Abstract
Importance
Some eyes have persistent diabetic macular edema (DME) following anti–vascular endothelial growth factor (anti-VEGF) therapy for DME. Subsequently adding intravitreous corticosteroids to the treatment regimen might result in better outcomes than continued anti-VEGF therapy alone.
Objective
To compare continued intravitreous ranibizumab alone with ranibizumab plus intravitreous dexamethasone implant in eyes with persistent DME.
Design, Setting, and Participants
Phase 2 multicenter randomized clinical trial conducted at 40 US sites in 129 eyes from 116 adults with diabetes between February 2014 and December 2016. Eyes had persistent DME, with visual acuity of 20/32 to 20/320 after at least 3 anti-VEGF injections before a run-in phase, which included an additional 3 monthly 0.3-mg ranibizumab injections. Data analysis was according to intent to treat.
Interventions
Following the run-in phase, study eyes that had persistent DME and were otherwise eligible were randomly assigned to receive 700 μg of dexamethasone (combination group, 65 eyes) or sham treatment (ranibizumab group, 64 eyes) in addition to continued 0.3-mg ranibizumab in both treatment arms as often as every 4 weeks based on a structured re-treatment protocol.
Main Outcomes and Measures
The primary outcome was change in mean visual acuity letter score at 24 weeks as measured by the electronic Early Treatment Diabetic Retinopathy Study (E-ETDRS). The principal secondary outcome was change in mean central subfield thickness as measured with the use of optical coherence tomography.
Results
Of the 116 randomized patients, median age was 65 years (interquartile range [IQR], 58-71 years); 50.9% were female and 60.3% were white. Mean (SD) improvement in visual acuity from randomization was 2.7 (9.8) letters in the combination group and 3.0 (7.1) letters in the ranibizumab group, with the adjusted treatment group difference (combination minus ranibizumab) of –0.5 letters (95% CI, −3.6 to 2.5; 2-sided P = .73). Mean (SD) change in central subfield thickness in the combination group was –110 (86) μm compared with –62 (97) μm for the ranibizumab group (adjusted difference, –52; 95% CI, −82 to −22; 2-sided P < .001). Nineteen eyes (29%) in the combination group experienced increased intraocular pressure or initiated treatment with antihypertensive eyedrops compared with 0 in the ranibizumab group (2-sided P < .001).
Conclusions and Relevance
Although its use is more likely to reduce retinal thickness and increase intraocular pressure, the addition of intravitreous dexamethasone to continued ranibizumab therapy does not improve visual acuity at 24 weeks more than continued ranibizumab therapy alone among eyes with persistent DME following anti-VEGF therapy.
Trial Registration
clinicaltrials.gov Identifier: NCT01945866
In this phase 2 randomized clinical trial, the Diabetic Retinopathy Clinical Research Network compares continued ranibizumab treatment alone vs ranibizumab plus dexamethasone in patients with persistent diabetic macular edema.
Key Points
Question
Does the addition of intravitreous dexamethasone provide benefits to eyes receiving continued intravitreous ranibizumab therapy for persistent diabetic macular edema?
Findings
In this phase 2 randomized clinical trial that included 129 eyes with persistent diabetic macular edema, improvement in visual acuity at 24 weeks was not significantly different between combination therapy and ranibizumab alone.
Meaning
For eyes with persistent diabetic macular edema, the addition of intravitreous dexamethasone to continued ranibizumab therapy reduces retinal thickness but does not improve visual acuity more than continued ranibizumab therapy alone.
Introduction
Studies have demonstrated the benefit of intravitreous anti–vascular endothelial growth factor (VEGF) injections for improving visual acuity and decreasing retinal thickening in eyes with diabetic macular edema (DME). Nevertheless, in 32% to 66% of eyes treated with at least 6 monthly injections, edema persisted, often with reduced visual acuity. Thus, there is a need for additional treatments for eyes with suboptimal response to anti-VEGF therapy.
Corticosteroids decrease inflammation, reduce breakdown of the blood-retinal barrier, and have antiangiogenic properties, possibly owing to downregulation of VEGF. Intravitreous corticosteroid treatment for DME results in superior visual acuity compared with sham treatment, although not compared with laser photocoagulation or intravitreous anti-VEGF treatment in phakic eyes. Because corticosteroids have consistently been shown to reduce retinal thickening, they might be beneficial in eyes with persistent DME and vision loss despite previous anti-VEGF therapy if visual acuity outcomes are also improved. The Diabetic Retinopathy Clinical Research Network (DRCR.net) conducted a randomized clinical trial to compare continued ranibizumab therapy alone vs continued ranibizumab plus intravitreous dexamethasone in eyes with persistent DME and visual acuity of 20/32 to 20/320 despite previous anti-VEGF therapy.
Methods
Forty clinical sites across the United States participated in this multicenter phase 2 randomized clinical trial. The study adhered to the tenets of the Declaration of Helsinki and was approved by multiple institutional review boards. Study participants provided written informed consent. An independent data and safety monitoring committee conducted the study oversight. The full trial protocol is available at http://www.drcr.net and in Supplement 1, and a list of the participating institutions and institutional review boards are available in eAppendixes 1 and 2 in Supplement 2.
