This cross-sectional study assesses the incidence of and characterizes factors contributing to overdiagnosis of acute optic neuritis.
Key Points
Question
What is the overdiagnosis rate of optic neuritis and what are some causes of overdiagnosis?
Findings
In this cross-sectional study of 122 patients referred for optic neuritis, optic neuritis was overdiagnosed in 73 patients (59.8%). The most common errors appeared to result from overreliance on a single item of history or failure to consider alternative diagnoses.
Meaning
Eye pain should be considered in context with other symptoms, and headache, nonarteritic ischemic optic neuropathy, functional visual loss, and other alternative diagnoses should be considered in the differential diagnosis.
Abstract
Importance
Diagnostic error is an important source of medical error. Overdiagnosis of optic neuritis may prompt unnecessary and costly diagnostic tests, procedures, and treatments.
Objective
To assess the incidence of and characterize factors contributing to overdiagnosis of acute optic neuritis.
Design, Setting, and Participants
In this retrospective clinic-based cross-sectional study of new patient encounters, 122 patients referred for acute optic neuritis at a university-based Midwestern neuro-ophthalmology clinic between January 2014 and October 2016 were studied. Data were analyzed from September 2016 to July 2017.
Interventions
Definite diagnosis was determined by neuro-ophthalmologists. For patients with alterative diagnoses, the Diagnosis Error Evaluation and Research taxonomy tool was applied to categorize the type of diagnostic error.
Main Outcomes and Measures
The primary outcome was the primary type of diagnostic error in patients erroneously diagnosed as having optic neuritis. Secondary outcomes included final diagnosis and interventions undergone prior to referral.
Results
A total of 122 patients were referred with acute optic neuritis during the study period; 88 (72.1%) were female, and the mean (SD) age was 42.6 (14.0) years. Of these, 49 patients (40.2%; 95% CI, 31.4-49.4) were confirmed to have optic neuritis, and 73 (59.8%; 95% CI, 50.6-68.6) had an alternative diagnosis. The most common alternative diagnoses were headache and eye pain, functional visual loss, and other optic neuropathies, particularly nonarteritic anterior ischemic optic neuropathy. The most common diagnostic error was eliciting or interpreting critical elements of history, which occurred in 24 of 73 patients (33%) with alternative diagnoses. Other common errors included errors weighing or considering alternative diagnoses (23 patients [32%]), errors weighing or interpreting physical examination findings (15 patients [21%]), and misinterpreting diagnostic test results (11 patients [15%]). In patients with alterative diagnoses, 12 (16%) had normal magnetic resonance imaging findings preceding the referral, 12 (16%) had received a lumbar puncture, and 8 (11%) had received unnecessary treatment with intravenous steroids.
Conclusions and Relevance
These data suggest that nearly 60% (95% CI, 50.6-68.6) of patients referred for optic neuritis have an alternative diagnosis, with the most common errors being overreliance on a single item of history and failure to consider alternative diagnoses. Understanding pitfalls leading to overdiagnosis of optic neuritis may improve clinicians’ diagnostic process.
Introduction
Optic neuritis is an acute inflammatory demyelinating optic neuropathy that can occur in isolation or herald other underlying diseases, such as multiple sclerosis (MS) or neuromyelitis optica. Optic neuritis classically presents with acute to subacute central visual loss, pain with eye movements, and dyschromatopsia. Diagnosis is based on presenting symptoms, time course, and examination findings consistent with optic neuropathy, such as abnormal visual acuity, visual fields, color vision, or the presence of a new relative afferent pupillary defect (APD). Diagnosis may be supported by neuroimaging studies, such as magnetic resonance imaging (MRI). Optic disc swelling may be present but is not mandatory for diagnosis; the inflammation is retrobulbar in two-thirds of patients. Treatment with intravenous corticosteroids or megadose oral corticosteroids hastens visual recovery, and further diagnostic investigation with MRI or lumbar puncture is often performed to stratify the risk of progression to MS.
