This population-based cohort study evaluates the association of primary tumor site with mortality among patients receiving systemic chemotherapy and biologic therapy for metastatic colorectal cancer.
Key Points
Question
What is the association of tumor origin (right or left side) with mortality among patients receiving systemic chemotherapy and bevacizumab or cetuximab for metastatic colorectal cancer?
Findings
In this population-based cohort study of 11 905 patients with metastatic colorectal cancer, those with left-sided tumors who were treated with systemic chemotherapy plus bevacizumab or cetuximab experienced significantly improved survival, whereas those with right-sided tumors who were treated with cetuximab experienced worse survival. Among patients with wild-type KRAS tumors, treatment with cetuximab benefited only those with left-sided disease and was associated with significantly poorer survival among those with right-sided disease.
Meaning
These findings support use of tumor origin in stratification for research and guidelines for treatment of metastatic colorectal cancer.
Abstract
Importance
Biologic therapy (BT) (eg, bevacizumab or cetuximab) is increasingly used to treat metastatic colorectal cancer (mCRC). Recent investigations have suggested that right- or left-sided primary tumor origin affects survival and response to BT.
Objective
To evaluate the association of tumor origin with mortality in a diverse population-based data set of patients receiving systemic chemotherapy (SC) and bevacizumab or cetuximab for mCRC.
Design, Setting, and Participants
This population-based nonconcurrent cohort study of statewide California Cancer Registry data included all patients aged 40 to 85 years diagnosed with mCRC and treated with SC only or SC plus bevacizumab or cetuximab from January 1, 2004, through December 31, 2014. Patients were stratified by tumor origin in the left vs right sides.
Interventions
Treatment with SC or SC plus bevacizumab or cetuximab.
Main Outcomes and Measures
Mortality hazards by tumor origin (right vs left sides) were assessed for patients receiving SC alone or SC plus bevacizumab or cetuximab. Subgroup analysis for patients with wild-type KRAS tumors was also performed.
Results
A total of 11 905 patients with mCRC (6713 men [56.4%] and 5192 women [43.6%]; mean [SD] age, 60.0 [10.9] years) were eligible for the study. Among these, 4632 patients received SC and BT. Compared with SC alone, SC plus bevacizumab reduced mortality among patients with right- and left-sided mCRC, whereas SC plus cetuximab reduced mortality only among patients with left-sided tumors and was associated with significantly higher mortality for right-sided tumors (hazard ratio [HR], 1.31; 95% CI, 1.14-1.51; P < .001). Among patients treated with SC plus BT, right-sided tumor origin was associated with higher mortality among patients receiving bevacizumab (HR, 1.31; 95% CI, 1.25-1.36; P < .001) and cetuximab (HR, 1.88; 95% CI, 1.68-2.12; P < .001) BT, compared with left-sided tumor origin. In patients with wild-type KRAS tumors (n = 668), cetuximab was associated with reduced mortality among only patients with left-sided mCRC compared with bevacizumab (HR, 0.75; 95% CI, 0.63-0.90; P = .002), whereas patients with right-sided mCRC had more than double the mortality compared with those with left-sided mCRC (HR, 2.44; 95% CI, 1.83-3.25, P < .001).
Conclusions and Relevance
Primary tumor site is associated with response to BT in mCRC. Right-sided primary tumor location is associated with higher mortality regardless of BT type. In patients with wild-type KRAS tumors, treatment with cetuximab benefited only those with left-sided mCRC and was associated with significantly poorer survival among those with right-sided mCRC. Our results underscore the importance of stratification by tumor site for current treatment guidelines and future clinical trials.
Introduction
Colorectal cancers (CRCs) are molecularly heterogeneous with carcinogenic pathways that are, in part, associated with embryologic origin. The right side of the colon, anatomically defined from the cecum through the proximal two-thirds of the transverse colon, develops embryonically from the midgut, whereas the left colon, the distal one-third of transverse colon through the rectum, arises from the hindgut. Increasing evidence demonstrates that right- and left-sided CRCs have distinct genomic patterns owing to genetic and epigenetic alterations. Right-sided tumors have been found to have a higher incidence of mucinous histologic characteristics, increased microsatellite instability, and greater frequency of KRAS (OMIM 190070) and BRAF mutations, whereas left-sided CRCs have higher expression of c-myc, RAS, and VEGFA; amplification of EGFR and ERBB2 (formerly HER2); and more TP53 gene mutations.
