Key Points
Question
Do patients with head and neck cancer (HNC) have short- or long-term neurocognitive deficits after treatment?
Findings
In this prospective 2-year longitudinal study that included 80 newly diagnosed patients with HNC, 38% of patients showed neurocognitive decline at 24 months.
Meaning
People treated for HNC are at risk of neurocognitive sequelae for at least 2 years after treatment; further research is warranted in search of strategies to avoid and reduce the risk for decline.
This prospective, longitudinal study assesses objective neurocognitive function before and after definitive radiation therapy for patients with head and neck cancer.
Abstract
Importance
Neurocognitive deficits (NCD) have been observed in noncentral nervous system cancers, yet short- and long-term neurocognitive data on patients treated for head and neck cancer (HNC) are lacking.
Objective
To assess objective neurocognitive function before and after definitive radiation therapy for HNC.
Design, Setting, and Participants
In a prospective, longitudinal study, neurocognitive function and self-reported symptoms were assessed in 80 patients with histologically proven HNC requiring definitive chemoradiotherapy or radiotherapy and in 40 healthy controls 4 times (baseline, 6, 12, and 24 months after baseline) prior to commencing treatment at Princess Margaret Cancer Centre, Toronto, Canada.
Main Outcomes and Measures
Neurocognitive test scores were converted to age-corrected z scores (mean, 0; standard deviation, 1) and reported as mean scores, standardized regression-based scores, and frequencies of impairments in intellectual capacity, concentration, memory, executive function, processing speed, and motor dexterity. Multivariable analysis was used to identify factors associated with NCD 2 years after treatment.
Results
Eighty patients and 40 healthy controls enrolled. Analyses revealed significant differences between patient and control mean performance in some domains, with patient deficits increasing over time: intellectual capacity (Cohen d, effect sizes [95% CIs] of −0.46 [−0.64 to 0.30], −0.51 [−0.72 to −0.30], and −0.70 [−0.92 to −0.49] for time points 6, 12, and 24 months, respectively); concentration/short-term attention span (−0.19 [−0.37 to 0.00], −0.38 [−0.55 to −0.21], −0.54 [−0.71 to −0.37]); verbal memory (−0.16 [−0.33 to 0.02], −0.38 [−0.64 to −0.12], −0.53 [−0.74 to −0.32]); executive function (−0.14 [−0.27 to 0.00], −0.34 [−0.52 to −0.16], −0.43 [−0.64 to −0.22]), and global cognitive function composite (−0.38 [−0.55 to −0.22], −0.75 [−0.92 to −0.58], −1.06 [−1.26 to −0.86]). There was an increased rate of impaired global neurocognitive functioning among patients (38%) at 24 months compared with controls (0%). Neurocognitive deficits were not associated with baseline cytokines.
Conclusions and Relevance
Head and neck cancer survivors have neurocognitive sequelae up to 2 years after definitive chemoradiotherapy or radiation treatment. Patients and health care teams should know about such potential risks. Further research is warranted in search of strategies to avoid, reduce, and compensate for declines.
Introduction
As cancer survival rates continue to improve, long-term adverse effects of treatment on health and quality of life are increasingly important considerations for clinicians, patients, and family members. Neurocognitive deficits (NCD) are well established as a common adverse effect in brain tumor survivors, owing to the tumor itself, but also localized brain irradiation treatment. Prophylactic brain radiation for other cancers is also associated with delayed cognitive impairment. More recent evidence suggests NCD after treatment for breast cancer and hematological malignant abnormalities and deficits can include concentration and/or short-term attention span, memory, processing speed, and executive function.
Head and neck cancer (HNC) incidence is rising, secondary to an epidemic of human papillomavirus (HPV). Fortunately, cure rates of HNC are rising as well, and the 5-year overall survival rate across all stages is now above 64%. However, we know very little about long-term neurocognitive functioning in these survivors. They may be at risk for NCD related to the increasing role of chemoradiation to achieve organ preservation and the involvement of central nervous system (CNS) structures in the radiation field. In addition, a higher susceptibility for NCD in this population might be present because HNC risk factors, such as alcohol consumption and tobacco smoking, are also associated with neurocognitive impairment.
A few studies have assessed neurocognitive function in HNC patients. They suggest that HNC patients may be at risk of cognitive impairment after treatment, but several design limitations made findings ambiguous. The International Cognition and Cancer Task Force issued several recommendations to strengthen interpretability of these types of studies, including implementation of a longitudinal design that includes pretreatment assessment, objective and self-report measures, and a comparison group to estimate expected practice effects. Use of more than 1 method to analyze longitudinal data, including standardized regression-based models and frequencies of deficits over time, also is recommended.
In accordance with these recommendations, we conducted a prospective, longitudinal study using a comprehensive battery of validated and standardized measures to assess neurocognitive function in patients with HNC undergoing definitive chemoradiation or radiation and in noncancer controls.
Methods
Participants
People with newly diagnosed, histologically proven, nonmetastatic squamous cell HNCs (other than nasopharyngeal cancers) were enrolled prior to commencing definitive chemoradiation or radiation at Princess Margaret Cancer Center, Toronto, Canada. Noncancer participants were recruited by patients nominating a friend or family member within 5 years of their age or through advertisements posted on public bulletin boards in and around the hospital. They were offered a stipend for participation. The study was approved by the University Health Network Research Ethics Board. All participants provided written informed consent.
