
With use of an EAT-10 score of 3 or more as indicative of abnormal swallowing according to published norms, the prevalence of dysphagia over time across the 3 groups is depicted in Figure 1. At baseline, the prevalence of dysphagia was 29%, and this increased to 95% postoperatively, with a subsequent decrease to 75% at long-term follow-up. Significant group differences were found at only the 6- to 12-month follow-up (χ2 = 8.23; Cohen w = 0.37; 95% CI, 0.06-0.60), at which the TORS-only group had the lowest prevalence of dysphagia (60%) compared with the TORS+RT (72%) and TORS+CRT (90%) groups; this finding suggests a more rapid rate of recovery in the TOR-only group. Scores for the PSS-HN, EAT-10, and HNQOL were compared with baseline scores in the 3 treatment groups (Figure 2 and Figure 3). For the clinician-rated normalcy of diet score of the PSS-HN, all 3 groups had significant dysphagia postoperatively at 7 to 21 days, with subsequent improvement of scores at 6 to 12 months. For the PSS–Eating in Public score, similarly there was a significant decrease across all groups at 7 to 21 days, with subsequent recovery at 6 to 12 months. At long-term follow-up, the scores for all 3 groups were no longer significantly different from baseline on the PSS-Diet subscale. For the PSS–Eating in Public subscale scores, there was no significant difference in long-term scores in the TORS only or the TORS+RT groups, but scores in the TORS+CRT group remained significantly below baseline. At long-term follow-up, the percentage of individuals eating an unrestricted diet (PSS-Diet score of 100) was 62.5% overall and varied significantly by group as follows: 86% (TORS only), 62% (TORS+RT), and 45% (TORS+CRT). The percentage of individuals able to eat in public without restriction (PSS–Eating in Public score of 100) was 50% overall and similarly varied significantly by group (80% [TORS only], 45% [TORS+RT], and 35% [TORS+CRT]). For the PSS-Speech subscale, scores were relatively stable across all 3 time points, with mean scores slightly below baseline at long-term follow-up in all 3 groups. Most individuals (73%) achieved a score of 100, indicating that their speech was always understandable, and this did not differ significantly by group.
Figure 1. Prevalence of Dysphagia by Treatment Group Over Time.
Prevalence of dysphagia over time by group using a 10-item Eating Assessment Tool score of 3 or more as indicative of the presence of dysphagia. The postoperative period was 7 to 21 days after transoral robotic surgery (TORS); short-term follow-up, 6 to 12 months; and long-term follow-up, 12-36 months. CRT indicates chemoradiotherapy; RT, radiotherapy.
Figure 2. The 10-Item Eating Assessment Tool (EAT-10) and Performance Status Scale (PSS) Scores by Treatment Group Over Time.
Mean scores are shown, with error bars indicating SD. CRT indicates chemoradiotherapy; NA, not applicable; RT, radiotherapy; and TORS, transoral robotic surgery.
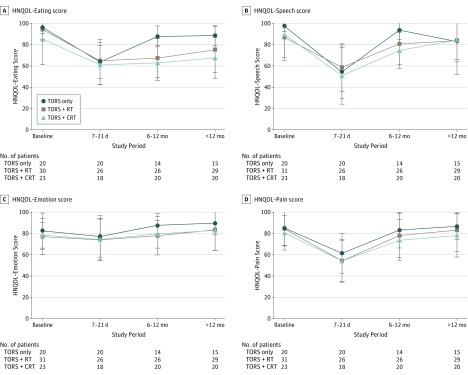
Figure 3. Head and Neck Quality of Life (HNQOL) Scores by Treatment Group Over Time.
Mean scores are shown, with error bars indicating SD. CRT indicates chemoradiotherapy; NA, not applicable; RT, radiotherapy; and TORS, transoral robotic surgery.
