Key Points
Question
Can a systematic weaning process (SWP) be safely and effectively implemented to gradually wean children off of thickened fluids?
Findings
In a case series of 50 pediatric patients with aspiration who underwent the SWP, most patients tolerated a reduction in thickeners and a full wean to a thin-fluid diet. Children required a mean of 0.7 videofluoroscopic swallow studies from the time of presentation to completion of the SWP.
Meaning
The SWP uses incremental steps to gradually reduce fluid thickness based on a patient’s clinical response, which may allow the swallowing mechanism to adapt to consistent change over time.
This case series describes the experience of a systematic weaning process in children who received thickened liquids due to oropharyngeal dysphagia and identified risk of aspiration.
Abstract
Importance
Thickening of fluids is a common strategy for feeding patients with oropharyngeal dysphagia but has known risks and should be stopped once it is safe to do so. Weaning children from thickened fluids safely can be challenging, and novel methods are required.
Objective
To describe the use of a systematic weaning process (SWP) for children who received thickened liquids owing to oropharyngeal dysphagia and identified risk of aspiration.
Design, Setting, and Participants
Retrospective case series (2010 to 2015) at a tertiary care center of 50 children with documented aspiration by clinical swallowing assessment, airway evaluation, and videofluoroscopic swallow study with at least 4 months of follow-up. All patients were initially receiving thickened fluids. A 10% reduction in thickness was made every 2 weeks based on clinical symptoms. Caregivers progressed to the next incremental level if there were no signs or symptoms of aspiration.
Main Outcomes and Measures
Number of patients weaned to a thin-fluid diet.
Results
Of 50 children (32 [64%] male; median [interquartile range] age, 0.7 [1.0] y at presentation and 1.8 [1.3] y at start of wean) using the SWP, 44 (88%) were able to reduce the amount of thickener used. A successful wean from thickened fluids to thin fluids was completed in 39 (78%). The mean (SD) duration of a successful wean was 0.9 (0.6) years. Five patients tolerated a reduction in thickener but not a full wean to thin fluids. For 6 patients, weaning failed and they continued to receive thickened fluids. Of those whose weaning failed, 2 patients developed pneumonia. Of the 39 successfully weaned patients, 14 (36%) experienced a temporary stall but eventually tolerated thin fluids. Only 2 (5%) developed pneumonia while all other successfully weaned patients (n = 37 [95%]) did not experience any substantial respiratory issues. Overall, 46 (92%) of children required 2 or fewer videofluoroscopic swallow study evaluations.
Conclusions and Relevance
Patients with oropharyngeal dysphagia and aspiration should be gradually weaned off of thickened fluids. The SWP uses small incremental steps to gradually reduce the amount of thickener. Using this method, most children tolerated a reduction in thickeners and a thin-fluid diet. The SWP presents a safe and effective way of gradually returning children to a more normal diet.
Introduction
Swallowing is a complex neuromuscular process that requires the development and coordination of voluntary and involuntary processes. Impairment in any component may lead to dysphagia and aspiration. The incidence of pediatric patients with aspiration as a result of oropharyngeal dysphagia is increasing. This is likely due to improvements in perinatal care, particularly in infants with premature birth, low birth weight, and medically complex conditions. Aspiration may present with cough, wheeze, apneas, or respiratory issues. Alternatively, aspiration may be “silent,” without any obvious signs or symptoms. Aspiration of fluids into the lungs can result in pneumonia, bronchiectasis, and respiratory compromise.