Study Population
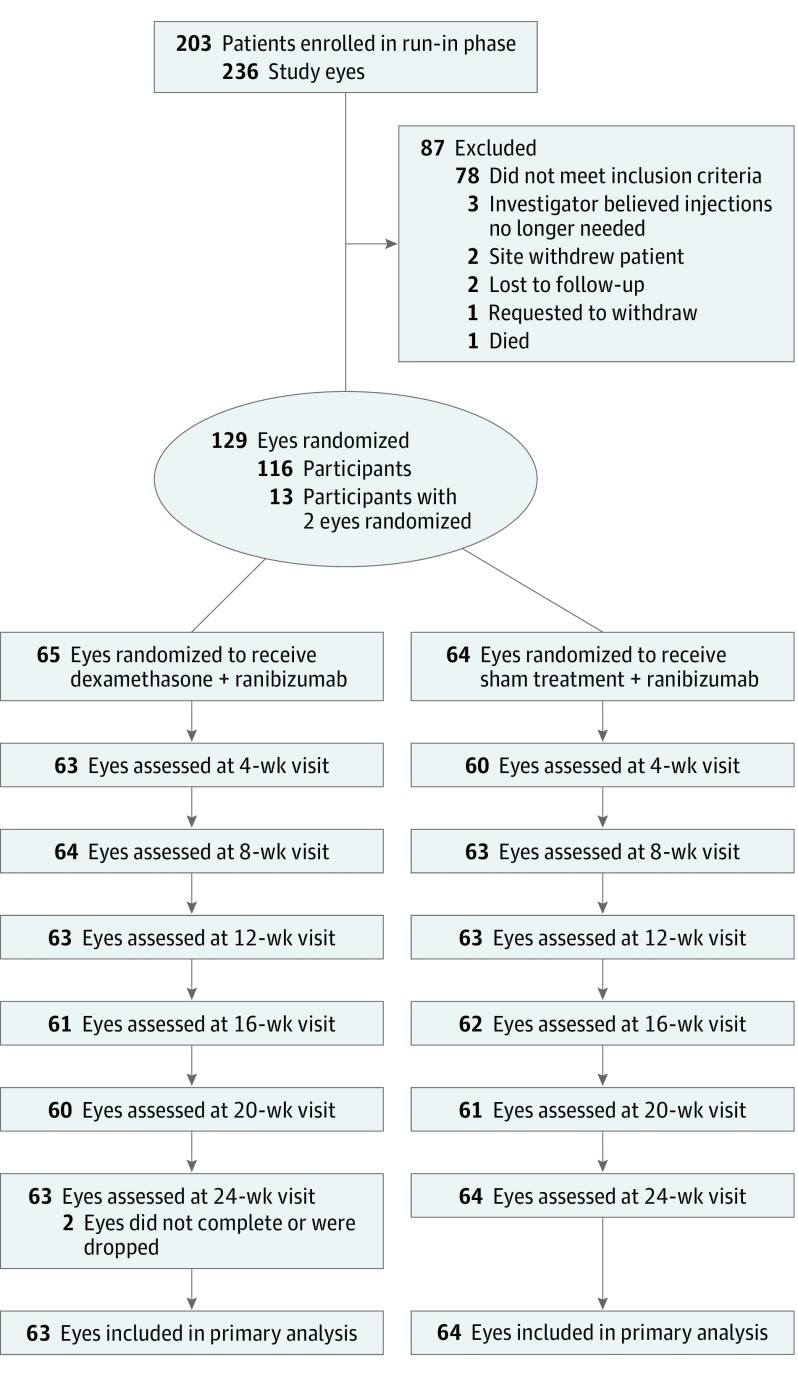
Participants were 18 years or older, had type 1 or type 2 diabetes, had 1 or both eyes with a best-corrected visual acuity letter score of 78 to 24 (approximate Snellen equivalent, 20/32 to 20/320), had an optical coherence tomography (OCT)–measured central subfield thickness (CST) above protocol-defined thresholds (eTable 1 in Supplement 2), and had received treatment with at least 3 anti-VEGF injections for DME (aflibercept, bevacizumab, or ranibizumab) within the previous 20 weeks (Figure 1). Initially, only pseudophakic eyes were eligible for the study. However, because only 17 participants with pseudophakia were randomized from February 2014 to July 2015, eligibility criteria were expanded to permit enrollment of phakic eyes.
Figure 1. Completion of Follow-up.
Protocol-specified visits occurred at baseline (randomization) and every 4 weeks (±1 week) at 4, 8, 12, 16, 20, and 24 weeks after randomization. For the primary analysis, a visit conducted between 20 and 30 weeks was included for the 24-week visit. For other protocol-specified follow-up visits, an analysis window of plus or minus 4 weeks was defined.
A 12-week run-in phase was conducted DME was still persistent in eyes with DME after additional anti-VEGF injections. During the run-in phase, each eye was required to receive 3 additional anti-VEGF injections using intravitreous ranibizumab, 0.3 mg, at enrollment, 4 weeks, and 8 weeks (in addition to the ≥3 anti-VEGF injections needed for eligibility). At week 12 of the run-in phase, eyes that had received all run-in injections and continued to meet the visual acuity criteria above with an OCT CST greater than the threshold values described in eTable 1 in Supplement 2 were eligible for randomization. eTable 1 in Supplement 2 details eligibility criteria for the run-in and randomization phases.
Study Design
Randomization was performed on the study website (http://www.drcr.net) using a permuted-block design. A participant could have 1 or 2 study eyes. Participants with 1 study eye were randomly assigned with equal probability, stratified by presence of improvement in visual acuity and retinal thickness during the run-in phase, to receive either a combination of ranibizumab, 0.3 mg (Lucentis; Genentech), and intravitreous sustained dexamethasone drug-delivery system (Ozurdex; Allergan), 700 μg, injection (combination group) or sham and ranibizumab, 0.3 mg, injections (ranibizumab group). Participants with 2 study eyes had 1 eye randomly assigned to each group.
Follow-up visits occurred every 4 weeks through 24 weeks, with the primary outcome visit at 24 weeks. At each visit, certified personnel performed an electronic Early Treatment Diabetic Retinopathy Study (E-ETDRS) best-corrected visual acuity test, spectral-domain OCT imaging, intraocular pressure (IOP) assessment, and an ocular examination. Values from OCT scans were converted to time-domain equivalent values for analysis and reporting.