In many cases, diagnostic error does not reflect gaps in knowledge but rather reasoning errors or cognitive biases, such as premature closure and anchoring bias. One prior study reported up to 74% of diagnostic errors may be caused by cognitive factors. Other studies have shown that alternative diagnoses, such as other optic neuropathies, ocular surface disease, and primary headache disorders, may be mistaken for optic neuritis. Previously reported rates of overdiagnosis in patients referred for optic neuritis range from 9% to 37%. Overdiagnosis in patients with alternative conditions may lead to unnecessary MRIs, lumbar punctures, treatments, loss of time, and expense.
The objective of this study is to assess the incidence of and characterize factors contributing to overdiagnosis of optic neuritis in the neuro-ophthalmology outpatient clinic at a tertiary care center. The intent is both to determine the diagnoses most likely to be mistaken for optic neuritis and to identify common pitfalls in assessing for optic neuritis to improve the diagnostic process and minimize medical errors.
Methods
All new patient encounters in neuro-ophthalmology at the Washington University in St Louis Department of Ophthalmology and Visual Sciences Center for Advanced Medicine Eye Center, a university-based Midwestern clinic in the United States, scheduled between January 2014 and October 2016 were reviewed to identify patients referred with “optic neuritis” in our electronic medical record scheduling comments. Referral materials and patient encounter notes were then independently and systematically reviewed by L.S., N.H.K., and G.P.V.S. Patients were included in the study if the referral notes indicated that the referral to neuro-ophthalmology was prompted by the clinician’s concern that acute optic neuritis was the most likely diagnosis. Patients were excluded if, despite the scheduling comment being “optic neuritis,” review of the referral notes revealed that the referral was in fact for optic pallor, a remote episode of optic neuritis, or an alternative diagnosis. Patients with insufficient outside records to determine the diagnosis on referral were also excluded. Institutional review board approval was obtained from the Washington University in St Louis Human Research Protection Office. The research adhered to the tenets of the Declaration of Helsinki. Informed consent was waived because data were deidentified.
Demographic data, including age, sex, specialty of referring clinician, and prior established diagnosis of MS or neuromyelitis optica, were collected. Initial symptoms, examination findings, diagnostic testing, and treatments prior to referral to neuro-ophthalmology were also recorded.
Definite diagnosis of optic neuritis was extracted from medical records and had been determined by a neuro-ophthalmologist (G.P.V.S. or C.M.M.) using history, a structured clinical examination (including acuity, color, and the presence or absence of a relative APD), visual fields, funduscopy, and MRI when available or clinically indicated.
For erroneously diagnosed cases of optic neuritis, the Diagnosis Error Evaluation and Research (DEER) taxonomy tool was applied to identify the type of diagnostic error and to evaluate where in the diagnostic process the problem occurred. The DEER classification was assigned by consensus based on the major cause of diagnostic error.
Statistical Methods
The mean, standard deviation, and range are reported for continuous measures. Comparisons between the group diagnosed as having optic neuritis and group not diagnosed as having optic neuritis were conducted using 2-group t tests. For categorical measures, percentages are reported, and the comparisons are conducted using either the χ2 test or the Fisher exact test. Analysis of the overdiagnosis rate was restricted to ophthalmology, optometry, and neurology; family medicine and neuro-ophthalmology had insufficient sample sizes for this analysis. Statistical analyses were performed using SAS version 9.4 (SAS Institute). All P values were 2-tailed, and statistical significance was set at P < .05.