Several reports have demonstrated significant differences in survival between right- and left-sided CRCs in nonmetastatic and metastatic settings. In an analysis that combined data from 3 trials (PROVETTA [Evaluation of VEGF Polymorphism as Predictive Factor in Metastatic Colorectal Cancer Treated With Folfiri Plus Bevacizumab], AVF2017g [Study to Evaluate Avastin in Combination With Standard Chemotherapy to Treat Colorectal Cancer], and NO16966 [Study of Capecitabine (Xeloda) and Bevacizumab as a First-line Therapy in Patients With Metastatic Colorectal Cancer]), Loupakis et al found that patients with left-sided metastatic CRC (mCRC) experienced better overall survival compared with those with right-sided mCRC. Evidence suggests that the association of primary tumor site with the observed outcome differences in mCRC may be attributable in part to differences in response to systemic treatment.
Since approval by the US Food and Drug Administration in 2004, biologic therapies (BTs) that target vascular endothelial growth factor (eg, bevacizumab) and epidermal growth factor receptor (EGFR) (eg, cetuximab) have been used in addition to systemic chemotherapy (SC) for treatment of mCRC. Recent investigations have confirmed the influence of primary tumor location on the effectiveness of BT. The CALGB/SWOG 80405 (Cetuximab and/or Bevacizumab Combined With Combination Chemotherapy in Treating Patients With Metastatic Colorectal Cancer) trial showed that in first-line treatment of mCRC, all patients with wild-type RAS right-sided primary tumors had better survival when treated with bevacizumab compared with cetuximab, whereas patients with left-sided primary tumors had better survival when treated with cetuximab (hazard ratio [HR], 0.77; 95% CI, 0.59-0.99; P = .04).
Owing to mounting evidence confirming the influence of primary tumor site on response to BT, National Comprehensive Cancer Network (NCCN) recommendations have been revised to include the primary tumor site for first-line treatment of unresectable mCRC. Anti-EGFR therapy with cetuximab or panitumumab is recommended for wild-type RAS and left-sided tumors only. However, the NCCN does not currently recommend stratification by primary tumor site in consideration for subsequent treatment with BT agents.
Most studies that have evaluated the influence of primary tumor site on response to BT in mCRC are post hoc analyses of large prospective randomized clinical trials or single-institution analyses of small patient data sets. We sought to evaluate the association of tumor origin (right or left side) and BT (bevacizumab or cetuximab) with mCRC survival using a large and diverse population-based data set.
Methods
The California Cancer Registry (CCR) is the state-mandated cancer surveillance system for approximately 39 million residents and consists of the 3 most populous Surveillance Epidemiology and End Results program registries of the United States. Since 1988, information regarding cancer occurrence, diagnosis date, morphologic type of cancer, anatomic subsite origin, stage at diagnosis, behavior, vital status, date of death, underlying cause of death, and other information has been confidentially reported to the CCR. Mutant and wild-type KRAS mutation status as has been available in CCR data since 2010. In addition to electronically analyzable data fields, CCR data include multiple text fields containing additional treatment information, such as BT type (bevacizumab or cetuximab). The institutional review board of Loma Linda University approved this study and waived the need for informed consent for use of the deidentified CCR data with no patient contact.
Study Design and Population
We performed a population-based nonconcurrent cohort study of statewide California Surveillance Epidemiology and End Results program data assessing the association of 2 BT agents (bevacizumab or cetuximab) with all-cause mortality for right-sided (appendix, cecum, ascending colon, hepatic flexure, or transverse colon) vs left-sided (splenic flexure, descending colon, sigmoid colon, rectosigmoid junction, or rectum) stage IV (metastatic) CRC. Histologic definitions for adenocarcinoma included the following codes from International Classification of Diseases for Oncology: M-8140, M-8210-8211, M-8255, M-8260-8261, M-8263, M-8323, M-8470, M-8480-8481, and M-8490. Although the seventh edition of the American Joint Committee on Cancer Staging manual replaced the sixth edition rules beginning in 2010, this research used the sixth edition staging rules, ensuring continuity of staging categories throughout the study period.
Study participant selection included California residents aged 40 to 85 years at diagnosis of mCRC with a single primary tumor diagnosed from January 1, 2004, through December 31, 2014, who received SC alone or SC with bevacizumab or cetuximab. We extracted BT type from text fields. Overall survival was calculated from the diagnosis date for mCRC to death or censorship. Potential demographic confounding factors considered for inclusion in the analyses were year of diagnosis, age (continuous), sex (female or male), race/ethnicity (Asian or other, non-Hispanic black, Hispanic, or non-Hispanic white), quintile for neighborhood socioeconomic status (SES), and marital status (married; single; or separated, divorced, or widowed). In addition, mucinous (codes M-8470 and M-8480-8481) (yes or no) and signet ring (code M-8490) (yes or no) histologic types were included in the analyses.