Inclusion criteria for all participants were age 18 years or older; Eastern Cooperative Oncology Group (ECOG) performance status 0 to 1; adequate organ function (bilirubin below upper limit of normal [ULN]), and liver function (aspartate aminotransaminase, alanine aminotransaminase, alkaline phosphatase), and creatinine within 2.5× ULN; and ability to provide informed consent.
Exclusion criteria were uncontrolled infection, diagnosed neurological or psychiatric condition affecting cognition (eg, stroke, dementia, moderate or severe traumatic brain injury, concussion within past 5 years, schizophrenia), previous systemic chemotherapy, diagnosis of other malignant disease, or insufficient hearing, vision, or English language skills to complete assessment.
Anticancer treatments, at the treating physician’s discretion, included standard therapy (radiation alone or combined with cisplatin, according to tumor site, stage, and comorbidities). Some patients received accelerated fractionation radiation concurrently with the antiepidermal growth factor receptor antibody panitumumab in the context of an investigational therapy.
Procedures
Participant characteristics, including age, sex (male vs female), ECOG performance status (0 vs 1), HPV status (positive vs negative), disease stage (II/III vs IV), disease site (oropharynx vs others), smoking history (never vs ≤10 pack years vs >10 pack years), alcohol intake (≤10 units vs >10 units per week), comorbidities (heart disease, hypertension, diabetes mellitus, all yes vs no), remote concussion (yes vs no), and education (<high school, high school, university, postgraduate) were based on participants’ self-reporting or medical records.
Details of diagnosis, radiation dose to the tumor, radiation exposure of CNS, type of systemic treatment, and cumulative dose of cisplatin or panitumumab were collected from medical records.
Neurocognitive assessments were done at 4 time points: baseline (within 2 weeks prior to start of treatment), end of treatment (6 months after baseline evaluation), 12 months after baseline, and 24 months after baseline. Controls completed assessments at similar intervals.
Participants provided blood samples at the 4 time points for measurement of 20 variables including hemoglobin, thyroid stimulating hormone, and vitamin B12. Plasma levels of 10 cytokines (interleukin [IL]-1b, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α, IFN-γ, GM-CSF) were assessed at baseline and 24 months using LiquiChip Human10-cytokine kit (Qiagen). The serum parameter details are available in the eMethods section of the Supplement.
Measures
Details of the 90-minute neurocognitive test battery are shown in eTable 1 in the Supplement. The battery is feasible in HNC and includes instruments recommended for use in cancer populations. Each cognitive domain was assessed by at least 2 objective measures to improve validity of interpretation. To attenuate practice-related improvements from serial retesting, psychometrically matched alternate forms (equivalent reliability, validity, difficulty) were used when available and switched at each assessment, with half of all participants starting with 1 version. Domains assessed were: intellectual capacity, concentration/short-term attention span, visual memory, verbal memory, processing speed, executive function, and motor dexterity. A Global Cognitive Function (GCF) composite score was calculated using the mean score across domains. Participants also completed self-reported questionnaires assessing cognitive function (Functional Assessment of Cancer Therapy [FACT-COG3] total), head and neck specific symptoms (FACT-HN for patients), fatigue (Functional Assessment of Chronic Illness Therapy [FACIT-fatigue]), depression and anxiety (Hospital Anxiety Depression scale [HADS]).
Statistical Analysis
A pragmatic sample size of 80 patients and 40 healthy, noncancer controls was selected to ensure sufficient power to detect a moderate or larger effect; eg, a 2-sample (α = .05, 2-sided) t test would have at least 80% power to detect a moderate effect size of .55 or more between groups, or accounting for expected drop-outs and lost to follow-up patients, a large effect size of .70 or more.
Neurocognitive raw test scores were normalized to age-corrected z scores using published normative data. A z score of 0 equates to test performance equivalent to that obtained by the mean (50th percentile) of that individual’s age group, while a z score of −1.64 and 1.64 corresponds to 5th and 95th percentiles, respectively. The z scores of tests measuring the same cognitive domain were averaged to generate a domain score.
A random effects model was used to examine cohort and time effects. Effect sizes along with 95% CIs were calculated to evaluate differences in demographics, domain scores and frequencies of cognitive decline at each time point between patients and controls. Cohen d and odds ratios were calculated for continuous and categorical characteristics respectively. Because several variables are nonsymmetric, Spearman rank correlations were used to examine associations between neurocognitive performance, self-report symptoms, and cytokine levels. Effect sizes and CIs were calculated based on ranked data. Exploratory linear regression was used to test for baseline demographic and disease characteristics prognostic for subsequent NCD in univariable and multivariable models.
Three statistical approaches were undertaken to evaluate and compare patient and control data. The first compared the mean z scores of patients and controls at each time point. The second used standardized regression-based (SRB) models that delineate normal, expected change across assessments based on the control cohort, and yield standardized z scores, representative of the number of standardized units above or below the expected change, adjusted for baseline performance, age, education, and depression scores. The SRB scores control for practice effects that occur with repeated testing and enable determination of whether patients perform differently than expected over time. This method is appropriate with serial neuropsychological testing owing to retest-related improvements, and is suitable when the cohorts are not matched on factors that may impact learning, such as age and education. The third method analyzed frequencies of neurocognitive decline (defined as percentage of participants who drop SRB score >1.64 from baseline) for each domain and at each time point. This provides an estimate of the likelihood that an individual will decline.
Effects of missing data and withdrawal were explored through supportive analyses using only those patients who completed all 4 neurocognitive assessments. All CIs were 2-sided. Given the exploratory nature of these analyses, no adjustments were performed for multiple comparisons.
