Key Points
Question
What is the growth curve of thyroid cancer found by ultrasonography screening among children and young adults?
Findings
In this observational study, thyroid cancer in young patients screened by ultrasonography after a nuclear accident in Fukushima, Japan, did not grow linearly and fell into a growth arrest pattern after the initial proliferative phase.
Meaning
It is important to avoid overdiagnosis and overtreatment to monitor the natural course of suspected noninvasive thyroid cancer in young patients without immediate diagnosis.
Abstract
Importance
Thyroid cancer generally grows at a very slow rate in adults, and overdiagnosis is a global issue. However, the detection of early-stage thyroid cancer by screening is not well described in young patients. To prevent overdiagnosis, it is essential to understand the natural course of thyroid cancer growth detection by ultrasonography screening in young patients.
Objective
To evaluate the natural progression of thyroid cancer in young patients.
Design, Setting, and Participants
An observational study evaluated changes in the diameter of malignant or suspected malignant thyroid tumors on 2 occasions. Changes in malignant thyroid tumor diameter were estimated during the observation period between the screening and confirmatory examinations in the first-round thyroid ultrasonography examination of the Fukushima Health Management Survey in patients younger than 21 years after a nuclear accident at a power plant in Fukushima, Japan. In total, 116 patients cytologically diagnosed with or suspected to have thyroid cancer were classified into 3 groups based on a greater than 10% reduction, a change of −10% to +10% in diameter, and a greater than 10% increase in tumor diameter. The association between tumor growth rate and tumor diameter was analyzed. The study was conducted from March 1, 2016, to August 6, 2017.
Main Outcomes and Measures
Tumor volume changes, the coefficient of growth of thyroid cancer in young patients, and the association between the observation period or tumor diameter and them.
Results
Of 116 patients, 77 were female; the mean age was 16.9 years (median, 17.5 years). The mean observation period was 0.488 (range, 0.077-1.632) years. No significant differences in age, sex, tumor diameter, observation period, or serum levels of thyrotropin and thyroglobulin were observed among the groups. Whereas tumor volume changes were not linearly correlated with the observation period (Pearson R = 0.121; 95% CI, −0.062 to 0.297), the coefficient of growth was significantly and negatively correlated with the tumor diameter in the screening examination (Spearman ρ = −0.183; 95% CI, −0.354 to −0.001), suggesting growth arrest after the initial proliferation phase.
Conclusions and Relevance
Ultrasonography screening could reveal asymptomatic thyroid cancer that is falling into a growth arrest pattern in many young patients. Considering the long life expectancy, prevention of overdiagnosis necessitates careful long-term monitoring without immediate diagnosis for suspected noninvasive thyroid cancer.
This observational study evaluates the change in diameter and volume of malignant thyroid tumors in children after a nuclear accident in Fukushima, Japan.
Introduction
The technology of early cancer detection has progressed with new developments in imaging and biomarkers. Early diagnosis has improved the prognosis of several cancers but has also resulted in overdiagnosis of some cancers during screening, especially thyroid, breast, and prostate cancers. One reason for this problem is that the natural history of early cancer is not well known regarding whether it will develop to life-threatening clinical cancer. The incidence of thyroid cancer, a predominantly small papillary carcinoma, has increased significantly in recent decades, although thyroid cancer–related mortality rates have not changed substantially. In autopsy studies, a markedly high prevalence of papillary thyroid cancer has been observed compared with the expected number of thyroid cancers in cancer registries, and this thyroid cancer includes tumors larger than microcarcinomas. Furthermore, the prevalence of these latent thyroid cancers in patients of early middle age is as high as that found in older patients, suggesting that many latent thyroid cancers progress to a detectable level from young to early middle age.
In South Korea, the incidence of thyroid cancer in 2011 was 15 times higher than that in 1993 before thyroid ultrasonography screening started. Active surveillance of adult papillary thyroid microcarcinoma (≤10 mm) in Japan since approximately 1993 has shown that more than 90% of papillary thyroid microcarcinomas do not increase in size during an average observation period. Other ultrasonography studies have also revealed no significant growth of differentiated papillary cancer during observation. These data suggest that many differentiated papillary thyroid carcinomas in adults are virtually in growth arrest, although they proliferate before reaching a certain size.
