Key Points
Question
Does the addition of adjuvant radiotherapy improve locoregional recurrence–free survival compared with chemotherapy alone for locally advanced bladder cancer after radical cystectomy?
Findings
A phase 2 trial randomized patients with locally advanced bladder cancer with negative margins after radical cystectomy to adjuvant sequential chemotherapy plus radiotherapy (n = 75) vs adjuvant chemotherapy alone (n = 45). The addition of adjuvant radiotherapy significantly improved locoregional recurrence–free survival compared with chemotherapy alone.
Meaning
Adjuvant chemotherapy plus radiotherapy is associated with significant improvements in locoregional recurrence–free survival and favorable cancer control outcomes compared with chemotherapy alone.
Abstract
Importance
Locoregional failure for patients with locally advanced bladder cancer (LABC) after radical cystectomy (RC) is common even with chemotherapy and is associated with high morbidity and mortality. Adjuvant radiotherapy (RT) can decrease locoregional failure but has not been studied in the chemotherapy era.
Objective
To investigate if adjuvant sequential RT plus chemotherapy can improve locoregional recurrence–free survival (LRFS) compared with adjuvant chemotherapy alone.
Design, Setting, and Participants
A randomized phase 3 trial was opened to compare adjuvant RT vs sequential chemotherapy plus RT after RC for LABC, but a third arm was added later as a randomized phase 2 trial to compare chemotherapy plus RT vs adjuvant chemotherapy alone, an emerging standard. The intent-to-treat phase 2 trial reported herein enrolled patients from December 2002 to July 2008. Data were analyzed from August 3, 2015, to January 6, 2016. Routine follow-up and surveillance pelvic computed tomographic (CT) scans every 6 months during the first 2 years were performed. The setting was an academic center. Patients with bladder cancer 70 years or younger having 1 or more risk factors (≥pT3b, grade 3, or positive nodes) with negative margins after radical cystectomy plus pelvic lymph node dissection were eligible. Patients had Eastern Cooperative Oncology Group performance status of 0 to 2, no evidence of distant metastases on CT scan of the abdomen and pelvis or on chest imaging, and adequate renal, hepatic, and hematologic function. Ninety-one percent (109 of 120) had ≥ pT3 disease.
Interventions
Chemotherapy plus RT included 2 cycles of gemcitabine (1000 mg/m2 intravenously on days 1, 8, and 15) and cisplatin (70 mg/m2 intravenously on day 2) before and after RT to 4500 cGy in 150 cGy twice-daily fractions over 3 weeks using 3-dimensional conformal techniques. Chemotherapy alone included 4 cycles of gemcitabine and cisplatin.
Main Outcome and Measure
Locoregional recurrence–free survival.
Results
The chemotherapy plus RT arm accrued 75 patients, and the chemotherapy-alone arm accrued 45 patients, with a weighted randomization to speed accrual. Fifty-three percent (64 of 120) had urothelial carcinoma, and 46.7% (56 of 120) had squamous cell carcinoma or other. The arms were balanced except for age (median, 52 vs 55 years; P = .04) and tumor size (mean, 4.9 vs 5.8 cm; P < .01), both favoring chemotherapy plus RT. Two-year outcomes and overall adjusted hazard ratios (HRs) for chemotherapy plus RT vs chemotherapy alone were 96% vs 69% (HR, 0.08; 95% CI, 0.02-0.39; P < .01) for LRFS, 68% vs 56% (HR, 0.53; 95% CI, 0.27-1.06; P = .07) for disease-free survival, and 71% vs 60% (HR, 0.61; 95% CI, 0.33-1.11; P = .11) for overall survival (OS). Five patients (7%) had RT-associated late grade 3 gastrointestinal tract adverse effects in the chemotherapy plus RT arm.
Conclusions and Relevance
Adjuvant chemotherapy plus RT was reasonably well tolerated and was associated with significant improvements in LRFS and marginal improvements in disease-free survival vs chemotherapy alone in LABC. The addition of adjuvant RT should be considered for LABC. This regimen warrants further study in phase 3 trials.
Trial Registration
clinicaltrials.gov Identifier: NCT01734798
This randomized phase 2 trial investigates if adjuvant sequential radiotherapy plus chemotherapy can improve locoregional recurrence–free survival compared with adjuvant chemotherapy alone.
Introduction
Patients having bladder cancer with ≥ pT3 disease at the time of radical cystectomy (RC) have an estimated 5-year overall survival (OS) of only 10% to 40%. Approximately one-third will develop a pelvic recurrence as the initial site of failure after surgery. Perioperative chemotherapy does not reduce pelvic recurrence rates, salvage of such failures is rarely successful, and the median survival after locoregional failure (LF) is only approximately 9 months. Postoperative radiotherapy (RT) has been attempted, and evidence has shown that it reduces local recurrence and significantly improves disease-free survival (DFS). A previous randomized clinical trial of postoperative RT vs observation conducted in the 1980s at the National Cancer Institute (NCI) in Cairo, Egypt, among 236 patients reported not only a significant improvement in local control but also a significant improvement in DFS for patients treated with RT. Eighty percent had squamous cell carcinoma (SCC), and only 20% had urothelial carcinoma, but the outcomes were the same regardless of histology. That trial, which used older 2-dimensional RT techniques, established adjuvant RT as a standard treatment for locally advanced disease in Egypt; however, postoperative RT has no defined role outside of the Middle East, largely because of the adverse effects reported in several small series using pre-1980s treatment techniques and concerns about the applicability of the Egyptian trial for urothelial carcinoma.