Results
Cohort Characteristics
Eighty patients with HNC and 40 noncancer controls completed baseline evaluation. Baseline characteristics, including demographics, and patient clinical and treatment details are summarized in Table 1. Age range of patients and controls were identical (41-75 years), although patient group mean age was 3.7 years older. Patients and controls who completed all 4 assessments were equivalent in age (eTable 2 in the Supplement). Patients were less educated and had higher cigarette and alcohol consumption.
Table 1. Participant Characteristics.
| Baseline Characteristic | Patient Group | Control Group | Difference (95% CI) |
|---|---|---|---|
| No. (%) | 80 | 40 | |
| Age | |||
| Median (range) | 59 (41-76) | 54.5 (41-75) | |
| Mean (SD) | 58.3 (7.6) | 54.6 (8.4) | 3.7 (0.6 to 6.8) |
| Distribution (%) | |||
| <50 | 11 (14) | 11 (28) | |
| 50-59 | 34 (43) | 16 (40) | |
| 60-69 | 30 (38) | 12 (30) | |
| ≥70 | 5 (6) | 1 (3) | |
| Sex | |||
| Male, No. (%) | 68 (85) | 35 (88) | −2.5 (−15.3 to 14.0) |
| Education (coded no.) | −30.0 (−47.5 to −9.8)a | ||
| <High school | 17 (21) | 3 (8) | |
| High school with or without some college | 39 (49) | 13 (33) | |
| University degree | 15 (19) | 17 (43) | |
| Postgraduate degree | 9 (11) | 7 (18) | |
| ECOG performance status | |||
| 0 | 44 (55) | NA | NA |
| 1 | 32 (40) | ||
| 2 | 1 (1) | ||
| Missing | 3 (4) | ||
| Cigarette history (pack-years) | |||
| Median (range) | 17.5 (0-80) | 0 (0-56) | 14.5 (9.0 to 20.0) |
| Smoking status | |||
| Never smoker | 29 (36) | 24 (60) | 26.3 (6.2 to 42.5)b |
| ≤10 Pack-years | 10 (13) | 6 (15) | |
| >10 Pack-years | 41 (51) | 10 (25) | |
| Alcohol units per week | |||
| Mean (SD) | 16.2 (19) | 6.9 (5) | 16.3 (−3.4 to 32.7)c |
| Median (range) | 9 (0-140) | 9 (0-20) | |
| ≤10 | 47 (59) | 30 (75) | |
| >10 | 33 (41) | 10 (25) | |
| Comorbidities (heart disease, renal disease, hypertension, diabetes), No. | |||
| 0 | 49 (61) | 36 (90) | 28.8 (10.8 to 42.2)d |
| 1 | 16 (20) | 4 (10) | |
| ≥2 | 15 (19) | 0 (0) | |
| Remote concussion, No. (%) | 15 (19) | 8 (20) | −1.3 (−19.1 to 13.6) |
| Disease and treatment | |||
| Site | NA | NA | |
| Hypopharynx | 5 (6) | ||
| Oropharynx | 61 (76) | ||
| Laryngeal | 7 (9) | ||
| Nasal cavity | 2 (3) | ||
| Unknown primary | 5 (6) | ||
| HPV status | NA | NA | |
| Positive | 46 (58) | ||
| Negative | 11 (14) | ||
| Unknown | 23 (29) | ||
| Stage | NA | NA | |
| II | 3 (4) | ||
| III | 5 (6) | ||
| IVa | 70 (87) | ||
| IVb | 2 (3) | ||
| Max radiation dose to target | NA | NA | |
| 70 Gy in 35 fractions | 71 (89) | ||
| Other | 9 (11) | ||
| Max radiation dose to brain | |||
| Median highest drug concentration, Gy (range) | 56 (0.6-77.3) | ||
| Systemic treatment | NA | NA | |
| Cisplatin | 49 (61) | ||
| Carboplatin with or without fluorouracil | 2 (3) | ||
| Panitumumab | 18 (22) | ||
| None | 11 (14) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NA, not applicable.
At least university education versus less than university.
>10 Pack-years of smoking versus ≤10 pack-years of smoking.
>10 Units of alcohol per week versus ≤10 units of alcohol per week.
≥1 Comorbidity versus 0 comorbidities.
Most patients had oropharyngeal SCC (76%); most had stage IVa and received cisplatin-based chemoradiation (61%).
By 24 months, 8 participants had died (7 patients) and 14 had withdrawn (10 patients) (eFigure in the Supplement).
Baseline Neurocognitive Results
Neurocognitive domain z scores and standard deviations at baseline and subsequent time points are shown in Table 2. Details of baseline results will be published elsewhere (Razak et al, under review), but to summarize, patients and controls were broadly comparable with age-matched population norms (z score, −1.34 to 1.34, which corresponds to 9th-91st percentiles) on all domains. Patients had less formal education and were slightly older, but they performed similarly to controls at baseline in all domains except intellectual capacity, with patients performing better (z score, 0.55 vs 0.19; d = 0.45; 95% CI, 0.31 to 0.60).
