Abstract
Impaired epithelial regeneration is a crucial pathophysiological feature of ulcerative colitis (UC). Yes-associated protein (YAP1) appears to control cell proliferation and differentiation. In this study, we sought to identify the roles of YAP in intestinal epithelial cell (IEC) self-renewal, regeneration and tumorigenesis. We first observed that YAP was significantly reduced in 62.5% (45/72) of human UC tissues and it was dramatically enhanced during epithelial regeneration in a murine colitis model. Using lentiviral infection, we established a YAP-overexpression (YAPWT) mouse model. We then found that after tissue injury, YAPWT mice had increased epithelial cell self-renewal capacity and drastically restored intestinal crypt structure. Strikingly, these mice were more susceptible to colitis-associated cancer (CAC) in chemically induced carcinoma. Mechanistically, YAP and β-catenin showed increased nuclear co-localization during regeneration after inflammation. Overexpressing YAP significantly improved IEC ‘wound-healing’ ability and increased the expression of both β-catenin and the transcriptional targets of Wnt signalling Lgr5 and cyclin D1, whereas silencing β-catenin in YAPWT cells attenuated this effect. Remarkably, we observed that YAP could directly interact with β-catenin in the nucleus and formed a transcriptional YAP/β-catenin/TCF4 complex; Lgr5 and cyclin D1 were confirmed to be the target genes of this complex. In contrast, cancer cell proliferation and tumour development were suppressed by the phospho-mimetic YAP mutant. In summary, nuclear YAP-driven IEC proliferation could control epithelial regeneration after inflammation and may serve as a potential therapeutic target in UC. However, excessive YAP activation promoted CAC development.
Introduction1
One essential function of the Intestinal epithelium is to act as a physical barrier that hampers luminal antigens such as the intestinal microbiota interacting with the internal milieu, while maintaining the absorption of nutrients and ions1,2. A contiguous layer of cells has a pivotal role in the epithelial barrier function. During homeostasis, intestinal epithelial cells (IECs) self-renew every 3–5 days3. Upon surface damage, an intricate repair process is triggered to restore the structural and functional integrity of the impaired tissue within several minutes. This process is also called ‘epithelial restitution’, is particularly important in the resealing of the damaged epithelium4, following by intestinal stem cells (ISCs) activation, proliferation and differentiation5.
Ulcerative colitis (UC) is a subcategory of inflammatory bowel disease (IBD) with high-risk of colitis-associated cancer (CAC)6. Two key pathophysiological features of this disease are the dysregulation of innate immune system and impaired epithelial regeneration7. Although, immunosuppressive therapies in treatment of UC are advancing, the poor clinical consequences and variable complications still remain UC as a challenge to physicians. For both pathophysiological features, mucosal healing has recently been regarded as a key treatment goal to UC8.
Multiple pathways including Wnt/β-catenin, Notch, Hippo/Yes-associated protein and transforming growth factor-β (TGF-β) are involved in the regenerative epithelial process9,10. The Hippo pathway is particularly essential in control of organ size and tissue growth11,12. YAP (YAP1 or YAP65) is a core component of the Hippo pathway and a candidate oncogene in humans13. When Hippo is active, the LATS1/2 kinases of the Hippo pathway phosphorylate YAP, leading to YAP cytoplasmic retention and degradation by β-TrCP ubiquitin ligase11,14. Upon nuclear translocation, YAP binds to transcription factors (mainly TEAD family proteins) and acts as a transcriptional co-activator to initiate the transcription of target genes4,15. YAP regulates EGFR-dependent regenerative program via elevated Areg (acts as a YAP/TEAD target gene) after exposure to irradiation16. Meanwhile, biological activation of the Notch pathway, which is important in epithelial cell proliferation/differentiation17, was alternative driven via YAP interaction with Notch ligands18.
In addition to YAP in epithelial cells, the Wnt signalling pathway is a critical regulator of stem cell maintenance, cell fate and proliferation19,20. When inactive, cytosolic β-catenin is recruited into the adenomatous polyposis coli (APC)/Axin/GSK3/CK1 destruction complex, resulting in its phosphorylation by GSK3 and CK1 and degradation by the β-Trcp ubiquitin ligase. When activated, β-catenin escapes the regulation of the destruction complex and triggers transcription by subsequent nuclear accumulation and interaction with TCF/LEF transcription factors21,22. Establishing the ISC compartment requires Wnt signalling that deletion of TCF4 expression caused foetal mice to lack proliferative crypts23. Moreover, the conditional knockout of β-catenin or TCF4 results in crypt proliferation deficiency in adult mice, which suggested that maintenance of IECs proliferation was Wnt-dependent24,25.
The overlap between YAP and Wnt regulation in IEC proliferation imply that these factors do not work independently and might have a potential link, but the available mechanism has not been investigated yet. In this study, we demonstrate that YAP triggers Wnt/β-catenin signalling, which stimulates epithelial cell proliferation and not only facilitates enterocyte self-renewal and crypt regeneration after colitis, but also promotes CAC development through chronic inflammation and excessive regeneration.
Materials and methods
Human colon samples
Two groups of human specimens were procured with approval from the Institute Research Medical Ethics Committee of Nanfang Hospital. One group comprised paraffin-embedded sections of biopsy specimens (n = 72 UC and n = 21 normal tissues) from the Department of Pathology at Nanfang Hospital. These sections were used for analysing immunohistochemical (IHC) expression. The other matched specimens (n = 15) were routine colonoscopy biopsies procured from the Department of Gastroenterology at Nanfang Hospital. These fresh specimens were used for Western blotting and RT-PCR analysis. The corresponding clinical data were recorded from the medical records. Patient characteristics and histological data are shown in Table 1.
Table 1.
Patient characteristics
| Characteristic | Normal control (n = 21) | Inactive UC (n = 30) | Active UC (n = 42) |
|---|---|---|---|
| Gender | |||
| Male | 13 | 19 | 22 |
| Female | 8 | 11 | 20 |
| Age (years ± SD) | 44.3 ± 12.8 | 44.7 ± 19.2 | 47.9 ± 12.2 |
| Disease duration (years ± SD) | _ | 3.2 ± 5.3 | 4.2 ± 4.8 |
| Extent of disease | |||
| Extensive colitis | _ | 10 | 17 |
| Left-sided colitis | _ | 12 | 12 |
| Proctitis | _ | 8 | 13 |
| Treatment | |||
| TNF antagonist use | _ | 5 | 7 |
| Aminosalicylates | _ | 20 | 28 |
| Immune modulator use | _ | 4 | 8 |
| Corticosteroids | _ | 11 | 20 |
| None | _ | 3 | 3 |
| Pathological severity | |||
| Grade 0–1 | _ | 30 | _ |
| Grade 2–5 | _ | _ | 42 |
Reagents and antibodies
Recombinant Wnt-3a and DKK1 were purchased from R&D Systems (Abingdon, UK); AOM (Azoxymethane), fluorescein isothiocyanate (FITC)-dextran (40,000 kDa) and 5-bromo-2′-deoxyuridine (Brdu) were from Sigma (St. Louis, MO); and dextran sodium sulfate (DSS; molecular weight (MW) 40,000–50,000 kDa) was from MP Biomedicals (Santa Ana, CA). The following antibodies were purchased: YAP, phosphor-YAP S127, β-catenin, β-Trcp, Brdu and Ki67 (Cell Signaling Technology, Boston, MA); cyclin D1, LATS2, DVL2 Caspase-3 and PCNA (Proteintech, Rosemont, USA); Lgr5 and TCF4 (Abcam, Cambridge, MA); and c-myc, CK20, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and histone H3 (Santa Cruz, Dallas, Texas).