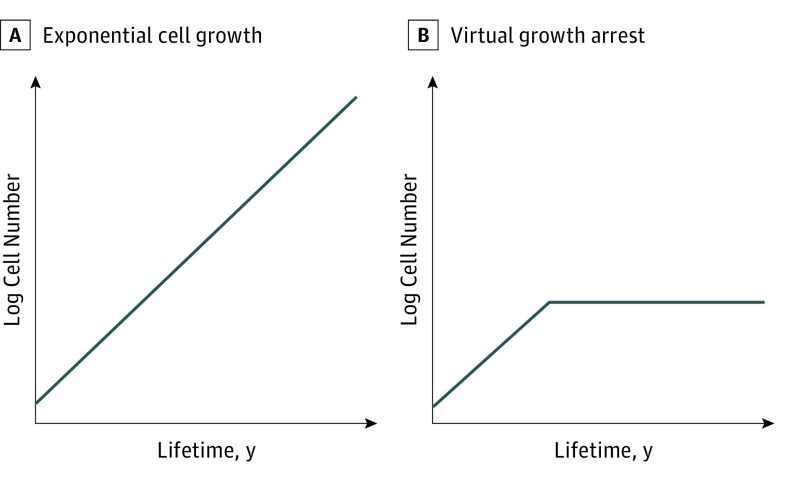
Welch and Black reviewed the heterogeneity of cancer progression and reported that some cancers grow very slowly or do not progress beyond a certain size, thus remaining asymptomatic. This finding differs from the ordinary near-exponential growth model and short doubling time of many other cancers (Figure 1A). It is possible that many thyroid cancers that are not in the advanced stage, including both microcarcinomas and larger cancers, grow to a certain size in younger individuals and subsequently enter growth arrest (Figure 1B).
Figure 1. Two Hypothesized Patterns of Thyroid Cancer Progression.
A, Linear semilogarithmic progression (exponential cell growth) pattern. B, Linear semilogarithmic progression and virtual growth arrest (relatively rapid growth in the initial phase with subsequent growth arrest) pattern.
In response to the Fukushima Daiichi Nuclear Power Plant accident that occurred on March 11, 2011, thyroid ultrasonographic examinations within the Fukushima Health Management Survey were conducted among children and young adults. The preliminary baseline survey from fiscal years 2011 to 2013 evaluated data before the period in which slight radiation effects on the thyroid gland might occur. In total, 116 individuals were diagnosed with malignant or suspected malignant tumors in this preliminary baseline survey, exceeding the cancer registry data in Japan. To understand the natural progression of thyroid cancer in young patients, we examined changes in the diameter of malignant or suspected malignant thyroid tumors among individuals from the Fukushima Health Management Survey between the screening and confirmatory examinations and analyzed the growth pattern of thyroid cancer in young patients.
Methods
Thyroid Ultrasound Examination in the Fukushima Health Management Survey
The thyroid examination program consisted of 2 stages. In the screening examination, participants with nodules larger than 5.0 mm in diameter were primarily screened using ultrasonography equipment. In the confirmatory examination, fine-needle aspiration cytology was performed only when necessary according to the Japanese guideline for adult thyroid nodules to confirm the pathologic diagnosis in addition to ultrasonographic examination and blood tests. These examinations were then repeated every 2 to 5 years. The first-round Thyroid Ultrasound Examination (preliminary baseline survey) was intended to capture baseline data prior to the period during which the radiation effects on the thyroid gland were assumed to become apparent. The survey included approximately 360 000 children 18 years or younger at the time of the accident, with a participation rate of 81.7% as of March 31, 2016. A total of 2086 participants underwent a confirmatory examination. Of these patients, 116 were diagnosed with malignant or suspected malignant thyroid tumors and enrolled in the present study. The study was conducted from March 1, 2016, to August 6, 2017, at Fukushima Medical University, Fukushima, Japan.
The study was approved by the Fukushima Medical University Ethics Committee. Participants or their guardians provided written consent, and they did not receive financial compensation.