Whereas children with severe dysphagia may require alternative feeding methods such as nasogastric or gastrostomy tube feeding, fluid thickeners may be used to facilitate enteral nutrition in less severe cases. Thickening fluids improves swallowing by increasing oral transit time, normalizes swallowing patterns during respiration, and enhances sensory input. In this capacity, use of fluid thickeners serves as a useful strategy to reduce the risk of aspiration while physiological processes mature or definitive management is carried out. Common thickening agents include commercial thickeners, such as those made from modified cornstarch, gum-based products (eg, xanthan gum), or food products (eg, infant cereals). The National Dysphagia Diet taskforce describes viscosity ranges for standardized clinical thickness levels: spoon-thick (>1750 cP), honey-like (351-1750 cP), nectar-like (51-350 cP), and thin (1-50 cP).(pp1-20) Although this strategy may reduce the risk of aspiration and laryngeal penetration, use of thickeners should not be thought of as an end point. Using thickeners over a prolonged period can alter physiologic swallowing patterns, negatively affect quality of life, and pose a financial burden to families. Moreover, thickened fluids may increase the amount of postswallow residue in the pharynx and put the patient at risk for postswallow aspiration. For these reasons, children should be transitioned to nonthickened diets as soon as it is safe to do so.
At our institution and others, patients were traditionally prescribed the safest appropriate thickness of fluids following videofluoroscopic swallow study (VFSS). Patients would continue to receive fluids of a specified thickness until repeated VFSS demonstrated safe swallowing at the next gross category. As such, children with dysphagia often require frequent VFSS, which involves exposure to ionizing radiation. Moreover, “step-down” methods that progress between standardized viscosities require children to tolerate large changes in their diet. For example, a child may tolerate honey-thick fluids easily but nectar-thick consistencies still pose a risk for aspiration. In such instances, progress is halted. Recent research efforts have demonstrated that patients are able to discriminate between small steps in thickness, which supports using unconventional intervening thicknesses during a wean.
In 2010, a systematic weaning process (SWP) in which thickeners are systematically reduced in small, 10% increments to allow a more gradual, controlled adjustment process was proposed by the senior author (R.R.). Whereas the concept of weaning is not new and has been described for transitioning from enteral tube feeding to oral feeding, to our knowledge, weaning off thickened fluids for oral feeding has not yet been described. The objective of this study was to describe the impact of the SWP on infants and children who received thickened liquids due to oropharyngeal dysphagia and identified risk of aspiration.
Methods
A retrospective review was performed of patients evaluated at our multidisciplinary Center for Airway Disorders (CAD) who had undergone the SWP between 2010 and 2015. The study was approved by the Boston Children’s Hospital Institutional Review Board. Informed consent was waived due to the retrospective nature of this study. Patients were included if they had documentation of aspiration with liquids (thin or nectar-thick) based on initial VFSS results, a multidisciplinary evaluation by an otolaryngologist and speech language pathologist including endoscopic evaluation of the airway, and a lack of interval improvement from initial VFSS to repeated VFSS. All patients received thickened liquids using infant rice cereal or SimplyThick (SimplyThick, LLC). Patients were excluded from the study if they had a contraindication to the SWP, such as unstable respiratory status, severe aspiration requiring tube feeding, or unreliable follow-up. Patients were excluded if they did not have at least 4 months of follow-up from the time they concluded the SWP.
The SWP represents a method for transitioning from thickened liquids to a normal fluid diet. Patients and caregivers were given a written SWP regimen outlining a 10% reduction in thickness every 2 weeks. Patients and caregivers were instructed on both overt and subtle signs and symptoms of aspiration. Caregivers progressed to the next defined incremental level if there were no signs or symptoms of aspiration. This new consistency was maintained for 2 weeks until the next incremental change. Thickened liquid recipe calculations and a timeline for weaning were individually outlined for each child based on the type of thickening product being used and the severity of dysphagia. A wean was conducted by keeping the amount of thickening agent constant while increasing the amount of fluid or keeping the amount of fluid constant and decreasing the amount of thickening agent (Table 1). The method and type of thickener was tailored to each patient but followed the same gradation. Clinical assessment in the CAD occurred every 4 to 8 weeks during the SWP but was more frequent for families requiring greater assistance. For example, children with silent aspiration were not excluded from the study and in these cases more frequent follow-up was planned. A wean was considered successful once the patient transitioned to thin liquids while maintaining stable respiratory health without any clinical signs or symptoms of aspiration. If any signs or symptoms of aspiration were detected, parents were instructed to halt the SWP and resume the previously tolerated consistency until further evaluation by the CAD team. This was defined as a “stall” for the purposes of this study. If, after CAD evaluation, the patient could safely resume the SWP and was ultimately successful, this was considered a “temporary stall.” If the patient could not make further progress using the SWP, this was considered a “permanent stall.” Patients who could not tolerate any reduction in their fluid thickness or had a significant respiratory event during the SWP were defined as experiencing “failure.” Occasionally, additional therapeutic feeding and swallowing interventions were implemented to maintain safe and efficient oral feeding. These included changes in positioning, flow rate, bolus delivery method, pacing, or targeting oral-motor skills to improve bolus control.