Plasmids, siRNA and transfection
The lentiviral expression vectors overexpressing wild-type YAP protein and the YAP mutant were purchased from Genechem (Shanghai, China). The vectors were GV358, which contained the component order Ubi-MCS-3FLAG-SV40-EGFP-IRES-puromycin. The primer sequences for YAP1 were as follows: Human YAP1 forward: 5′-GAGGATCCCC
GGGTACCGGTCGCCACCATGGATCCCGGGCAGCAGCCGC-3′ and reverse: 5′-TCCTTGTAGTCCATACCTAACCATGTAAGAAAGCTTTC-3′. Mouse YAP1 forward: 5′-GAGGATCCCCGGGT ACCGGTCGCCACCATGGAGCCCGCGCAACAG-3′ and reverse: 5′-TCCTTGTAGTCC
ATACCTAACCACGTGAGAAAGCT-3′. Both YAP fragments harbouring the S127D (serine to alanine at residue 127) and the S112D (serine to alanine at residue 112, corresponding to S127 of human YAP) mutations were inserted into the vector GV358 to construct the recombinant lentivirus plasmid. Stable YAP-WT-, YAP-mutant- and empty control vector-transfected clones were selected using medium containing puromycin (2 μg/ml) for 2–3 weeks. The pcDNA3.1-β-catenin-myc plasmid was kindly provided by Dr. Gengtai Ye (Southern Medical University, Guangzhou, China). Specific YAP and β-catenin small interfering RNAs (siRNAs) and control siRNAs were obtained from Genepharma (Shanghai, China). The sequences of siRNAs were detailed in Supplementary Table 2. Transfection with plasmids or siRNAs was completed using Lipofectamine 3000 (Invitrogen, Carlsbad, CA) as described by the manufacturer. Infection of the above cells was confirmed by immunoblot analysis.
Cell culture
The FHC, DLD1, HT29 and HEK293T cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37 °C with 5% CO2. For DSS-induced inflammation in vitro, FHC cells were treated with 1% DSS for 0 or 4 h after an overnight starvation period in serum-free medium, followed by 1 h (4 + 1 h), 2 h (4 + 2 h) or 4 h (4 + 4 h) of incubation in reduced serum culture medium; Wnt activation was accomplished by treating FHC cells with Wnt3a (100 ng/ml) for the indicated time.
Nuclear and cytosol fractionation
Nuclear and cytosol fractions were obtained using a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, China) according to the manufacturer’s instructions. Briefly, 1 × 107 cells were washed with phosphate-buffered saline (PBS), collected in 200 µl of cytoplasmic protein extraction agent A/protease inhibitor buffer and then left on ice for 10–15 min. Cells were then incubated in 10 µl of cytoplasmic protein extraction agent B, followed by centrifugation at 12,000 g for 10 min at 4 °C to pellet nuclei away from the cytoplasm. The nuclei were resuspended in 50 µl of nuclear protein extraction buffer and agitated for 30 min on ice. After centrifugation at 12 000 g for 10 min at 4 °C, the supernatant was collected as the nuclear extract for subsequent analysis.
Co-immunoprecipitation and western blot analysis
Equal amounts of protein samples were loaded onto 10 to 12% SDS-polyacrylamide gel electrophoresis gels and transferred onto polyvinylidene difluoride membranes (Bio-Rad). Protein blots were incubated with the indicated primary antibody and then with the appropriate secondary antibody, followed by detection with the enhanced chemiluminescence detection system.
For immunoprecipitation, cells were lysed in buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride and cocktail protease inhibitors followed by centrifugation at 12,000 g for 30 min at 4 °C. Lysates (200 μg) were then precipitated with 5 µg of the indicated antibody or control IgG plus 20 µl of protein A/G agarose (Santa Cruz) for 2 h. After extensive shaking, immune complexes were then washed three times in lysis buffer and subjected to western blot analysis using the indicated antibodies.
Chromatin immunoprecipitation assays
Quantitative chromatin immunoprecipitation was performed with a Pierce Agarose ChIP Kit (Thermo Fisher Scientific) according to the protocol, FHC-EV, FHC-YAPWT and FHC-YAPS127D cells were crosslinked for 10 min using a final concentration of 1% formaldehyde in the media. After sonication, the chromatin solution was diluted in chromatin immunoprecipiattion (ChIP) lysate and incubated with either anti-TCF4 antibody or normal IgG. ChIP Grade Protein A/G plus Agarose (20 μl) was added to each fraction and rotated at 4 °C. Then the antibody/protein/DNA complex was washed using elution buffer. The DNA–protein crosslinks were reversed by heating at 65 °C for 4 h. DNA was finally purified and subjected to real-time PCR. The sequences of the PCR primers used were as follows: Lgr5 forward 5′-GGAGGGGACAAGTGGAGGG-3′ and reverse 5′-CAGTGGCGGTGCGCC-3′, and cyclin D1 forward 5′-AAGAGTCTCCAGGCTAGAAGGACA-3′ and reverse 5′-AGTTAAAGGGATTTCAGCTTAGCATG-3′.
Confocal microscopy
FHC cells were plated at sterile coverslips in culture dishes. Twelve hours later, cultures were subjected to overnight starvation with serum-free medium prior to treatment with 1% DSS for 4 h. Cells were washed thrice with PBS, followed by fixation with 4% paraformaldehyde for 15 min at room temperature. The fixed cells were washed three times and permeabilized with 0.5% Triton X-100 in PBS at room temperature for 10 minutes. Cells were then left in 3% bovine serum albumin/PBS blocking buffer for 30 min, followed by an incubation with primary antibody overnight at 4 °C. Then with three times washing, cells were incubated with secondary fluorescent antibodies for 1 h and counterstained with DAPI (4',6-diamidino-2-phenylindole). Cells were visualized with an FV1000 confocal laser scanning microscope (Olympus, Japan). Images were analysed with FV10-ASW 3.0 Viewer software.
Quantitative real-time PCR
Total RNA was extracted from cells and tissues using TRIzol (Takara, Japan), and cDNA synthesis was performed with a reverse transcriptase kit (Takara) according to the manufacturer’s protocol. Then, SYBR Green Premix Ex Taq was used for subsequent quantitative real-time PCR amplification on an LC480 system. GAPDH was used as the internal control. Gene-specific primer sequences are listed in Supplementary Table 1.
Histochemistry and immunohistochemistry
Mouse colon samples were fixed in 4% paraformaldehyde for 24 h and embedded in paraffin; 5 μm-thick sections were used for haematoxylin and eosin or IHC staining. For IHC, deparaffinized sections were performed quenching of endogenous peroxidase activity, antigen retrieval and subsequent blocking procedures. Slices were incubated in the primary antibodies of interest at 4 °C overnight, followed by incubation with biotinylated secondary antibody for 2 h at room temperature. The immunosignal was visualized via a DAB kit. The histological inflammation score and tumour classification were independently analysed by two senior pathologists.
Cell proliferation, wound healing and colony-formation assays
Cell proliferation was measured using a Cell Counting Kit-8 (Dojindo, Tokyo) according to the manufacturer’s instruction. Briefly, 100 µl of cells were plated at a concentration of 2000 cells/well in 96-well plates. CCK-8 working buffer (10 μl) was added into the cultures and incubated at 37 °C for 1 h. Colorimetric measurements were performed at 450 nm on a microplate reader.
For scratch assays, cell migratory activity was visualized after transfection with targeted plasmids and (or) siRNAs. The cell monolayer was scratched with a 10 μl pipette tip. Cells that migrated towards the scratches were imaged at the indicated times.
To study colony formation, 500 cells were seeded in 6-well plates. After 7-day incubation, colonies were obtained, fixed with 4% paraformaldehyde and then stained with 0.05% crystal violet; the number of colonies was calculated, followed by extensive washing in PBS.
Cell apoptosis
Assessment of APC Annexin V-labelled cell apoptosis was measured according to the manufacturer’s protocol (KeyGEN BioTECH, Nangjing, China). FHC-EV and FHCWT Cells were seeded on six-well plates and treated with 1% DSS for 12 h, then cells were suspended in 500 μl binding buffer containing 5 μl APC Annexin-V and 5 μl Propidium Iodide (PI). Cells were analysed by a fluorescence-activated cell sorting (BD Biosciences). A total of 10,000 cells were analysed per determination. Cells were considered apoptotic when they manifested early apoptosis (Annexin-V positive, PI negative) or late apoptosis (Annexin-V positive, PI positive).