Measurement and Analysis of Thyroid Cancers
The maximum tumor diameter was measured on the same comparable cross section that was selected by 2 endocrinologists (S.M. and A.O.) in agreement. The mean diameter was calculated from tumor diameter measured by 2 experienced medical technologists independently in both the screening and confirmatory examinations. Changes in diameter were calculated by subtracting the comparable maximum diameter at the confirmatory examination from that at the screening examination. The change in diameter per year was estimated by dividing the change in diameter by the number of observation periods. The percentage change was calculated from dividing changes in diameter by the diameter at the screening examination. The 116 patients with a thyroid cancer diagnosis or suspicion of having thyroid cancer were classified into 3 groups: group 1, more than 10% reduction in diameter; group 2, –10% to +10% change in diameter; and group 3, more than 10% increase in diameter. These thresholds were chosen because previous studies revealed that any ultrasonography-measured differences of less than 7.3% in diameter should not be considered as a real change in size.
Tumor Growth Models
Tumor growth models were developed to evaluate the tumor growth rate. Fujita suggested that tumor cell numbers show exponential growth until a growth arrest pattern occurs and can be explained by the number of years since development of the tumor. In this study, we assumed that tumor cell numbers were proportional to the tumor volume, and the tumor volume was proportional to the cube of the tumor diameter. The tumor growth rate can be described as follows:
| log10Y3 = a × X + b, |
where Y is the tumor diameter (millimeters), X is the number of years since development of the tumor (year), a is the coefficient of growth (year −1), and b is log10 Y3 at X = 0.
From the above equation, the observations at the screening and confirmatory examinations can be explained as follows:
| log10 (Y2/Y1)3 = a × T, |
where T is the observation period (X2 − X1) (year) and 1 and 2 represent the screening and confirmatory examinations, respectively.
Statistical Analysis
The χ2 test or Kruskal-Wallis and Dunn-Bonferroni post hoc tests were used to assess age and tumor diameter at the screening examination; sex; observation period; percentage change in tumor diameter; tumor volume change; coefficient of growth; ultrasonographic findings, such as microcalcifications, irregularity of surface, and heterogeneity of echogenicity; and serum levels of thyrotropin and thyroglobulin among the 3 groups. Pearson correlation coefficient R was used for testing between observation periods and for the percentage change in tumor diameter or tumor volume change to investigate whether tumor growth can be explained by only the linear semilogarithmic progression pattern, following Eq. 2. Spearman correlation coefficient ρ was tested between the age and the percentage change in tumor diameter or tumor volume change and between the coefficient of growth and the tumor diameter at the screening examination to investigate which growth pattern fit (Figure 1). The 95% CIs of correlation coefficients were estimated by using Fisher z-transformation.
For separate estimation of the growth rate in a linear semilogarithmic progression pattern and a growth arrest pattern, the data were divided into 2 groups according to the threshold tumor diameter YT in the screening examination. The growth rate in both patterns and YT were estimated using the least-squares method. Furthermore, the mean tumor volume (log10 Y3) in the growth arrest pattern in the patients whose tumor diameters exceeded YT was regarded as the representative tumor volume at the growth arrest point (log10 YAP3) because the coefficient of growth in the growth arrest pattern was almost zero.
The mean period from the time point at which the tumor measured 5 mm in diameter to the growth arrest point was then estimated. The value of 5 mm was used as the minimum clinically observed tumor diameter because the screening criterion was set at a tumor diameter of more than 5 mm. In addition, 95% CIs were estimated by Monte Carlo simulation using commercially available software (Crystal Ball, version 11.1.2.3.500; Oracle). The arithmetic means and sample SEs of the coefficients of growth and log10 YAP3 were used as input in the simulation, assuming that the data followed a normal distribution. The simulation was performed 100 000 times. Data were analyzed using JMP, version 12 (SAS Institute Japan) or SPSS, version 22 (IBM Corp).