Statistical Analysis
Descriptive statistics were calculated for demographic and tumor characteristics. Unadjusted (χ2 and Fisher exact) tests of dependent variable associations for right- and left-sided mCRC were performed for SC only and separately for SC with BT agents. Kaplan-Meier survival plots with log-rank tests were used to assess unadjusted differences in all-cause mortality.
Multivariable analyses used purposeful variable selection for model building. Age, diagnosis year, and socioeconomic status variables were retained and subsequently used in a logistic regression model to generate a propensity score for treatment probability. In a second step, we used Cox proportional hazard analyses adjusted for propensity score and tumor characteristic to estimate mortality HRs for tumor origin (right or left) and compared SC and bevacizumab or SC and cetuximab vs SC alone. In addition, in the group of patients treated with SC and BT, Cox regression was used to evaluate all-cause mortality HR for the interaction between tumor origin (right or left side) and treatment (SC + bevacizumab or SC + cetuximab). The attributable proportion was used to measure the fraction of excess risk resulting from the interaction between tumor side and BT type. Proportionality assumptions were assessed using the Schoenfield residuals correlation and log-log survival plot. All tests were 2-sided (α = .05) and were performed using SAS software (version 9.4; SAS Institute). These analyes were performed separately for patients with wild-type KRAS mCRC treated with radiation therapy who were diagnosed from January 1, 2010, through December 31, 2014.
Results
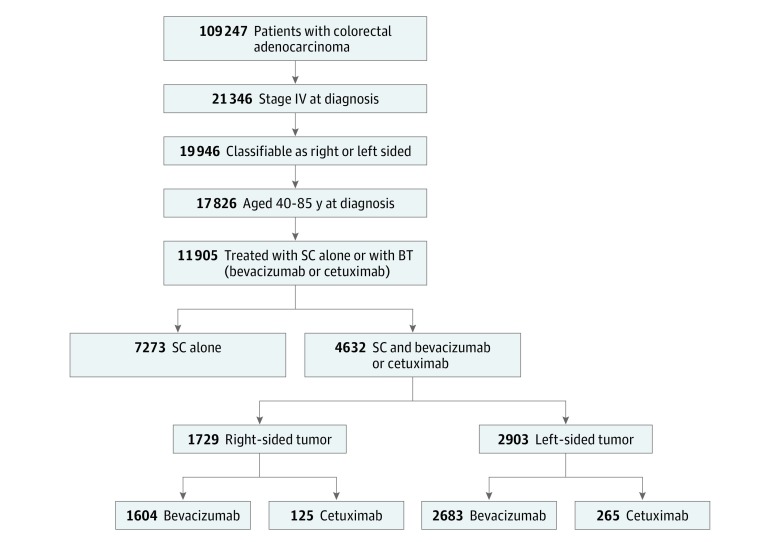
A total of 11 905 patients with mCRC (6713 men [56.4%] and 5192 women [43.6%]; mean [SD] age, 60.0 [10.9] years) aged 40 to 85 years and diagnosed from January 1, 2004, through December 31, 2014, who were classified as having right- or left-sided mCRC, and who underwent treatment with SC alone (n = 7273) or SC and BT (n = 4632) were included in this study (Figure 1). Table 1 includes demographic and tumor characteristics stratified by treatment type (SC only and SC + BT) and tumor origin (right or left side). Significant associations were seen between right- vs left-sided mCRC for age (1184 [44.2%] vs 1454 [31.6%] for those aged 66-85 years; P < .001), mucinous (414 [15.5%] vs 273 [5.9%]; P < .001) and signet ring (116 [4.3%] vs 83 [1.8%]; P < .001) histologic findings, and socioeconomic status (568 [21.2%] vs 879 [19.1%] for highest SES; P = .02) among patients treated with SC only (Table 1). Similar associations were seen for right- vs left-sided mCRC in patients treated with SC and BT, with the exception of socioeconomic status (450 [26.0%] vs 716 [24.7%] for highest SES; P = .37).
Figure 1. Patient Selection Flowchart.
All patient data were obtained from the California Cancer Registry as part of the Surveillance Epidemiology and End Results program registries of the United States. BT indicates biologic therapy; SC, systemic chemotherapy.