Comparison of the subjective scores on the EAT-10 showed that all 3 groups had a significant increase in dysphagia symptoms postoperatively compared with baseline levels and remained significantly more impaired than at baseline at 6 to 12 months. At long-term follow-up, the EAT-10 score in the TORS-only group no longer significantly differed from their baseline value, whereas there continued to be significantly greater levels of dysphagia compared with baseline in the TORS+RT and TORS+CRT groups. The HNQOL-Eating subscale similarly demonstrated significant short-term worsening postoperatively in all 3 groups compared with baseline scores. All 3 groups continued to have significantly worse scores on the HNQOL scale at the 6- to 12-month time point, but descriptively there appeared to be a more rapid recovery in the TORS-only group, which had the highest mean score at this point. All 3 groups remained significantly worse at long-term follow-up compared with baseline, although the TORS-only group continued to have the least reduction. The HNQOL-Speech scale demonstrated a significant decrease in scores postoperatively, and scores in the TORS+CRT group remained significantly below baseline scores at 6 to 12 months. At long-term follow-up, scores in all 3 groups were no longer significantly different from baseline for this measure. This pattern mirrored that of the HNQOL-Pain scale, in which pain was significantly worse postoperatively in all 3 groups but not thereafter. Finally, HNQOL-Emotion scores were relatively stable over time, showing no significant difference from baseline for any treatment group. Correlation among these various QOL outcome measures is detailed in the eTable in the Supplement.
Long-term follow-up scores were compared by group (Table 2). Median time postoperatively at follow-up was 21 months (range, 12-36 months) and did not differ significantly by group. No significant correlation was found between length of time at long-term follow-up and any of the outcome measures (as discussed in the Statistical Analysis subsection of the Methods section), suggesting that outcome measures may have stabilized by this time. This finding is consistent with previous longitudinal research in the population with head and neck cancer that has found few significant improvements in status after 1 year. Between-group comparison confirmed that there were significant between-group differences for the PSS-Eating and HNQOL-Eating subscales. There was a clinically meaningful difference in weight loss at long-term follow-up, and post hoc testing confirmed that the TORS-only group had less weight loss than did the TORS+RT group (mean difference, −16.10; 97.5% CI, −29.79 to −2.41). The difference in weight loss between the TORS-only and the TORS+CRT group was also clinically meaningful (mean difference, −14.58; 97.5% CI, −29.19 to 0.03). The lower bounds of the CIs for both comparisons suggest that the weight loss difference with the use of adjuvant therapy compared with TORS only could be as great as 30 lb. In addition, the TORS-only group had significantly better scores than the TORS+CRT group on the PSS–Eating in Public scale and on the HNQOL-Eating subscale.
Table 2. Comparison of Long-term Outcomes by Groupa.
| Outcome Measure | All (N = 64) |
TORS Only (n = 15) |
TORS + RT (n = 29) |
TORS + CRT (n = 20) |
Effect Size, η2b | Post Hoc Comparisons, Mean Difference (95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| TORS Only vs TORS + RT | TORS Only vs TORS + CRT | TORS + RT vs TORS + CRT | ||||||
| Time postoperatively, mo | 21 (12 to 36) | 22.50 (12 to 36) | 20 (13 to 36) | 22 (14 to 33) | .004 | 1.16 (−4.76 to 7.09) | −0.01 (−6.35 to 6.34) | −1.16 (−6.46 to 4.13) |
| Weight loss, mean (SD), lbc | 19.70 (17.03) | 7.92 (9.24) | 24.02 (16.69) | 22.50 (18.51) | .153d | −16.10 (−29.79 to −2.41)e | −14.58 (−29.19 to .03) | 1.52 (−11.01 to 14.04) |
| PSS–Normalcy of Diet | 100 (10 to 100) | 100 (60 to 100) | 100 (50 to 100) | 90 (10 to 100) | .102 | 7.72 (−10.00 to 25.45) | 18.00 (−1.30 to 37.03) | 10.28 (−5.92 to 26.47) |