Results
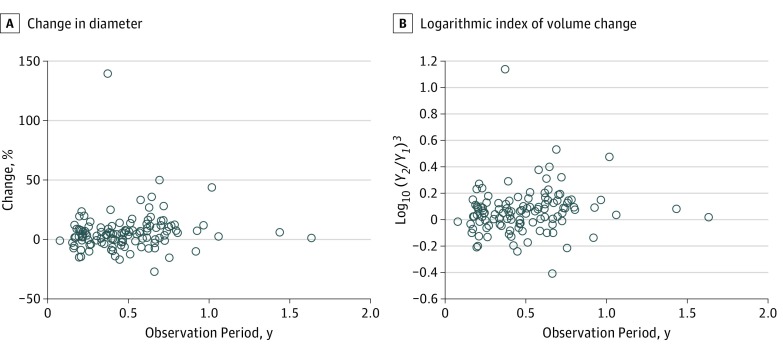
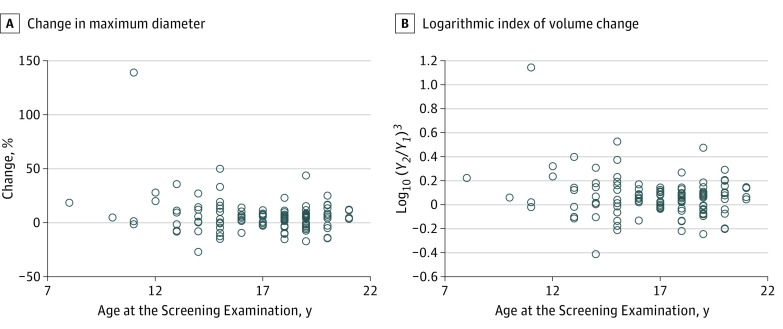
The mean age of the 116 patients (39 male, 77 female) was 16.9 (median, 17.5) years, and the mean observation period between the screening and confirmatory examinations was 0.488 years (median, 0.461; range, 0.077-1.632 years). The mean tumor diameters in the screening and confirmatory examinations were 13.5 (median, 11.1) mm and 14.2 (median, 11.6) mm, respectively. There was no significant correlation between age and tumor diameter. Figure 2 shows the scatterplot of the percentage change in tumor diameter or the logarithmic index of volume change by the length of the observation period between the screening and confirmatory examinations. There was no significant correlation between the observation period and either the percentage change in tumor diameter (Pearson R = 0.098; 95% CI, –0.085 to 0.276) or the logarithmic index of volume change (Pearson R = 0.121; 95% CI, –0.062 to 0.297). Furthermore, a scatterplot of age and the percentage change in tumor diameter at the screening examination showed no significant correlation (Figure 3) (Spearman ρ = –0.065; 95% CI, –0.245 to 0.118).
Figure 2. Correlation Between Observation Period and Tumor Growth.
A, Percentage change in tumor diameter. B, Logarithmic index of volume change by length of observation period. Y1 indicates tumor diameter in the screening examination (millimeters); Y2, tumor diameter in the confirmatory examination (millimeters). There were no significant correlations between the observation period and either the percentage change of the tumor diameter (Pearson R = 0.098; 95% CI, –0.085 to 0.276) or the logarithmic index of volume change (Pearson R = 0.121; 95% CI, –0.062 to 0.297). The logarithmic index of volume change well reflects the percent change in tumor diameter. The tumor growth rate is not exponential.
Figure 3. Correlation Between Age and Tumor Growth.
A, Percentage change in maximum tumor diameter at the screening examination. B, Logarithmic index of volume change. Y1 indicates tumor diameter in the screening examination (millimeters); Y2, tumor diameter in the confirmatory examination (millimeters). There were no significant correlations (Spearman ρ = –0.065; 95% CI, –0.245 to 0.118).
Group analysis showed no significant differences in age; sex; observation period; tumor diameter; ultrasonographic findings, such as microcalcifications, irregularity of surface, and heterogeneity of echogenicity; and serum levels of thyrotropin and thyroglobulin among group 1 (reduced diameter), group 2 (no change in diameter), and group 3 (increased diameter) (Table). However, significant differences were observed in the changes in diameter, percentage change, and change per year between all pairs.
Table. Clinical Characteristics Among 3 Groups According to Change In Tumor Diameter.
| Characteristic | Mean (SD) | ||
|---|---|---|---|
| Group 1 (n = 7) | Group 2 (n = 81) | Group 3 (n = 28) | |
| Sex, No, (%) | |||
| Female | 6 (85.7) | 53 (65.4) | 18 (64.3) |
| Male | 1 (14.3) | 28 (34.6) | 10 (34.7) |
| Age, y | 17.3 (3) | 17.2 (2.4) | 16.1 (3.4) |
| Observation period, y | 0.457 (0.211) | 0.469 (0.270) | 0.550 (0.227) |
| Tumor diameter, mm | 12.1 (5.2) | 13.9 (8.5) | 12.8 (7.9) |
| Change of diameter, mma | −2.0 (1.1) | 0.2 (0.8) | 3.0 (3.2) |
| % Changea | −16.5 (4.8) | 1.7 (5.5) | 24.1 (24.9) |
| Changed value/y, mm/ya | −5.0 (2.7) | 0.2 (2.7) | 6.4 (7.2) |
| Thyrotropin, mIU/L | 1.62 (1.43) | 1.29 (0.68) | 1.33 (0.64) |
| Thyroglobulin, ng/mL | 23.4 (24.3) | 43.1 (89.1) | 37.3 (65.8) |
SI conversion factor: To convert thyroglobulin to micrograms per liter, multiply by 1.