Mouse work
BALB/c mice were purchased from the Medical Experimental Animal Centre of Southern Medical University. All experiments were performed with the approval of the Animal Experimentation Committee of the Southern Medical University (2016023) and in accordance with institutional regulations. For the YAP genetically manipulated model, 6- to 8-week-old male mice were infected with mouse YAPWT or YAPS112D lentivirus (1 × 107 units per mouse) in a total volume of 100 µl by intraperitoneal injections, the empty vector (EV) was used as the internal control. Three days later, gene expression in mice was first analysed to insure the successful infection and then different model constructions were subsequently performed. For chronic model, mice were treated with lentivirus intraperitoneally twice a week.
For DSS-induced colitis and regeneration, mice underwent intestinal inflammation by receiving water with 3% DSS (MW 40,000–50,000, MP Biomedicals) for 5 consecutive days (5d), and followed by 5 days normal drinking water (5 + 5d), which was defined as regeneration after colitis. In this model, body weight and disease activity index (DAI) of mice were monitored every day. Mice were killed at the time points indicated in the text.
To generate chemically induced colorectal carcinoma, mice were intraperitoneally injected with 10 mg/kg body weight AOM (Sigma). One week later, mice were treated with 3% DSS in distilled water for 7 days, followed by 14 days of normal drinking water as previously described26. This cycle was repeated three times. Mice were killed at the time points indicated in the text.
Isolation of primary colonic epithelial cells from mice
After the mice were killed according to the previously related protocol, the colons were obtained, cut the colon open lengthwise and rinsed with ice-cold PBS. Crypts were obtained after incubating intestines at 37 °C in 5 mM EDTA for 20 min with extensive shaking at 200 r.p.m. FBS/DMEM (10%) was added to neutralize the digestion, then passed buffer/crypt solution through a 70 μm cell strainer and collected in a 50 ml centrifuge tube. The pellet was collected by centrifuging the filtered crypts at 1,000 g for 10 min at 4 °C.
Histological analysis
Human UC severity was graded from 0 to 5 according to Geboes criteria27. Immunoreactivity was assessed from immunoreactive scores (IRSs). In addition, the histological scores of mice were assessed in a blinded manner according to the previously described criteria28. Each quantitative measurement was evaluated from 3–5 fields of view in each independent specimen.
Brdu-labelling assays
Mice received intraperitoneal injections of 50 mg/kg Brdu before killing; then, mouse colons were collected for paraffin sections and anti-Brdu IHC staining. The number and location of Brdu-positive cells were assessed in the different crypt samples.
Intestinal permeability assay
Intestinal permeability was assessed through the oral administration of FITC-labelled dextran as previously described29.
Statistics
Data are expressed as the means ± SEMs. Significance was determined using Student’s t-test, one-way or two-way analysis of variance, as appropriate, for multiple comparisons by Prism 5 (GraphPad Software, USA). Statistical significance was defined as a P-value < 0.05.
Results
YAP expression was decreased in human UC tissues
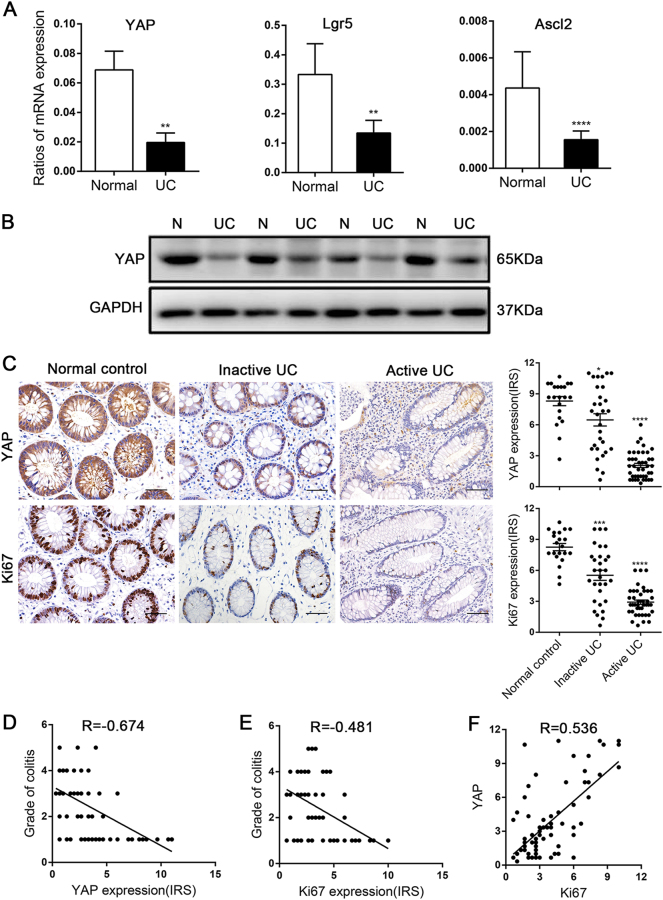
We first examined the expression of proliferation-related genes in fresh specimens obtained from colonoscopies of UC tissues and found that the YAP mRNA level was decreased in 80% (12/15) of paired UC tissues, concomitant with declined ISC signature genes, Lgr5 and ascl2 (Fig. 1a). Meanwhile, total YAP protein expression was also lower in UC tissues than in the matched normal tissues (Fig. 1b). IHC analysis of YAP localization in human normal colons showed that it accumulated in both the nucleus and cytoplasm of epithelial cells. YAP was significantly reduced in 62.5% (45/72) of UC tissues, especially in active UC, and was undetectable in 18.1% (13/72) of these specimens. In parallel, Ki67 expression in patients with active UC showed a significant decrease compared with that in patients with inactive UC or in control patients (p < 0.0001, Fig. 1c). In addition, Spearman’s correlation analyses revealed that both YAP and Ki67 expression in epithelial cells correlated negatively with colitis severity (R = – 0.674, p < 0.0001; R = – 0.481, p < 0.0001, Fig. 1d,e) and YAP accumulation was positively associated with Ki67 expression in UC tissues (R = 0.536, p < 0.0001, Fig. 1f). Patients in long-term remission with no endoscopic signs of colitis were defined as inactive UC (Geboes histology score 0 or 1). Patients in the acute stage of the disease with endoscopically visible signs of colitis were defined as active UC (Geboes histology score ≥ 2)30. These data demonstrated that epithelial cell proliferation was obstructed in the development of human UC and could be mediated by YAP.
Fig. 1. YAP expression was down-regulated in human ulcerative colitis (UC).