Table 1. Characteristics of Patients With mCRC by Tumor Origin and Targeted BTa.
| Characteristic | Treatment Group | |||||
|---|---|---|---|---|---|---|
| SC Alone | SC + BTb | |||||
| No. (%) | P Valuec | No. (%) | P Valuec | |||
| Right-Sided Tumor (n = 2679) |
Left-Sided Tumor (n = 4594) |
Right-Sided Tumor (n = 1729) |
Left-Sided Tumor (n = 2903) |
|||
| Age, y | ||||||
| 40-50 | 373 (13.9) | 998 (21.7) | <.001 | 324 (18.7) | 786 (27.1) | <.001 |
| 51-65 | 1122 (41.9) | 2142 (46.6) | 786 (45.5) | 1443 (49.7) | ||
| 66-85 | 1184 (44.2) | 1454 (31.6) | 619 (35.8) | 674 (23.2) | ||
| Mucinous histologic type | ||||||
| Yes | 414 (15.5) | 273 (5.9) | <.001 | 209 (12.1) | 160 (5.5) | <.001 |
| No | 2265 (84.5) | 4321 (94.1) | 1520 (87.9) | 2743 (94.5) | ||
| Signet ring histologic type | ||||||
| Yes | 116 (4.3) | 83 (1.8) | <.001 | 59 (3.4) | 34 (1.2) | <.001 |
| No | 2563 (95.7) | 4511 (98.2) | 1670 (96.6) | 2869 (98.8) | ||
| SES quintiled | ||||||
| 1 (Lowest) | 387 (14.4) | 763 (16.6) | .02 | 217 (12.6) | 423 (14.6) | .37 |
| 2 | 495 (18.5) | 924 (20.1) | 321 (18.6) | 521 (17.9) | ||
| 3 | 599 (22.4) | 1002 (21.8) | 323 (18.7) | 546 (18.8) | ||
| 4 | 630 (23.5) | 1026 (22.3) | 418 (24.2) | 697 (24.0) | ||
| 5 (Highest) | 568 (21.2) | 879 (19.1) | 450 (26.0) | 716 (24.7) | ||
| Diagnosis year | ||||||
| 2004-2007 | 1031 (38.5) | 1684 (36.7) | .12 | 529 (30.6) | 829 (28.6) | .18 |
| 2008-2010 | 704 (26.3) | 1184 (25.8) | 521 (30.1) | 858 (29.6) | ||
| 2011-2013 | 944 (35.2) | 1726 (37.6) | 679 (39.3) | 1216 (41.9) | ||
Abbreviations: BT, biologic therapy; mCRC, metastatic colorectal cancer; SC, systemic chemotherapy; SES, socioeconomic status.
Data were obtained from the California Cancer Registry as part of the Surveillance Epidemiology and End Results program registries of the United States. Percentages have been rounded and may not total 100.
Includes bevacizumab or cetuximab.
Calculated using the global χ2 test.
Quintiles based on the US 2000 census data (2004-2005) and American Community Survey Data (2006-2014).
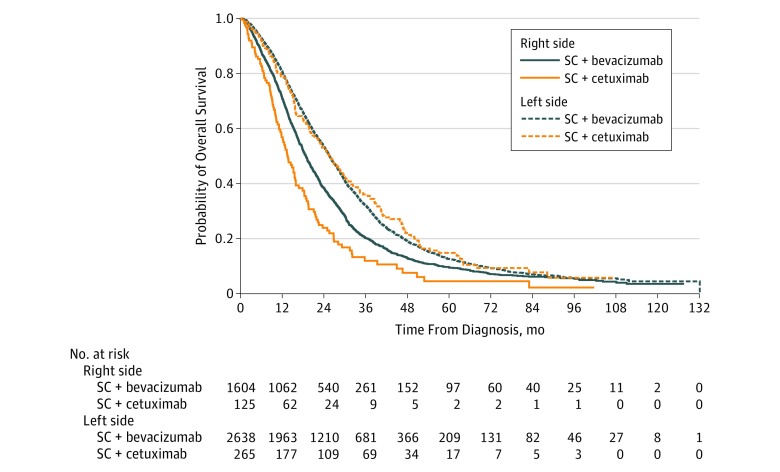
Median follow-up time in this study was 75 months (95% CI, 74-78 months) for all patients with mCRC treated with SC and bevacizumab or cetuximab. Median overall survival was significantly poorer among patients with right-sided mCRC treated with SC and bevacizumab or SC and cetuximab (19 months [95% CI, 18-20 months] and 14 months [95% CI, 12-16 months], respectively) compared with patients with left-sided mCRC treated with SC and bevacizumab or SC with cetuximab (26 months [95% CI, 25-27 months] and 26 months [21-29 months], respectively; P < .001) (Figure 2).
Figure 2. Kaplan-Meier Survival Curves for Overall Survival.
Patients are stratified by treatment with systemic chemotherapy (SC) and bevacizumab or cetuximab and by side of the primary colorectal tumor.
When compared with SC alone, treatment with SC plus bevacizumab was associated with decreased mortality for right-sided (n = 1604) (HR, 0.93; 95% CI, 0.88-0.97; P = .02) and left-sided (n = 2683) (HR, 0.88; 95% CI, 0.84-0.91; P < .001) mCRC. However, SC plus cetuximab was associated with decreased mortality only among patients with left-sided mCRC (n = 265) (HR, 0.82; 95% CI, 0.74-0.92; P < .001) (Table 2). Patients with right-sided mCRC treated with SC plus cetuximab (n = 125) had significantly higher mortality (HR, 1.31; 95% CI, 1.14-1.51; P < .001) compared those treated with SC alone (Table 2).