Study participants and the medical monitor, who reviewed all adverse events, were masked to treatment group assignments. Refractionists, visual acuity testers, and OCT technicians were masked at the 24-week primary outcome visit. Investigators and study coordinators were not masked.
Treatment Protocol
The DRCR.net anti-VEGF injection procedure was reported previously. At randomization, all participants received a ranibizumab injection. Sham or dexamethasone injections were required within 0 to 8 days of the ranibizumab injection. When combination injections were given on the same day, the ranibizumab injection was given first in the dexamethasone group but last in the sham group. In both groups, povidone iodine was required to be reapplied to the conjunctiva before the second injection. There was no minimum required time between injections except that at least 30 seconds had to have elapsed following the second povidone iodine application. Initially, sham applicators were identical to the dexamethasone applicator but without a needle and produced an audible click when activated (93 injections). However, these applicators expired April 1, 2016. Subsequent sham injections were performed by pressing the hub of a needleless syringe to the conjunctival surface (36 injections).
At each follow-up visit, investigators evaluated the study eye for re-treatment based on visual acuity and OCT findings. An injection of ranibizumab was administered if the visual acuity letter score was less than 84 (approximate Snellen equivalent of 20/25 or worse) or the OCT CST was at or above the protocol-defined, sex-specific OCT cutoff. At the 4-week and 8-week visits, only ranibizumab injections were permitted. At 12 weeks and continuing through 20 weeks, if re-treatment criteria were met, participants were re-treated with the same treatment they were randomized to receive initially: either ranibizumab plus dexamethasone or ranibizumab plus sham. In both groups, a maximum of 2 injections of either dexamethasone or sham treatment were given in each eye.
Statistical Analysis
The primary outcome measure was mean change in visual acuity letter score from randomization to the 24-week visit. Treatment group comparisons were performed using a linear mixed model including fixed effects for visual acuity at the randomization visit and randomization stratification factors. In addition, a random subject effect was included to account for between-eye correlation for participants with 2 study eyes. With a sample size of 150 eyes, the trial had 90% power to identify a statistically significant difference between treatments, assuming a true difference of 5 letters with an SD of 9 letters and 10% loss to follow-up.
The primary analysis followed the intent-to-treat principle, including all eyes randomized into the study. Multiple imputation using the Markov chain Monte Carlo method was performed to impute missing 24-week data (2 participants). Changes at 24 weeks were truncated to 3 SDs from the mean to minimize the impact of statistical outliers (no visual acuity values and 2 OCT CST values in the ranibizumab group [1 value on each end]). Subgroup analyses were performed by adding an interaction between subgroup and treatment to the primary mixed model. Safety analyses were conducted using the Fisher exact test, with P < .01 prespecified to be considered of interest. All 95% CIs and P values are 2-sided. SAS, version 9.4 (SAS Institute Inc) was used for all statistical analyses.
Results
Study Participants
Between February 2014 and December 2016, 236 eyes from 203 participants were enrolled in the run-in phase, after which 129 eyes of 116 participants met eligibility criteria and were randomly assigned to the dexamethasone + ranibizumab group (combination, 65 eyes) or the sham + ranibizumab group (ranibizumab, 64 eyes). Median age of the 116 randomized participants was 65 years (interquartile range, 58-71), 50.9% were women, 60.3% were white, 94.8% had type 2 diabetes, and median hemoglobin A1c level was 7.4% (interquartile range, 6.5%-8.3%). Baseline characteristics by treatment group are presented in Table 1 and Table 2. Sixty percent of eyes in the combination group were phakic and 50% were phakic in the ranibizumab group. During the run-in phase, mean (SD) visual acuity improvement was 3 (6) letters in the combination group and 3 (7) letters in the ranibizumab group, and the mean (SD) visual acuity letter scores at randomization were 63 (12) (approximately 20/63) in the combination group and 63 (13) in the ranibizumab group. Mean (SD) reduction in CST during the run-in phase was −58 (83) μm in the combination group and −50 (102) μm in the ranibizumab group, and mean (SD) CST at randomization was 375 (97) μm in the combination group and 396 (122) μm in the ranibizumab group. The 24-week primary outcome visit was completed by 114 of the 116 participants (98.3%). The 2 participants who did not complete the visit were both in the combination group; 1 participant was lost to follow-up and 1 participant missed the visit (Figure 1).
Table 1. Baseline Patient Characteristics.
| Characteristic | Dexamethasone + Ranibizumab (N = 65) | Sham Treatment + Ranibizumab (N = 64) |
|---|---|---|
| Women, No. (%) | 31 (48) | 36 (56) |
| Age, median (IQR), y | 64 (59-69) | 66 (59-71) |
| Race/ethnicity, No. (%) | ||
| White | 39 (60) | 35 (55) |
| Black/African American | 6 (9) | 9 (14) |
| Hispanic or Latino | 13 (20) | 16 (25) |
| Asian | 6 (9) | 2 (3) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (2) |
| American Indian or Alaskan Native | 0 | 0 |
| >1 Race | 0 | 0 |
| Unknown / not reported | 1 (2) | 1 (2) |
| Diabetes type, No. (%) | ||
| Type 1 | 2 (3) | 2 (3) |
| Type 2 | 62 (95) | 61 (95) |
| Uncertain | 1 (2) | 1 (2) |
| Duration of diabetes, median (IQR), y | 15 (10-21) | 19 (10-26) |
| Insulin used, No. (%) | 40 (62) | 39 (61) |
| HbA1c, median (IQR), %a | 7.1 (6.4-8.3) | 7.4 (6.6-8.2) |
| Arterial blood pressure, median (IQR), mm Hg | 97 (87-106) | 98 (87-106) |
| Smoking status, No. (%) | ||
| Never | 44 (68) | 43 (67) |
| Prior | 18 (28) | 14 (22) |
| Current | 3 (5) | 7 (11) |
| BMI, median (IQR)b | 32 (29-37) | 33 (29-37) |
Abbreviations: BMI (calculated as weight in kilograms divided by height in meters squared); HbA1c, hemoglobin A1c; IQR, interquartile range.