Table 1. Examples of the Systematic Weaning Process Regimen Given to Parentsa.
| Decreasing Thickness | Recipe | |
|---|---|---|
| Nectar-Thick Recipe = 1 Tablespoon Infant Rice Cereal per 2 oz Fluidb | Thickener, tsp | Fluid, oz |
| Nectar thick | 9 | 6 |
| Less than nectar thick | 8 | 6 |
| 7 | 6 | |
| 6 | 6 | |
| Half-strength (50%) nectar thick | 5 | 6 |
| Less than half strength | 4 | 6 |
| 3 | 6 | |
| 2 | 6 | |
| 1 | 6 | |
| Thin liquid | 0 | NA |
| Nectar-Thick Recipe = 1 Packet SimplyThick (Nectar) per 4 oz Fluidc | Thickener, Packets | Fluid, oz |
| Nectar thick | 1 | 4 |
| Less than nectar thick | 1 | 5 |
| 1 | 6 | |
| 1 | 7 | |
| Half-strength (50%) nectar thick | 1 | 8 |
| Less than half strength | 1 | 9 |
| 1 | 10 | |
| 1 | 11 | |
| 1 | 12 | |
| Thin liquid | 0 | NA |
Abbreviations: NA, not applicable; oz, ounce; tsp, teaspoon.
The type of wean is tailored to the patient and family in each case.
The fluid volume is kept consistent, whereas the amount of cereal is systematically reduced.
The amount of SimplyThick is kept consistent and the fluid is systematically increased.
Results
During the study period from 2010 through 2015, 642 new patients were seen in the CAD clinic. Of those patients, 346 underwent feeding and swallowing assessment by the speech language pathologist. Ninety-one patients participated in the SWP, and 50 of these patients had follow-up of at least 4 months and were eligible to be included in this study. These patients had a mean (SD) follow-up of 1.52 (0.16) years (Table 2). The most common etiology of swallowing dysfunction was anatomic abnormalities (15 [30%]), which included laryngeal cleft, laryngomalacia, or vocal cord dysfunction. The next most common etiologies were cardiopulmonary (10 [20%]) and syndromic (9 [18%]), which included trisomy 21, Turner syndrome, and Goldenhar syndrome. The mean (SD) age at the start of the SWP was 1.8 (1.3) years.
Table 2. Demographic Characteristics.
| Characteristic | Value (N = 50) |
|---|---|
| Sex, No. (%) | |
| Male | 32 (64) |
| Female | 18 (36) |
| Age, median (IQR), y | |
| At presentation | 0.7 (1.0) |
| At start of wean | 1.8 (1.3) |
| Follow-up, mean (SD), y | 1.52 (0.16) |
| Etiology, No. (%) | |
| Anatomic | 15 (30) |
| Cardiopulmonary | 10 (20) |
| Syndromic | 9 (18) |
| Prematurity | 8 (16) |
| Neuromuscular | 3 (6) |
| Gastrointestinal | 1 (2) |
| Other | 4 (8) |
Overall, 39 (78%) patients were successfully weaned such that they could tolerate a thin-fluid diet. The mean (SD) duration of a successful wean was 0.9 (0.6) years. Temporary stalls were documented in 14 (36%) of successfully weaned patients during the SWP process. These patients ultimately completed the wean to thin fluids and were deemed to have experienced successful weaning. Temporary stalls occurred a median (SD) of 0.51 (0.12) years after the onset of the SWP. Temporary stalls were the result of cough or concern by speech language pathologist on VFSS (7 of 14), parental choice (5 of 14), or pneumonia (2 of 14). The median duration of a temporary stall was 9 (interquartile range, 6.9-24.1) weeks. It is unclear from the medical record review whether the pneumonia was directly related to swallowing, but aspiration was not documented and these patients were deemed safe to continue the SWP.