Kruskal-Wallis and Dunn-Bonferroni post hoc test between all pairs, P < .01.
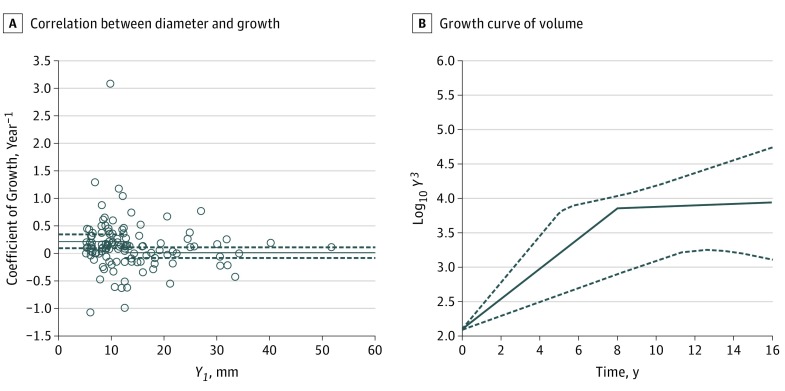
To further analyze the tumor growth pattern, we compared the coefficient of growth with the tumor diameter. As shown in Figure 4A, the coefficient of growth was significantly and negatively correlated with the tumor diameter (Spearman ρ = –0.183; 95% CI, −0.354 to −0.001), suggesting a reduction of the growth rate with time.
Figure 4. Coefficient of Growth by Tumor Diameter and Growth Curve of Tumor Volume.
A, Correlation between tumor diameter and coefficient of growth. There was a significant negative correlation between the coefficient of growth and the tumor diameter (Spearman ρ = −0.183; 95% CI, –0.354 to –0.001). The data were divided into 2 groups according to tumor size (small and large). The threshold tumor diameter YT in the screening examination was estimated as 12.4 mm. The coefficient of growth was almost zero (0.010 year−1) in the large tumor group (n = 47). B, Growth curve of tumor volume in a linear semilogarithmic progression period and a growth arrest period. The 95% CIs were estimated by Monte Carlo simulation. The mean number of years during which the tumor grew from a diameter of 5 mm until reaching the growth arrest point was calculated as approximately 8.0 (range, 5.1–17.6) years. Solid lines and broken lines represent mean and 95% CI, respectively.
The data were statistically divided into 2 groups according to tumor size (small and large) by using the least-squares method as described in the Statistical Analysis section. The estimated threshold tumor diameter YT in the screening examination was calculated to be 12.4 mm. The coefficient of growth was 0.220 (95% CI, 0.097 to 0.343) year−1 in the small tumor group (n = 69) (significantly higher than zero), and the diameter was less than 12.4 mm in the screening examination. In contrast, the coefficient of growth was almost zero (0.010; 95% CI, –0.088 to 0.108) year−1 in the large tumor group (n = 47), and the diameter was larger than 12.4 mm in the screening examination. The representative tumor volume in the large tumor group was 3.86 (range, 3.72-4.00). We then estimated the mean change in tumor growth (Figure 4B). The mean number of years during which the tumor grew from a diameter of 5 mm to the growth arrest point was estimated by Monte Carlo simulation using the coefficients of growth and the representative tumor volume at the growth arrest point described above. The mean number of years was calculated to be 8.0 (range, 5.1-17.6) years.
Discussion
The natural progression of cancer varies according to the type of malignancy. Some cancers progress rapidly, including most types of leukemia and sarcoma, while others show relatively slow progression. Some cancers may even exhibit shrinking or senescence. In 1978, Fujita summarized the natural progression of early gastric cancer from pathologic studies. The study found that gastric cancer grows relatively rapidly in the initial phase but then remains as a localized-stage cancer for a prolonged time without further growth in most cases (ie, very slow growth or virtual growth arrest). The duration of this very slow growth or virtual growth arrest is reportedly 14 to 21 years, and advanced gastric cancer subsequently reproliferates after this phase. Although the molecular carcinogenic mechanism of gastric cancer differs from that of papillary thyroid carcinoma, this model of the natural progression of cancer appears to be similar among various types of cancer and does not appear to follow a model in which semilogarithmic proliferation persists from the onset.