HbA1c was missing for 3 participants in the dexamethasone + ranibizumab group and 4 in the sham treatment + ranibizumab group.
Body mass index was missing for 17 participants in the dexamethasone + ranibizumab group and 16 in the sham treatment + ranibizumab group.
Table 2. Ocular Characteristics at Randomization.
| Characteristic | Dexamethasone + Ranibizumab (N = 65) | Sham Treatment + Ranibizumab (N = 64) |
|---|---|---|
| Participants with 2 study eyes, No. (%) | 13 (20) | 13 (20) |
| Prior macular laser treatment for DME, No. (%) | 31 (48) | 31 (48) |
| Prior anti-VEGF treatment for DME, No. (%) | ||
| Aflibercept only | 7 (11) | 8 (13) |
| Bevacizumab only | 48 (74) | 49 (77) |
| Ranibizumab only | 3 (5) | 6 (9) |
| Both aflibercept and bevacizumab | 4 (6) | 0 |
| Both aflibercept and ranibizumab | 1 (2) | 0 |
| Both bevacizumab and ranibizumab | 2 (3) | 1 (2) |
| Total anti-VEGF injections for DME within the 20 wk before run-in phase, No. (%)a | ||
| 3 | 34 (56) | 44 (70) |
| 4 | 17 (28) | 16 (25) |
| 5 | 7 (11) | 2 (3) |
| 6 | 1 (2) | 0 |
| 8 | 2 (3) | 1 (2) |
| Median (IQR) | 3 (3-4) | 3 (3-4) |
| Prior intravitreal corticosteroid treatment for DME, No. (%) | 9 (14) | 10 (16) |
| Prior PRP, No. (%) | 21 (32) | 21 (33) |
| Intraocular pressure, median (IQR), mm Hg | 15 (13-17) | 16 (14-18) |
| Lens status at clinical examination, No. (%) | ||
| Pseudophakic | 26 (40) | 32 (50) |
| Phakic | 39 (60) | 32 (50) |
| Randomization visual acuity letter score (approximate Snellen equivalent), No. (%) | ||
| 24-28 (20/320) | 0 | 1 (2) |
| 29-33 (20/250) | 1 (2) | 0 |
| 34-38 (20/200) | 1 (2) | 3 (5) |
| 39-43 (20/160) | 4 (6) | 3 (5) |
| 44-48 (20/125) | 0 | 1 (2) |
| 49-53 (20/100) | 7 (11) | 6 (9) |
| 54-58 (20/80) | 6 (9) | 6 (9) |
| 59-63 (20/63) | 9 (14) | 7 (11) |
| 64-68 (20/50) | 8 (12) | 8 (13) |
| 69-73 (20/40) | 15 (23) | 14 (22) |
| 74-78 (20/32) | 14 (22) | 15 (23) |
| Mean (SD) letter score | 63 (12) | 63 (13) |
| Snellen equivalent | 20/63 | 20/63 |
| Change in visual acuity letter score from enrollment to randomization | ||
| Median (IQR) letters | 3 (−1 to 6) | 2 (−2 to 6) |
| Mean (SD) letters | 3 (6) | 3 (7) |
| Randomization CST (Stratus equivalent), No. (%)b | ||
| <350 μm | 34 (52) | 32 (50) |
| 350-449 μm | 17 (26) | 15 (23) |
| ≥450 μm | 14 (22) | 17 (27) |
| Mean (SD), μm | 375 (97) | 396 (122) |
| Change in CST from enrollment to randomization CST, μm | ||
| Median (IQR) | −39 (−111 to −5) | −37 (−93 to −3) |
| Mean (SD) | −58 (83) | −50 (102) |
| Improvement in VA and OCT CST during run-in phase, No. (%)c | ||
| Neither VA nor OCT CST is improved | 15 (23) | 12 (19) |
| VA and OCT CST are both improved | 22 (34) | 22 (34) |
| VA is not improved but OCT CST is improved | 16 (25) | 16 (25) |
| VA is improved but OCT CST is not improved | 12 (18) | 14 (22) |
| Randomization retinal volume (Stratus equivalent), mm3d | ||
| Median (IQR) | 8.0 (7.2 to 9.1) | 8.1 (7.3 to 9.1) |
| Mean (SD) | 8.3 (1.6) | 8.6 (2.0) |
| Randomization diabetic retinopathy severity level at clinical examination, No. (%) | ||
| Mild/moderate NPDR | 30 (46) | 28 (44) |
| Severe NPDR | 10 (15) | 12 (19) |
| PDR and/or prior scatter | 25 (38) | 24 (38) |
Abbreviations: CST, central subfield thickness; DME, diabetic macular edema; IQR, interquartile range; NPDR, nonproliferative diabetic retinopathy; OCT, optical coherence tomography; PDR, proliferative diabetic retinopathy; PRP, panretinal photocoagulation; VA, visual acuity; VEGF, vascular endothelial growth factor.
Data were not shown for 4 dexamethasone + ranibizumab participants and 1 sham treatment + ranibizumab participant, who reported a number of anti-VEGF injections within the prior 36 weeks instead.
Optical coherence tomography CST values obtained by spectral-domain OCT were converted to time-domain equivalent values for analysis and reporting as follows: −43.12 + 1.01 × Zeiss Cirrus; −72.76 + 1.03 × Spectralis.