Results
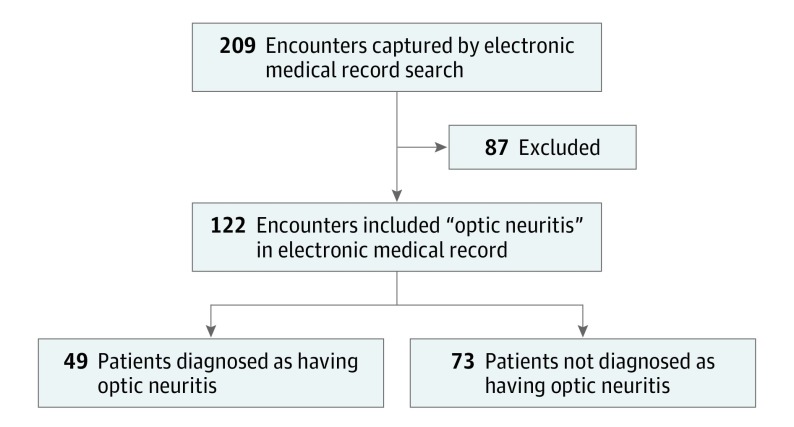
A total of 219 patient encounters were initially captured using the search term optic neuritis. Of these, 87 were excluded because the referral notes indicated that the referrals were for conditions other than acute optic neuritis (Figure). Of the 122 included patients who were referred for optic neuritis, 49 (40.2%; 95% CI, 31.4-49.4) were ultimately diagnosed as having optic neuritis, and 73 (59.8%; 95% CI, 50.6-68.6) did not have optic neuritis (Figure).
Figure. Flow Diagram of Patient Encounters.
The mean age of the patients diagnosed as having optic neuritis was younger than patients found to have alternative diagnoses, and more of the patients diagnosed as having optic neuritis had a known diagnosis of MS; otherwise, there were no significant demographic differences between the 2 groups (Table 1). There was not a significant difference in overdiagnosis by referral source (Table 2).
Table 1. Demographic Information.
| Characteristic | Diagnosed as Having Optic Neuritis (n = 49) | Not Diagnosed as Having Optic Neuritis (n = 73) | P Value |
|---|---|---|---|
| Age, y | .01 | ||
| Mean (SD) | 38.8 (10.6) | 45.1 (15.5) | NA |
| Median (range) | 39 (14-64) | 45.0 (14-85) | NA |
| >50 y, No. (%) | 6 (12) | 30 (41) | <.001 |
| Female, No. (%) | 34 (69) | 54 (74) | .58 |
| Prior diagnosis of MS, No. (%) | 9 (18) | 3 (4) | .01 |
Abbreviations: MS, multiple sclerosis; NA, not applicable.
Table 2. Overdiagnosis Rates by Specialty of Referral Source.
| Specialty | No./Total No. (%) | 95% CI | |
|---|---|---|---|
| Referrals | Overdiagnosis Rate | ||
| Ophthalmology | 57/122 (46.7) | 36/56 (64) | 49.3-75.6 |
| Optometry | 43/122 (35.2) | 25/43 (58) | 42.1-73.0 |
| Neurology | 19/122 (15.6) | 11/19 (58) | 33.5-79.8 |
| Family medicine | 2/122 (1.6) | 0/2 (0) | NA |
| Neuro-ophthalmology (2nd opinion) | 1/122 (0.8) | 1/1 (100) | NA |
| Total, No. | 122 | 73 | 50.6-68.6a |
Abbreviation: NA, not applicable.
P = .85. The P value refers to a χ2 test comparing misdiagnosis rates among Ophthalmology (64%), optometry (58%), and neurology (58%), indicating that there is no significant difference in overdiagnosis among these 3 specialties. Family medicine and neuro-ophthalmology were excluded from the analysis, as they had referred only 2 and 1 patients, respectively.
Table 3 details the alternative diagnoses assigned to patients who were not diagnosed as having optic neuritis. The most common alternative diagnosis was primary headache disorder with associated eye pain and/or visual symptoms (16 patients [22%]). Other common alternative diagnoses included functional visual loss (14 [19%]); other optic neuropathies (12 [16%]), in particular nonarteritic anterior ischemic optic neuropathy (NAION; 9 [12%]); and retinopathies (11 [15%]), such as neuroretinitis, central retinal artery occlusion, or branch retinal artery occlusion. Less frequent alternative diagnoses included ocular surface disease (3 [4%]), congenital disc abnormalities (3 [4%]), and optic nerve sheath meningioma (3 [4%]).