A second randomized trial was conducted at the NCI in Cairo from December 2002 to July 2008 that was initially designed to compare adjuvant RT alone vs sequential sandwich chemotherapy plus RT using modern 3-dimensional (3-D) conformal techniques. After the trial opened, adjuvant chemotherapy had emerged as a standard treatment option, and a third arm, adjuvant chemotherapy alone, was added later as part of a randomized phase 2 trial comparing adjuvant chemotherapy plus RT vs chemotherapy alone. In this phase 2 trial, we hypothesized that the addition of adjuvant RT would significantly improve locoregional recurrence–free survival (LRFS) compared with adjuvant chemotherapy alone in patients with locally advanced bladder cancer (LABC). Herein, we report the results of the randomized phase 2 trial.
Methods
Study Design
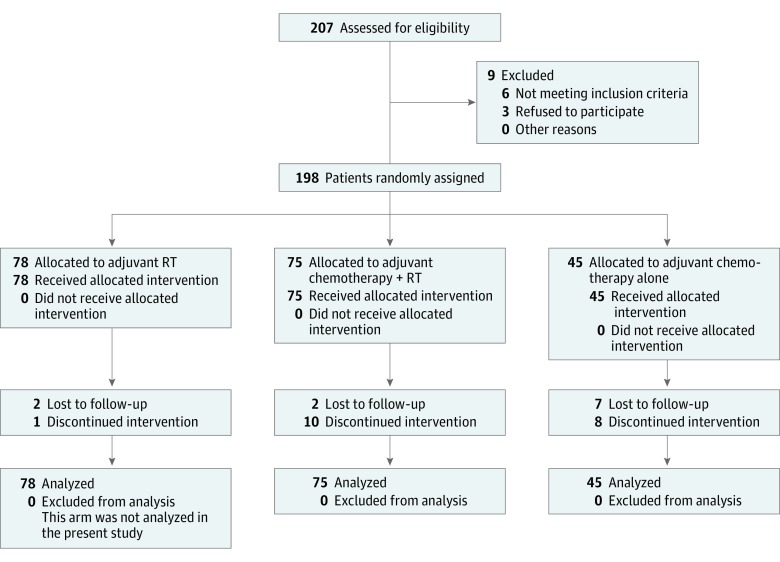
This NCI in Cairo institutional review board–approved trial (NCT01734798) enrolled patients with bladder cancer who underwent RC with negative surgical margins and 1 or more of the following risk factors: stage ≥ pT3b (American Joint Committee on Cancer's Cancer Staging Manual, Fourth Edition), grade 3, or positive pathologic lymph nodes (Figure 1). All patients provided written informed consent. The study was originally designed as a phase 3 trial comparing sequential adjuvant chemotherapy plus RT vs adjuvant RT alone. A third arm, adjuvant chemotherapy alone, was added later as part of a randomized phase 2 trial to compare the emerging standard of adjuvant chemotherapy alone vs sequential adjuvant chemotherapy plus RT. The chemotherapy plus RT arm received 2 cycles of gemcitabine and cisplatin before and after RT, with a 1-week break between chemotherapy and RT. The arm receiving chemotherapy alone had 4 cycles of gemcitabine and cisplatin (eFigure 1 in the Supplement).
Figure 1. Study Flow Diagram.
RT indicates radiotherapy.
Patients
All patients underwent RC and standard bilateral pelvic lymphadenectomy (up to but not including the common iliac nodes) at the NCI in Cairo from 2002 to 2008. The setting was an academic center. Inclusion criteria were treatment-naive patients with bladder cancer 70 years or younger, Eastern Cooperative Oncology Group performance status of 0 to 2, and adequate renal, hepatic, and hematologic function. All patients had no evidence of distant metastases or second cancer on physical examination, computed tomography (CT) scans of the abdomen and pelvis, or chest imaging (either chest radiograph or CT scan). Patients with neobladders were not enrolled.
Randomization
Patients (n = 198) were randomly assigned to 1 of the following 3 adjuvant treatments: RT alone, sequential sandwich chemotherapy plus RT, or chemotherapy alone. Participants were registered within 6 weeks of RC and were randomized within 8 weeks after surgery. The chemotherapy-alone arm was opened in 2007; to speed accrual, patients were enrolled in a 1:1:4 randomization weighted toward the chemotherapy arm. Only patients in the chemotherapy plus RT and chemotherapy-alone arms are included in this analysis.