Presence or absence of improvement in retinal thickness during the run-in phase was defined as reduction in CST by 10% at any run-in visit compared with the prior visit. Presence or absence of improvement in visual acuity during the run-in phase was defined as a gain of 5 or more letters in visual acuity at any run-in visit compared with the prior visit.
Optical coherence tomography retinal volume measurement was missing for 2 in the sham treatment + ranibizumab group.
DME Treatment
The mean (SD) number of ranibizumab injections between randomization and 24 weeks (maximum possible injections, 6) was 5.6 (0.7) in the combination group and 5.7 (0.7) in the ranibizumab group. Through 20 weeks, 63 of the 65 eyes (97%) in the combination group and 63 of the 64 eyes (98%) in the ranibizumab group received a second dexamethasone or sham injection. Dexamethasone/sham injections were performed almost always on the same day as ranibizumab injection (122 of the 128 injections in the combination group [95.3%] and 124 of the 129 injections in the ranibizumab group [96.1%]). No eye received nonprotocol DME treatment.
Effect of Treatment
Visual Acuity
At 24 weeks, the mean (SD) visual acuity letter scores were 66 (13) in the combination group (Snellen equivalent, 20/50) and 66 (15) in the ranibizumab group. Mean (SD) improvement in visual acuity from randomization was 2.7 (9.8) letters in the combination group and 3.0 (7.1) letters in the ranibizumab group, with the adjusted treatment group difference (combination minus ranibizumab) being −0.5 letters (95% CI, −3.6 to 2.5; P = .73) (Table 3). Similar results were observed when adjusting for prespecified potential baseline confounders (lens status, age, duration of diabetes, hemoglobin A1c level, retinal thickening on OCT, and diabetic retinopathy severity on clinical examination) and in a per-protocol analysis (eTable 2 in Supplement 2).
Table 3. 24-Week Study Outcomesa.
| Outcome | Dexamethasone + Ranibizumabb | Sham Treatment + Ranibizumabb | Adjusted Difference: Combination − Ranibizumab (95% CI)c | P Valued |
|---|---|---|---|---|
| No. of eyes | 63 | 64 | ||
| Visual acuity | ||||
| Mean (SD) letter score | 66 (13.4) | 66 (15.1) | ||
| Snellen equivalent, mean | 20/50 | 20/50 | ||
| 20/20 or better (letter score ≥84), No. (%) | 4 (6) | 3 (5) | 2 (−6 to 9) | .70 |
| 20/40 or better (letter score ≥69), No. (%) | 32 (51) | 33 (52) | −2 (−19 to 15) | .80 |
| 20/200 or worse (letter score ≤38), No. (%) | 4 (6) | 3 (5) | 2 (−6 to 9) | .70 |
| Change at 24 wk from randomization visit | ||||
| Median (IQR) letters | 2 (−1 to 8) | 4 (0 to 9) | ||
| Mean (SD) letters | 2.7 (9.8) | 3.0 (7.1) | −0.5 (−3.6 to 2.5) | .73e |
| ≥15-Letter improvement, No. (%) | 7 (11) | 1 (2) | 9 (1 to 17) | .03 |
| ≥10-Letter improvement, No. (%) | 14 (22) | 9 (14) | 6 (−6 to 18) | .34 |
| ≥10-Letter worsening, No. (%) | 8 (13) | 4 (6) | 7 (−1 to 16) | .09 |
| ≥15-Letter worsening, No. (%) | 4 (6) | 3 (5) | 2 (−5 to 9) | .62 |
| Area under the curve across 24 wkf | ||||
| Median (IQR) letters | 0.9 (−1.9 to 6.5) | 2.9 (0.4 to 5.1) | ||
| Mean (SD) letters | 1.9 (6.3) | 2.5 (4.4) | −0.3 (−2.3 to 1.7)b | .76 |
| Central subfield thickness, μmg | ||||
| Median (IQR) | 256 (191 to 316) | 282 (238 to 395) | ||
| Mean (SD) | 264 (95) | 333 (137) | ||
| CST below sex- and OCT machine-specific values, No. (%)h | 32 (52) | 20 (31) | 20 (3 to 37)b | .02 |
| Change at 24 wk from randomization visiti | ||||
| Median (IQR), μm | −88 (−148 to −50) | −43 (−105 to −11) | ||
| Mean (SD), μm | −110 (86) | −62 (97) | −52 (−82 to −22) | <.001 |
| ≥1 LogOCT step improvement, No. (%) | 34 (55) | 22 (34) | 23 (7 to 39) | .004 |
| ≥2 LogOCT steps improvement, No. (%) | 14 (23) | 8 (13) | 9 (−3 to 22) | .15 |
| ≥1 LogOCT step worsening, No. (%) | 0 | 1 (2) | −1 (−5 to 2) | .41 |
| ≥2 LogOCT steps worsening, No. (%) | 0 | 1 (2) | −2 (−8 to 4) | .37 |
| Area under the curve over 24 wkf | ||||
| Median (IQR), μm | −67.6 (−112.4 to −42.8) | −28.3 (−63.1 to −6.4) | ||
| Mean (SD), μm | −86.9 (65.6) | −33.5 (56.8) | −54.9 (−78.4 to −31.4)b | <.001 |
Abbreviations: CST, central subfield thickness; IQR, interquartile range; OCT, optical coherence tomography.
All outcomes are prespecified. Empty cells indicate not applicable.
Observed data only, including participants who completed the 24-week visit.