Table 3. Alternative Diagnoses in Patients Who Were Not Diagnosed as Having Optic Neuritis.
| Diagnosis | Patients, No. (%) (n = 73) |
|---|---|
| Headache with eye pain and/or visual symptoms | 16 (22) |
| Functional visual loss | 14 (19) |
| Optic neuropathies | 12 (16) |
| Nonarteritic anterior ischemic optic neuropathy | 9 (12) |
| Arteritic ischemic optic neuropathy | 1 (1) |
| Traumatic optic neuropathy | 1 (1) |
| Unclear etiology | 1 (1) |
| Retinal/macular | 11 (15) |
| Neuroretinitis | 3 (4) |
| Central or branch retinal artery occlusion | 2 (3) |
| Acute zonal occult outer retinopathy | 2 (3) |
| Maculopathy/macular edema | 2 (3) |
| Other | 2 (3) |
| Neoplastic | 4 (5) |
| Optic nerve sheath meningioma | 3 (4) |
| Orbital tumor | 1 (1) |
| Ocular surface disease | 3 (4) |
| Congenital disc abnormalities | 3 (4) |
| Inflammatory | 3 (4) |
| Trochleitis | 1 (1) |
| Uveitis | 2 (3) |
| Other/uncertain | 8 (11) |
Table 4 details the diagnostic errors assigned based on DEER criteria. The most common diagnostic errors were in eliciting or interpreting critical elements of history, which occurred in 24 of 73 patients (33%). Examples of failure to elicit critical elements of the history included failure to elicit that episodes of vision loss were stereotyped, recurrent, or isolated events or that the vision loss was bilateral. Examples of failure to weigh items of the history included overreliance on a known diagnosis of MS or a previous episode of optic neuritis. However, one of the more common examples of failure to weigh elements of history was overweighing the presence of eye pain or pain with eye movements. This was the critical diagnostic error in 9 patients (12%) found to have an alternative diagnosis. Overall, eye pain or pain with eye movements was documented in the referral notes for 21 patients (29%) who were found to have an alternative diagnosis.
Table 4. Categorization of Types of Diagnostic Error in Patients Not Diagnosed as Having Optic Neuritis.
| Type of Diagnostic Error | Patients, No. (%) (n = 73) |
|---|---|
| Error in eliciting or interpreting critical elements of history | 24 (33) |
| Failure to elicit critical piece of history | 5 (7) |
| Inaccurate/misinterpreted history | 2 (3) |
| Failure in weighing history | 17 (23) |
| Error in weighing or interpreting physical examination findings | 15 (21) |
| Failure to elicit critical examination finding | 2 (3) |
| Inaccurate/misinterpreted examination | 7 (10) |
| Failure in weighing examination findings | 6 (8) |
| Error in diagnostic testing | 11 (15) |
| Failure to order necessary test | 2 (3) |
| Failure to perform necessary test | 1 (1) |
| Error in laboratory or radiology read | 6 (8) |
| Error in physician interpretation of test | 2 (3) |
| Error in considering other diagnoses | 23 (32) |
| Failure to consider diagnosis | 6 (8) |
| Too much weight on optic neuritis diagnosis | 4 (5) |
| Too little weight on alternative diagnosis | 13 (18) |
| Total, No. | 73 |
The second most common category of error was in weighing or considering alternative diagnoses, which occurred in 23 patients (32%). Most commonly, this was because of failure to consider common diagnoses, such as NAION, or functional visual loss, but in some cases, the error was due to a failure to consider rare diagnoses, such as neuroretinitis or optic nerve sheath meningioma.
