Abstract
Using the National Cancer Database, this study examines the association of treatment at high-volume facilities with survival in patients receiving chemotherapy for nasopharyngeal cancer.
Similar to other head and neck cancers, successful delivery of radiation therapy (RT) in nasopharyngeal cancer (NPC) is challenging. Reasons include technical aspects, such as treatment planning (especially in the era of intensity-modulated RT and simultaneous integrated boosting), physician experience in target delineation (eg, knowing routes of subclinical nodal spread), available technologies (eg, image-guided RT), coordination of multimodality therapy, and treatment of acute and late toxic effects.
Thus, there may be advantages to administering chemoradiotherapy (CRT) at high-volume facilities (HVFs). For similar reasons as the aforementioned, treatment at HVFs and/or from more experienced clinicians have been associated with improved outcomes in the surgical literature. Analogously, we hypothesized that treatment at an HVF could improve outcomes in a nonsurgical disease such as NPC, which we evaluated in this study. The National Cancer Database (NCDB) provides a unique resource with which to address this novel and clinically important issue and has been proven to be of great utility for similar studies in other tumor types.
Methods
Details about the NCDB are presented elsewhere. This study was exempt from institutional review board review because all data used were deidentified, and thus informed consent was not required. Race of patients was reported in accordance with coded variables defined in the NCDB. Inclusion criteria were patients with newly-diagnosed T2N0 and above NPC (owing to recommended receipt of CRT). Radiation therapy referred to total doses of at least 66 Gy. The definition of an HVF was similar to other work in that facility volume was dichotomized into HVF or lower-volume facility (LVF) based on an a priori threshold corresponding to the 80th percentile of patient numbers treated per facility. Similar to published work, this cutoff was used to evaluate a roughly 1:1 ratio of patients in HVF vs LVF, because the top 20% of facilities treat nearly half of patients. However, to evaluate whether this a priori definition was significant at other cutoffs, sensitivity analysis was performed to evaluate whether altering the HVF definition affected the association with OS.
Statistics were carried out using STATA statistical software (version 14, STATA Corp) included multivariable logistic regression to discern which characteristics were independently associated with treatment at an HVF. Kaplan-Meier analysis evaluated OS (date of diagnosis to death or last contact). Multivariable Cox proportional hazards modeling evaluated predictors of OS.
Results
From 2004 to 2013, there were 12 389 patients with newly diagnosed NPC in the NCDB. Exclusion criteria were applied corresponding to the following: incomplete staging and/or T1N0 disease (n=3140), metastatic (M1) disease (n=1045), unknown and/or improper radiation dose (n=2291), unknown or no chemotherapy (n=1260), pharyngectomy or unknown surgery (n=142), and palliative care treatment (n=42).
Of the remaining 4469 patients treated at 934 different institutions, 2553 (57%) patients were treated at an LVF and 1916 (43%) at an HVF. The Table displays notable clinical characteristics of both cohorts. Factors independently associated with treatment at an HVF included metro residence (urban: odds ratio [OR], 0.18; 95% CI, 0.15-0.23; vs rural: OR, 0.15; 95% CI, 0.08-0.28).
Table. Characteristics of the Overall Cohort, Factors Independently Associated With Treatment at an HVF (Logistic Regression), and Factors Independently Associated With Overall Survival (Cox Proportional Hazards Model)a.