| PSS–Eating in Public | 87.50 (0 to 100) | 100 (75 to 100) | 75 (50 to 100) | 75 (0 to 100) | .143f | 9.66 (−6.58 to 25.89) | 21.75 (4.32 to 39.18)d | 12.09 (−2.74 to 26.93) |
| PSS–Speech Understandability | 100 (25 to 100) | 100 (75 to 100) | 100 (50 to 100) | 100 (25 to 100) | .009 | 0.29 (−13.12 to 13.70) | −0.83 (−15.24 to 13.57) | −1.12 (−13.38 to 11.13) |
| EAT-10 | 7 (0 to 36) | 4.50 (0 to 22) | 9.50 (0 to 36) | 9.50 (0 to 32) | .080 | −5.72 (−14.34 to 2.89) | −8.05 (−17.30 to 1.20) | −2.33 (−10.20 to 5.55) |
| HNQOL-Eating | 83.33 (25 to 100) | 89.59 (66.67 to 100) | 83.33 (25 to 100) | 68.34 (33.33 to 95.83) | .165f | 13.18 (−2.76 to 29.12) | 21.15 (4.04 to 38.27)e | 7.97 (−6.59 to 22.53) |
| HNQOL-Speech | 93.75 (0 to 100) | 100 (0 to 100) | 93.75 (25 to 100) | 84.38 (37.50 to 100) | .017 | −1.04 (−20.11 to 18.67) | 0.72 (−21.86 to 19.78) | 1.04 (−18.04 to 17.40) |
| HNQOL-Emotion | 91.67 (33.33 to 100) | 93.75 (66.67 to 100) | 91.67 (37.50 to 100) | 87.50 (33.33 to 100) | .011 | 6.12 (−8.73 to 20.97) | 6.81 (−9.14 to 22.76) | 0.69 (−12.88 to 14.26) |
| HNQOL-Pain | 87.50 (25 to 100) | 90.63 (62.50 to 100) | 90.63 (25 to 100) | 84.38 (37.50 to 100) | .023 | 3.69 (−12.16 to 19.55) | 8.54 (−8.48 to 25.57) | 4.85 (−9.64 to 19.34) |
Abbreviations: CRT, chemoradiotherapy; EAT-10, 10-Item Eating Assessment Tool; HNQOL, Head and Neck Quality of Life; PSS, Performance Status Scale; RT, radiotherapy; TORS, transoral robotic surgery.
Data are presented as mean score (95% CI) unless otherwise indicated.
F tests for determining effect sizes for means.
To convert to kilograms, multiply by 0.45.
Significant difference using analysis of variance with α adjustment for multiple comparisons.
Significant between-group difference.
Significant difference using Kruskal-Wallis test with α adjustment for multiple comparisons.
Discussion
Our study describes one of the largest prospective studies of patients undergoing TORS with outcomes stratified based on treatment modality to assess the association of the treatment with long-term QOL outcomes. To date, a wide variety of measures have been used to track outcomes after TORS, including the MD Anderson Dysphagia Inventory, the University of Washington Quality of Life Questionnaire, and the Head and Neck Cancer Inventory. To our knowledge, the present study is the first to use the HNQOL and EAT-10. The HNQOL-Eating subscale similarly demonstrated significant short-term worsening postoperatively, with a more rapid and complete recovery in the TORS-only group, but scores in all 3 groups remained significantly below baseline at long-term follow-up. The HNQOL–Speech and Pain subscales also demonstrated a significant decrease in scores postoperatively, followed by a subsequent recovery to levels no longer significantly different from baseline. Finally, the HNQOL-Emotion scores were relatively stable over time. These findings are fairly consistent with those of Choby et al, who reported statistically significant improvement in pain, chewing, and swallowing scores on the University of Washington Quality of Life Questionnaire during the first year after treatment and remaining fairly stable after that time. Dziegielewski et al reported that poorer QOL scores were associated with RT, CRT, and older age on the Head and Neck Cancer Inventory.
We found strong correlations between the EAT-10 and the HNQOL-Eating subscale and with the clinician-rated normalcy of diet and eating in public subscales of the PSS-HN (eTable in the Supplement), which suggests its validity for use in this population. Similar associations have been previously reported between the MD Anderson Dysphagia Inventory and the PSS-HN. At long-term follow-up, however, the TORS-only group had EAT-10 scores that were not significantly different from baseline despite scores remaining significantly below baseline on the HNQOL-Eating subscale. Some of the head and neck cancer–specific items on the latter measure might explain this discrepancy, and EAT-10, as a generic measure, may not be as sensitive to some of these changes.