Procedures
All patients in the chemotherapy plus RT arm underwent CT scan simulation for RT treatment planning. Intravenous contrast was used unless contraindicated. Radiotherapy was delivered using 3-D conformal techniques with 6-mV or 14-mV photons to the cystectomy bed and bilateral pelvic lymph nodes with the patient supine. Most patients were treated with a 3-field beam arrangement with 1 anterior beam and 2 posterior oblique beams. The entire true pelvis was included in the field. Superiorly, the field extended to the top of S2 in most patients and to the top of S1 in patients thought to be at higher risk for nodal involvement. Inferiorly, the lower border extended to the lower two-thirds of the obturator foramen, anteriorly to the pubic symphysis, posteriorly to the anterior one-third of the rectal wall, and laterally 1 to 1.5 cm beyond the edge of the pelvic brim. Patients were treated to a total dose of 4500 cGy in 150-cGy fractions delivered twice daily over 3 weeks.
Chemotherapy was given in 28-day cycles, including gemcitabine (1000 mg/m2 intravenously over 30 minutes) on days 1, 8, and 15 and cisplatin (70 mg/m2 intravenously over 30 minutes) on day 2. Doses of chemotherapy were adjusted if toxic effects occurred.
Follow-up
Patients were seen for follow-up every 2 months in the first 2 years and then every 6 months thereafter. Surveillance pelvic CT scan was performed every 6 months during the first 2 years and then yearly thereafter unless otherwise clinically indicated. Data were analyzed from August 3, 2015, to January 6, 2016.
Study End Points
The primary end point was LRFS. Secondary end points were DFS, distant metastasis–free survival (DMFS), OS, and adverse effects. Locoregional recurrence–free survival was defined as the time from RC to any recurrence in the pelvic lymph nodes or soft tissues before or within 3 months of evidence of distant failure. Other events were censored. Recurrences cephalad to the iliac bifurcation or within the inguinal nodes were scored as distant metastases. Acute toxicities observed within 90 days were recorded using the World Health Organization grading system. Late GI tract adverse events potentially associated with RT and observed more than 90 days after completion of therapy were scored using European Organization for Research and the Treatment of Cancer (EORTC) version 3.0 in both arms. If a potential late GI tract adverse effect was observed cosynchronously with a pelvic recurrence, the effect was attributed to the disease recurrence.
Statistical Analysis
This study was designed as a randomized phase 2 comparison using LRFS to screen the original sequential chemotherapy plus RT arm against the chemotherapy-alone arm, which had emerged as a standard of care. The original 2-arm study was designed to accrue 75 patients in each arm. With a 1:1:4 weighted accrual in favor of the chemotherapy-alone arm, the target accrual was 44 patients in the third arm. The trial was powered to detect a 25% improvement in 5-year LRFS (α = .05, β = .20) for chemotherapy plus RT (90% vs 65%). The 90% 5-year LRFS was based on the LRFS from the prior NCI study in Cairo. Analysis was intent to treat. Survival end points were measured from the date of RC. Cox proportional hazards regression models were used to adjust for covariates and to evaluate interaction terms in which LRFS, DFS, DMFS, and OS were the end points. Kaplan-Meier analysis was used. A 2-sided P < .05 was considered significant for all end points. Comparisons were adjusted for covariates that were not balanced between the arms or were associated (P < .10) with the outcome of interest on univariate analysis. Cox proportional hazards regression multivariate analysis was performed to evaluate factors that independently predict LRFS, DFS, DMFS, and OS. Statistical analysis was performed using R (version 3; R Foundation). Acute adverse effects were not formally compared because the duration of the treatment period and the follow-up schedule differed between the 2 arms. Late effects were not formally compared because there were too few events.
Results
Patient Characteristics
One hundred twenty patients were enrolled in the chemotherapy plus RT (n = 75) and chemotherapy alone (n = 45) arms. Patient characteristics are summarized in Table 1. Their median age was 54 years (range, 27-69 years), and the male to female ratio was 4:1. Fifty-three percent (64 of 120) had urothelial carcinoma, and 46.7% (56 of 120) had SCC or other. Ninety-two percent (69 of 75) of patients in the chemotherapy plus RT arm and 88.9% (40 of 45) of patients in the chemotherapy-alone arm had ≥ pT3 disease. Only 3 patients with grade 3 disease without ≥ pT3 or pN+ disease were enrolled. The chemotherapy plus RT and chemotherapy-alone arms were well balanced except for age (median, 52 vs 55 years; P = .04) and tumor size (mean, 4.9 vs 5.8 cm; P < .01), both favoring chemotherapy plus RT.
Table 1. Patient Characteristics in the Chemotherapy Plus Radiotherapy (RT) Arm and the Chemotherapy-Alone Arm.