Table 2. Adjusted Hazards for Tumor Origin by Treatment Type.
| Tumor Origin | Treatment | ||
|---|---|---|---|
| SC Alone | SC + Bevacizumab | SC + Cetuximab | |
| Right side | |||
| HR (95% CI)a | 1 [Reference] | 0.93 (0.88-0.97) | 1.31 (1.14-1.51) |
| P value | NA | .002 | <.001 |
| Left side | |||
| HR (95% CI)a | 1 [Reference] | 0.88 (0.84-0.91) | 0.82 (0.74-0.92) |
| P value | NA | <.001 | <.001 |
Abbreviations: SC, systemic chemotherapy; HR, hazard ratio; NA, not applicable.
Adjusted for age, diagnosis year, mucinous and signet ring histologic types, and socioeconomic status using propensity score.
Among all patients receiving SC plus BT, those with right-sided mCRC had higher mortality compared with those with left-sided mCRC treated with SC and bevacizumab (HR, 1.31; 95% CI, 1.25-1.36, P < .001) and SC and cetuximab (HR, 1.88; 95% CI, 1.68-2.12; P < .001). In patients with left-sided mCRC, we found no significant difference in mortality by BT type (HR, 0.97; 95% CI, 0.88-1.05; P = .43). However, patients with right-sided mCRC treated with SC and cetuximab had significantly higher mortality compared with those treated with SC and bevacizumab (HR, 1.43; 95% CI 1.26-1.61; P < .001). Compared with patients with left-sided mCRC treated with SC plus cetuximab, patients with right-sided tumors had a nearly 2-fold higher mortality (HR, 1.93; 95% CI, 1.66-2.25; P < .001). One-third of the excess risk was attributable to the interaction between tumor site and BT (attributable proportion, 0.33) (Table 3).
Table 3. Interaction Between Tumor Origin and Selected Treatment Type on Mortality Hazards for mCRC.
| Tumor Origin | Treatment Type, HR (95% CI) | Treatment Type Within Strata of Tumor Origin, HR (95% CI) | |
|---|---|---|---|
| SC + Bevacizumab | SC + Cetuximab | ||
| All Patients Treated With BT, 2004-2014a | |||
| Right side | |||
| HR (95% CI) | 1.31 (1.25-1.36) | 1.88 (1.68-2.12) | 1.43 (1.26-1.61)b |
| P value | <.001 | <.001 | <.001 |
| Left side | |||
| HR (95% CI) | 1 [Reference] | 0.97 (0.88-1.05) | 0.97 (0.88-1.05)c |
| P value | .43 | .43 | |
| Right vs left sides within strata of treatment typed | |||
| HR (95% CI) | 1.31 (1.25-1.36)c | 1.93 (1.66-2.25)e | NA |
| P value | <.001 | <.001 | NA |
| Patients With Wild-type KRAS Tumors Treated With BT, 2010-2014f | |||
| Right side | |||
| HR (95% CI) | 1.66 (1.46-1.89) | 2.08 (1.64-2.64) | 1.28 (1.00-1.65)b |
| P value | <.001 | <.001 | .05 |
| Left side | |||
| HR (95% CI) | 1 [Reference] | 0.75 (0.63-0.90) | 0.75 (0.63-0.90)c |
| P value | NA | .002 | .002 |
| Right vs left sides within strata of treatment typeg | |||
| HR (95% CI) | 1.66 (1.46-1.89)c | 2.44 (1.83-3.25)e | NA |
| P value | <.001 | <.001 | NA |
Abbreviations: BT, biologic therapy; HR, hazard ratio; mCRC, metastatic colorectal cancer; SC, systemic chemotherapy.
Adjusted for age, diagnosis year, mucinous and signet ring histologic types, and socioeconomic status using propensity score.
Referent category is right-sided tumor treated with SC and bevacizumab.
Referent category is left-sided tumor treated with SC and bevacizumab.
For the measure of interaction on an additive scale, the attributable proportion was 0.33 (95% CI, 0.24-0.41; P < .001).
Referent category is left-sided tumor treated with SC and cetuximab.
Adjusted for age, diagnosis year, and the part that socioeconomic status played using propensity score.
For the measure of interaction on additive scale, the attributable proportion was 0.32 (95% CI, 0.14-0.50; P = .01).