(a) mRNA expression levels in ulcerative colitis and matched normal tissues (n = 15), as determined by real-time PCR. GAPDH mRNA expression was used as the internal control. Data represent the means ± SEMs. **P < 0.01, ****P < 0.0001. (b) Western blot analysis of total YAP protein expression level in UC and corresponding normal tissues (n = 15). N, normal colon; UC, ulcerative colitis. (c) Immunohistochemical staining of total YAP in normal controls (n = 21), inactive UC (n = 30) and active UC (n = 42) tissues (× 400). Data for the scatter diagrams were generated from immunoreactive scores (IRSs). Scale bars = 20 μm. Data represent the means ± SEMs. *P < 0.05, ***P < 0.001, ****P < 0.0001. (d) The Spearmen’s correlation between YAP expression and colitis severity (r = – 0.674, p < 0.0001). (e) The Spearman’s correlation between Ki67 expression and colitis severity (r = – 0.481, p < 0.0001). (f) The Spearman’s correlation between YAP and Ki67 expression in epithelial cells of patients with UC (r = 0.536, P < 0.0001)
YAP expression was upregulated during epithelium regeneration in murine colitis model
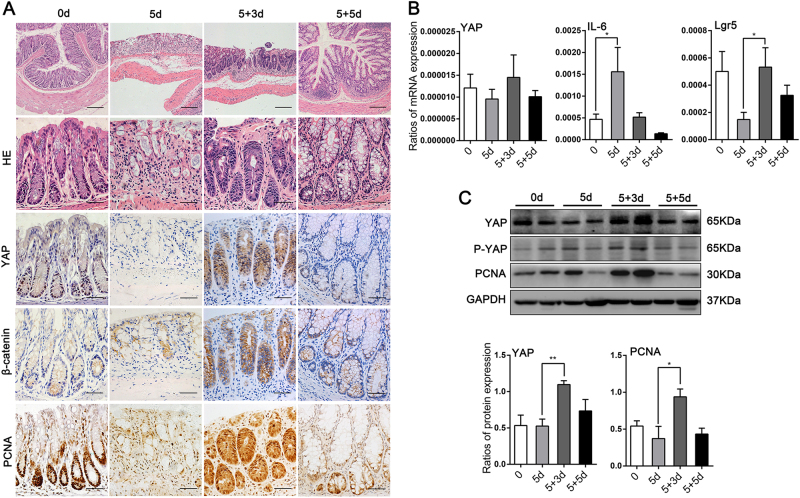
As previously reported, YAP is particularly important in hepatocyte regeneration after liver injury31. To examine the role of YAP during intestinal homeostasis, inflammation and regeneration, we took advantage of the well-established DSS-induced colitis and repair model. During homeostasis, active IEC proliferation was displayed at the crypt base. Five days of 3% DSS exposure led to the destruction of most colonic crypts with severe inflammatory cells infiltration. However, after 3 days of DSS withdrawal, crypts consisted of hyperproliferative epithelial cells that extended the entire crypt length and the ulcer area was clearly reduced. Crypt structure and the distribution of differentiated cells were largely restored 5 days after the removal of DSS (Fig. 2a).
Fig. 2. YAP expression was enhanced during epithelial regeneration following DSS-induced colitis in mice.
(a) H&E (upper × 100, bottom × 400) and immunohistochemical staining (× 400) in colons of BALB/c mice at day 0 and 5 days after treatment with 3% DSS, followed by 3 days (5 + 3d) or 5 days (5 + 5d) of normal drinking water (n = 4 each). Scale bars = 200 μm. (b) mRNA expression levels were detected at the indicated times by real-time PCR. Data represent the means ± SEMs. *P < 0.05. (c) Immunoblot (IB) analysis of the indicated proteins in primary colon cells prepared from mice killed at the indicated times, followed by densitometric analysis of the blots. n = 4. Data represent the means ± SEMs. *P < 0.05, **P < 0.01
In this murine model, we examined the temporal and spatial modulation of YAP in colonic mucosa. During homeostasis, YAP was specifically present in the colonic crypt base. After DSS treatment, the expression of YAP was dramatically diminished in crypts, with certain cells displaying weak cytoplasmic localization. After DSS withdraw for 3 days, a tremendous restoration of YAP expression was detected, which extended throughout the whole crypt with both cytoplasmic and nuclear accumulation (Fig. 2a), and proliferation-related markers were up-regulated (Fig. 2b,c). This upregulation in YAP protein expression might be due to post-transcriptional control since there was not a significant increase in YAP mRNA expression during regeneration (Fig. 2b). By 5 days, YAP expression was detected in the crypt base again, at almost identical levels as those in normal colon tissue (Fig. 2a). Therefore, the real-time loss and regain of YAP in crypt cells upon DSS-induced colitis suggested its particular role in IECs proliferation, especially in the course of epithelial regeneration.
YAP accelerated self-renewal, protected enterocytes from DSS-induced colitis and promoted epithelial regeneration after mucosal injury
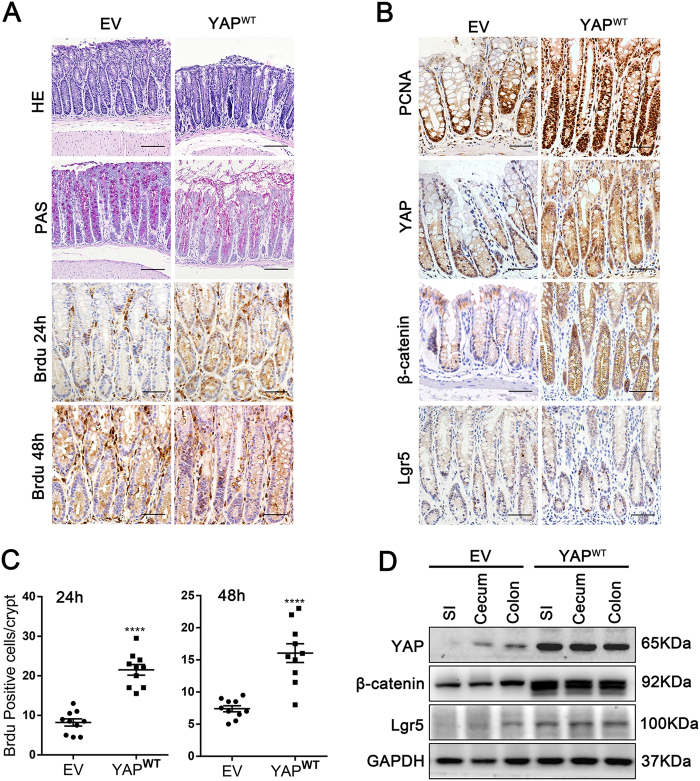
To specifically validate YAP function, based on the markedly increased YAP expression in restored crypts during intestinal regeneration, we constructed a YAP overexpression model through intraperitoneal injections of YAP-wild-type lentivirus (1 × 107 units once) in adult mice. We observed the increased YAP expression of mucosal epithelium including small intestine, caecum and colon 14 days after infection (Fig. 3b,d). In homeostasis, YAP overexpression caused a notable decrease in cells of the secretory lineage4, but no significant difference of crypt lengths (Fig. 3a). Notably, we did not observe the death of mice after infection with YAPWT or EV lentirvirus for 24 weeks. A time-course study of Brdu labelling (24 and 48 h) showed not only an increase in the number of proliferating crypt cells (p < 0.0001) but also the activation of cell migration along the crypt axis in the YAPWT mice (Fig. 3a,c). Moreover, compared with mice injected with the EV, YAPWT mice also had an increased expression of proliferation-related proteins β-catenin and Lgr5 (Fig. 3b,d).
Fig. 3. YAP overexpression increased the IEC self-renewal of mouse colonic crypts.