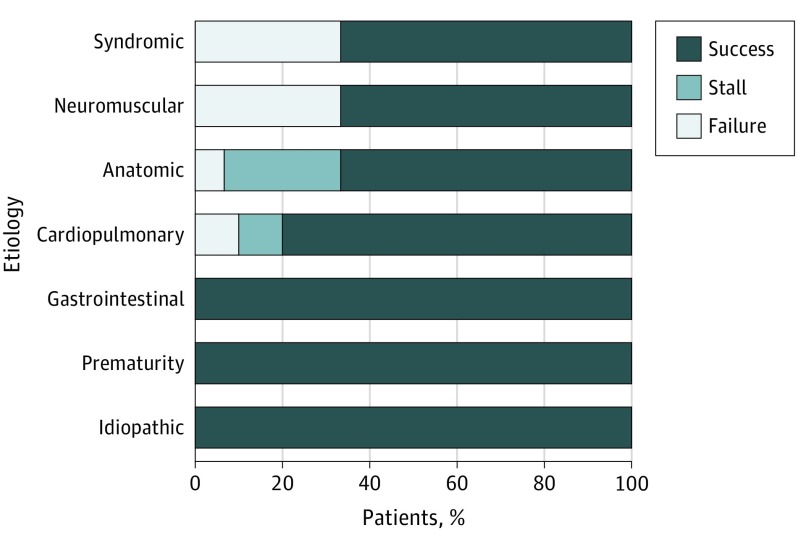
In addition to the 39 patients who experienced successful weaning, 5 (10%) patients experienced a permanent stall, yielding a total of 44 (88%) patients who tolerated a permanent reduction in fluid thickness. Permanent stalls occurred early, with a mean (SD) time to stall of 0.35 (0.08) years. Four of the 5 children who experienced a permanent stall had an anatomic etiology for their swallowing dysfunction (Figure). All 5 patients who experienced a permanent stall were initially receiving liquids with honey or nectar-thick consistencies at the outset of the SWP and were still able to decrease to 50% nectar-thick at the time of the stall without further difficulty. None of the permanently stalled patients developed pneumonia.
Figure. Systematic Weaning Process Outcome Results by Etiology of Dysphagia.
For the remaining 6 (12%) patients, weaning failed and they resumed consumption of their original fluid thickness. The mean (SD) duration from the start of the SWP to failure was 0.32 (0.07) years. Of the 6 patients whose wean failed, 2 (33%) developed pneumonia. Neither patient required hospitalization. The remaining 4 (67%) patients whose wean failed were found to have persistent aspiration on VFSS but did not develop pneumonia while undergoing the SWP. Patients whose wean failed had etiologies that were syndromic (n = 3 [50%]), anatomic (n = 1 [17%]), cardiopulmonary (n = 1 [17%]), or neuromuscular (n = 1 [17%]). Children required a mean of 0.7 VFSSs (range, 0-4) during the SWP. Overall, 46 (92%) of children required 2 or fewer VFSS evaluations. Successfully weaned children did not routinely undergo VFSS at the end of the study. Videofluoroscopic swallow study was performed in 17 (44%) successfully weaned children at the end of the study when prompted by parental or care team concern. Silent aspiration was not seen in any of these studies.
Discussion
Dysphagia occurs in 25% to 45% of the general pediatric population but can be found in as many as 80% of children with developmental delay. Aspiration, which may occur as the result of dysphagia, is the inhalation of foreign material into the airway. The goal of dysphagia management is to establish safe and adequate nutrition and hydration for the child, and in the case of aspiration, to avoid chronic irritation of the lower respiratory tract. Conservative management strategies, such as changes in feeding position, bolus flow rate, and bolus delivery methods, may be beneficial; however, enteral tube feeding strategies are required in severe cases. Alternatively, when compensatory strategies are insufficient, the use of thickened liquids to increase fluid viscosity is commonly recommended.