The next most common category of error was in weighing or interpreting physical examination findings, which occurred in 15 patients (21%). There were several examples of misinterpretation of an anomalous disc. In addition, 2 errors (3%) were due to underweighing the fact that a patient had normal examination findings (in these patients, a normal examination was documented by the referring physician). Three errors (4%) were due to overemphasis on red cap desaturation in a patient who had otherwise normal examination findings.
The presence of an APD was also statistically significantly associated with the correct diagnosis of optic neuritis. Patients for whom referral notes documented a positive APD were diagnosed as having optic neuritis in 27 of 40 patients (68%). Patients for whom referral notes documented that an APD was not found were diagnosed as having optic neuritis in 10 of 47 patients (21%). Patients for whom referral notes did not document whether an APD was checked were diagnosed as having optic neuritis in 12 of 25 patients (48%).
Other errors were due to misinterpretation of diagnostic test results, which occurred in 11 patients (15%). Most commonly, this was an erroneous read by the radiologist that the optic nerve was enhancing or concerning for optic neuritis, which occurred in 6 patients (8%). Of the 73 patients who were not diagnosed as having optic neuritis, 12 (16%) had normal MRI results preceding the referral, 12 (16%) had received a lumbar puncture, and 8 (11%) had received unnecessary treatment with intravenous steroids.
Discussion
These data, if representative of other neuro-ophthalmology practices, suggest that between 51% and 69% of patients referred for optic neuritis may have an alternative diagnosis, regardless of whether the patient was referred by an optometrist, ophthalmologist, or neurologist. There were several notable patterns of diagnostic error and cognitive bias that emerged in this study. While symptoms of eye pain and pain with eye movements should raise concern for optic neuritis, eye pain, particularly without associated visual loss, may also be caused by a primary headache disorder. Pain with eye movements is one nonspecific clinical feature of optic neuritis and should not be overweighed. The time course of pain and visual loss is particularly important to achieving a correct diagnosis. Several patients in our study described discrete, stereotyped episodes of visual loss most consistent with migraine aura, a pattern that would be extraordinarily unusual for optic neuritis.
Patients with a known diagnosis of MS were more likely to have optic neuritis. However, 4% of the patients with known MS who were referred for optic neuritis had an alternative diagnosis. Patients with a known diagnosis of MS who present with eye pain or vision loss may actually have an alternative diagnosis, which may be delayed if physicians do not consider other conditions because of overreliance on the history of MS to make a diagnosis of optic neuritis.
Discounting normal examination findings was a common source of diagnostic error in this study. Normal examination findings are reassuring and virtually exclude the diagnosis of acute optic neuritis. Red desaturation is subjective and should not be overweighed, particularly if the examination results are otherwise normal. Optic discs with an anomalous appearance may mimic optic disc edema and lead to an erroneous diagnosis of optic neuritis.
Importantly, an APD was one of the more consistent examination findings that correlated with a true diagnosis of optic neuritis. The lack of an APD strongly argues against a diagnosis of acute optic neuritis unless there is bilateral optic nerve involvement.
Overweighing or misinterpreting MRI findings led to a substantial portion of the diagnostic errors. In these cases, an MRI read as having possible optic nerve enhancement was overweighed despite an overall clinical picture that did not support a diagnosis of optic neuritis. It is even more striking that 17% of patients falsely diagnosed as having optic neuritis already had normal MRI results before the referral. If MRI is performed, T2 hyperintensity and/or enhancement of the optic nerve should be seen in nearly all cases of true optic neuritis, although sensitivity is dependent on the quality of the images and the magnet strength. Gadolinium-enhanced fat-suppressed MRI of the orbits has been shown to be up to 94% sensitive for the detection of optic neuritis.