Treatment group differences in mean change in visual acuity, central subfield thickness, and area under the curve outcomes and the corresponding 95% CIs and P values were obtained using a linear mixed-effects model, including fixed effects for baseline visual acuity (for visual acuity outcomes) or OCT (for OCT outcomes) at the randomization visit; laterality, presence or absence of improvement in visual acuity (for visual acuity outcomes) or OCT (for OCT outcomes) during the run-in phase; and a random participant effect to account for the correlation between 2 study eyes of the same participant, with Markov chain Monte Carlo multiple imputation (100 imputations) for missing data unless otherwise indicated. Binary outcomes were analyzed using binomial regression models with generalized estimating equations to account for correlation between eyes of participants with 2 study eyes, adjusting for the same covariates in the primary linear mixed-effects model. When the binomial regression model failed to converge, a hierarchy was applied to the model to remove covariates: laterality, presence or absence of improvement during the run-in phase, and baseline value at the randomization visit. The Barnard unconditional test was performed when the binomial regression model failed to converge without any covariates.
All P values are 2-sided.
P = .69 for comparing mean change in visual acuity letter score at 24 weeks between treatment groups, with additional adjustment for potential baseline confounders, including lens status, age, duration of diabetes, hemoglobin A1c level, retinal thickening on OCT, and diabetic retinopathy severity on clinical examination.
No imputation for missing data was performed.
Optical coherence tomography values obtained by spectral-domain OCT were converted to time-domain equivalent values for analysis and reporting as follows: −43.12 + 1.01 × Zeiss Cirrus; −72.76 + 1.03 × Spectralis. One central subfield thickness value measured by OCT was missing for the combination group owing to low resolution.
Sex- and OCT machine-specific values for OCT central subfield thickness (in micrometers) are defined as less than 290 in women and less than 305 in men in Zeiss Cirrus; less than 305 in women and less than 320 in men in Heidelberg Spectralis.
Change in OCT central subfield thickness (in micrometers) was truncated to 3 SDs from the mean (–372 to 201; calculated using observed changes at 24 weeks and combining all treatment groups), to minimize the effect of outliers. Two values were truncated in the sham treatment + ranibizumab group: 1 on the negative end, and 1 on the positive end.
Mean change in visual acuity over 24 weeks (area under the curve) was 1.9 letters for combination and 2.5 letters for ranibizumab therapy (adjusted difference, −0.3; 95% CI, −2.3 to 1.7; P = .76) (Figure 2A). Improvement of 10 letters or more between randomization and 24 weeks was observed in 14 of the 63 eyes (22%) in the combination group and 9 of the 64 eyes (14%) in the ranibizumab group (adjusted difference, 6%; 95 CI, −6% to 18%; P = .34). Improvement of 15 letters or more was seen in 7 eyes (11%) in the combination group and 1 eye (2%) in the ranibizumab group (adjusted difference, 9%; 95 CI, 1% to 17%; P = .03). In the combination group, 8 eyes (13%) lost 10 letters or more compared with 4 (6%) for the ranibizumab group (adjusted difference, 7%; 95% CI, −1% to 16%; P = .09); worsening of 15 letters or more was seen in 4 eyes (6%) in the combination group and 3 eyes (5%) in the ranibizumab group (adjusted difference, 2%; 95 CI, −5% to 9%; P = .62). Table 3 and eFigure 1 in Supplement 2 show the full distribution of visual acuity and visual acuity changes at 24 weeks.
Figure 2. Mean Change in Visual Acuity and Central Subfield Thickness (CST) on Optical Coherence Tomography (OCT) Across 24 Weeks.
A, Mean change in visual acuity. B, Mean change in CST on OCT. Error bars indicate 95% CIs. Outlying values were truncated to 3 SDs from the mean.
Subgroup analyses are presented in eTables 3 and 4 and eFigures 2 through 4 in Supplement 2. For baseline lens status (P = .08 for interaction), the adjusted mean difference in visual acuity at the 24-week visit between the combination group and the ranibizumab group was 3.1 letters (95% CI, −2.1 to 8.3) for pseudophakic eyes and −3.0 letters (95% CI, −7.7 to 1.7) for phakic eyes.
Retinal Thickening
At 24 weeks, 32 of the 62 eyes (52%) in the combination group and 20 of the 64 (31%) eyes in the ranibizumab group had normal CST values based on the sex-specific spectral-domain OCT threshold norms (P = .02) (Table 3). Mean (SD) change in CST in the combination group was −110 (86) μm compared with −62 (97) μm for the ranibizumab group (adjusted difference, −52; 95% CI, −82 to −22; P < .001) (Table 3). A per-protocol analysis suggested similar conclusions (eTable 2 in Supplement 2). The mean (SD) change in OCT CST over 24 weeks (area under the curve) was greater with combination therapy (−86.9 [65.6] μm) compared with ranibizumab (−33.5 [56.8] μm; combination minus ranibizumab, −54.9; 95% CI, −78.4 to −31.4; P < .001) (Figure 2B). Additional OCT outcomes are presented in Table 3. No significant interactions (eTable 3 in Supplement 2) were seen for CST changes according to baseline lens status (P = .17 for interaction; eFigure 5 in Supplement 2), improvement in visual acuity during the run-in phase (P = .33 for interaction; eFigure 6 in Supplement 2), or improvement in CST during the run-in phase (P = .15 for interaction; eFigure 7 in Supplement 2).
Safety
There were no cases of endophthalmitis. In the combination group, 19 of 65 eyes (29%) experienced increased IOP or initiated IOP-lowering eyedrops compared with 0 of 64 in the ranibizumab group (P < .001, eTable 5 in Supplement 2). Fifteen eyes in the combination group (23%) experienced increases of 10 mm Hg or more in IOP compared with 0 in the ranibizumab group. In the combination group, 3 of 65 eyes (5%) received postrandomization cataract extractions compared with 0 of 64 eyes in the ranibizumab group (P = .24). Additional ocular outcomes are reported in eTable 5 in Supplement 2. Rates of systemic adverse events did not appear to be substantially different between the groups (eTables 6 and 7 in Supplement 2).