| Parameter | No. (%) | Multivariable Logistic Regression OR (95% CI) | Multivariable Cox Proportional Hazards Model HR (95% CI) | |
|---|---|---|---|---|
| LVF (n = 2553) |
HVF (n = 1916) |
|||
| Age, y | ||||
| ≤55 | 1338 (52) | 1150 (60) | 1 [Reference] | |
| >55 | 1215 (48) | 766 (40) | 1.64 (1.44-1.87) | |
| Race | ||||
| White | 1713 (67) | 967 (50) | 1 [Reference] | |
| Black | 381 (15) | 256 (13) | 0.98 (0.84-1.16) | |
| Other | 459 (18) | 693 (36) | 0.73 (0.63-0.86) | |
| Insurance type | ||||
| Private | 1334 (52) | 1048 (55) | 0.66 (0.52-0.82) | |
| Medicare | 582 (23) | 339 (18) | 1.17 (0.92-1.50) | |
| Medicaid | 329 (13) | 347 (18) | 1.03 (0.80-1.33) | |
| Other governmental | 57 (2) | 32 (2) | 1.01 (0.65-1.57) | |
| Uninsured | 200 (8) | 113 (6) | 1 [Reference] | |
| Unknown | 51 (2) | 37 (2) | ||
| Annual income | ||||
| <$38 000 | 540 (21) | 307 (16) | 1 [Reference] | |
| $38 000-$47 999 | 638 (25) | 401 (21) | ||
| $48 000-$62 999 | 656 (26) | 485 (25) | 0.84 (0.75-0.95) | |
| ≥$63 000 | 689 (27) | 699 (36) | ||
| Unknown | 30 (1) | 24 (1) | ||
| Location | ||||
| Metro | 2039 (80) | 1702 (89) | 1 [Reference] | |
| Urban | 405 (16) | 136 (7) | 0.18 (0.15-0.23) | |
| Rural | 37 (1) | 25 (1) | 0.15 (0.08-0.28) | |
| Unknown | 72 (3) | 53 (3) | 1.49 (0.89-2.48) | |
| Year of diagnosis | 0.82 (0.73-0.93)b | |||
| 2004-2008 | 1013 (40) | 777 (41) | ||
| 2009-2013 | 1540 (60) | 1139 (59) | ||
| Charlson-Deyo Score | ||||
| 0 | 2179 (85) | 1700 (89) | 1 [Reference] | 1 [Reference] |
| 1 | 294 (12) | 177 (9) | 0.75 (0.61-0.92) | 1.23 (1.04-1.46) |
| ≥2 | 80 (3) | 39 (2) | 0.83 (0.56-1.21) | 1.52 (1.15-2.01) |
| Tumor grade | ||||
| Well differentiated | 50 (2) | 34 (2) | 1 [Reference] | 1 [Reference] |
| Moderately differentiated | 339 (13) | 174 (9) | ||
| Poorly differentiated | 953 (37) | 575 (30) | 1.33 (1.11-1.60) | 0.72 (0.62-0.83) |
| Undifferentiated | 534 (21) | 590 (31) | ||
| Unknown | 677 (27) | 543 (28) | ||
| Clinical stage | ||||
| II | 352 (14) | 186 (10) | 1 [Reference] | |
| III | 1066 (42) | 821 (43) | 1.44 (1.09-1.90) | |
| IV (substage unspecified) | 46 (2) | 45 (2) | 2.03 (1.31-3.15) | |
| IVA | 713 (28) | 598 (31) | 2.12 (1.60-2.82) | |
| IVB | 376 (15) | 266 (14) | 2.55 (1.90-3.41) | |
| Treatment at HVF | ||||
| Yes | 0 | 1916 | NA | 0.85 (0.75-0.96) |
| No | 2553 | 0 | NA | 1 [Reference] |
Abbreviations: HVF, high-volume facility; LVF, lower-volume facility; NA, not applicable.
Percentages may not add up to 100% owing to rounding. Multivariable analyses contain factors present in the final model.
Parameters were analyzed as a continuous variable for multivariable analysis.