Failure to consider or appropriately weigh alternative diagnoses was the second most common type of error. Most notably, this was failure to consider headache or NAION. Strikingly, patients who were older than 50 years were more likely to have been overdiagnosed than younger patients. This may reflect the higher incidence of NAION in patients older than 50 years. It is important to consider NAION as a possible diagnosis in patients older than 50 years who present with vision loss and optic nerve edema.
Limitations
This study has limitations. The assignment of DEER categories is inherently subjective. In many cases, there were multiple sources of diagnostic error, and there could be arguments made for more than one of the DEER categories to be assigned as the primary source of error. Similarly, the determination of the source of diagnostic error was dependent on the detail of available referral records. By necessity, our study was limited by the accuracy of the information in the referral documents available. Additionally, the study was affected by the limitations of our electronic medical record. We identified potential patients based on having “optic neuritis” in the scheduling comments. When we searched our database of final diagnoses, we found 12 additional patients within the time span of our study who were diagnosed as having optic neuritis but who had not been captured by our scheduling comment search, indicating that our search for patients with “optic neuritis” in the scheduling comments did not capture every patient who was ultimately diagnosed as having optic neuritis in our clinic. (These 12 patients were not included in the study, as the aim of the study was to evaluate for overdiagnosis of optic neuritis.) Also, race/ethnicity data were not collected. Finally, our study was limited to a single, university-based Midwestern tertiary care neuro-ophthalmology clinic. It is unknown to what extent this generalizes to other clinic settings or other geographic areas. For example, it is possible that some patients were referred to this tertiary center because their cases were particularly ambiguous or challenging. Therefore, this study may not necessarily reflect the rate of overdiagnosis of all cases of optic neuritis.
Conclusions
The primary aim of this study was to identify pitfalls or cognitive biases that may lead to an incorrect diagnosis of optic neuritis to help physicians avoid similar clinical errors and prevent unnecessary testing or treatment with costly and invasive procedures, such as lumbar puncture, MRI, or treatment with high-dose steroids. An overdiagnosis rate of nearly 60% for those referred with acute optic neuritis was higher than previously published in the literature. Overdiagnosis was most commonly caused by errors in taking the history and errors in generating a complete differential diagnosis.
Physicians may implement these findings into their clinical practice by making note of common pitfalls that may arise in the diagnosis of optic neuritis. For example, it is important to consider eye pain in context with other symptoms, to elicit a detailed history with special emphasis on the time course of symptoms, and to avoid premature closure on a diagnosis of optic neuritis by considering headache, NAION, functional visual loss, and other alternative diagnoses.
References
- 1.Optic Neuritis Study Group The clinical profile of optic neuritis: experience of the Optic Neuritis Treatment Trial. Arch Ophthalmol. 1991;109(12):1673-1678. [DOI] [PubMed] [Google Scholar]
- 2.Hoorbakht H, Bagherkashi F. Optic neuritis, its differential diagnosis and management. Open Ophthalmol J. 2012;6:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balcer LJ. Clinical practice: optic neuritis. N Engl J Med. 2006;354(12):1273-1280. [DOI] [PubMed] [Google Scholar]
- 4.Petzold A, Wattjes MP, Costello F, et al. The investigation of acute optic neuritis: a review and proposed protocol. Nat Rev Neurol. 2014;10(8):447-458. [DOI] [PubMed] [Google Scholar]
- 5.Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol. 2014;13(1):83-99. [DOI] [PubMed] [Google Scholar]