Table 3 also presents findings for subgroup analyses for patients with wild-type KRAS mCRC diagnosed from 2010 through 2014 (n = 668). Those with left-sided wild-type KRAS tumors who received SC plus cetuximab had reduced mortality compared with patients who received SC plus bevacizumab (HR, 0.75; 95% CI, 0.63-0.90; P = .002). In contrast, patients with right-sided wild-type KRAS tumors who were treated with SC plus cetuximab had mortality nearly 2.5 times that of patients with left-sided wild-type KRAS tumors (HR, 2.44; 95% CI, 1.83-3.25; P < .001).
Discussion
We assessed the association of tumor origin and response to treatment with bevacizumab or cetuximab with mortality among patients with mCRC using a large and diverse population-based data set. Our results demonstrated that primary tumor site may be associated with response to BT and survival in mCRC. Patients with right-sided mCRC, regardless of the BT type, showed poorer overall survival compared with patients with left-sided mCRC, highlighting the association of primary tumor location with outcomes in mCRC. We observed a modest improvement in survival among patients with right- and left-sided mCRC with the addition of bevacizumab compared with SC alone. The beneficial influence of bevacizumab in first-line treatment for mCRC has been demonstrated in a phase 3 trial, AVF2107g, in which addition of bevacizumab to irinotecan hydrochloride, fluorouracil, and leucovorin calcium was associated with improved survival among patients with right- and left-sided mCRC. In our study, in patients treated with SC and bevacizumab, primary tumor site had a significant association with outcome, with right-sided mCRC being associated with significantly higher mortality than left-sided mCRC. Other investigators have reported similar outcome differences with bevacizumab treatment for right- and left-sided mCRC.
One of the key observations of our study was the interaction between the primary tumor location and response to cetuximab treatment. Patients with right-sided mCRC who were treated with cetuximab had significantly decreased survival, compared with those treated with SC alone or SC plus bevacizumab despite the relatively small sample size receiving cetuximab. In contrast, patients with left-sided mCRC had improved survival. The observed lack of benefit with cetuximab treatment in right-sided mCRC was further amplified in patients with wild-type KRAS tumors. In contrast, cetuximab was associated with improved survival compared with bevacizumab in patients with wild-type KRAS left-sided mCRC.
Our results are consistent with the findings reported in a recent retrospective analysis of the CALGB/SWOG 80405 trial. Venook et al analyzed the association of primary tumor location with survival outcomes in patients with wild-type RAS tumors who were treated with leucovorin (folic acid), fluorouracil, and irinotecan (FOLFIRI) plus bevacizumab or with FOLFIRI plus cetuximab as first-line treatment for mCRC. The authors reported that overall survival was better for cetuximab compared with bevacizumab treatment for left-sided mCRC (39.3 vs 32.6 months) and was poorer for both BT groups with right-sided primary tumors (13.6 vs 29.2 months). Similar retrospective analyses of other randomized clinical trials by various authors have consistently shown that primary tumor location in the right side is associated with lack of response to anti-EGFR therapy despite wild-type RAS status. In light of this evidence, the NCCN has recently revised its recommendations for the use of anti-EGFR therapy for first-line treatment in mCRC. Treatment with cetuximab or panitumumab is now recommended only for wild-type RAS and left-sided primary tumors. However, stratification by primary tumor location has not been extended to subsequent treatment with BT. In the present study, we found that primary tumor site was associated with response regardless of whether BT agents were used as first-line or a subsequent line of treatment in mCRC. Similar findings were reported in a study by Chen et al, in which the primary tumor site was found to be associated with cetuximab efficacy in third-line and salvage treatment of wild-type KRAS mCRC.
Reasons for survival differences between right- and left-sided mCRC may result from known and unknown genetic factors. Missiaglia et al found higher prevalence of an EGFR inhibitor–sensitive phenotype in left-sided metastatic colon tumors, with right-sided primary tumors characterized by more heterogeneous phenotypes, including poor sensitivity to EGFR inhibitors. Right-sided tumors have also been noted to have more microsatellite instability and BRAF mutations previously associated with poor overall survival. A recent retrospective analysis of tumor samples from patients with CRC treated with cetuximab found that ephrin A2 expression was associated with poorer prognosis, whereas expression of amphiregulin and epiregulin was associated with improved survival. These mechanisms, in addition to other unknown factors, may account, in part, for observed survival differences for BT agents in right- and left-sided mCRC.
Limitations
Our study has several limitations that are inherent to a retrospective analysis of a large population data set. We classified all transverse colon tumors as right sided because current reporting does not distinguish tumors in the proximal two-thirds and distal one-third of the transverse colon. Inclusion of distal transverse colon tumors as right sided could have affected our results, although this effect would arguably be minimal given the small proportion of CRC cases in the transverse colon. Furthermore, eliminating transverse colon cases from the analyses did not alter the initial interpretations of findings presented in Table 2 and Table 3 and eTables 1 and 2 in the Supplement.