Table 2. Neurocognitive Function Mean (SD) z Scores for Patients and Controls at Each Time Pointa.
| Neurocognitive Domain | Baseline | 6 Months | 12 Months | 24 Months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P (SD) | C (SD) | d (CI) | P (SD) | C (SD) | d (CI) | P (SD) | C (SD) | d (CI) | P (SD) | C (SD) | d (CI) | |
| Intellectual capacity | 0.55 (0.80) | 0.19 (0.80) | 0.45 (0.31 to 0.60) |
0.45 (0.79) | 0.39 (0.74) | 0.08 (−0.06 to 0.22) |
0.53 (0.74) | 0.57 (0.71) | −0.06 (−0.19 to 0.08) |
0.40 (0.89) | 0.58 (0.69) | −0.22 (−0.38 to −0.06) |
| Concentration/short-term attention span | −0.30 (0.62) | −0.41 (0.56) | 0.19 (0.08 to 0.29) |
−0.28 (0.71) | 0.36 (0.58) | −0.96 (−1.09 to −0.84) |
−0.26 (0.62) | −0.24 (0.66) | −0.03 (−0.15 to 0.09) |
−0.35 (0.77) | −0.20 (0.69) | −0.20 (−0.35 to −0.06) |
| Verbal memory | −1.03 (1.27) | −1.04 (1.08) | 0.01 (−0.21 to 0.22) |
−1.01 (1.25) | −0.76 (1.08) | −0.21 (−0.43 to 0.01) |
−1.08 (1.26) | −0.59 (0.84) | −0.44 (−0.65 to −0.22) |
−1.08 (1.27) | −0.46 (0.94) | −0.54 (−0.77 to −0.31) |
| Visual memory | −0.96 (1.02) | −1.02 (1.05) | 0.06 (−0.12 to 0.24) |
−0.76 (0.92) | −0.96 (0.97) | 0.21 (0.05 to 0.39) |
−0.44 (1.22) | −0.46 (1.08) | 0.02 (−0.21 to 0.24) |
−0.66 (1.11) | −0.34 (1.01) | −0.30 (−0.51 to −0.09) |
| Processing speed | 0.07 (0.77) | 0.08 (0.82) | −0.01 (−0.15 to 0.13) |
0.13 (0.83) | 0.12 (0.82) | 0.01 (−0.14 to 0.16) |
0.13 (0.86) | 0.19 (0.81) | −0.07 (−0.23 to 0.09) |
0.17 (0.88) | 0.25 (0.83) | −0.09 (−0.26 to 0.08) |
| Executive function | 0.09 (0.81) | 0.01 (0.82) | 0.10 (−0.05 to 0.24) |
0.14 (0.73) | 0.03 (0.84) | 0.15 (0.01 to 0.28) |
0.15 (0.78) | 0.16 (0.80) | −0.01 (−0.16 to 0.14) |
0.08 (0.81) | 0.15 (0.78) | −0.09 (−0.25 to 0.07) |
| Motor dexterity | −0.75 (0.85) | −0.53 (0.89) | −0.26 (−0.41 to −0.10) |
−0.69 (0.96) | −0.32 (0.95) | −0.39 (−0.56 to −0.22) |
−0.64 (0.97) | −0.22 (0.95) | −0.44 (−0.62 to −0.26) |
−0.68 (1.03) | −0.18 (0.90) | −0.51 (−0.71 to −0.32) |
| Global cognitive function composite score | −0.33 (0.63) | −0.39 (0.52) | 0.10 (−0.00 to 0.21) |
−0.29 (0.59) | −0.27 (0.53) | −0.04 (−0.14 to 0.07) |
−0.23 (0.63) | −0.08 (0.52) | −0.25 (−0.37 to −0.14) |
−0.31 (0.70) | −0.03 (0.54) | −0.44 (−0.56 to −0.31) |
Abbreviations: C, control group; OR, odds ratio; P, patients; UND, undefined; d, Cohen d effect size.
A significant neurocognitive decline is defined as SRB score decrease of ≥1.64 from baseline.
Longitudinal Neurocognitive Assessment
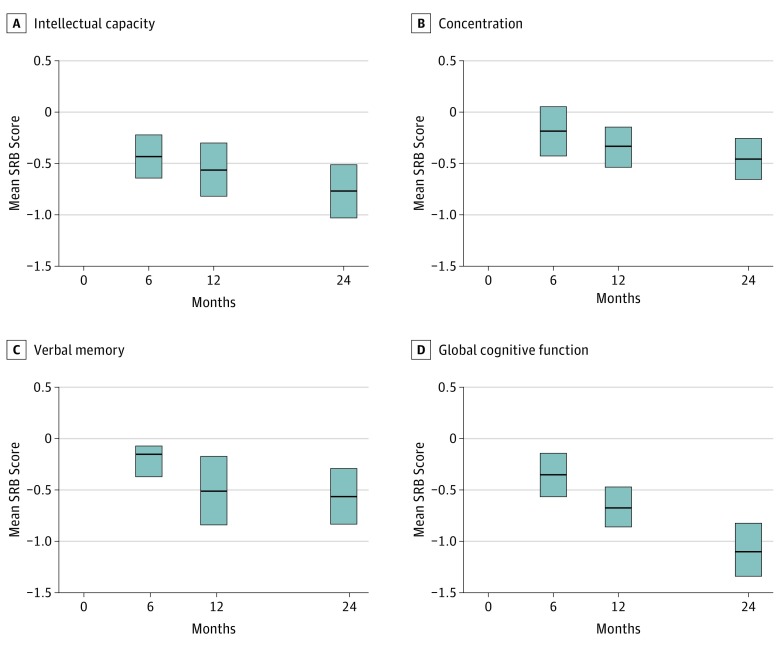
Interactions were observed between cohort and time in the random effects model for intellectual capacity, concentration, executive function, verbal memory, visual memory, and global cognitive function composite score. Mean SRB results adjusted for baseline performance, age, and education for patients are shown in Figure 1 and for all participants in eTables 3 and 4 in the Supplement at all time points separately. Patients demonstrated declines in GCF (d = −0.38; 95% CI, −0.55 to −0.22; d = −0.75; 95% CI, −0.92 to −0.58; and d = −1.06; 95% CI, −1.26 to −0.86 at time points 6 months, 12 months, and 24 months, respectively), intellectual capacity (d = −0.46; 95% CI, −0.64 to −0.30; d = −0.51; 95% CI, −0.72 to −0.30; and d = −0.70; 95% CI, −0.92 