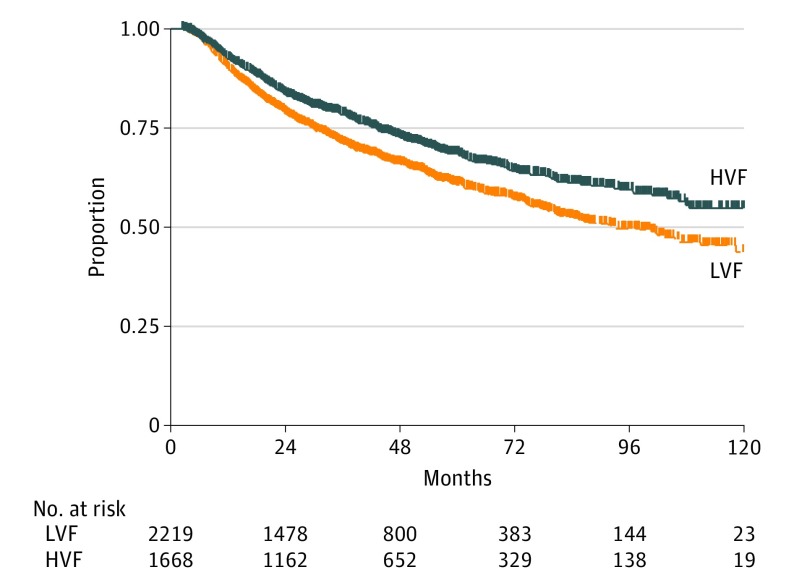
Median follow-up was 45 (range, 3-130) months. The difference in 3- and 5-year OS was 6% (95% CI, 3%-11%), and the difference in 5-year OS was 7% (95% CI, 3%-13%) in favor of patients receiving care at an HVF (Figure).
Figure. Overall Survival Between Populations Based on Facility Volume.
Abbreviations: HVF, high-volume facility; LVF, lower-volume facility.
Independent characteristics associated with higher OS on multivariable analysis (Table) included younger age (continuous variable; hazard ratio [HR], 1.64; 95% CI, 1.44-1.87), private insurance (HR, 0.66; 95% CI, 0.52-0.82), higher income (HR, 0.84; 95% CI, 0.75-0.95), fewer comorbidities, lower clinical stage, diagnosis in more recent years (HR, 0.82; 95% CI, 0.73-0.93), and nonwhite/black race (HR, 0.73; 95% CI, 0.63-0.86). Treatment at an HVF was an independent predictor of higher OS (HR, 0.85; 95% CI, 0.75-0.96).
On sensitivity analysis, increasing the cutoff of HVF definition from the 80th percentile (11 patients per facility over the time period) was still associated with an independent impact on the multivariable model. As the cutoff was lowered, the strength of association slightly decreased (for 10 patients: HR, 0.89; 95% CI, 0.79-1.00; for 9 patients: HR, 0.91; 95% CI, 0.81-1.03; for 8 patients: HR, 0.92; 95% CI, 0.82-1.04).
Discussion
Multimodality treatment of NPC is challenging for many reasons, and in this investigation we demonstrate that treatment at a high-volume center is independently associated with improved prognosis. These data have notable ramifications for multidisciplinary oncologic providers, in addition to patient counseling by both referring and treating clinicians.
There are likely multiple reasons to explain improved outcomes at an HVF. Streamlined diagnostic processes at HVFs could prevent treatment delays, which may influence prognosis in NPC. Treatment planning is another potential advantage because considerable skill and experience is required in ensuring adequately high doses to clinical and subclinical disease—amidst an exquisitely unique anatomic area with proximity to numerous critical organs-at-risk. Clinicians at HVFs may use more evidence-based approaches and may have more experience in coordinating multimodality and intraspecialty care. Greater ancillary support staff for services, such as nutritional support, or for more aggressive clinical monitoring could contribute, and closer monitoring of toxic effects may be correlated with fewer missed RT/chemotherapy sessions. In addition, the quality of RT delivered at HVFs may correlate with outcomes in locally-advanced head and neck neoplasms because deficiencies in treatment planning are more common at LVFs and impact survival. Finally, the availability of clinical trials and/or willingness to reirradiate locoregional failures could play into more effective salvage therapies at HVFs.