Our study did not allow evaluation of differences in extent of metastatic disease or metastectomy for right- vs left-sided mCRC. Because the extent of metastatic disease and metastectomy is likely to be associated with survival among patients in our study, absence of this information may have confounded our findings and persists as a limitation of our study. Furthermore, CCR data do not include information about duration of therapies that could affect our findings.
Differences in baseline characteristics exist between treatment groups. To minimize these differences, adjustment for propensity score was performed in all multivariable analyses. Colorectal cancer–specific mortality hazard analyses showed findings similar to those for all-cause mortality, limiting the potential for bias from competing causes of death in the all-cause mortality findings. In addition, our analyses did not distinguish between patients who received oxaliplatin- vs irinotecan-based therapy. Recent data have suggested the potential for interactions between type of SC backbone and BT. These interactions could influence our results.
Although KRAS mutation status was not available in the CCR data until 2010, a subgroup analysis confined to patients with wild-type KRAS tumors produced findings similar to those presented in the full analysis. Other molecular markers for mCRC prognosis such as BRAF and microsatellite instability status were unavailable in our study and could have altered our results. Factors that have been shown to affect survival in mCRC, such as the extent of metastatic disease and surgical treatment of metastatic disease, were also not available for analysis.
Conclusions
Despite these limitations, our study results revealed an association of of primary tumor site with response to BT in mCRC. Patients with right-sided mCRC had poorer survival than those with left-sided mCRC regardless of the type of BT and KRAS status. We found that bevacizumab was associated with improved overall survival in right- and left-sided mCRC. However, in wild-type KRAS tumors, use of cetuximab benefited only the patients with left-sided mCRC and was associated with significantly higher mortality among patients with right-sided mCRC. We demonstrated that treatment with cetuximab may be associated with decreased survival among patients with wild-type KRAS right-sided mCRC. Our results have important treatment and health care cost implications. Selection of appropriate BT based on primary tumor site and response may help eliminate ineffective and expensive treatment in patients with mCRC.
To the best of our knowledge, our study is the first US population-based investigation that has validated the findings from clinical trials evaluating the effects of bevacizumab and cetuximab by tumor origin on overall survival in mCRC. This evidence provides further support for translation of clinical trial findings into CRC treatment practice and the need for stratification by primary tumor site in current clinical practice recommendations beyond first-line treatment with BT. Primary tumor site should be routinely incorporated as a stratification factor in future clinical trials to better elucidate potential benefits and harms predicted by tumor origin in mCRC treatment. Further research is needed to define the molecular basis for differences in BT efficacies for right- and left-sided mCRC.
eTable 1. Adjusted Hazard Ratios for Tumor Origin by Treatment Type (n = 11 269)
eTable 2. Interaction between Tumor Origin and Selected Treatment Type on
Mortality Hazards for Metastatic Colorectal Cancer
References
- 1.Bullard Dunn KM, Rothenberger DA. Colon, rectum, and anus In: Brunicardi FC, Andersen DK, Billiar TR, et al. , eds. Schwartz’s Principles of Surgery. 10th ed. New York, NY: McGraw-Hill Education; 2014. [Google Scholar]
- 2.Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113(10):779-788. [DOI] [PubMed] [Google Scholar]
- 3.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101(5):403-408. [DOI] [PubMed] [Google Scholar]
- 4.Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117(20):4623-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Missiaglia E, Jacobs B, D’Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25(10):1995-2001. [DOI] [PubMed] [Google Scholar]
- 6.Bendardaf R, Buhmeida A, Hilska M, et al. VEGF-1 expression in colorectal cancer is associated with disease localization, stage, and long-term disease-specific survival. Anticancer Res. 2008;28(6B):3865-3870. [PubMed] [Google Scholar]
- 7.Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol. 2008;15(9):2388-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H; Colon/Rectum Carcinomas (Primary Tumor) Study Group . Comparison of 17 641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53(1):57-64. [DOI] [PubMed] [Google Scholar]
- 9.Weiss JM, Pfau PR, O’Connor ES, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of Surveillance, Epidemiology, and End Results–Medicare data. J Clin Oncol. 2011;29(33):4401-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrelli F, Tomasello G, Borgonovo K, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis [published online October 27, 2016]. JAMA Oncol. doi:10.1001/jamaoncol.2016.4227 [DOI] [PubMed] [Google Scholar]