to −0.49, respectively), concentration/short-term attention span (d = −0.19; 95% CI, −0.37 to 0.00; d = −0.38; 95% CI, −0.55 to −0.21; and d = −0.54; 95% CI, −0.71 to −0.37, respectively), verbal memory (d = −0.16; 95% CI, −0.33 to 0.02; d = −0.38; 95% CI, −0.64 to −0.12; and d = −0.53; 95% CI, −0.74 to −0.32, respectively), executive function (d = −0.14; 95% CI, −0.27 to −0.00; d = −0.34; 95% CI, −0.52 to −0.16; and d = −0.43; 95% CI, −0.64 to −0.22, respectively), and motor dexterity (d = −0.22; 95% CI, −0.40 to −0.04; d = −0.51; 95% CI, −0.69 to −0.33; and d = −0.28; 95% CI, −0.49 to −0.06, respectively). Patients performed as well as controls at all time points in processing speed and visual memory. Results were similar after adjusting for baseline depression scores (eTable 3 in the Supplement).
Figure 1. Standardized Regression Based (SRB) Performance Scores Over Time, Adjusted for Baseline Scores, Age, and Education.
A, Intellectual capacity; B, concentration/short-term attention span; C, verbal memory; D, global cognitive function (GCF). Control group SRBs are 0 by definition. Bars show the 95% CIs.
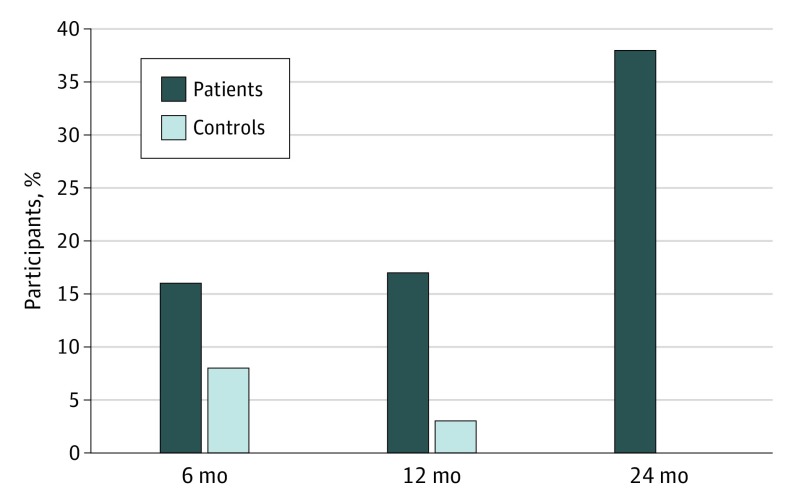
The frequency of individuals with declines at different time points was higher in the patient cohort, as shown in Table 3 and Figure 2. At 6 months the cohorts had comparable frequencies of decline in all domains, whereas at 24 months more patients declined in intellectual capacity (27% patients vs 3% controls; odds ratio[OR], 13.0; 95% CI, 1.6 to 102.7), verbal memory (21% vs 3%; OR, 9.3; 95% CI, 1.1 to 74.9), processing speed (12% vs 0%; OR, undefined), executive function (26% vs 6%; OR, 5.8; 95% CI, 1.2 to 26.9), motor dexterity (10% vs 0%; OR, undefined), and global cognitive function (38% vs 0%, OR, undefined).
Table 3. Number of Patients and Controls With Significant Neurocognitive Function Decline From Baseline.
| Neurocognitive Domain | 6 Months | 12 Months | 24 Months | ||||||
| P (%) | C (%) | OR (95% CI) | P (%) | C (%) | OR (95% CI) | P (%) | C (%) | OR (95% CI) | |
| Intellectual capacity | 12/66 (18) | 2/38 (5) | 4.0 (0.8-18.9) | 16/63 (25) | 1/35 (3) | 11.6 (1.5-91.5) | 16/58 (27) | 1/35 (3) | 13.0 (1.6-102.7) |
| Concentration/short-term attention span | 6/66 (9) | 1/38 (2) | 3.7 (0.4-32.0) | 4/63 (6) | 2/35 (6) | 1.1 (0.2-6.4) | 4/58 (7) | 1/35 (3) | 2.5 (0.3-23.5) |
| Verbal memory | 8/64 (12) | 2/38 (5) | 2.6 (0.5-12.8) | 19/62 (30) | 2/35 (6) | 7.3 (1.6-33.5) | 12/56 (21) | 1/35 (3) | 9.3 (1.1-74.9) |
| Visual memory | 3/66 (4) | 3/38 (8) | 0.6 (0.1-2.9) | 4/63 (6) | 0/35 | UND | 3/57 (5) | 1/35 (3) | 1.9 (0.2-18.9) |
| Processing speed | 8/66 (12) | 1/38 (2) | 5.1 (0.6-42.5) | 3/63 (5) | 1/35 (3) | 1.7 (0.2-17.0) | 7/58 (12) | 0/35 | UND |
| Executive function | 1/66 (1) | 2/38 (5) | 0.3 (0.0-3.2) | 7/63 (10) | 2/35 (6) | 2.1 (0.4-10.5) | 15/58 (26) | 2/35 (6) | 5.8 (1.2-26.9) |
| Motor dexterity | 6/64 (9) | 0/38 | UND | 5/62 (8) | 2/35 (6) | 1.4 (0.3-7.9) | 6/57 (10) | 0/35 | UND |
| Global cognitive function composite score | 11/66 (16) | 3/38 (8) | 2.3 (0.6-9.0) | 11/63 (17) | 1/35 (3) | 7.2 (0.9-58.3) | 22/58 (38) | 0/35 | UND |
Abbreviations: C, Control group; OR, odds ratio; NA, not applicable; P, patients; UND, undefined; d, Cohen d effect size.