In contrast to these metrics, few studies to date have reported longitudinal functional outcomes using the PSS-HN scale. Leonhardt et al compared longitudinal outcomes in a cohort of 38 patients who underwent TORS with or without adjuvant treatment using the PSS-HN. For the PSS-Eating and PSS-Diet subscales, they found significant decreases at 6 months after treatment but not at 12 months. They also found significant decreases in PSS-Speech scores at 6 and 12 months. When they examined outcomes based on the extent of treatment, the authors concluded that adjuvant CRT was association with significantly lower scores on the PSS-Eating and PSS-Diet subscales at 6 and 12 months and incomplete long-term recovery. Genden et al published a prospective observational study that compared outcomes between 30 patients with OPSCC treated with TORS and adjuvant RT and 26 treated with concurrent CRT. The scores in the TORS group returned to baseline on all scales of the PSS-HN within 9 months after surgery, but the CRT group continued to have PSS-Diet scores significantly below baseline at 12 months after treatment. In our study, normalcy of diet and eating in public scores of the PSS-HN in all 3 groups demonstrated significant dysphagia postoperatively with subsequent recovery, but both groups that had received adjuvant therapy had scores significantly below baseline on the PSS-Eating subscale.
The known toxic effects associated with definitive CRT combined with the increasing incidence of HPV-related tumors have led us to rethink the treatment of oropharyngeal cancer. A previous study found that although acute toxic effects are equivalent between HPV-positive and HPV-negative patients treated with CRT, the development of late toxic effects is decreased in HPV-positive patients. Patients with HPV-positive OPSCC also have improved survival compared with HPV-negative patients regardless of treatment modality. This finding has led a movement toward so-called deescalation therapy for the treatment of patients with HPV-positive OPSCC, and multiple trials are under way, most notably the Eastern Cooperative Oncology Group Trial 3311. The advantage of TORS for the treatment of OPSCC is that the total dose of adjuvant RT, if necessary, can be decreased and TORS can obviate the need for adjuvant chemotherapy. The feasibility of TORS from an oncologic standpoint has been well established, and the focus has shifted to long-term functional outcomes.
Tracheostomy dependence and feeding tube dependence have been the most commonly reported functional outcomes after TORS. The use of short-term NG tubes varies widely by disease stage and institutional practice, and placement rates of 3% to 100% have been reported, with mean durations ranging from 2 to 13 days. According to one review, short-term G tube rates range from 18% to 39%, typically during adjuvant treatment, with long-term G tube dependency rates of 0% to 7%. One study found that older age, more extensive TORS, and higher pathologic T stage were associated with greater risk of G tube dependency. Postoperative chemotherapy was found to be associated with increased risk of G tube dependency for more than 3 months in another series. Reported tracheostomy rates have ranged from 0% to 31%, with permanent tracheostomy dependence occurring rarely (0.5%). Compared with previous reports, the mean duration of NG tube placement (9.84 days) in our study was toward the upper end of previously reported ranges. Although nearly 50% of patients in our study had their NG tube removed before discharge, most of the remainder of the patients had their NG tubes removed at their first follow-up visit 7 to 10 days after discharge. However, this time lag to follow-up necessarily increased the mean time for NG tube use. The rates of short-term G tube placement (9%), long-term G tube dependency (1%), short-term tracheostomy placement (1%), and long-term tracheostomy dependency (0%) in our study are at the lower limits of previously reported ranges. Our data do not necessarily speak to NG tube or G tube management; in particular, NG tubes are only present for a couple of weeks at most, whereas our data are focused on longer-term outcomes. However, these data are useful for patient counseling, particularly when primarily deciding on treatment modality for oropharynx carcinoma.