From these considerations, we hypothesized 2 patterns of thyroid cancer progression in young patients: linear semilogarithmic progression (exponential cell growth) and virtual growth arrest (relatively rapid growth in the initial phase with subsequent growth arrest) as shown in Figure 1. The expected outcomes of the 2 patterns are summarized as follows. First, the linear semilogarithmic progression pattern seems to show a positive correlation between age and tumor volume, but the growth arrest pattern does not. Second, a positive correlation is considered to exist between the tumor volume change and examination period in the linear semilogarithmic progression pattern, while the growth arrest pattern shows no such correlation. Third, if we assume that the hypothesis of the linear progression pattern is correct, the coefficients of growth are constant irrespective of the tumor diameter. However, for occurrence of the growth arrest pattern, the coefficients of growth decrease as the tumor diameter increases.
We demonstrated that 76% of thyroid cancers or suspected cancers did not increase in diameter during each observation period. No correlation was observed between age and tumor diameter, change in tumor diameter and observation period, or age and percentage change, supporting the growth arrest pattern hypothesis. Although Group 3 comprised 24% of thyroid cancers, the coefficient of growth significantly decreased as the tumor diameter increased. The coefficient of growth in the small tumor diameter group was significantly higher than zero, whereas it was almost zero in the large tumor diameter group; this finding suggests that more tumors in group 3 were in the initial phase than in groups 1 and 2. All of these results support the occurrence of a growth arrest pattern rather than only a linear semilogarithmic progression pattern.
According to the data regarding active surveillance without immediate surgery in Japan, papillary thyroid microcarcinomas in younger patients (<40 years) have a relatively higher tumor growth rate than in older patients (>60 years). Some reports have suggested that thyroid cancer in children exhibits relatively more aggressive behavior despite its better prognosis. However, some of these observations might also be made at the beginning of the growth phase, prior to the growth arrest phase in young patients.
In the present study, many thyroid cancers that were screened by ultrasonography did not grow linearly and instead entered growth arrest, even in young patients. Furthermore, the mean duration during which the tumor grew from a diameter of 5 mm to the growth arrest point was 8.0 (range, 5.1-17.6) years. Recent studies of active surveillance in adults in Japan have revealed that more than 90% of papillary thyroid microcarcinomas do not increase in size during an average observation period. These findings may be beneficial when considering treatment, including active surveillance of thyroid cancer incidentally discovered by ultrasonographic examination at a young age. Furthermore, these findings help to determine how to manage various cancers for which overdiagnosis is a global problem. However, observation of the clinical course of cancer, including thyroid cancer, as an active surveillance protocol for a prolonged period may lead to a negative risk perception of the disease and a psychological burden for children and young adults and their families. This issue may be difficult to resolve by active surveillance alone for thyroid cancers diagnosed by screening following a nuclear accident.
Limitations
There were some limitations to this study. First, the observation period was short (mean, 0.488 years). Although most thyroid cancers showed slow growth with linear semilogarithmic progression, cancers with this slower progression might have been erroneously classified into group 2 (no change in diameter). Second, we evaluated the tumor diameter as a measure of tumor progression; we were unable to measure the tumors in 3 dimensions because only comparable 2-dimensional images were available. Changes in tumor diameter do not accurately reflect changes in tumor volume or tumor cell proliferation. Third, not all 116 patients with cancer or suspected cancer in this study underwent surgery; the final pathologic diagnosis was obtained only in the 102 surgical patients (papillary thyroid cancer, 100; poorly differentiated cancer, 1; and benign, 1). A pathologic diagnosis in the remaining 14 patients who did not undergo surgery was therefore not obtained. Finally, 6% of the tumors showed a greater than 10% reduction in tumor diameter in this study. O’Kane et al performed a Ukraine-American cohort study and found that the size of more than half of the tumors decreased during a 2- to 8-year observation period and that the change in tumor size was not different between benign nodules and papillary thyroid carcinomas in young patients. If the rate of tumor shrinkage increases with longer observation periods, the analysis results may differ from the present results.