A significant neurocognitive decline is defined as SRB score decrease of ≥1.64 from baseline.
Figure 2. Percentage of Participants With Decline in Global Cognitive Functioning .
Decline defined as change in standardized score of −1.64 or more from baseline.
A subgroup analysis of participants who completed all 4 assessments (50 patients, 35 controls) was performed. The results supported these findings in both SRB and frequency analyses, with similar outcomes (eTable 4 in the Supplement).
Self-reported Outcomes
Self-reported symptoms of cognitive function, fatigue, anxiety, depression, and head and neck symptoms are reported in eTable 5 in the Supplement. At baseline, patients reported worse cognition (mean FACT-COG of 102.1 vs 116.9; d = −0.71; 95% CI, 0.34 to 1.13), and higher fatigue (mean FACIT-F of 41.5 vs 37.5; d = 0.92; 95% CI, 0.52 to 1.31), depression (mean 3.86 vs 1.33; d = 0.76; 95% CI, 0.37 to 1.16), and anxiety (mean, 6.99 vs 3.35; d = 1.00; 95% CI, 0.60 to 1.40) than controls, and patients continued to express greater symptoms than controls across all time points. Head and neck cancer-specific symptoms (only assessed in patients) were greater at all posttreatment assessments than at baseline (mean FACT-HN of 10.48, 18.12, 15.98, and 15.52 at baseline, 6, 12, and 24 months, respectively). Standardized regression-based analyses to identify change in symptoms in patients suggested no clinically meaningful changes in self-reported symptoms of cognition, fatigue, depression, or anxiety at the follow-up assessments.
Prognostic Factors of Neurocognitive Decline
Separate exploratory multivariable analyses assessing possible prognostic factors of NCD were conducted for 4 different subsets of baseline variables: clinical/demographic (age, sex, education, smoking, alcohol consumption, medical comorbidities, concussion history), self-reported symptoms, and serum markers (baseline cytokines as described, TSH, folic acid, and sex hormones) (eTable 6 in the Supplement).
Lower education and higher baseline depression were the only patient characteristics prognostic for patients’ NCD across multiple domains. Education was prognostic for intellectual capacity (estimate, 0.52; 95% CI, 0.35 to 0.69), verbal memory (estimate, 0.41; 95% CI, 0.22 to 0.62), visual memory (estimate, 0.26; 95% CI 0.10 to 0.43), executive function (estimate, 0.26; 95% CI, 0.08 to 0.44), and GCF (estimate, 0.46; 95% CI, 0.26 to 0.65), and baseline depression level was prognostic for intellectual capacity (estimate, −0.07; 95% CI, −0.02 to −0.12), concentration (estimate, −0.10; 95% CI, −0.05 to −0.15), processing speed (estimate, −0.12; 95% CI, −0.07 to −0.16), executive function (estimate, −0.07; 95% CI, −0.02 to −0.12), and GCF (estimate, −0.12; 95% CI, −0.07 to −0.17).
Correlations between treatment parameters and neurocognitive outcomes were examined. We noticed no consistent pattern to suggest reliable risk between receiving any particular chemotherapy regimen or radiation dose and having greater NCD.
Discussion
In this prospective longitudinal study of neurocognitive function in patients with HNC and noncancer controls, findings indicate that neurocognitive function, although not immediately affected after treatment, progressively declines in the 2 years after definitive treatment with chemotherapy or radiation. In all domains (except visual memory) decline was documented by the SRB score, frequency of decliners, or both. The GCF frequency results are especially concerning, suggesting that patients are at high risk of suffering from delayed and progressive neurocognitive sequelae 2 years after treatment.
Efforts were made to recruit participants in the control group with similar demographic and socioeconomic factors, yet, of the participants who completed all assessments, the control group was more educated, and had less alcohol consumption history. Despite this potential imbalance that favors the control group, the groups performed similarly at baseline in all cognitive domains except intellectual capacity, where the patient group performed better. The longitudinal test results were analyzed using 3 complementary methods to ensure that the results from them are concordant: comparisons of mean z scores at each time point; baseline-, age-, education-, and depression-adjusted SRB scores; and percentage of participants who declined from their baseline. The SRB model accounts for practice effects, based on the performance of the control group; hence although the mean z score in the patient group might seem unchanged or even improved across time points, there is an impairment when there are no practice-related improvements.