In accordance with the conclusions of Leonhardt and colleagues, the development of new treatment techniques to manage OPSCC requires an understanding of the potential effect on not only oncologic but also functional and QOL outcomes. The improvement in disease control and survival through the use of concurrent CRT has also been associated with an increased risk of late complications, including dysphagia. As new protocols are developed, it is imperative that longitudinal functional and QOL outcomes be assessed to avoid unnecessary, life-limiting alterations in voice, speech, respiration, swallowing, and cosmesis. Unlike Sinclair et al, we did not find evidence that there was improvement in outcomes after the 12-month follow-up, suggesting that outcomes were stable after this time.
This study describes functional outcomes based on treatment modality and does not directly address pathologic upstaging or downstaging based on current staging guidelines. However, the functional data presented in this study highlight the importance of patient selection and decision making. Kim et al reported the utility of up-front robotic surgery to tailor adjuvant therapy. Up-front TORS was associated with deintensified adjuvant therapy; 76% of patients with stage I/II and 46% of patients with stage III/IV disease did not receive CRT. Conversely, pathologic staging resulted in 33% of patients receiving adjuvant CRT who would have received RT only based on current clinical staging.
Furthermore, our findings suggest that individuals who undergo triple-modality intervention with surgery and CRT are at increased risk of significantly poorer long-term function. Triple-modality intervention in this patient population should be avoided; currently, the primary driver of adjuvant CRT in these patients is the presence of extranodal extension on pathologic assessment of cervical lymph nodes. It can be challenging to detect extranodal extension preoperatively; however, a study by Geltzeiler et al determined that the presence of 3 or more radiologically suspicious lymph nodes on computed tomography has a 91% positive predictive value for any histologic evidence of extranodal extension. In the short term, for patients with a high likelihood of requiring adjuvant CRT, TORS should be avoided and definitive CRT should be administered. In the coming years, extranodal extension may no longer be an absolute indication for adjuvant CRT. Recent evidence suggests that in HPV-positive OPSCC, extranodal extension is not a significant prognostic indicator, and these data have been incorporated in the upcoming eighth edition of the American Joint Committee on Cancer staging manual. Most important, results from the Eastern Cooperative Oncology Trial 3311 and Abnormal Doppler Enteral Prescription trials will better define which patients with HPV-positive OPSCC truly need adjuvant CRT. Our data support the drive toward deintensification of treatment in HPV-positive OPSCC and highlight the importance of including robust QOL measures during a long period of follow-up in future clinical trials.
Limitations
This study has several limitations. First, the time points for follow-up were relatively broad. Although duration of follow-up did not differ significantly between groups, this might have affected our ability to assess change over time, particularly at long-term follow-up. Second, similar to many other large medical centers, OHSU draws patients from a wide geographic area. Consequently, some patients received adjuvant therapy at outside facilities, which may have influenced total radiation dose and practices regarding G tube placement. Only 2 patients had G tubes placed prophylactically by the surgical team for advanced tumor stage or significant preoperative dysphagia; the remaining 5 G tubes were placed at the discretion of the treating radiation oncologist. Although reactive G tube placement is the standard at our institution, many outside radiation oncologists place G tubes prophylactically, which would increase the overall G tube rate. However, our long-term G tube rate was low (1%), occurring in a patient who received adjuvant CRT and who declined to participate in dysphagia rehabilitation. Third, patients who lived closer to OHSU may have been able to return more frequently for knowledgeable care and rehabilitation than those living in more remote areas, which may have affected outcomes. Fourth, the 3 treatment groups differed, as expected, in the nature and extent of their disease; consequently, it is not possible to differentiate the relative effect of treatment from the effects of the disease itself. There may also have been other a priori differences between the groups that were not accounted for in our data collection, and our study was not sufficiently powered to control for other extraneous variables that could have affected outcomes.