(a) Paraffin-embedded colon sections from mice infected with the YAPWT or empty vector (EV) lentivirus (1 × 107 units per mouse) were analysed by H&E (n = 10 each, × 200), PAS (× 200) and Brdu staining at 24 and 48 h (× 400). Scale bars = 200 μm. (b) Immunohistochemical staining of PCNA, YAP, β-catenin and Lgr5 in YAPWT- and EV-infected mouse colons (n = 5 each, × 400). (c) Brdu-positive cells were counted in each colonic crypt after 24 and 48 h of infection. Data represent the means ± SEMs. ****P < 0.0001. (d) Western blot analysis of the indicated proteins in mucosal epithelium prepared from YAPWT and EV mice (n = 4 each). SI, small intestine
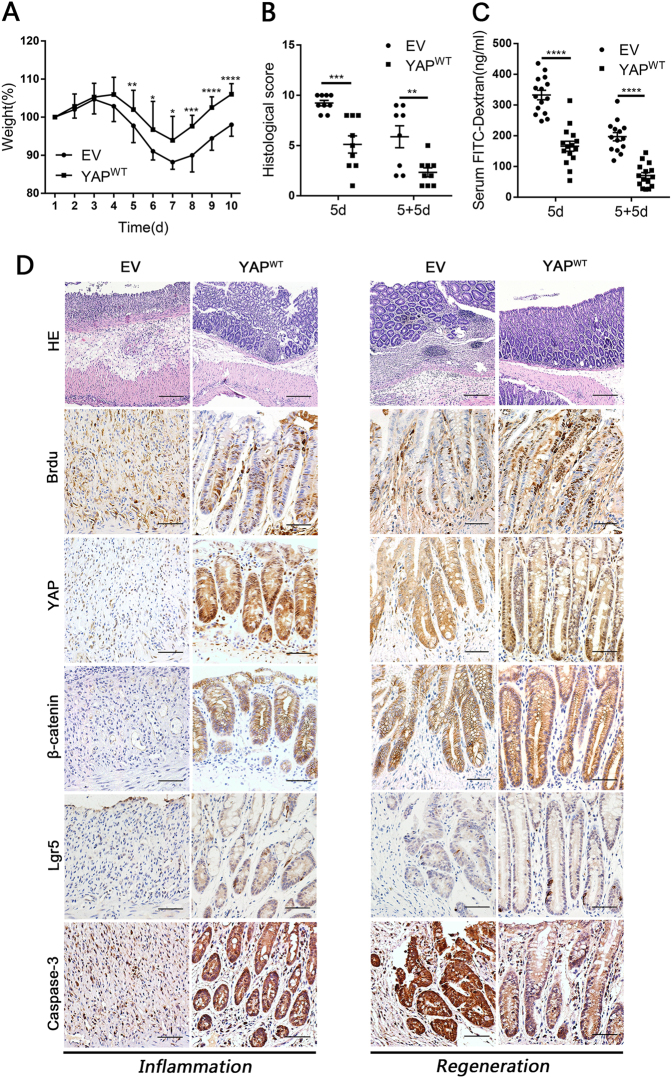
Next, we assessed the susceptibility of YAPWT mice to acute DSS-induced colitis. The overall DAI was significantly lower in YAPWT mice than in EV mice, which presented with bloody stools (Supplementary Figure 1B). On average, YAPWT mice had a colon length 9-mm longer (Supplementary Figure 1A) and a higher body weight than mice injected with EV (Fig. 4a). Histological assessments demonstrated that YAPWT mouse crypts exhibited better structural integrity; in contrast, the control littermates presented with catastrophic crypt loss and widespread ulcers (Fig. 4b,d).
Fig. 4. YAP overexpression protected epithelial cells against DSS-induced colitis and promoted intestinal regeneration in mice.
(a) Body weight curves of YAPWT and EV mice during the successive 5-d DSS treatment, followed by 5 days of normal drinking water. Mice were killed on 5d (n = 9 each) and 5 + 5d (EV n = 8, YAPWT n = 9). Data represent the means ± SDs. (b) Histological scores in YAPWT and EV mice on 5d (n = 9 each) and 5 + 5d (EV n = 8, YAPWT n = 9). Data represent the means ± SEM. **P < 0.01, ***P < 0.001. (c) Serum FITC-dextran in YAPWT and EV mice on 5d (n = 15 each) and 5 + 5d (EV n = 14, YAPWT n = 15). Data represent the means ± SEM. ****P < 0.0001. (d) Paraffin-embedded sections of YAPWT and EV mice colons were analysed by H&E (× 100) and immunohistochemical staining (× 400) at 5d (Left: inflammation) and 5 + 5d (Right: regeneration). Scale bars = 200 μm
More importantly, YAPWT mice rapidly regained body weight after the removal of DSS, while mice injected with the EV showed a delayed recovery (Fig. 4a). In addition, 5 days after DSS withdrawal, the structure of crypts and the gut barrier function in YAPWT mice were almost restored (Fig. 4c). Moreover, colonic crypts of YAPWT mice contained more Brdu and PCNA-positive proliferating cells and fewer apoptotic cells than those in EV mice (Fig. 4d and Supplementary Figure 1C and D). Collectively, these observations suggested that YAP was an essential regulator of epithelial cell proliferation that maintained normal intestinal homeostasis and mediated protection against colitis. Notably, after chemical exposure, overexpressing YAP drastically accelerated epithelial regeneration to restore the intestinal mucosal barrier in the murine model.
YAP triggered Wnt/β-catenin signalling during epithelial cell inflammation and regeneration
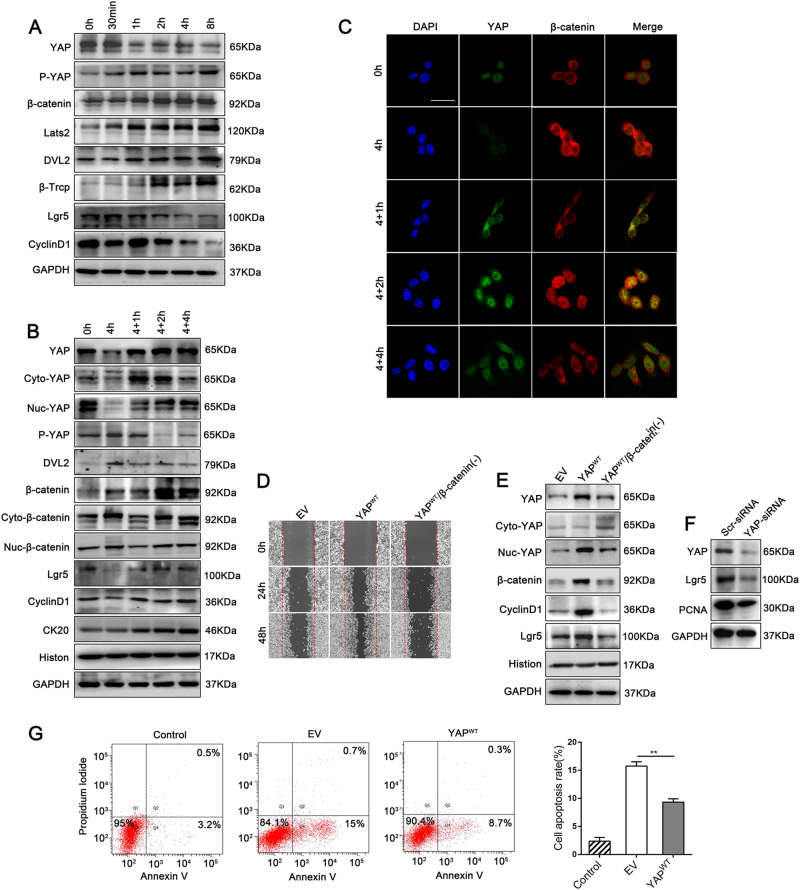
As the Wnt/β-catenin signalling pathway has a particularly important role in hepatobiliary repair after chemically-induced hepatocyte injury32,33, to examine the relationship between YAP and β-catenin in intestinal inflammation and regeneration, we used DSS to induce epithelial cell injury in vitro, which has been done previously34. A time course of 1% DSS treatment in normal colonic FHC cells resulted in a progressive increase in the expression of LATS2 kinase, YAP S127 phosphorylation and E3 ubiquitin ligase β-Trcp. In contrast, YAP expression was significantly reduced, which suggested that the Hippo pathway was activated after DSS administration, leading to YAP protein degradation by the ubiquitin proteasome pathway (Fig. 5a). Strikingly, DSS triggered the activation of Wnt signalling by inducing Dvl2 and β-catenin stimulation (similar to Wnt3a stimulation in cells, Supplementary Figure 2A) but had no effect on Lgr5 and cyclin D1 accumulation (Fig. 5a). These data indicated that increasing the protein expression level of β-catenin alone was insufficient to initiate the transcription of Wnt signalling genes, and the loss of YAP expression caused by Hippo activation might participate in this process.
Fig. 5. YAP expression was significantly enhanced during DSS-induced regeneration and triggered Wnt/β-catenin signalling in cell proliferation in vitro.