| Variable | Chemotherapy + RT (n = 75) |
Chemotherapy Alone (n = 45) |
P Value |
|---|---|---|---|
| Age, median, y | 52 | 55 | .04 |
| Sex, No. (%) | |||
| Male | 60 (80.0) | 37 (82.2) | .76 |
| Female | 15 (20.0) | 8 (17.8) | |
| Tumor histology, No. (%) | |||
| Urothelial | 41 (54.7) | 23 (51.1) | .71 |
| SCC or other | 34 (45.3) | 22 (48.9) | |
| Tumor grade, No. (%) | |||
| 1 | 1 (1.3) | 3 (6.7) | .12 |
| 2 | 40 (53.3) | 27 (60.0) | |
| 3 | 34 (45.3) | 15 (33.3) | |
| Pathologic T stage, No. (%) | |||
| pT2 | 6 (8.0) | 5 (11.1) | .10 |
| pT3 | 61 (81.3) | 29 (64.4) | |
| pT4a | 8 (10.7) | 11 (24.4) | |
| Tumor size, cm | |||
| Mean | 4.9 | 5.8 | <.01 |
| Median | 5 | 5 | NA |
| Pathologic nodal disease, No. (%) | |||
| Positive | 35 (46.7) | 17 (37.8) | .34 |
| Negative | 40 (53.3) | 28 (62.2) | |
| No. of involved lymph nodes, mean | 1.1 | 0.9 | .29 |
| No. of lymph nodes removed, median | 12 | 12 | .17 |
| ≥10 Lymph nodes removed, No. (%) | |||
| Yes | 54 (72.0) | 27 (60.0) | .17 |
| No | 21 (28.0) | 18 (40.0) | |
| Type of urinary tract diversion, No. (%) | |||
| Ileal conduit | 37 (49.3) | 25 (55.6) | .22 |
| Ureterocolic | 11 (14.7) | 6 (13.3) | |
| Ureterocutaneous | 11 (14.7) | 3 (6.7) | |
| Rectal bladder | 9 (12.0) | 10 (22.2) | |
| Ileocecal conduit | 7 (9.3) | 1 (2.2) | |
| Events, No. (%) | |||
| LF | 2 (2.7) | 13 (28.9) | NA |
| LF only | 1 (1.3) | 12 (26.7) | |
| LF and synchronous DM | 1 (1.3) | 1 (2.2) | |
| DM | 15 (20.0) | 6 (13.3) | |
| Recurrence or death due to progression | 19 (25.3) | 18 (40.0) | |
| Death | 26 (34.7) | 23 (51.1) |
Abbreviations: DM, distant metastasis; LF, locoregional failure; NA, not applicable; SCC, squamous cell carcinoma.
Completion of the Prescribed Therapy
Ten of 75 (13.3%) patients in the chemotherapy plus RT arm did not complete the prescribed treatment. All finished the initial chemotherapy cycles, but 5 did not complete RT. Of the 5, one stopped RT owing to grade 4 diarrhea, another received only 30 Gy before resuming chemotherapy, and 3 stopped RT after only a few fractions because of adverse effects attributed to surgery or chemotherapy, with one dying of renal failure. An additional 5 patients completed RT but did not complete the final 2 cycles of chemotherapy, with one dying of severe dehydration and renal failure and another developing bone metastasis.
Eight of 45 (17.8%) patients in the chemotherapy arm failed to complete all cycles. Of these, 4 dropped out at the patient’s request and were lost to follow-up, 2 after the third cycle and 1 each after the first and second cycles. Of the remaining 4 patients, 3 died of renal failure during chemotherapy (2 after the second cycle and 1 after the first cycle), and 1 died of an intestinal obstruction after the second cycle.
Efficacy
The median follow-up for patients who completed treatment was 24 months after chemotherapy plus RT (range, 5-127 months) and 27 months after chemotherapy alone (range, 3-76 months). There were 2 LFs in the chemotherapy plus RT arm and 13 LFs in the chemotherapy-alone arm, with one patient in each arm having synchronous local and distant failure.
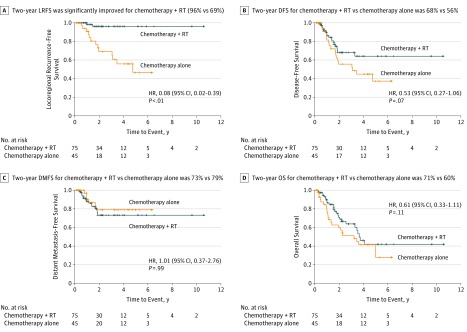
On univariate analysis, significant predictors of LRFS included treatment with chemotherapy plus RT and the number of lymph nodes removed (eTable 1 in the Supplement). Marginal predictors included age and tumor size. Sex, tumor histology (urothelial vs SCC or other), tumor grade, pathologic T stage, pathologic nodal disease, and the number of involved lymph nodes were not predictive. While pathologic T stage was not a statistically significant covariate, we included it as an adjustment factor because of its well-known association with the outcome of interest in the literature (LRFS). In the covariate-adjusted model (Table 2), treatment with chemotherapy plus RT was a significant independent predictor of LRFS (hazard ratio [HR], 0.08; 95% CI, 0.02-0.39; P < .01). Age was also a significant independent predictor of LRFS (HR, 0.93; 95% CI, 0.88-0.98; P = .01). In the post hoc analysis, 2-year LRFS was significantly improved for the chemotherapy plus RT arm vs the chemotherapy-alone arm (96%; 95% CI, 91%-100% vs 69%; 95% CI, 54%-88%; P < .01) (Figure 2A). Eleven of 15 patients with LF died, with a median survival of 2 months (range, 0-6 months) from the time of LF. In an unplanned analysis of patients with urothelial-only tumor histology, LRFS was significantly improved for the chemotherapy plus RT arm (n = 41) vs the chemotherapy-alone arm (n = 23), with 2-year LRFS of 100% vs 67% (P < .01) (eFigure 2 in the Supplement).