- 11.Loupakis F, Yang D, Yau L, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107(3):dju427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials[published online October 10, 2016]. JAMA Oncol. doi:10.1001/jamaoncol.2016.3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brulé SY, Jonker DJ, Karapetis CS, et al. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer. 2015;51(11):1405-1414. [DOI] [PubMed] [Google Scholar]
- 14.Botrel TE, Clark LG, Paladini L, Clark OA. Efficacy and safety of bevacizumab plus chemotherapy compared to chemotherapy alone in previously untreated advanced or metastatic colorectal cancer: a systematic review and meta-analysis. BMC Cancer. 2016;16:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubicka S, Greil R, André T, et al. ; ML18147 study investigators including AIO, GERCOR, FFCD, UNICANCER GI, TTD, BGDO, GEMCAD, and AGMT groups . Bevacizumab plus chemotherapy continued beyond first progression in patients with metastatic colorectal cancer previously treated with bevacizumab plus chemotherapy: ML18147 study KRAS subgroup findings. Ann Oncol. 2013;24(9):2342-2349. [DOI] [PubMed] [Google Scholar]
- 16.Wong HL, Lee B, Field K, et al. Impact of primary tumor site on bevacizumab efficacy in metastatic colorectal cancer. Clin Colorectal Cancer. 2016;15(2):e9-e15. [DOI] [PubMed] [Google Scholar]
- 17.Moosmann N, von Weikersthal LF, Vehling-Kaiser U, et al. Cetuximab plus capecitabine and irinotecan compared with cetuximab plus capecitabine and oxaliplatin as first-line treatment for patients with metastatic colorectal cancer: AIO KRK-0104—a randomized trial of the German AIO CRC study group. J Clin Oncol. 2011;29(8):1050-1058. [DOI] [PubMed] [Google Scholar]
- 18.Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011-2019. [DOI] [PubMed] [Google Scholar]
- 19.Lenz H. Outcome in CALGB 80405. Paper presented at: Special Session of the European Society for Medical Oncology 2014 Congress; September 29, 2014; Madrid, Spain. [Google Scholar]
- 20.Burt RW, Barthel JS, Dunn KB, et al. ; NCCN . NCCN clinical practice guidelines in oncology: colorectal cancer screening. J Natl Compr Canc Netw. 2010;8(1):8-61. [DOI] [PubMed] [Google Scholar]
- 21.California Department of Public Health California Cancer Registry. http://www.ccrcal.org/index.shtml December 24, 2014. Accessed February 26, 2017.
- 22.US Census Bureau. Quick Facts California Population. http://www.census.gov/quickfacts/fact/table/CA/PST045216. Accessed February 26, 2017.
- 23.Greene F, Page DL, Fleming ID, et al. In: Manual CS, ed. American Joint Committee on Cancer. 6th ed New York, NY: Springer; 2002. [Google Scholar]
- 24.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC Cancer Staging Manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471-1474. [DOI] [PubMed] [Google Scholar]
- 25.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. 3rd ed Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 26.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703-711. [DOI] [PubMed] [Google Scholar]
- 27.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41(2):514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boisen MK, Johansen JS, Dehlendorff C, et al. Primary tumor location and bevacizumab effectiveness in patients with metastatic colorectal cancer. Ann Oncol. 2013;24(10):2554-2559. [DOI] [PubMed] [Google Scholar]
- 30.Venook AP, Niedzwiecki D, Innocenti F, et al. Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2016;34(15)(suppl):3504. [Google Scholar]
- 31.von Einem JC, Heinemann V, von Weikersthal LF, et al. Left-sided primary tumors are associated with favorable prognosis in patients with KRAS codon 12/13 wild-type metastatic colorectal cancer treated with cetuximab plus chemotherapy: an analysis of the AIO KRK-0104 trial. J Cancer Res Clin Oncol. 2014;140(9):1607-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen KH, Shao YY, Chen HM, et al. Primary tumor site is a useful predictor of cetuximab efficacy in the third-line or salvage treatment of KRAS wild-type (exon 2 non-mutant) metastatic colorectal cancer: a nationwide cohort study. BMC Cancer. 2016;16:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strimpakos A, Pentheroudakis G, Kotoula V, et al. The prognostic role of ephrin A2 and endothelial growth factor receptor pathway mediators in patients with advanced colorectal cancer treated with cetuximab. Clin Colorectal Cancer. 2013;12(4):267-274.e2. [DOI] [PubMed] [Google Scholar]
- 34.Zhou SW, Huang YY, Wei Y, et al. No survival benefit from adding cetuximab or panitumumab to oxaliplatin-based chemotherapy in the first-line treatment of metastatic colorectal cancer in KRAS wild type patients: a meta-analysis. PLoS One. 2012;7(11):e50925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Adjusted Hazard Ratios for Tumor Origin by Treatment Type (n = 11 269)
eTable 2. Interaction between Tumor Origin and Selected Treatment Type on
Mortality Hazards for Metastatic Colorectal Cancer