(a) IB analysis of total YAP, PS127-YAP and Wnt/β-catenin signalling associated proteins in FHC cells. Cells were serum-starved overnight and then stimulated with 1% DSS for the indicated time. (b) IB analysis of total YAP, PS127-YAP and Wnt/β-catenin signalling associated proteins in FHC cell lines. Cells were serum-starved overnight and then stimulated with 1% DSS for 0 or 4 h, followed by 1 h (4 + 1 h), 2 h (4 + 2 h) or 4 h (4 + 4 h) of incubation in reduced serum culture medium. Cyto, cytoplasm; Nuc, nucleus. from three independent experiments. (c) Immunofluorescence staining for YAP and β-catenin in FHC cell lines, as determined by confocal laser scanning microscopy. Scale bars = 50 μm. (d) Detection of wound healing ability in YAPWT and β-catenin-silenced with YAPWT expression (YAPWT /β-catenin( – )) in FHC cells at 0, 24 and 48 h. (e) IB analysis of Wnt/β-catenin related proteins expression in YAPWT and YAPWT/β-catenin( – ) FHC cells. (f) IB analysis of Lgr5 and PCNA expression levels in Scr-siRNA- and YAP-siRNA-transfected HT29 cells. (g) Representative dot blots of APC Annexin-V versus PI. Flow cytometry analysis of apoptosis in FHC-EV and FHC-YAPWT cells after incubation with 1% DSS for 12 h
During the regeneration process, an abundant expression of cytoplasmic YAP rapidly occurred after termination of DSS administration and was gradually translocated into the nucleus; by 2 h post DSS cessation, YAP expression reached the highest amount of nuclear accumulation, and by 4 h it became a weak immune-signal again. Meanwhile, β-catenin showed clear nuclear localization, which was accompanied by up-regulated total protein expression levels of β-catenin, Lgr5 and cyclin D1 by 2 h DSS withdrawn (Fig. 5b,c), and mRNA levels of the ISC makers Lgr5 and Olfm4 were also clearly increased (Supplementary Figure 2B) in this period. These results suggested that in the presence of nuclear YAP, Wnt/β-catenin signalling that including expression and transcriptional function of β-catenin were concurrently triggered during epithelium cell regeneration.
By using western blotting, we screened expression of YAP protein in several cell lines, to determine which cell line should be applied to the subsequent lentivirus plasmids (YAP-Low) or siRNAs (YAP-High) transfection (Supplementary Figure 3A). We then investigated the interaction between YAP and β-catenin in FHC clones stably overexpressing YAP (YAPWT mainly expressed in the nucleus, Figs. 5e and 6g) or EV control. Compared with that of cells infected with an EV, YAPWT increased wound healing and significantly suppressed apoptosis after DSS treatment in FHC cells (Fig. 5d,g). The expression of Wnt-associated proteins including β-catenin, Lgr5 and cyclin D1 was upregulated in YAPWT cells compared with that in EV cells (Fig. 5e). Moreover, silencing YAP by siRNA caused a decrease in the expression of Lgr5 in HT29 cells (Fig. 5f). However, silencing β-catenin impeded monolayer wound healing and reversed the up-regulated Wnt transcription in YAPWT FHC cells (Fig. 5d,e). These data indicated that nuclear YAP triggered Wnt/β-catenin signalling to drive IEC proliferation.
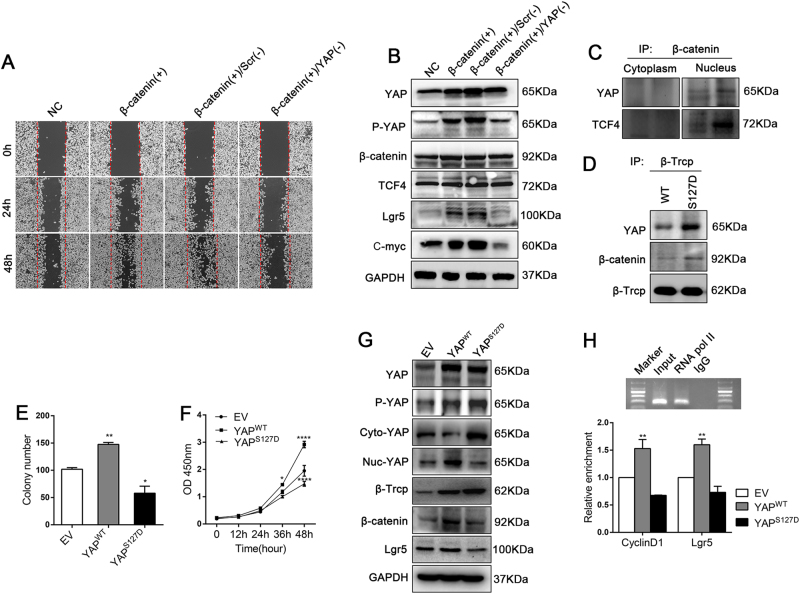
Fig. 6. Nuclear YAP and β-catenin/TCF4 formed a transcriptional complex; Lgr5 and cyclin D1 were targets of this complex in epithelial cells.
(a) Wound-healing detection in FHC cells transfected with negative control (NC) plasmids, β-catenin-overexpressing (β-catenin( + )) plasmids or β-catenin-overexpressing plasmids either plus Scr-siRNA (β-catenin( + )/Scr( – )) or YAP-siRNA (β-catenin( + )/YAP( – )). (b) IB analysis of total YAP, PS127-YAP and Wnt/β-catenin signalling associated proteins from the above FHC cells after the different treatments. (c) IB analysis of endogenous YAP and TCF4 expression in co-IP assay with anti-β-catenin antibody in nuclear and cytosol lysates of FHC cells. (d) IB analysis of exogenous YAP and β-catenin expression in co-IP assays with anti-β-Trcp antibody in stable 293T-YAPWT and 293T-YAPS127D cells. (e) Cell Counting Kit-8 assay in stable DLD1-EV, DLD1-YAPWT and DLD1-YAPS127D cells. (f) Colony formation assay in stable DLD1-EV, DLD1-YAPWT and DLD1-YAPS127D cells from three independent experiments. *P < 0.05, **P < 0.01, ****P < 0.0001. (g) IB analysis of total YAP, PS127-YAP and Wnt/β-catenin signalling associated proteins in stable DLD1-EV, DLD1-YAPWTand DLD1-YAPS127D cells. (h) Real-time PCR analysis of cyclin D1 and Lgr5 chromatin-IP with anti-TCF4 antibody from stable FHC-EV, FHC-YAPWT and FHC -YAPS127D cells. Data represent the means ± SEMs. **P < 0.01
Nuclear YAP and β-catenin/TCF4 formed a transcriptional complex; Lgr5 and cyclin D1 were targets of this complex in epithelial cells
As stated above, YAP effectively promoted the transcriptional activation of Wnt/β-catenin signalling molecules, whereas increased β-catenin was loss of function when YAP was deficient upon inflammation. This prompted us to investigate whether YAP is a component of the β-catenin-regulated transcriptional complex. To test this hypothesis, we first transfected FHC β-catenin-overexpressing cells with YAP-specific siRNA. The result showed that β-catenin overexpression enhanced ‘wound’ closure, but silencing YAP in FHC-β-catenin ( + ) cells attenuated this effect (Fig. 6a). Furthermore, protein expression levels of Wnt targets such as Lgr5 and C-myc were increased in FHC-β-catenin ( + ) cells, but this was completely reversed in FHC-β-catenin ( + )/YAP( – ) cells (Fig. 6b). Moreover, endogenous co-immunoprecipitation (IP) analysis indicated that YAP directly interacted with β-catenin in the cell nucleus, but not in the cytoplasm (Fig. 6c). Chromatin IP detection showed that Lgr5 and cyclin D1 expression was clearly increased in FHC-YAPWT cells (Fig. 6h). Thus, we speculated that YAP is a dispensable part of the β-catenin/TCF4 complex that drives IEC proliferation; Lgr5 and cyclin D1 were confirmed as the targets of this complex.