Table 2. Multivariate Analysis of Factors Predictive of Locoregional Recurrence–Free Survival, Disease-Free Survival, Distant Metastasis–Free Survival, and Overall Survival.
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Locoregional Recurrence–Free Survival | ||
| Treatment with chemotherapy + RT | 0.08 (0.02-0.39) | <.01 |
| Age | 0.93 (0.88-0.98) | .01 |
| Tumor size | 1.01 (0.72-1.42) | .95 |
| No. of lymph nodes removed | 0.93 (0.84-1.02) | .13 |
| Pathologic T stage | ||
| pT2 | 1.19 (0.12-12.01) | .88 |
| pT4 | 1.59 (0.40-6.38) | .51 |
| Disease-Free Survival | ||
| Treatment with chemotherapy + RT | 0.53 (0.27-1.06) | .07 |
| Age | 0.97 (0.93-0.99) | .04 |
| Tumor size | 1.03 (0.83-1.27) | .80 |
| Pathologic T stage | ||
| pT2 | 0.24 (0.03-1.78) | .16 |
| pT4 | 1.30 (0.58-2.94) | .52 |
| Distant Metastasis–Free Survival | ||
| Treatment with chemotherapy + RT | 1.01 (0.37-2.76) | .99 |
| Age | 0.96 (0.92-1.01) | .12 |
| Tumor size | 0.93 (0.69-1.25) | .63 |
| No. of lymph nodes removed | 1.08 (1.00-1.16) | .05 |
| Pathologic T stage | ||
| pT2 | 1 [Reference] | NA |
| pT4 | 1.47 (0.48-4.54) | .50 |
| Overall Survival | ||
| Treatment with chemotherapy + RT | 0.61 (0.33-1.11) | .11 |
| Age | 0.98 (0.95-1.01) | .27 |
| Tumor size | 0.98 (0.81-1.18) | .83 |
| Pathologic T stage | ||
| pT2 | 0.18 (0.02-1.34) | .10 |
| pT4 | 1.29 (0.63-2.64) | .49 |
Abbreviations: NA, not applicable; RT, radiotherapy.
Figure 2. Kaplan-Meier Curves Comparing Adjuvant Sequential Sandwich Chemotherapy Plus Radiotherapy (RT) vs Adjuvant Chemotherapy Alone.
A, Locoregional recurrence–free survival (LRFS). B, Disease-free survival (DFS). C, Distant metastasis–free survival (DMFS). D, Overall survival (OS). HR indicates hazard ratio.
There were no significant predictors of DFS on univariate analysis. Marginal predictors included treatment with chemotherapy plus RT and age (eTable 2 in the Supplement). In the covariate-adjusted model, age was a significant predictor of DFS (HR, 0.97; 95% CI, 0.93-0.97; P < .05). Treatment with chemotherapy plus RT was a marginal predictor of DFS (HR, 0.53; 95% CI, 0.27-1.06; P = .07) (Table 2). Two-year DFS for the chemotherapy plus RT arm vs the chemotherapy-alone arm was 68% (95% CI, 56%-81%) vs 56% (95% CI, 38%-73%) (Figure 2B).
There were no significant predictors of DMFS on univariate analysis. Marginal predictors included age and the number of lymph nodes removed (eTable 3 in the Supplement). In the covariate-adjusted model, the number of lymph nodes removed was a significant independent predictor of DMFS (HR, 1.08; 95% CI, 1.00-1.16; P = .05). Treatment with chemotherapy plus RT was not statistically significant. Two-year DMFS for the chemotherapy plus RT arm vs the chemotherapy-alone arm was 73% (95% CI, 62%-86%) vs 79% (95% CI, 65%-96%) (Figure 2C).
There were no significant predictors of OS on univariate analysis. Marginal predictors included pathologic T stage (eTable 4 in the Supplement). There were no significant predictors of OS on multivariate analysis (eTable 5 in the Supplement). Treatment with chemotherapy plus RT (HR, 0.61; 95% CI, 0.33-1.11; P = .11) and pathologic T stage (HR, 0.18; 95% CI, 0.02-1.34; P = .10) were not statistically significant. Two-year OS for the chemotherapy plus RT arm vs the chemotherapy-alone arm was 71% (95% CI, 65%-77%) vs 60% (95% CI, 52%-68%) (Figure 2D).
Adverse Effects
Acute adverse effects for the 2 arms are summarized in Table 3. Late grade 3 GI tract adverse effects were observed in 5 patients (6.7%) in the chemotherapy plus RT arm, 2 with an intestinal obstruction and 1 each with abdominal pain, colitis, and bowel fistula. Late grade 3 GI tract adverse effects were observed in 1 patient (2.2%) in the chemotherapy-alone arm who had an intestinal obstruction. No late grade 4 GI toxicity was observed in either arm. There was no late grade 3 or higher obstructive uropathy in either arm.