Conclusions
In patients with OPSCC, treatment with TORS only was associated with improved long-term functional outcomes in swallowing and speech, with QOL metrics returning to near baseline. Patients who received adjuvant treatment did not recover as quickly, and those who underwent CRT in addition to TORS had the greatest risk for poor long-term outcomes.
eTable. Correlations Among Outcome Measures
References
- 1.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62(2):118-128. doi: 10.3322/caac.20141 [DOI] [PubMed] [Google Scholar]
- 2.Davidson J, Freeman J, Birt D. Mandibulotomy in the irradiated patient. Arch Otolaryngol Head Neck Surg. 1989;115(4):497-499. doi: 10.1001/archotol.1989.01860280095024 [DOI] [PubMed] [Google Scholar]
- 3.Shaha AR. Mandibulotomy and mandibulectomy in difficult tumors of the base of the tongue and oropharynx. Semin Surg Oncol. 1991;7(1):25-30. [DOI] [PubMed] [Google Scholar]
- 4.O’Hara J, MacKenzie K. Surgical versus non-surgical management of early stage oropharyngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2011;268(3):437-442. doi: 10.1007/s00405-010-1362-4 [DOI] [PubMed] [Google Scholar]
- 5.Pignon J-P, le Maître A, Maillard E, Bourhis J; MACH-NC Collaborative Group . Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4-14. doi: 10.1016/j.radonc.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 6.Monnier Y, Simon C. Surgery versus radiotherapy for early oropharyngeal tumors: a never-ending debate. Curr Treat Options Oncol. 2015;16(9):42. doi: 10.1007/s11864-015-0362-4 [DOI] [PubMed] [Google Scholar]
- 7.Mowry SE, Ho A, Lotempio MM, Sadeghi A, Blackwell KE, Wang MB. Quality of life in advanced oropharyngeal carcinoma after chemoradiation versus surgery and radiation. Laryngoscope. 2006;116(9):1589-1593. doi: 10.1097/01.mlg.0000233244.18901.44 [DOI] [PubMed] [Google Scholar]
- 8.Peponi E, Glanzmann C, Willi B, Huber G, Studer G. Dysphagia in head and neck cancer patients following intensity modulated radiotherapy (IMRT). Radiat Oncol. 2011;6:1-8. doi: 10.1186/1748-717X-6-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstein GS, O’Malley BW Jr, Cohen MA, Quon H. Transoral robotic surgery for advanced oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2010;136(11):1079-1085. doi: 10.1001/archoto.2010.191 [DOI] [PubMed] [Google Scholar]
- 10.Ford SE, Brandwein-Gensler M, Carroll WR, Rosenthal EL, Magnuson JS. Transoral robotic versus open surgical approaches to oropharyngeal squamous cell carcinoma by human papillomavirus status. Otolaryngol Head Neck Surg. 2014;151(4):606-611. doi: 10.1177/0194599814542939 [DOI] [PubMed] [Google Scholar]
- 11.Dziegielewski PT, Teknos TN, Durmus K, et al. Transoral robotic surgery for oropharyngeal cancer: long-term quality of life and functional outcomes. JAMA Otolaryngol Head Neck Surg. 2013;139(11):1099-1108. doi: 10.1001/jamaoto.2013.2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iseli TA, Kulbersh BD, Iseli CE, Carroll WR, Rosenthal EL, Magnuson JS. Functional outcomes after transoral robotic surgery for head and neck cancer. Otolaryngol Head Neck Surg. 2009;141(2):166-171. doi: 10.1016/j.otohns.2009.05.014 [DOI] [PubMed] [Google Scholar]
- 13.Lee SY, Park YM, Byeon HK, Choi EC, Kim S-H. Comparison of oncologic and functional outcomes after transoral robotic lateral oropharyngectomy versus conventional surgery for T1 to T3 tonsillar cancer. Head Neck. 2014;36(8):1138-1145. doi: 10.1002/hed.23424 [DOI] [PubMed] [Google Scholar]
- 14.Leonhardt FD, Quon H, Abrahão M, O’Malley BW Jr, Weinstein GS. Transoral robotic surgery for oropharyngeal carcinoma and its impact on patient-reported quality of life and function. Head Neck. 2012;34(2):146-154. doi: 10.1002/hed [DOI] [PubMed] [Google Scholar]