Thickening of fluids is a simple, well-accepted strategy to reduce the risk of aspiration of fluids. It is critical that thickeners be used as a bridging strategy until swallowing skills improve or definitive management is carried out, rather than as an end-point treatment. Despite the widespread use of thickeners to prevent aspiration, there are a number of downsides to prolonged use related to financial cost, impaired quality of life, altered swallow physiology, altered nutritional composition, and even the risk of postswallow aspiration of pharyngeal residue. For these reasons, patients, caregivers, and clinicians are eager to discontinue or reduce thickener use whenever possible. Typical weaning strategies involve a step-down reduction in the thickness of the fluids according to predetermined viscosities, for example, spoon-thick, honey-like, nectar-like, and thin fluids. Historically, at our institution and others, a child is initially evaluated for dysphagia or signs or symptoms of aspiration and may be prescribed thickened liquids following VFSS. They continue to receive liquids at this thickness, sometimes with initiation of dysphagia therapy, until a repeated VFSS is performed to ensure a safe transition to a thinner consistency in a step-down fashion. The child may continue a modified feeding regimen with serial VFSS, with time frames varying from 6 weeks to months. If the results of the repeated instrumental assessment are unchanged, the patient continues to receive liquids of the same thickness. If the results are improved, the patient may take an incremental step-down to the next safest level of thickness (eg, from honey-thick to nectar-thick).
However, children may have difficulty adapting to the large, conventional changes in thickness during the step-down approach (eg, honey-thick to nectar-thick). In dysphagia management, a child may demonstrate signs of aspiration when ingesting nectar-thick but not honey-thick fluids. As a result, he or she would continue to receive honey-thick consistencies longer than necessary. Additionally, a child might manage a consistency well during instrumental assessment but have increased difficulty with larger volumes at home. Recent work has demonstrated that patients are able to discriminate between small changes in fluid viscosity; thus, more narrowly defined categories of thickened liquids may benefit dysphagia management.
A large aspiration event, or even chronic aspiration of small volumes of fluid into the lungs, carries substantial risks for patients. As such, the cessation of thickener use must be carried out with caution. In 2010, our institution altered its clinical practice for pediatric patients with dysphagia who were being managed with thickened liquids in our CAD clinic. This study describes our initial experience with a systematic process for weaning children off of thickened liquids, whereby small 10% incremental reductions in fluid thickness are made biweekly and are guided by the patients’ clinical signs and symptoms. We hypothesized that slower and more subtle changes in viscosity over time may better allow the sensory-motor system to adapt to thinner consistencies, rather than attempting a large step-down process between broad viscosity categories.
In our study, 78% of children with aspiration who underwent the SWP were successfully weaned from thickened fluids to a normal, thin-fluid diet. During the SWP, 95% of successful patients were weaned without a significant respiratory event, while the remaining 5% (n = 2) developed pneumonia. The SWP was temporarily stalled in 14 patients but was resumed after clinical assessment. In the majority of cases, progress was stalled due to parental concerns or findings on the VFSS, and conservative feeding strategies were implemented to augment the SWP. Temporary stalls usually occurred at approximately 6 months or later. Permanent stalls occurred earlier on in the SWP, at approximately 4 months from the start of the SWP. Although these numbers were too small for statistical comparison, in this series, a greater proportion of successfully weaned children had gastrointestinal issues or were premature infants (Figure). In our experience, these issues tend to improve over time and it is possible that their success was related to natural history. However, a gradual weaning process that implements small changes may be well suited to such conditions. The large changes in fluid consistency levels in standard step-down processes may overwhelm the comparatively slower physiological improvements and lead to aspiration. Most patients with anatomic and cardiopulmonary etiologies also fared well with the SWP. Of patients who experienced a permanent stall, 80% had anatomical issues, and there were no notable differences in outcome between patients who underwent corrective surgical procedures and those who did not. All patients whose weaning process stalled were able to tolerate a reduction in fluid consistency. Interestingly, their progress was stalled at a consistency that was 50% that of a nectar-thick fluid. Using a traditional step-down process, they likely would have failed a test of thin liquids and would have resumed receiving nectar-thick consistencies.