- 6.Söderström M. The clinical and paraclinical profile of optic neuritis: a prospective study. Ital J Neurol Sci. 1995;16(3):167-176. [DOI] [PubMed] [Google Scholar]
- 7.Foroozan R, Buono LM, Savino PJ, Sergott RC. Acute demyelinating optic neuritis. Curr Opin Ophthalmol. 2002;13(6):375-380. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins TM, Toosy AT. New developments in the treatment of optic neuritis. Eye Brain. 2010;2:83-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck RW, Cleary PA, Anderson MM Jr, et al. ; The Optic Neuritis Study Group . A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med. 1992;326(9):581-588. [DOI] [PubMed] [Google Scholar]
- 10.Sellebjerg F, Nielsen HS, Frederiksen JL, Olesen J. A randomized, controlled trial of oral high-dose methylprednisolone in acute optic neuritis. Neurology. 1999;52(7):1479-1484. [DOI] [PubMed] [Google Scholar]
- 11.Du Y, Li JJ, Zhang YJ, Li K, He JF. Risk factors for idiopathic optic neuritis recurrence. PLoS One. 2014;9(9):e108580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrow SA, Fraser JA, Day C, et al. Recovery of demyelinating optic neuritis after treatment with bioequivalent high doses of oral vs. intravenous corticosteroids: a randomized single blinded clinical trial. Poster presented at: 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); September 15, 2016; London, England. [Google Scholar]
- 13.Söderström M, Ya-Ping J, Hillert J, Link H. Optic neuritis: prognosis for multiple sclerosis from MRI, CSF, and HLA findings. Neurology. 1998;50(3):708-714. [DOI] [PubMed] [Google Scholar]
- 14.Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. [DOI] [PubMed] [Google Scholar]
- 15.Norman G, Young M, Brooks L. Non-analytical models of clinical reasoning: the role of experience. Med Educ. 2007;41(12):1140-1145. [DOI] [PubMed] [Google Scholar]
- 16.Eva KW, Cunnington JP. The difficulty with experience: does practice increase susceptibility to premature closure? J Contin Educ Health Prof. 2006;26(3):192-198. [DOI] [PubMed] [Google Scholar]
- 17.Voytovich AE, Rippey RM, Suffredini A. Premature conclusions in diagnostic reasoning. J Med Educ. 1985;60(4):302-307. [DOI] [PubMed] [Google Scholar]
- 18.Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. [DOI] [PubMed] [Google Scholar]
- 19.Croskerry P. A universal model of diagnostic reasoning. Acad Med. 2009;84(8):1022-1028. [DOI] [PubMed] [Google Scholar]
- 20.Vickrey BG, Samuels MA, Ropper AH. How neurologists think: a cognitive psychology perspective on missed diagnoses. Ann Neurol. 2010;67(4):425-433. [DOI] [PubMed] [Google Scholar]
- 21.Siuko M, Tienari PJ, Saastamoinen KP, et al. Neuromyelitis optica and aquaporin-4 (AQP4) autoantibodies in consecutive optic neuritis patients in southern Finland. Acta Ophthalmol. 2014;92(4):387-391. [DOI] [PubMed] [Google Scholar]
- 22.Horwitz H, Friis T, Modvig S, et al. Differential diagnoses to MS: experiences from an optic neuritis clinic. J Neurol. 2014;261(1):98-105. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Liang X, Wei S, Li H. Differential diagnosis for multiple sclerosis-related optic neuritis. Eye Sci. 2015;30(1):23-28. [PubMed] [Google Scholar]
- 24.Fisayo A, Bruce BB, Newman NJ, Biousse V. Overdiagnosis of idiopathic intracranial hypertension. Neurology. 2016;86(4):341-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Concepts and Methodology. Rockville, MD: Agency for Healthcare Research and Quality; 2005:255-278. Advances in Patient Safety: from Research to Implementation; vol 2. [PubMed] [Google Scholar]
- 26.Kelman L. Migraine pain location: a tertiary care study of 1283 migraineurs. Headache. 2005;45(8):1038-1047. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe S, Van Stavern G. Characteristics of patients presenting with ocular pain. Can J Ophthalmol. 2008;43(4):432-434. [DOI] [PubMed] [Google Scholar]
- 28.Kupersmith MJ, Alban T, Zeiffer B, Lefton D. Contrast-enhanced MRI in acute optic neuritis: relationship to visual performance. Brain. 2002;125(pt 4):812-822. [DOI] [PubMed] [Google Scholar]