Discussion
In this phase 2 randomized clinical trial, the addition of dexamethasone treatment to an eye receiving ranibizumab therapy for persistent DME did not result in superior visual acuity gains, on average, compared with continuing ranibizumab treatment alone through 24 weeks. The combination group had a significantly greater reduction in retinal thickening on OCT. Consistent with other trials evaluating corticosteroids, increased IOP developed in more eyes in the combination group than in the ranibizumab-only group. The 6-month duration of this study was insufficient to evaluate for differences in cataract extraction, and no standardized measurement of cataract development was performed.
There was a suggestion that more eyes in the combination group had visual acuity improvement by a clinically relevant amount (≥15-letter gain from randomization) but also a suggestion that more eyes in the combination group worsened visual acuity by a clinically relevant amount (≥10-letter loss from randomization). The small number of eyes that had clinically relevant changes in vision do not allow definitive conclusions about treatment differences. It is possible that vision loss in some phakic eyes in the combination group that had not undergone cataract surgery was due to early cataract formation. Among the eyes with at least a 2-line visual acuity loss, 7 of 8 eyes in the combination group and 1 of 4 eyes in the ranibizumab group were phakic.
The results from this study are consistent with previous trials of similar cohorts in which improvement in CST after switching to or adding corticosteroid treatment was greater but concomitant improvement in visual acuity was not greater than continued anti-VEGF treatment alone. Several earlier studies of DME also have shown discordance between the treatment effects, with improvement in retinal thickening and no improvement in visual acuity, as seen in this study. Because a long-term benefit of reducing retinal thickening in the absence of visual acuity improvement has not been demonstrated, we hesitate to recommend a treatment approach on the basis of anatomic response alone.
Participants had at least 3 anti-VEGF injections before they were considered by the investigator to have persistent DME, and they were given 3 additional ranibizumab injections during the run-in phase, which addresses potential concerns that suboptimal responses were initially a result of insufficient anti-VEGF treatment. The continued ranibizumab treatment in the control group addresses the potential confounder of time, which might have contributed to improvement in edema noted in previous case series that evaluated a switch in therapies for eyes judged to have inadequate anti-VEGF levels. Approximately one-third of the eyes during the run-in phase became ineligible for the randomized trial because persistent DME resolved. Such improvement in DME is consistent with previous reports indicating that, in many eyes given continued anti-VEGF with a single agent, DME continued to resolve beyond 3 injections. Most eyes in the ranibizumab group received 9 study injections, totaling at least 12 anti-VEGF injections. On average, these eyes gained 6 letters over 9 months from the 3-month run-in phase through the end of the 6-month randomization phase.
Intraocular pressure increases of 10 mm Hg or more after the first 2 dexamethasone injections were found in 23% of participants receiving dexamethasone in the present study, a similar proportion as in the MEAD Study (approximately 21%). In the MEAD study, dexamethasone treatment was administered every 6 months; in the present study, treatment was permitted as often as every 3 months. In the DRCR.net Protocol I, the proportion of eyes with an increase in IOP at 6 months after 1 or 2 intravitreous triamcinolone acetonide injections (24%; Adam Glassman, MS, e-mail, September 25, 2017) was similar to the present trial with intravitreous dexamethasone. The present study confirmed no large safety concern from administering combination treatment on the same day; a moderate or small safety concern cannot be ruled out.
The study was designed originally to include only pseudophakic eyes because of the known effect of corticosteroids on cataract formation. However, owing to slow recruitment, phakic eyes subsequently were allowed, constituting about half of the study eyes. A prespecified subgroup analysis suggested that pseudophakic eyes, on average, had a better visual acuity outcome with combination treatment than with ranibizumab therapy alone and that phakic eyes, on average, had a better outcome with ranibizumab therapy alone than with the combination treatment. Other trials evaluating corticosteroid treatment for DME also have shown an interaction between treatment and lens status on visual acuity. Nevertheless, the magnitude of the effect at 24 weeks was relatively small in each subgroup (combination group, 3.1 and ranibizumab group, –3.0 letters) and even smaller in the pseudophakic eyes at earlier visits. Furthermore, as a general rule, when the primary analysis does not show a statistically significant treatment group difference, as in this study, subgroup results must be viewed with extreme caution and should be viewed as hypothesis generating only. Given the challenges faced by the DRCR.net in enrolling an adequate number of pseudophakic eyes, it seems unlikely that a study of sufficient size with only pseudophakic eyes is feasible.
Strengths and Limitations
The strengths of this study include randomization, participant masking to avoid bias, confirmation via standardized run-in phase that the eye had persistent DME, a standardized treatment protocol, and a high retention rate. However, there are several limitations. As a phase 2 study, the sample size was not as large as would be needed for a phase 3 study, and treatment duration was relatively short. Inclusion of phakic eyes could have confounded visual acuity results if cataract progression occurred more frequently in the combination group. By chance, there were more phakic eyes in the combination group (60% phakic) than the ranibizumab group (50%); however, adjusting for this potential confounder did not alter the results. Although the sham procedure changed during the trial, when analyses were stratified according to the type of sham procedure, results were similar to the overall results. The effects of other corticosteroid formulations, other means of delivery, or other anti-VEGF agents cannot be determined from this study.
Conclusions
In this randomized clinical trial of eyes with persistent DME after multiple anti-VEGF injections, the addition of dexamethasone with continued ranibizumab injections produced no better improvement in mean visual acuity at 6 months than ranibizumab therapy alone, even though, on average, there was a significantly greater reduction in retinal thickness in the combination group. The study is of insufficient size to determine whether treatment responses would be different in phakic and pseudophakic eyes.