Most patients whose SWP failed and who had to resume their original fluid thickness did so at approximately 4 months from the outset of the wean. Most of these patients had syndromic or neuromuscular etiologies. Considering the substantial sensorimotor impact that syndromic and neuromuscular etiologies can have on swallowing, this finding is not surprising. However, these conditions did not preclude all affected patients from completing the SWP because 67% of patients with syndromic or neuromuscular etiologies were successfully weaned to thin fluids (Figure). At the outset of the study, we considered severe cardiopulmonary issues, severe aspiration, and unreliable caregiver follow-up as contraindications to the SWP. Based on these findings, we would also suggest that careful consideration be made prior to attempting the SWP in children who are syndromic or have neuromuscular conditions and close monitoring be implemented to ensure their safety. The gradation and time frame can be titrated to suit the patient’s progress. In all cases, the clinical team must work closely with patients and caregivers to ensure a safe weaning process.
An additional benefit of the SWP is that it may help reduce the number of VFSSs required during dysphagia management. Patients with dysphagia commonly undergo multiple VFSSs that involve variable doses of ionizing radiation, and it is well accepted that this exposure carries long-term consequences. Considering that more than 90% of children with dysphagia have additional comorbidities, they are likely subject to radiation exposure from multiple sources. Functional endoscopic evaluations of swallowing offers one such option. However, it is not available in all settings, can be challenging in toddlers, and may alter natural swallowing secondary to the presence of the fiber-optic scope. The SWP provides a viable alternative to minimize cumulative radiation exposure in these patients, given that gradual alterations in fluid thickness can be made on a clinical basis and instrumental assessments are reserved for when there is concern. In our study, a mean of 0.7 (range, 0-4) studies were needed during the wean, with 92% of patients requiring 2 studies or fewer. Patients with laryngeal cleft required a mean of 1.2 (range, 0-4) VFSSs, whereas a previous study reported a mean of 3.2 studies (range, 1-10) over the course of treatment in this patient population. Although these findings are encouraging, a definitive conclusion cannot be made because we did not include a control group in our study.
Limitations
This study was intended to describe our initial experience with this novel weaning method. As such, statistical analysis was limited by the low number of patients who underwent the SWP. Although the sample size was small, patients were observed for a mean of 1.5 years, and during that time no patients who successfully completed the SWP developed recurrence or were hospitalized for respiratory issues. We hope to improve on this as our clinical experience with the SWP expands. Moreover, we did not have a control group to compare the SWP with the traditional step-down methods. Finally, it should be noted that children in this study were observed clinically, with VFSS only being used when prompted by clinical suspicion, in an effort to reduce exposure to ionizing radiation. However, whereas children did not undergo instrumental assessment routinely on conclusion of the SWP, 44% of successfully weaned patients were evaluated by VFSS on the basis of clinical or parental suspicion. None of those cases demonstrated silent aspiration; however, it remains possible that following the completion of the SWP, children who appeared to have completely resolved their swallowing issues could have silent aspiration. As such, although children may seem to fare well following the SWP, diligent follow-up is essential, with consideration for a VFSS if there are any concerns such as changes in respiratory status or feeding patterns. Future investigations will further elucidate the benefits of using the SWP, particularly with regard to safety, duration of therapy, and the ability to reduce the number of VFSSs. Finally, our patient population was heterogenic; however, this may reflect the range of etiologies that can result in dysphagia and aspiration. As our work continues, we hope to determine which patient populations are best suited to undergo the SWP.