- 15.Hutcheson KA, Holsinger FC, Kupferman ME, Lewin JS. Functional outcomes after TORS for oropharyngeal cancer: a systematic review. Eur Arch Otorhinolaryngol. 2015;272(2):463-471. doi: 10.1007/s00405-014-2985-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper JS, Pajak TF, Forastiere AA, et al. ; Radiation Therapy Oncology Group 9501/Intergroup . Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937-1944. [DOI] [PubMed] [Google Scholar]
- 17.Terrell JE, Nanavati KA, Esclamado RM, Bishop JK, Bradford CR, Wolf GT. Head and neck cancer-specific quality of life: instrument validation. Arch Otolaryngol Head Neck Surg. 1997;123(10):1125-1132. [DOI] [PubMed] [Google Scholar]
- 18.Genden EM, Kotz T, Tong CCL, et al. Transoral robotic resection and reconstruction for head and neck cancer. Laryngoscope. 2011;121(8):1668-1674. doi: 10.1002/lary.21845 [DOI] [PubMed] [Google Scholar]
- 19.Belafsky PC, Mouadeb DA, Rees CJ, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919-924. [DOI] [PubMed] [Google Scholar]
- 20.Ojo B, Genden EM, Teng MS, Milbury K, Misiukiewicz KJ, Badr H. A systematic review of head and neck cancer quality of life assessment instruments. Oral Oncol. 2012;48(10):923-937. doi: 10.1016/j.oraloncology.2012.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edge SB; American Joint Committee on Cancer . AJCC Cancer Staging Manual. 7th ed New York, NY: Springer International Publishing; 2011. [Google Scholar]
- 22.Weymuller EA, Yueh B, Deleyiannis FW, Kuntz AL, Alsarraf R, Coltrera MD. Quality of life in patients with head and neck cancer: lessons learned from 549 prospectively evaluated patients. Arch Otolaryngol Head Neck Surg. 2000;126(3):329-335. [DOI] [PubMed] [Google Scholar]
- 23.Hammerlid E, Silander E, Hörnestam L, Sullivan M. Health-related quality of life three years after diagnosis of head and neck cancer: a longitudinal study. Head Neck. 2001;23(2):113-125. [DOI] [PubMed] [Google Scholar]
- 24.Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127(7):870-876. [PubMed] [Google Scholar]
- 25.Sinclair CF, McColloch NL, Carroll WR, Rosenthal EL, Desmond RA, Magnuson JS. Patient-perceived and objective functional outcomes following transoral robotic surgery for early oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2011;137(11):1112-1116. doi: 10.1001/archoto.2011.172 [DOI] [PubMed] [Google Scholar]
- 26.More YI, Tsue TT, Girod DA, et al. Functional swallowing outcomes following transoral robotic surgery vs primary chemoradiotherapy in patients with advanced-stage oropharynx and supraglottis cancers. JAMA Otolaryngol Head Neck Surg. 2013;139(1):43-48. doi: 10.1001/jamaoto.2013.1074 [DOI] [PubMed] [Google Scholar]
- 27.Choby GW, Kim J, Ling DC, et al. Transoral robotic surgery alone for oropharyngeal cancer: quality-of-life outcomes. JAMA Otolaryngol Head Neck Surg. 2015;141(6):499-504. doi: 10.1001/jamaoto.2015.0347 [DOI] [PubMed] [Google Scholar]
- 28.Khan MK, Patterson J, Owen S, Rees S, Gamberini L, Paleri V; North of England Cancer Network Audit group . Comparing the Performance Status Scale and MD Anderson Dysphagia Inventory as swallowing outcome measures in head and neck cancer: a prospective cohort study. Clin Otolaryngol. 2015;40(4):321-326. doi: 10.1111/coa.12369 [DOI] [PubMed] [Google Scholar]
- 29.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26(21):3582-3589. doi: 10.1200/JCO.2007.14.8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivelli TG, Mak MP, Martins RE, da Costa e Silva VT, de Castro G Jr. Cisplatin based chemoradiation late toxicities in head and neck squamous cell carcinoma patients. Discov Med. 2015;20(108):57-66. [PubMed] [Google Scholar]