Conclusions
Thyroid cancers that are screened by ultrasonography might not be progressive in many children and young adults. Clinical background factors, such as age, sex, and tumor diameter at the time of cancer discovery, might not be associated with the progression of the tumor. However, the natural progression of thyroid cancer in young patients remains relatively unknown; therefore, the present study may be beneficial when considering the most appropriate follow-up actions for thyroid nodules, including cancers discovered incidentally or by screening in children and young adults.
References
- 1.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. [DOI] [PubMed] [Google Scholar]
- 2.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. ; US Preventive Services Task Force . Screening for thyroid cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2017;317(18):1882-1887. [DOI] [PubMed] [Google Scholar]
- 3.Lin JS, Bowles EJA, Williams SB, Morrison CC. Screening for thyroid cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317(18):1888-1903. [DOI] [PubMed] [Google Scholar]
- 4.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? the increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614-617. [DOI] [PubMed] [Google Scholar]
- 5.Furuya-Kanamori L, Bell KJ, Clark J, Glasziou P, Doi SA. Prevalence of differentiated thyroid cancer in autopsy studies over six decades: a meta-analysis. J Clin Oncol. 2016;34(30):3672-3679. [DOI] [PubMed] [Google Scholar]
- 6.Valle LA, Kloos RT. The prevalence of occult medullary thyroid carcinoma at autopsy. J Clin Endocrinol Metab. 2011;96(1):E109-E113. [DOI] [PubMed] [Google Scholar]
- 7.Bondeson L, Ljungberg O. Occult papillary thyroid carcinoma in the young and the aged. Cancer. 1984;53(8):1790-1792. [DOI] [PubMed] [Google Scholar]
- 8.Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”—screening and overdiagnosis. N Engl J Med. 2014;371(19):1765-1767. [DOI] [PubMed] [Google Scholar]
- 9.Miyauchi A. Clinical trials of active surveillance of papillary microcarcinoma of the thyroid. World J Surg. 2016;40(3):516-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010;34(6):1222-1231. [DOI] [PubMed] [Google Scholar]
- 11.Mihailescu DV, Collins BJ, Wilbur A, Malkin J, Schneider AB. Ultrasound-detected thyroid nodules in radiation-exposed patients: changes over time. Thyroid. 2005;15(2):127-133. [DOI] [PubMed] [Google Scholar]
- 12.Asanuma K, Kobayashi S, Shingu K, et al. . The rate of tumour growth does not distinguish between malignant and benign thyroid nodules. Eur J Surg. 2001;167(2):102-105. [DOI] [PubMed] [Google Scholar]
- 13.Laird AK. Dynamics of tumor growth. Br J Cancer. 1964;13(Sep):490-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartung N, Mollard S, Barbolosi D, et al. . Mathematical modeling of tumor growth and metastatic spreading: validation in tumor-bearing mice. Cancer Res. 2014;74(22):6397-6407. [DOI] [PubMed] [Google Scholar]
- 15.Barbara L, Benzi G, Gaiani S, et al. . Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology. 1992;16(1):132-137. [DOI] [PubMed] [Google Scholar]
- 16.Henschke CI, Yankelevitz DF, Yip R, et al. ; Writing Committee for the I-ELCAP Investigators . Lung cancers diagnosed at annual CT screening: volume doubling times. Radiology. 2012;263(2):578-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasumura S, Hosoya M, Yamashita S, et al. ; Fukushima Health Management Survey Group . Study protocol for the Fukushima Health Management Survey. J Epidemiol. 2012;22(5):375-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki S, Yamashita S, Fukushima T, et al. . The protocol and preliminary baseline survey of the thyroid ultrasound examination in Fukushima. Endocr J. 2016;63(3):315-321. [DOI] [PubMed] [Google Scholar]
- 19.Fukushima Medical University 2016. Proceedings of the 23rd Prefectural Oversight Committee Meeting for Fukushima Health Management Survey. http://fmu-global.jp/2016/06/07/proceedings-of-the-23rd-prefectural-oversight-committee-meeting-for-fukushima-health-management-survey/. Accessed May 14, 2017.