However, the above notions must be contextualized with potential confounders herein, namely that patients with higher comorbidities, along with those living in urban and/or rural areas (which may not have optimal oncologic follow-up), were less likely to be treated at HVFs. Although these factors are important, it is worth mentioning that there were no differences in tumor stage between cohorts noted in this study. It is also noteworthy that localized-stage (eg, II and some stage III) patients were included, implying that the benefit of facility volume is relatively generalizable, and not limited only to those patients with the most complex radiotherapy treatment volumes or advanced bulky disease (or larger amounts of uninvolved, electively-treated mucosal sites) that may receive a higher volume of high-dose radiotherapy.
With regard to a health disparity and health access standpoint, a major finding on multivariable logistic regression was that patients living in both urban and rural areas were less likely to receive treatment at an HVF; patients undergoing CRT at an HVF lived in metro areas and commuted farther distances. Although understandable that rural patients may have more limited access to an HVF, that many HVFs tend to be at major urban population centers should ideally signal further investigation into the root causes of whether some HVFs could be serving comparatively less of the urban population, and whether outreach programs could be developed to improve access. It is also important that we did not observe any racial or socioeconomic disparities on multivariable analysis impeding access to HVFs, which is a noteworthy finding in itself to affirm the programs specifically designed to address health disparities in NPC.
Limitations
This retrospective analysis is limited by the overall low numbers of treated patients (averaging just under 5 patients per institution in the studied time frame), especially compared with areas of the world where NPC is endemic. Hence, its applicability outside of the United States (where NPC is relatively rare) is limited; it can also be questioned whether such low case volumes are indeed competency maintaining or truly indicative of high-volume centers status. Nevertheless, the OS in HVFs herein is comparable to high-volume prospective data, and sensitivity analysis demonstrates that thresholds for facility volume also impacts OS, implying further advantages with the largest HVFs. Other shortcomings include the NCDB’s lack of information on Epstein-Barr virus status of the tumor cells; WHO classification of tumor histology; smoking history; chemotherapy type, amount, or timing (eg, induction); salvage therapies; coordination of multimodality care; diagnostic workup; and supportive services. Radiation technique also is not well defined in the database, including use of intensity-modulated radiotherapy between HVFs and LVFs, which may also associate with outcomes. Nevertheless, these data have notable ramifications for multidisciplinary oncologic providers, in addition to patient counseling by both referring and treating clinicians.
Conclusions
Using the National Cancer Database, this is the first investigation, to our knowledge, demonstrating that treatment at a high-volume center was a significant predictor of survival in patients with nasopharyngeal cancer, which persisted on matched analysis. These data have implications on multidisciplinary management as well as patient counseling by referring and treating clinicians.
References
- 1.Tuggle CT, Patel A, Broer N, Persing JA, Sosa JA, Au AF. Increased hospital volume is associated with improved outcomes following abdominal-based breast reconstruction. J Plast Surg Hand Surg. 2014;48(6):382-388. [DOI] [PubMed] [Google Scholar]
- 2.Chen YW, Mahal BA, Muralidhar V, et al. . Association between treatment at a high-volume facility and improved survival for radiation-treated men with high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2016;94(4):683-690. [DOI] [PubMed] [Google Scholar]
- 3.Haque W, Verma V, Butler EB, Teh BS. Definitive chemoradiation at high volume facilities is associated with improved survival in glioblastoma. J Neurooncol. 2017. doi: 10.1007/s11060-017-2563-0 [DOI] [PubMed] [Google Scholar]
- 4.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network Head and neck cancers. Version 1.2017. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed March 7, 2017. [DOI] [PMC free article] [PubMed]
- 6.Peters LJ, O’Sullivan B, Giralt J, et al. . Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J Clin Oncol. 2010;28(18):2996-3001. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard P, Lee A, Marguet S, et al. ; MAC-NPC Collaborative Group . Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645-655. [DOI] [PubMed] [Google Scholar]