Table 3. Acute Adverse Effects Measured Using World Health Organization Criteria for the Chemotherapy Plus Radiotherapy (RT) Arm and the Chemotherapy-Alone Arm.
| Variable | No. (%) | |
|---|---|---|
| Chemotherapy + RT (n = 75) |
Chemotherapy Alone (n = 45) |
|
| Nausea or vomiting | ||
| Grade 2 | 46 (61.3) | 11 (24.4) |
| Grade 3 | 28 (37.3) | 25 (55.6) |
| Grade 4 | 1 (1.3) | 0 |
| Diarrhea | ||
| Grade 2 | 44 (58.7) | 6 (13.3) |
| Grade 3 | 15 (20.0) | 9 (20.0) |
| Grade 4 | 1 (1.3) | 0 |
| Abdominal paina | ||
| Grade 2 | 48 (64.0) | NA |
| Grade 3 | 2 (2.7) | NA |
| Grade 4 | 0 | NA |
| Tenesmusa | ||
| Grade 2 | 15 (20.0) | NA |
| Grade 3 | 6 (8.0) | NA |
| Grade 4 | 4 (5.3) | NA |
| Liver injury | ||
| Grade 2 | 2 (2.7) | 2 (4.4) |
| Grade 3 | 0 | 0 |
| Grade 4 | 0 | 0 |
| Kidney injury | ||
| Grade 2 | 2 (2.7) | 9 (20.0) |
| Grade 3 | 4 (5.3) | 0 |
| Grade 4 | 0 | 0 |
| Anemia | ||
| Grade 2 | 28 (37.3) | 16 (35.6) |
| Grade 3 | 20 (26.7) | 4 (8.9) |
| Grade 4 | 3 (4.0) | 0 |
| Neutropenia | ||
| Grade 2 | 9 (12.0) | 9 (20.0) |
| Grade 3 | 1 (1.3) | 4 (8.9) |
| Grade 4 | 0 | 0 |
| Thrombocytopenia | ||
| Grade 2 | 1 (1.3) | 0 |
| Grade 3 | 2 (2.7) | 0 |
| Grade 4 | 0 | 0 |
Abbreviation: NA, not applicable.
Adverse effects not recorded for the chemotherapy-alone arm.
Discussion
Locoregional failures after RC for patients with LABC are common. In the Southwest Oncology Group (SWOG) 8710 trial of RC with or without neoadjuvant chemotherapy, the 5-year cumulative incidence of LF for patients with ≥ pT3 urothelial carcinoma was 32% and was even higher for similarly treated patients in the Medical Research Council (MRC) trial. Investigators have hypothesized that reducing LFs may lead to improved DFS and OS. This hypothesis is supported by several lines of evidence. Multiple retrospective surgical series have demonstrated improved survival with more extensive nodal dissections even in the absence of nodal metastasis, suggesting that eradicating occult nodal disease may improve survival by decreasing distant and local failure. There is also evidence that locoregional recurrence is an independent predictor of distant metastases and that LF often precedes, but uncommonly follows, the appearance of distant metastases, suggesting that LF may seed distant metastases.
If LFs have a role in the subsequent development of distant disease, then adjuvant therapy is needed to enhance local control. Chemotherapy does not appear to improve pelvic control. There was no reduction in LF with the addition of chemotherapy in the SWOG 8710 trial, the MRC trial, or the experience at the University of Pennsylvania, suggesting a potential role for adjuvant local therapy, such as RT, to reduce LF.
The need for adjuvant therapy to diminish LF is more compelling because salvage treatment is rarely successful, with a median survival of 9 months after the development of locoregional recurrence. High-dose RT to control grossly recurrent bladder cancer is usually precluded by the close proximity of critical normal structures, particularly bowel and the neobladder and urinary tract diversion. Even if there is no improvement in survival with adjuvant local therapy, such treatment can prevent the often substantial morbidity of pelvic recurrences, which can cause pain and ureteric, venous, and lymphatic obstruction.
To our knowledge, the randomized phase 2 trial we report herein is the first study to compare adjuvant RT plus chemotherapy vs adjuvant chemotherapy alone in LABC, a question that has drawn considerable interest recently in the genitourinary community. The renewed interest in adjuvant RT has been spurred by an increased awareness of the high rates of LF for ≥ pT3 disease, recent progress to stratify patients based on their LF risk and to map the patterns of pelvic failure, and improvements in RT treatment techniques that may reduce adverse effects. Several cooperative group trials of adjuvant RT have opened or are in development, including trials from France (GETUG) and the United Kingdom (NCRI), as well as trials at Tata Memorial Hospital (Mumbai, India) and Ghent University (Ghent, Belgium). The National Comprehensive Cancer Network (NCCN) revised its guidelines in 2016 to include adjuvant RT as an option to consider in patients with LABC. While the phase 2 trial herein also enrolled patients with pN+ or grade 3 disease, the trial was predominantly a study of ≥ pT3 disease (90.8% [109 of 120] of patients), which current research suggests is the factor most predictive of pelvic relapse in urothelial carcinoma. The present trial provides the only prospective evidence to date to support the NCCN’s current recommendation to consider adjuvant RT after R0 resection in patients with ≥ pT3 disease, positive nodes, or high-grade bladder cancer.