- 20.Katanoda K, Kamo K, Tsugane S. Quantification of the increase in thyroid cancer prevalence in Fukushima after the nuclear disaster in 2011—a potential overdiagnosis? Jpn J Clin Oncol. 2016;46(3):284-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohtsuru A, Midorikawa S, Suzuki S, Shimura H, Matsuzuka T, Yamashita S. Five-year interim report of thyroid ultrasound examinations in the Fukushima Health Management Survey In: Yamashita S, Thomas G, eds. Thyroid Cancer and Nuclear Accidents: Long-Term Aftereffects of Chernobyl and Fukushima. Amsterdam, the Netherlands: Academic Press, Elsevier; 2017:145-153. [Google Scholar]
- 22.Suzuki S, Suzuki S, Fukushima T, et al. . Comprehensive survey results of childhood thyroid ultrasound examinations in Fukushima in the first four years after the Fukushima Daiichi Nuclear Power Plant accident. Thyroid. 2016;26(6):843-851. [DOI] [PubMed] [Google Scholar]
- 23.Choi YJ, Baek JH, Hong MJ, Lee JH. Inter-observer variation in ultrasound measurement of the volume and diameter of thyroid nodules. Korean J Radiol. 2015;16(3):560-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita S. Biology of early gastric carcinoma. Pathol Res Pract. 1978;163(4):297-309. [DOI] [PubMed] [Google Scholar]
- 25.Mooi WJ, Peeper DS. Oncogene-induced cell senescence—halting on the road to cancer. N Engl J Med. 2006;355(10):1037-1046. [DOI] [PubMed] [Google Scholar]
- 26.Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427(6977):787. [DOI] [PubMed] [Google Scholar]
- 27.Serrano M. Cancer regression by senescence. N Engl J Med. 2007;356(19):1996-1997. [DOI] [PubMed] [Google Scholar]
- 28.Ito Y, Miyauchi A, Kobayashi K, Miya A. Prognosis and growth activity depend on patient age in clinical and subclinical papillary thyroid carcinoma. Endocr J. 2014;61(3):205-213. [DOI] [PubMed] [Google Scholar]
- 29.Wang JT, Huang R, Kuang AR. Comparison of presentation and clinical outcome between children and young adults with differentiated thyroid cancer. Asian Pac J Cancer Prev. 2014;15(17):7271-7275. [DOI] [PubMed] [Google Scholar]
- 30.Nies M, Klein Hesselink MS, Huizinga GA, et al. . Long-term quality of life in adult survivors of pediatric differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2016;102(49):1218-1226. [DOI] [PubMed] [Google Scholar]
- 31.Bresner L, Banach R, Rodin G, Thabane L, Ezzat S, Sawka AM. Cancer-related worry in Canadian thyroid cancer survivors. J Clin Endocrinol Metab. 2015;100(3):977-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Bergh RC, Korfage IJ, Bangma CH. Psychological aspects of active surveillance. Curr Opin Urol. 2012;22(3):237-242. [DOI] [PubMed] [Google Scholar]
- 33.Davies L, Hendrickson CD, Hanson GS. Experience of US patients who self-identify as having an overdiagnosed thyroid cancer: a qualitative analysis. JAMA Otolaryngol Head Neck Surg. 2017;143(7):663-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coon ER, Quinonez RA, Moyer VA, Schroeder AR. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134(5):1013-1023. [DOI] [PubMed] [Google Scholar]
- 35.Midorikawa S, Tanigawa K, Suzuki S, Ohtsuru A. Psychosocial issues related to thyroid examination after a radiation disaster. Asia Pac J Public Health. 2017;29(2 suppl):63S-73S. [DOI] [PubMed] [Google Scholar]
- 36.Midorikawa S, Ohtsuru A, Suzuki S, et al. . Psychosocial impact on the thyroid examination of the Fukushima Health Management Survey In: Yamashita S, Thomas G, eds. Thyroid Cancer and Nuclear Accidents: Long-Term Aftereffects of Chernobyl and Fukushima. Amsterdam, the Netherlands: Academic Press, Elsevier; 2017:165-173. [Google Scholar]
- 37.O’Kane P, Shelkovoy E, McConnell RJ, et al. . Frequency of undetected thyroid nodules in a large I-131–exposed population repeatedly screened by ultrasonography: results from the Ukrainian-American cohort study of thyroid cancer and other thyroid diseases following the Chornobyl accident. Thyroid. 2010;20(9):959-964. [DOI] [PMC free article] [PubMed] [Google Scholar]