- 31.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294-4301. doi: 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bledsoe TJ, Noble AR, Hunter GK, et al. Oropharyngeal squamous cell carcinoma with known human papillomavirus status treated with definitive chemoradiotherapy: patterns of failure and toxicity outcomes. Radiat Oncol. 2013;8:174-181. doi: 10.1186/1748-717X-8-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261-269. doi: 10.1093/jnci/djn011 [DOI] [PubMed] [Google Scholar]
- 34.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27(12):1992-1998. doi: 10.1200/JCO.2008.20.2853 [DOI] [PubMed] [Google Scholar]
- 35.Chin R-I, Spencer CR, DeWees T, et al. Reevaluation of postoperative radiation dose in the management of human papillomavirus-positive oropharyngeal cancer. Head Neck. 2016;38(11):1643-1649. doi: 10.1002/hed.24486 [DOI] [PubMed] [Google Scholar]
- 36.Masterson L, Moualed D, Liu ZW, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: a systematic review and meta-analysis of current clinical trials. Eur J Cancer. 2014;50(15):2636-2648. doi: 10.1016/j.ejca.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 37.Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94(11):2967-2980. doi: 10.1002/cncr.10567 [DOI] [PubMed] [Google Scholar]
- 38.Van Abel KM, Moore EJ, Carlson ML, et al. Transoral robotic surgery using the thulium:YAG laser: a prospective study. Arch Otolaryngol Head Neck Surg. 2012;138(2):158-166. doi: 10.1001/archoto.2011.1199 [DOI] [PubMed] [Google Scholar]
- 39.Moore EJ, Olsen KD, Kasperbauer JL. Transoral robotic surgery for oropharyngeal squamous cell carcinoma: a prospective study of feasibility and functional outcomes. Laryngoscope. 2009;119(11):2156-2164. doi: 10.1002/lary.20647 [DOI] [PubMed] [Google Scholar]
- 40.Gildener-Leapman N, Kim J, Abberbock S, et al. Utility of up-front transoral robotic surgery in tailoring adjuvant therapy. Head Neck. 2016;38(8):1201-1207. doi: 10.1002/hed.24390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maxwell JH, Rath TJ, Byrd JK, et al. Accuracy of computed tomography to predict extracapsular spread in p16-positive squamous cell carcinoma. Laryngoscope. 2015;125(7):1613-1618. doi: 10.1002/lary.25140 [DOI] [PubMed] [Google Scholar]
- 42.Chai RL, Rath TJ, Johnson JT, et al. Accuracy of computed tomography in the prediction of extracapsular spread of lymph node metastases in squamous cell carcinoma of the head and neck. JAMA Otolaryngol Head Neck Surg. 2013;139(11):1187-1194. doi: 10.1001/jamaoto.2013.4491 [DOI] [PubMed] [Google Scholar]
- 43.Geltzeiler M, Clayburgh D, Gleysteen J, et al. Predictors of extracapsular extension in HPV-associated oropharyngeal cancer treated surgically. Oral Oncol. 2017;65:89-93. doi: 10.1016/j.oraloncology.2016.12.025 [DOI] [PubMed] [Google Scholar]
- 44.Maxwell JH, Ferris RL, Gooding W, et al. Extracapsular spread in head and neck carcinoma: impact of site and human papillomavirus status. Cancer. 2013;119(18):3302-3308. doi: 10.1002/cncr.28169 [DOI] [PubMed] [Google Scholar]
- 45.Iyer NG, Dogan S, Palmer F, et al. Detailed analysis of clinicopathologic factors demonstrate distinct difference in outcome and prognostic factors between surgically treated HPV-positive and negative oropharyngeal cancer. Ann Surg Oncol. 2015;22(13):4411-4421. doi: 10.1245/s10434-015-4525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amin MB, Edge SB; American Joint Committee on Cancer . AJCC Cancer Staging Manual. 8th ed New York, NY: Springer International Publishing; 2017. [Google Scholar]
- 47.clinicaltrials.gov Post operative Adjuvant Therapy De-intensification Trial for Human Papillomavirus-Related, p16+ Oropharynx Cancer (ADEPT). https://clinicaltrials.gov/ct2/show/NCT01687413. Accessed September 7, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Correlations Among Outcome Measures