Conclusions
Thickening fluids is a common strategy for patients with oropharyngeal dysphagia and aspiration but imposes clinical risk. The SWP uses small incremental steps to gradually reduce the amount of thickener used. This allows the swallowing mechanism to adapt to subtle change over time. In this study, 88% of children tolerated a reduction in their thickeners, and 78% tolerated a normal thin-fluid diet. The SWP also allows therapists and trained parents to titrate the diminution of thickener to their child’s clinical response in real time. As such, if a child struggles with a small reduction in thickeners, the previous level can be resumed without substantial difficulty. The SWP presents a safe and effective way of gradually returning children to a more normal diet.
References
- 1.Lefton-Greif MA. Pediatric dysphagia. Phys Med Rehabil Clin N Am. 2008;19(4):837-851. [DOI] [PubMed] [Google Scholar]
- 2.Burklow KA, Phelps AN, Schultz JR, McConnell K, Rudolph C. Classifying complex pediatric feeding disorders. J Pediatr Gastroenterol Nutr. 1998;27(2):143-147. [DOI] [PubMed] [Google Scholar]
- 3.Lefton-Greif MA, Arvedson JC. Pediatric feeding and swallowing disorders: state of health, population trends, and application of the international classification of functioning, disability, and health. Semin Speech Lang. 2007;28(3):161-165. [DOI] [PubMed] [Google Scholar]
- 4.Reau NR, Senturia YD, Lebailly SA, Christoffel KK; Pediatric Practice Research Group . Infant and toddler feeding patterns and problems: normative data and a new direction. J Dev Behav Pediatr. 1996;17(3):149-153. [PubMed] [Google Scholar]
- 5.Weir K, McMahon S, Barry L, Masters IB, Chang AB. Clinical signs and symptoms of oropharyngeal aspiration and dysphagia in children. Eur Respir J. 2009;33(3):604-611. [DOI] [PubMed] [Google Scholar]
- 6.Gosa M, Schooling T, Coleman J. Thickened liquids as a treatment for children with dysphagia and associated adverse effects. Infant Child Adolesc Nutr. 2011;3(6):344-350. [Google Scholar]
- 7.Hammond CAS, Goldstein LB. Cough and aspiration of food and liquids due to oral-pharyngeal dysphagia: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1)(suppl):154S-168S. [DOI] [PubMed] [Google Scholar]
- 8.Smith CH, Jebson EM, Hanson B. Thickened fluids: investigation of users’ experiences and perceptions. Clin Nutr. 2014;33(1):171-174. [DOI] [PubMed] [Google Scholar]
- 9.Alper BS, Manno CJ. Dysphagia in infants and children with oral-motor deficits: assessment and management. Semin Speech Lang. 1996;17(4):283-310. [DOI] [PubMed] [Google Scholar]
- 10.Ylvisaker M, Logemann JA. Therapy for feeding and swallowing disorders after traumatic brain injury In: Ylvisaker M, ed. Head Injury Rehabilitation: Children and Adolescents. London, England: Taylor and Francis; 1985:195-215. [Google Scholar]
- 11.National Dysphagia Diet Task Force, American Dietetic Association National Dysphagia Diet: Standardization for Optimal Care. Chicago, IL: Diana Faulhaber; 2002. [Google Scholar]
- 12.Colodny N. Dysphagic independent feeders’ justifications for noncompliance with recommendations by a speech-language pathologist. Am J Speech Lang Pathol. 2005;14(1):61-70. [DOI] [PubMed] [Google Scholar]
- 13.Murray J, Miller M, Doeltgen S, Scholten I. Intake of thickened liquids by hospitalized adults with dysphagia after stroke. Int J Speech Lang Pathol. 2014;16(5):486-494. [DOI] [PubMed] [Google Scholar]
- 14.Clarke P, Robinson M. Feed thickeners and NEC: too risky to chance. J Perinatol. 2012;32(6):479. [DOI] [PubMed] [Google Scholar]