To further investigate the function of nuclear YAP in colon cells, we then constructed another stable clone in which YAP was phospho-mimetically mutated (YAPS127D) in DLD1 cells. Notably, in YAPS127D (serine to aspartic acid at residue 127), YAP is constitutively phosphorylated, leading to its functional cytoplasmic localization (Fig. 6g) and inability to promote transcriptional activation. In our study, we found that both YAP and β-catenin can directly bind to the E3 ubiquitin ligase β-Trcp, and YAPS127D recruited more β-catenin to β-Trcp, causing more β-catenin degradation than in stable 293T-YAPWT cells (Fig. 6d). YAPS127D significantly depressed DLD1 clone formation and cell proliferation (Fig. 6e,f and Supplementary Figure 3B). Accordingly, immunoblotting analysis verified the substantial suppression of β-catenin and Lgr5 protein expression in YAPS127D cells compared that in YAPWT or EV cells (Fig. 6g). In addition, the Wnt transcriptional targets Lgr5 and cyclin D1 were clearly down-regulated in YAPS127D cells (Fig. 6h). Thus, deficiency of nuclear YAP accumulation inactivated Wnt/β-catenin signalling, cytoplasmic phosphor-YAP even mediated suppressive effect. Collectively, nuclear YAP participated in β-catenin/TCF4 transcriptional complex to drive epithelial cell proliferation.
YAPWT and phospho-mimetic YAP controlled the development of colitis-associated carcinoma in opposite manners
Finally, we performed a classic CAC procedure in mice that were infected with YAPS112D (mouse S112D corresponding to S127D in humans) and YAPWT lentivirus to further probe the effect of YAP on chronic inflammation. After the first cycle, the colon length was 12 mm shorter in the YAPS112D mice than in the YAPWT-infected mice (Supplementary Figure 4D). In addition, after chemically induced injury, YAPS112D mice presented with worse intestinal barrier dysfunction than the YAPWT control mice (Supplementary Figure 4E). Moreover, YAPS112D mice exhibited a decrease in Wnt-related protein expression levels (Supplementary Figure 4A and F), combined with more severe destruction including the disappearance of crypt cells and inflammatory cell infiltration in the mucosa (Supplementary Figure 4B and C). Accordingly, compared with YAPWT mice, mutant YAP (YAPS112D) expression in the colonic crypts led to an increased susceptibility to colitis induced by short-term AOM/DSS exposure.
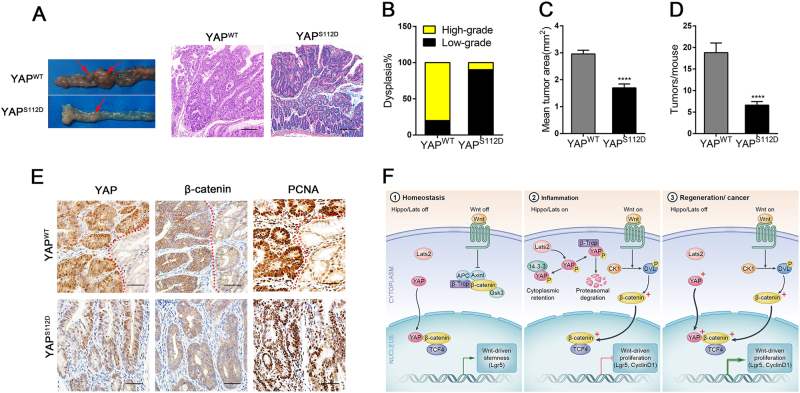
After the third cycle ended, we had successfully created a CAC model (Fig. 7a) and found a concomitant up-regulation in the nuclear staining of YAP and β-catenin in the neoplastic area of tissues (Fig. 7e). Interestingly, mice infected with YAPS112D developed significantly smaller tumour areas and tumour numbers than YAPWT mice (Fig. 7c,d). In addition, histological assessments showed that the tumours in YAPWT mice were usually identified as having high-grade dysplasia, but the colonic mucosa of YAPS112D mice presented with low-grade dysplasia (Fig. 7b). Therefore, we concluded that YAP promoted the development of associated neoplastic lesions during chronic mucosal injury and regeneration; conversely, phospho-YAP hindered IEC repair, thereby suppressing the excessive growth and development of CAC.
Fig. 7. YAPWT and phospho-mimetic YAP regulated the development of colitis-associated carcinoma in opposite manners.
YAPWT and mutant YAPS112D mice were obtained by classical CAC procedures and sacrificed at day 75 (n = 10 each). (a) Left: macroscopic tumours (arrow); right: colon mucosal histology (× 100); (b) percentage of mice with dysplasia; (c) mean tumour area; (d) number of tumours per mouse in YAPWT and YAPS112D mice at day 75. (e) Immunohistochemical staining (400 × ) in YAPWT and YAPS112D mice after 75 days of AOM/DSS administration. Red dashed lines represent the boundary between the normal mucosa and neoplastic regions. Data represent the means ± SEMs. Scale bars = 200 μm. ****P < 0.0001. (f) Model of how Hippo/YAP and the Wnt/β-catenin signalling pathway work during epithelial homeostasis, inflammation, regeneration and cancer
Discussion
YAP generally acts as a transcriptional co-activator of the Hippo pathway, which is essential for controlling organ size, tissue growth and tumour development.11,12 Normally, YAP is located at the crypt base near the ISCs, but its biological function in the intestine has not been clearly documented. UC is a subcategory of IBD and a prominent pathophysiological feature of this disease is impaired epithelial regeneration.7 In this study, we found that YAP was specifically downregulated in the colonic crypts of human UC and that mice overexpressing YAP increased IEC proliferation to maintain homeostasis and promote mucosal regeneration. However, YAP also increased colitis-associated carcinoma susceptibility, which is similar to the effect from STAT3 activation35,36. We concluded that YAP is a critical regulator of IEC self-renewal, crypt regeneration after inflammation and CAC growth.
Recent studies have investigated YAP as a core component of the Hippo pathway, and its interaction with TEAD family proteins triggers the transcription of target genes, such as CTGF and Areg, to promote proliferation and migration in multiple tumour types37–40. Based on the function of YAP in cancer, preceding studies have reported that YAP deficiency impaired DSS-induced intestinal regeneration, but the mechanism of this impairment was not determined41. Meanwhile, intestinal gp130 signalling facilitated epithelial cell proliferation and conferred resistance against mucosal invasion through the activation of YAP and Notch4. In our investigation, using the DSS-induced colitis and repair model, we found that YAP expression was markedly enhanced after the removal of DSS and was accompanied by actively proliferating colonic epithelial cells in crypts, which revealed that YAP has a crucial role in crypt cell regeneration. This YAP-driven effect on cell proliferation was also verified in YAP-overexpressing mice that YAPWT mice exhibited a greater IEC self-renewal ability and thus resistance to colitis damage than EV-infected mice. More importantly, YAP substantially promoted crypt regeneration after inflammatory stimulation.
Owing to the similar roles of YAP and Wnt in cell proliferation, we studied the relationship between YAP and Wnt/β-catenin signalling. Wnt is essential in stem cell maintenance, cell survival and proliferation19,42. The β-catenin/TCF4 complex40,43 has a central role in modulating the expression of its target genes. As reported, the deletion of Lgr5+ cells after radiation-induced damage and the subsequent regeneration period resulted in extensive crypt loss and the disappearance of villus structure44. In our study, during the regeneration following inflammation, we were amazed to find that the nuclear co-localization of YAP and β-catenin was increased. Overexpressing YAP significantly improved IEC ‘wound-healing’ ability and increased the expression of both β-catenin and the transcriptional targets of Wnt signalling, whereas silencing β-catenin attenuated these effects, indicating that YAP promoted IEC proliferation through the activation of Wnt/β-catenin signalling.