Trial Protocol.
eAppendix 1. Study Sites
eAppendix 2. Institutional Review Boards
eTable 1. Eligibility Criteria
eTable 2. Per-Protocol Analysis of Visual Acuity and Retinal Thickness
eTable 3. Pre-Planned Subgroup Analysis of Visual Acuity and Retinal Thickness
eTable 4. Post Hoc Subgroup Analysis of Visual Acuity
eTable 5. Ocular Adverse Events During Randomization Phase
eTable 6. Systemic Adverse Events During Randomization Phase
eTable 7. All Adverse Events During Randomization Phase by MedDRA System Organ Class
eFigure 1. Visual Acuity at 24 Weeks by Treatment Group Assignment
eFigure 2. Mean Change in Visual Acuity by Baseline Lens Status
eFigure 3. Mean Change in Visual Acuity by Improvement in Visual Acuity During Run-in Phase
eFigure 4. Mean Change in Visual Acuity by Improvement in Central Subfield Thickness During Run-in Phase
eFigure 5. Mean Change in Central Subfield Thickness by Baseline Lens Status
eFigure 6. Mean Change in Central Subfield Thickness by Improvement in Visual Acuity During Run-in Phase
eFigure 7. Mean Change in Central Subfield Thickness by Improvement in Central Subfield Thickness During Run-in Phase
References
- 1.Elman MJ, Aiello LP, Beck RW, et al. ; Diabetic Retinopathy Clinical Research Network . Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064-1077.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells JA, Glassman AR, Ayala AR, et al. ; Diabetic Retinopathy Clinical Research Network . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen QD, Brown DM, Marcus DM, et al. ; RISE and RIDE Research Group . Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. ; RESTORE Study Group . The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615-625. [DOI] [PubMed] [Google Scholar]
- 5.Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341(2-3):309-315. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J, Ingber DE. Angiostatic steroids: method of discovery and mechanism of action. Ann Surg. 1987;206(3):374-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Díaz-Flores L, Gutiérrez R, Varela H. Angiogenesis: an update. Histol Histopathol. 1994;9(4):807-843. [PubMed] [Google Scholar]
- 8.Diabetic Retinopathy Clinical Research Network A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115(9):1447-1449, 1449.e1-e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer DS, Yoon YH, Belfort R Jr, et al. ; Ozurdex MEAD Study Group . Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904-1914. [DOI] [PubMed] [Google Scholar]
- 10.Campochiaro PA, Brown DM, Pearson A, et al. ; FAME Study Group . Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626-635.e2. [DOI] [PubMed] [Google Scholar]
- 11.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 12.Maturi RK, Bleau L, Saunders J, Mubasher M, Stewart MW. A 12-month, single-masked, randomized controlled study of eyes with persistent diabetic macular edema after multiple anti-VEGF injections to assess the efficacy of the dexamethasone-delayed delivery system as an adjunct to bevacizumab compared with continued bevacizumab monotherapy. Retina. 2015;35(8):1604-1614. [DOI] [PubMed] [Google Scholar]
- 13.Shah SU, Harless A, Bleau L, Maturi RK. Prospective randomized subject-masked study of intravitreal bevacizumab monotherapy versus dexamethasone implant monotherapy in the treatment of persistent diabetic macular edema. Retina. 2016;36(10):1986-1996. [DOI] [PubMed] [Google Scholar]
- 14.Maturi RK, Pollack A, Uy HS, et al. ; Ozurdex MEAD Study Group . Intraocular pressure in patients with diabetic macular edema treated with dexamethasone intravitreal implant in the 3-year MEAD study. Retina. 2016;36(6):1143-1152. [DOI] [PubMed] [Google Scholar]
- 15.Ferris FL III, Maguire MG, Glassman AR, Ying GS, Martin DF. Evaluating effects of switching anti–vascular endothelial growth factor drugs for age-related macular degeneration and diabetic macular edema. JAMA Ophthalmol. 2017;135(2):145-149. doi: 10.1001/jamaophthalmol.2016.4820 [DOI] [PubMed] [Google Scholar]
- 16.Bressler SB, Ayala AR, Bressler NM, et al. ; Diabetic Retinopathy Clinical Research Network . Persistent macular thickening after ranibizumab treatment for diabetic macular edema with vision impairment. JAMA Ophthalmol. 2016;134(3):278-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bressler SB, Qin H, Beck RW, et al. ; Diabetic Retinopathy Clinical Research Network . Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol. 2012;130(9):1153-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eAppendix 1. Study Sites
eAppendix 2. Institutional Review Boards
eTable 1. Eligibility Criteria
eTable 2. Per-Protocol Analysis of Visual Acuity and Retinal Thickness
eTable 3. Pre-Planned Subgroup Analysis of Visual Acuity and Retinal Thickness
eTable 4. Post Hoc Subgroup Analysis of Visual Acuity
eTable 5. Ocular Adverse Events During Randomization Phase
eTable 6. Systemic Adverse Events During Randomization Phase
eTable 7. All Adverse Events During Randomization Phase by MedDRA System Organ Class
eFigure 1. Visual Acuity at 24 Weeks by Treatment Group Assignment
eFigure 2. Mean Change in Visual Acuity by Baseline Lens Status
eFigure 3. Mean Change in Visual Acuity by Improvement in Visual Acuity During Run-in Phase
eFigure 4. Mean Change in Visual Acuity by Improvement in Central Subfield Thickness During Run-in Phase
eFigure 5. Mean Change in Central Subfield Thickness by Baseline Lens Status
eFigure 6. Mean Change in Central Subfield Thickness by Improvement in Visual Acuity During Run-in Phase
eFigure 7. Mean Change in Central Subfield Thickness by Improvement in Central Subfield Thickness During Run-in Phase