Locoregional recurrence–free survival was selected as the primary end point herein owing to the high rate of micrometastasis in patients with apparently localized disease. The importance of locoregional recurrence as a clinical end point was leant additional credence by the high mortality after LF and low reported rates of synchronous distant disease (13.3% [2 of 15 failures] in our study). In the present study, in which most patients had urothelial carcinoma, the addition of RT was associated with a statistically significant improvement in LRFS, with a 27% absolute improvement in 2-year LRFS (96% vs 69%, P < .01). The magnitude of local control improvement with adjuvant RT arguably exceeds that reported for other cancers for which adjuvant RT is the standard of care, including breast, rectal, and vulvar cancer. The large benefit in locoregional control with the addition of RT translated to a marginal benefit in 2-year DFS (68% vs 56%, P = .07). Two-year OS favored chemotherapy plus RT, but the difference was not statistically significant (71% vs 60%, P = .11).
Most important, the addition of RT was associated with a statistically significant improvement in LRFS for patients with urothelial carcinoma in an unplanned subset analysis. The magnitude of the benefit was comparable to that observed in the entire cohort. Given the absence of published, randomized trials of adjuvant RT in Western populations or retrospective series, our study presents the best data to date on adjuvant RT for urothelial LABC.
Unlike the studies from the 1970s and 1980s in the United States using older RT techniques that reported relatively high late GI tract adverse effects, the present trial (the first to use modern 3-D conformal RT after RC) found that adjuvant RT was reasonably well tolerated. Acute and late grade 3 GI toxicity was not appreciably different between the arms, and a comparable percentage in each arm completed the prescribed therapy. Intensity-modulated RT was adopted in the NRG trial and the other cooperative group trials and may result in even lower rates of late GI toxicity based on a dosimetric analysis.
The present study, which excluded patients with positive margins, demonstrates that the addition of adjuvant RT can significantly improve local control even for patients with negative margins. The risk of LF is lower (ceteris paribus) among those with negative margins based on the University of Pennsylvania risk stratification.
Limitations
This study has several important limitations. The weighted randomization when the third arm was added resulted in imbalances between the chemotherapy plus RT arm and the chemotherapy-alone arm in terms of age and tumor size, which required an adjusted analysis to account for potential covariates for each outcome of interest. The small size of the patient cohort (N = 120) may have limited the ability to detect significant DFS and OS differences between the 2 arms. The heterogeneity of the tumor histology, with a sizable minority of patients having nonurothelial disease, may limit the applicability of the study to North American and European patients. The study also involved a standard pelvic lymph node dissection, rather than an extended dissection that includes the common iliac and presacral nodes, which may limit the study’s applicability for patients treated with an extended dissection. While the median number of lymph nodes removed was 12 in both arms, a sizable minority had less than 10 nodes removed, which has been identified in some series as a risk factor for pelvic recurrence, raising the possibility that adjuvant RT may be compensating for less extensive surgery. In addition, there was a time bias, with the chemotherapy plus RT arm generally treated in earlier years than the chemotherapy-alone arm. Late GI toxicities may have been underreported: if a potential late GI toxicity was observed at the same time as a pelvic recurrence, the effect was attributed to the recurrence. Finally, the study did not enroll patients with orthotopic neobladders and thus does not offer insight on the expected toxicity associated with adjuvant RT for patients with neobladders.
Conclusions
To our knowledge, this is the first prospective study comparing adjuvant chemotherapy plus RT with adjuvant chemotherapy alone in LABC. Adjuvant chemotherapy plus RT appears to be well tolerated and is associated with favorable cancer control outcomes. While not definitive, these results suggest that patients with negative margins and locally advanced disease after RC should be considered for referral to discuss adjuvant RT. Phase 3 trials of adjuvant RT for patients with urothelial carcinoma are warranted.