- 15.Gosa MM, Corkins MR. Necrotizing enterocolitis and the use of thickened liquids for infants with dysphagia. Perspect Swal Swal Dis (Dysph). 2015;24(2):44-49. [Google Scholar]
- 16.Steele CM, James DF, Hori S, Polacco RC, Yee C. Oral perceptual discrimination of viscosity differences for non-Newtonian liquids in the nectar- and honey-thick ranges. Dysphagia. 2014;29(3):355-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vilardell N, Rofes L, Arreola V, Speyer R, Clavé P. A Comparative study between modified starch and xanthan gum thickeners in post-stroke oropharyngeal dysphagia. Dysphagia. 2016;31(2):169-179. [DOI] [PubMed] [Google Scholar]
- 18.Ojha S, Ashland JE, Hersh C, Ramakrishna J, Maurer R, Hartnick CJ. Type 1 laryngeal cleft: a multidimensional management algorithm. JAMA Otolaryngol Head Neck Surg. 2014;140(1):34-40. [DOI] [PubMed] [Google Scholar]
- 19.Hersh C, Wentland C, Sally S, et al. Radiation exposure from videofluoroscopic swallow studies in children with a type 1 laryngeal cleft and pharyngeal dysphagia: a retrospective review. Int J Pediatr Otorhinolaryngol. 2016;89:92-96. [DOI] [PubMed] [Google Scholar]
- 20.Marinschek S, Dunitz-Scheer M, Pahsini K, Geher B, Scheer P. Weaning children off enteral nutrition by netcoaching versus onsite treatment: a comparative study. J Paediatr Child Health. 2014;50(11):902-907. [DOI] [PubMed] [Google Scholar]
- 21.Gardiner AY, Fuller DG, Vuillermin PJ. Tube-weaning infants and children: a survey of Australian and international practice. J Paediatr Child Health. 2014;50(8):626-631. [DOI] [PubMed] [Google Scholar]
- 22.Brown J, Kim C, Lim A, et al. Successful gastrostomy tube weaning program using an intensive multidisciplinary team approach. J Pediatr Gastroenterol Nutr. 2014;58(6):743-749. [DOI] [PubMed] [Google Scholar]
- 23.Linscheid TR. Behavioral treatments for pediatric feeding disorders. Behav Modif. 2006;30(1):6-23. [DOI] [PubMed] [Google Scholar]
- 24.Boesch RP, Daines C, Willging JP, et al. Advances in the diagnosis and management of chronic pulmonary aspiration in children. Eur Respir J. 2006;28(4):847-861. [DOI] [PubMed] [Google Scholar]
- 25.Sheikh S, Allen E, Shell R, et al. Chronic aspiration without gastroesophageal reflux as a cause of chronic respiratory symptoms in neurologically normal infants. Chest. 2001;120(4):1190-1195. [DOI] [PubMed] [Google Scholar]
- 26.Tutor JD, Gosa MM. Dysphagia and aspiration in children. Pediatr Pulmonol. 2012;47(4):321-337. [DOI] [PubMed] [Google Scholar]
- 27.Suleiman OH. Radiation doses in pediatric radiology: influence of regulations and standards. Pediatr Radiol. 2004;34(suppl 3):S242-S246. [DOI] [PubMed] [Google Scholar]
- 28.Willis CE, Slovis TL. The ALARA concept in pediatric CR and DR: dose reduction in pediatric radiographic exams—a white paper conference. AJR Am J Roentgenol. 2005;184(2):373-374. [DOI] [PubMed] [Google Scholar]
- 29.Kaste SC. Imaging gently: a call for awareness. J Am Osteopath Assoc. 2012;112(3):119-120. [PubMed] [Google Scholar]
- 30.Furlow B. Radiation protection in pediatric imaging. Radiol Technol. 2011;82(5):421-439. [PubMed] [Google Scholar]
- 31.Goske MJ, Frush DP, Schauer DA. Image Gently campaign promotes radiation protection for children. Radiat Prot Dosimetry. 2009;135(4):276. [DOI] [PubMed] [Google Scholar]
- 32.Adil E, Al Shemari H, Kacprowicz A, et al. Evaluation and management of chronic aspiration in children with normal upper airway anatomy. JAMA Otolaryngol Head Neck Surg. 2015;141(11):1006-1011. [DOI] [PubMed] [Google Scholar]