Preclinical and clinical data support a causal relationship of direct brain irradiation to the hippocampus and subcortical white matter with NCD. A retrospective study by our group suggested an association between radiation therapy for HNC and NCD and demonstrated an association of temporal lobe and cerebellum radiation dose with impaired memory and motor dexterity, respectively. Given the significant NCD found in the longitudinal data reported herein, dosimetry is being calculated in this cohort and a separate analysis will examine associations between CNS structures’ radiation doses with NCD. Chemotherapy-induced NCD have been documented in other cancer populations, and that literature indicates persisting but not necessarily delayed deficits after exposure to breast cancer and hematologic malignant disease regimens. Those studies involve multiple chemotherapy regimens that did not assess the effect of single-agent cisplatin on NCD in either the short or long term. In our exploratory multivariable analysis, results did not reveal a difference in neurocognitive outcomes between patients who were treated with or without plantinum-based chemotherapy (eTable 7 in the Supplement); however, the study was not designed statistically to answer this question.
Several potential mechanisms have been hypothesized to link chemotherapy and cognitive dysfunction. These include cytokine secretion. Published findings regarding cytokines and chemotherapy-associated NCD are heterogeneous, and data in HNC are lacking. We found no pattern of associations of baseline cytokine levels with subsequent neurocognitive outcomes. This may be because blood samples were collected at a time of day convenient for the participant since the association of cytokines and NCD was not a primary objective, so we cannot account for diurnal variations.
Exploratory analysis suggested that baseline prognostic factors of subsequent neurocognitive decline include lower education level and higher depression symptoms. Greater NCD in breast cancer patients with less formal education has been described before. Risk factors for vascular disease, such as hypertension, diabetes, and smoking, which previously have been found to correlate with radiation-induced cerebral vascular insufficiency and cognitive performance did not consistently predict NCD in our sample.
Clinical implications of NCD in the context of organ-sparing curative treatment for aggressive cancer might seem modest. However, cancer survivors with NCD are less likely to return to work, be involved in the community, and function socially. Potential strategies to avoid NCD include hippocampus-sparing radiation techniques, cognitive prehabilitation or rehabilitation, and development of neuroprotectors.
Strengths and Limitations
This study has some strengths and limitations. The prospective controlled design enabled the longitudinal analyses covering 4 time points over 2 years using a comprehensive battery of standardized neurocognitive tests as well as the correlative study of cytokines. To adjust for differences in age and education between the patients and controls we used age-corrected z scores as opposed to raw scores, as well as an adjunct statistical model (SRB adjusted for age, education, baseline depression, the correct method when there is imbalance). In addition, we analyzed data from the subsets of the patient and control group participants who completed all assessments (which were age equivalent) with very similar results. We also incorporated demographic and treatment related factors in exploratory multivariable analyses. Our sample did not have many radiation-only or patients who received less than 70 Gy or subgroups with balanced chemotherapy regimens, so interpretation regarding nonsignificant treatment risk factors at 24 months is tentative. In addition, patients and particpants in the control group may be different in unmeasurable ways, and there is no way to control for this, so we cannot prove cause and effect. We can conclude, however, that patients had more NCD and appeared to be at greater risk of declines in neurocognition over time than those in the noncancer control group. It remains unclear if the observed deficits are owing to cancer, treatment (lack of evidence does not imply zero association), other unmeasurable factors, or life changes.
Conclusions
This study, the first to our knowledge to comprehensively and longitudinally assess neurocognitive function in HNC from before treatment, suggests delayed NCD that progress over the 2 years after treatment. These adverse cognitive risks should be communicated to patients and families. Strategies to reduce toxic effects and cognitive rehabilitation options should be available for HNC survivors. Reassessment of these participants at 5 years is planned.
eMethods. Serum parameters
eTable 1. Neurocognitive domains, tests, and brief task description (with references)
eTable 2. Characteristics of participants who completed all assessments (baseline, 6, 12, and 24 months)
eTable 3. Standardized Regression Based (SRB) scores of all participants. The differences between patients (P) and controls (C), adjusted for baseline performance, depression, age, and education.
eTable 4. Standardized Regression Based scores of participants who completed all assessments (50 patients; 35 controls). The differences between patients (P) and controls (C), adjusted for baseline performance, age, and education.
eTable 5. Self reported Symptoms
eTable 6. Serum variables (Mean, effect size, and 95% confidence intervals)
eFigure 1. Study flow chart
References
- 1.Klein M, Heimans JJ, Aaronson NK, et al. . Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360(9343):1361-1368. [DOI] [PubMed] [Google Scholar]
- 2.Edelstein K, D’agostino N, Bernstein LJ, et al. . Long-term neurocognitive outcomes in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33(6):450-458. [DOI] [PubMed] [Google Scholar]
- 3.Stewart A, Bielajew C, Collins B, Parkinson M, Tomiak E. A meta-analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clin Neuropsychol. 2006;20(1):76-89. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein LJ, Catton PA, Tannock IF. Intra-individual variability in women with breast cancer. J Int Neuropsychol Soc. 2014;20(4):380-390. [DOI] [PubMed] [Google Scholar]
- 5.Edelstein K, Bernstein LJ. Cognitive dysfunction after chemotherapy for breast cancer. J Int Neuropsychol Soc. 2014;20(4):351-356. [DOI] [PubMed] [Google Scholar]
- 6.Ahles TA, Saykin AJ, Furstenberg CT, et al. . Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20(2):485-493. [DOI] [PubMed] [Google Scholar]
- 7.Panwar A, Batra R, Lydiatt WM, Ganti AK. Human papilloma virus positive oropharyngeal squamous cell carcinoma: a growing epidemic. Cancer Treat Rev. 2014;40(2):215-219. [DOI] [PubMed] [Google Scholar]
- 8.http://seer.cancer.gov/statfacts/html/oralcav.html S. 2014. Accessed September 26, 2017.