Next, to probe the regulation between YAP and β-catenin, the deeper mechanism of these effects was investigated. Previous literature has demonstrated that YAP/TAZ inhibited the CK1 δ/ε-mediated phosphorylation of DVL in the cytoplasm, thereby suppressing the activation of Wnt/β-catenin45. In addition, YAP/TAZ-TEAD induced the secretion of Wnt inhibitors such as Wnt 5A/B and suppressed Wnt/β-catenin activation46. However, nuclear YAP may also interact with β-catenin on Sox2 and Snai2 genes to promote cardiomyocyte proliferation and influence heart size47. In our research, we found the YAP could directly interact with β-catenin in the nucleus to form the YAP/β-catenin/TCF4 transcriptional complex in colon cells, moreover, Lgr5 and cyclin D1 were confirmed as the targets of this complex. To further clarify the function of nuclear YAP in IEC proliferation, we established a phospho-mimetic mutant of YAP (resulting in YAP retention in the cytoplasm) and found that it markedly inhibited Wnt activation by recruiting more β-Trcp to the β-catenin destruction complex. Meanwhile, cancer cell proliferation and tumour development were suppressed in the phosphor-YAP mutant. Therefore, nuclear YAP participated in the β-catenin/TCF4 transcriptional complex in colon cells and the activity of this complex was normal during homeostasis but was dramatically enhanced during colitis-induced regeneration and carcinoma (Fig. 7f).
In summary, insufficient regenerative proliferation increases the risk for disease including IBD and age-related tissue atrophy, while persistent and excessive proliferation may cause oncogenesis. Early mucosal healing is particularly important in the treatment of IBD. In this study, YAP-driven IEC proliferation regulated epithelial regeneration after inflammation, which might be caused by adaptive modulation of the nuclear YAP/β-catenin/TCF4 complex. Collectively, YAP has potential to serve as a preventative therapeutic tool for the treatment of UC. Conversely, the systemic delivery of mutant YAP to target tumours in Wnt-addicted cancers provides new selectivity for colon cancer therapy.
Electronic supplementary material
Acknowledgements
This study was sponsored by the National Natural Science Foundation of China (No. 81370502, 81402037 and 81302156).
Author contributions
FHD: draft of the manuscript, data acquisition and analysis. LP: study concept and design, and data analysis. ZJL, EBL and SBC: material and technological support. GT and XMZ: judgement of the histopathology images. FCZ: study concept and design, obtained funding and study supervision.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by P. Agoastinis.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41419-017-0244-8.
References
- 1.Paclik D, Lohse K, Wiedenmann B, Dignass AU, Sturm A. Galectin-2 and -4, but not Galectin-1, promote intestinal epithelial wound healing in vitro through a TGF-beta-independent mechanism. Inflamm. Bowel. Dis. 2008;14:1366–1372. doi: 10.1002/ibd.20499. [DOI] [PubMed] [Google Scholar]
- 2.Cader MZ, Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut. 2013;62:1653–1664. doi: 10.1136/gutjnl-2012-303955. [DOI] [PubMed] [Google Scholar]
- 3.Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2016;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taniguchi K, et al. Agp130–Src–YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J. Gastroenterol. 2008;14:348–353. doi: 10.3748/wjg.14.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat. Immunol. 2016;17:230–240. doi: 10.1038/ni.3384. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan K, Arnone B, Buchman A. Intestinal growth factors: Potential use in the treatment of inflammatory bowel disease and their role in mucosal healing. Inflamm. Bowel. Dis. 2011;17:410–422. doi: 10.1002/ibd.21316. [DOI] [PubMed] [Google Scholar]
- 8.Pineton DCG, Peyrin-Biroulet L, Lemann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat. Rev. Gastroenterol. Hepatol. 2010;7:15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 9.Baddour JA, Sousounis K, Tsonis PA. Organ repair and regeneration: an overview. Birth Defects Res. Part C Embryo Today. 2012;96:1–29. doi: 10.1002/bdrc.21006. [DOI] [PubMed] [Google Scholar]
- 10.Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug. Discov. 2013;13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu FX, Meng Z, Plouffe SW, Guan KL. Hippo pathway regulation of gastrointestinal tissues. Annu. Rev. Physiol. 2015;77:201–227. doi: 10.1146/annurev-physiol-021014-071733. [DOI] [PubMed] [Google Scholar]
- 12.Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tschaharganeh DF, et al. Yes-associated protein upregulates Jagged-1 and activates the NOTCH pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530–1542.e1512. doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF -TRCP. Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marti P, et al. YAP promotes proliferation, chemoresistance, and angiogenesis in human cholangiocarcinoma through TEAD transcription factors. Hepatology. 2015;62:1497–1510. doi: 10.1002/hep.27992. [DOI] [PubMed] [Google Scholar]
- 16.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5 + stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 17.Yimlamai D, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Cordero JB, Sansom OJ. Wnt signalling and its role in stem cell-driven intestinal regeneration and hyperplasia. Acta Physiol. 2012;204:137–143. doi: 10.1111/j.1748-1716.2011.02288.x. [DOI] [PubMed] [Google Scholar]
- 20.Causeret F, Sumia I, Pierani A. Kremen1 and Dickkopf1 control cell survival in a Wnt-independent manner. Cell Death Differ. 2016;23:323–332. doi: 10.1038/cdd.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 22.Azzolin L, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/ -catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell. Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Es JH, et al. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol. Cell. Biol. 2012;32:1918–1927. doi: 10.1128/MCB.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tao J, et al. Activation of β-catenin and Yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology. 2014;147:690–701. doi: 10.1053/j.gastro.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Gao Y, Yang M, Zhao Q, Wang G, Yang YM, et al. Identification of gene expression changes from colitis to CRC in the mouse CAC model. PLoS One 2014, 9(4): e95347. [DOI] [PMC free article] [PubMed]
- 27.Geboes K, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404–409. doi: 10.1136/gut.47.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dann SM, et al. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J. Immunol. 2008;180:6816–6826. doi: 10.4049/jimmunol.180.10.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guma M, et al. Constitutive intestinal NF-κB does not trigger destructive inflammation unless accompanied by MAPK activation. J. Exp. Med. 2011;208:1889–1900. doi: 10.1084/jem.20110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, et al. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut. 2010;59:227–235. doi: 10.1136/gut.2009.184176. [DOI] [PubMed] [Google Scholar]
- 31.Grijalva JL, Huizenga M, Mueller K, Rodriguez S, Brazzo J, Camargo F, et al. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am J Physiol Gastrointest Liver Physiol 2014, 307(2): G196-204. [DOI] [PubMed]
- 32.Okabe H, et al. Wnt signaling regulates hepatobiliary repair following cholestatic liver injury in mice. Hepatology. 2016;64:1652–1666. doi: 10.1002/hep.28774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson MD, Awuah P, Singh S, Monga SPS. Disparate cellular basis of improved liver repair in β-catenin-overexpressing mice after long-term exposure to 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Am. J. Pathol. 2010;177:1812–1822. doi: 10.2353/ajpath.2010.100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gadaleta RM, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–472. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 35.Bollrath J, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou D, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc. Natl Acad. Sci. 2011;108:E1312–E1320. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He C, et al. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol. Med. 2015;7:1426–1449. doi: 10.15252/emmm.201404976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen LT, et al. ERG activates the YAP1 transcriptional program and induces the development of age-related prostate tumors. Cancer Cell. 2015;27:797–808. doi: 10.1016/j.ccell.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, et al. Increased TEAD4 expression and nuclear localization in colorectal cancer promote epithelial-mesenchymal transition and metastasis in a YAP-independent manner. Oncogene. 2016;35:2789–2800. doi: 10.1038/onc.2015.342. [DOI] [PubMed] [Google Scholar]
- 41.Cai J, et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van de Wetering M, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/S0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 44.Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5 + stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14:149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Varelas X, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev. Cell. 2010;18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Park HW, et al. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heallen T, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.