- 9.Gan HK, Bernstein LJ, Brown J, et al. . Cognitive functioning after radiotherapy or chemoradiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81(1):126-134. [DOI] [PubMed] [Google Scholar]
- 10.Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry. 2009;17(7):542-555. [DOI] [PubMed] [Google Scholar]
- 11.Anstey KJ, von Sanden C, Salim A, O’Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166(4):367-378. [DOI] [PubMed] [Google Scholar]
- 12.Welsh LC, Dunlop AW, McGovern T, et al. . Neurocognitive function after (chemo)-radiotherapy for head and neck cancer. Clin Oncol (R Coll Radiol). 2014;26(12):765-775. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, Luo D, Rong X, Shi X, Peng Y. Psychological disorders, cognitive dysfunction and quality of life in nasopharyngeal carcinoma patients with radiation-induced brain injury. PLoS One. 2012;7(6):e36529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuen HK, Sharma AK, Logan WC, Gillespie MB, Day TA, Brooks JO. Radiation dose, driving performance, and cognitive function in patients with head and neck cancer. Radiother Oncol. 2008;87(2):304-307. [DOI] [PubMed] [Google Scholar]
- 15.Bond SM, Dietrich MS, Gilbert J, Ely EW, Jackson JC, Murphy BA. Neurocognitive function in patients with head and neck cancer undergoing primary or adjuvant chemoradiation treatment. Support Care Cancer. 2016;24(10):4433-4442. [DOI] [PubMed] [Google Scholar]
- 16.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703-708. [DOI] [PubMed] [Google Scholar]
- 17.Ouimet LA, Stewart A, Collins B, Schindler D, Bielajew C. Measuring neuropsychological change following breast cancer treatment: an analysis of statistical models. J Clin Exp Neuropsychol. 2009;31(1):73-89. [DOI] [PubMed] [Google Scholar]
- 18.Siu LL, Waldron JN, Chen BE, et al. . Effect of standard radiotherapy with cisplatin vs accelerated radiotherapy with panitumumab in locoregionally advanced squamous cell head and neck carcinoma: a randomized clinical trial. JAMA Oncol. 2017;3(2):220-226. [DOI] [PubMed] [Google Scholar]
- 19.Ringash J, Bernstein LJ, Cella D, et al. . Outcomes toolbox for head and neck cancer research. Head Neck. 2015;37(3):425-439. [DOI] [PubMed] [Google Scholar]
- 20.Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J Int Neuropsychol Soc. 2003;9(7):967-982. [DOI] [PubMed] [Google Scholar]
- 21.Yang D, Dalton JE A unified approach to measuring the effect size between two groups using SAS® SAS Global Forum Paper 335-2012. In:2012. http://support.sas.com/resources/papers/proceedings12/335-2012.pdf. Accessed September 26, 2017. [Google Scholar]
- 22.Duff K. Evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch Clin Neuropsychol. 2012;27(3):248-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingraham LJ, Aiken CB. An empirical approach to determining criteria for abnormality in test batteries with multiple measures. Neuropsychology. 1996;10(1):120-124. [Google Scholar]
- 24.Gibson E, Monje M. Effect of cancer therapy on neural stem cells: implications for cognitive function. Curr Opin Oncol. 2012;24(6):672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8(11):887-899. [DOI] [PubMed] [Google Scholar]
- 26.Cheung YT, Ng T, Shwe M, et al. . Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol. 2015;26(7):1446-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hopewell JW, Wright EA. The nature of latent cerebral irradiation damage and its modification by hypertension. Br J Radiol. 1970;43(507):161-167. [DOI] [PubMed] [Google Scholar]
- 28.McCready RA, Hyde GL, Bivins BA, Mattingly SS, Griffen WO Jr. Radiation-induced arterial injuries. Surgery. 1983;93(2):306-312. [PubMed] [Google Scholar]
- 29.Reid-Arndt SA, Yee A, Perry MC, Hsieh C. Cognitive and psychological factors associated with early posttreatment functional outcomes in breast cancer survivors. J Psychosoc Oncol. 2009;27(4):415-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunlop A, Welsh L, McQuaid D, et al. . Brain-sparing methods for IMRT of head and neck cancer. PLoS One. 2015;10(3):e0120141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dye NB, Gondi V, Mehta MP. Strategies for preservation of memory function in patients with brain metastases. Chin Clin Oncol. 2015;4(2):24. [DOI] [PubMed] [Google Scholar]
- 32.Senn S. Testing for baseline balance in clinical trials. Stat Med. 1994;13(17):1715-1726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Serum parameters
eTable 1. Neurocognitive domains, tests, and brief task description (with references)
eTable 2. Characteristics of participants who completed all assessments (baseline, 6, 12, and 24 months)
eTable 3. Standardized Regression Based (SRB) scores of all participants. The differences between patients (P) and controls (C), adjusted for baseline performance, depression, age, and education.
eTable 4. Standardized Regression Based scores of participants who completed all assessments (50 patients; 35 controls). The differences between patients (P) and controls (C), adjusted for baseline performance, age, and education.
eTable 5. Self reported Symptoms
eTable 6. Serum variables (Mean, effect size, and 95% confidence intervals)
eFigure 1. Study flow chart