eFigure 1. Schematic of the Clinical Trial Design
eFigure 2. Local-Regional Recurrence Free Survival for Chemo+RT vs Chemotherapy Alone for Patients With Urothelial Carcinoma
eTable 1. Univariate Analysis of Factors Predictive of Local Recurrence-Free Survival (LRFS)
eTable 2. Univariate Analysis of Factors Predictive of Disease-Free Survival (DFS)
eTable 3. Univariate Analysis of Factors Predictive of Distant Metastasis-Free Survival (DMFS)
eTable 4. Univariate Analysis of Factors Predictive of Overall Survival (OS)
eTable 5. Summary Comparison of LRFS, DFS, DMFS, and OS Between the Two Arms (Chemotherapy plus RT vs Chemotherapy Alone)
References
- 1.Christodouleas JP, Baumann BC, He J, et al. Optimizing bladder cancer locoregional failure risk stratification after radical cystectomy using SWOG 8710. Cancer. 2014;120(8):1272-1280. [DOI] [PubMed] [Google Scholar]
- 2.Baumann BC, Guzzo TJ, He J, et al. A novel risk stratification to predict local-regional failures in urothelial carcinoma of the bladder after radical cystectomy. Int J Radiat Oncol Biol Phys. 2013;85(1):81-88. [DOI] [PubMed] [Google Scholar]
- 3.Herr HW, Faulkner JR, Grossman HB, et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol. 2004;22(14):2781-2789. [DOI] [PubMed] [Google Scholar]
- 4.Visser O, Nieuwenhuijzen JA, Horenblas S; Members of the Urological Oncology Working Group of the Comprehensive Cancer Centre Amsterdam . Local recurrence after cystectomy and survival of patients with bladder cancer: a population based study in greater Amsterdam. J Urol. 2005;174(1):97-102. [DOI] [PubMed] [Google Scholar]
- 5.Volkmer BG, Kuefer R, Bartsch GC Jr, Gust K, Hautmann RE. Oncological followup after radical cystectomy for bladder cancer: is there any benefit? J Urol. 2009;181(4):1587-1593. [DOI] [PubMed] [Google Scholar]
- 6.Baumann BC, Guzzo TJ, He J, et al. Bladder cancer patterns of pelvic failure: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(2):363-369. [DOI] [PubMed] [Google Scholar]
- 7.Zaghloul MS, Awwad HK, Akoush HH, Omar S, Soliman O, el Attar I. Postoperative radiotherapy of carcinoma in bilharzial bladder: improved disease free survival through improving local control. Int J Radiat Oncol Biol Phys. 1992;23(3):511-517. [DOI] [PubMed] [Google Scholar]
- 8.Cozzarini C, Pellegrini D, Fallini M, et al. Reappraisal of the role of adjuvant radiotherapy in muscle-invasive transitional cell carcinoma of the bladder. Int J Radiat Oncol Biol Phys. 1999;45(3):221-222. Abstract 144. [Google Scholar]
- 9.Reisinger SA, Mohiuddin M, Mulholland SG. Combined pre- and postoperative adjuvant radiation therapy for bladder cancer: a ten year experience. Int J Radiat Oncol Biol Phys. 1992;24(3):463-468. [DOI] [PubMed] [Google Scholar]
- 10.Kopelson G, Heaney JA. Postoperative radiation therapy for muscle-invading bladder carcinoma. J Surg Oncol. 1983;23(4):263-268. [DOI] [PubMed] [Google Scholar]
- 11.Zaghloul MS, Christodouleas JP, Smith A, et al. A randomized clinical trial comparing adjuvant radiation versus chemo-RT versus chemotherapy alone after radical cystectomy for locally advanced bladder cancer [abstract 356]. J Clin Oncol. doi: 10.1200/jco.2016.34.2_suppl.356 [DOI] [Google Scholar]
- 12.World Health Organization WHO Handbook for Reporting Results of Cancer Treatment. Geneva, Switzerland: World Health Organization; 1979. WHO Offset Publication 48. [Google Scholar]
- 13.Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK; International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party (Now the National Cancer Research Institute Bladder Cancer Clinical Studies Group); European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group; Australian Bladder Cancer Study Group; National Cancer Institute of Canada Clinical Trials Group; Finnbladder; Norwegian Bladder Cancer Study Group; Club Urologico Espanol de Tratamiento Oncologico Group . International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29(16):2171-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skinner EC, Stein JP, Skinner DG. Surgical benchmarks for the treatment of invasive bladder cancer. Urol Oncol. 2007;25(1):66-71. [DOI] [PubMed] [Google Scholar]
- 15.Pollack A, Zagars GK, Cole CJ, Dinney CP, Swanson DA, Grossman HB. The relationship of local control to distant metastasis in muscle invasive bladder cancer. J Urol. 1995;154(6):2059-2063. [PubMed] [Google Scholar]
- 16.Ide H, Kikuchi E, Miyajima A, et al. The predictors of local recurrence after radical cystectomy in patients with invasive bladder cancer. Jpn J Clin Oncol. 2008;38(5):360-364. [DOI] [PubMed] [Google Scholar]
- 17.Baumann BC, Bosch WR, Bahl A, et al. Development and validation of consensus contouring guidelines for adjuvant radiation therapy for bladder cancer after radical cystectomy. Int J Radiat Oncol Biol Phys. 2016;96(1):78-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eapen LJ, Jones E, Kassouf W, et al. Enumerating pelvic recurrence following radical cystectomy for bladder cancer: a Canadian multi-institutional study. Can Urol Assoc J. 2016;10(3-4):90-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumann BC, Sargos P, Eapen LJ, et al. The rationale for post-operative radiation in localized bladder cancer. Bladder Cancer. 2017;3(1):19-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaghloul MS. Adjuvant and neoadjuvant radiotherapy for bladder cancer: revisited. Future Oncol. 2010;6(7):1177-1191. [DOI] [PubMed] [Google Scholar]
- 21.Ku JH, Kim M, Jeong CW, Kwak C, Kim HH. Risk prediction models of locoregional failure after radical cystectomy for urothelial carcinoma: external validation in a cohort of Korean patients. Int J Radiat Oncol Biol Phys. 2014;89(5):1032-1037. [DOI] [PubMed] [Google Scholar]
- 22.Novotny V, Froehner M, May M, et al. Risk stratification for locoregional recurrence after radical cystectomy for urothelial carcinoma of the bladder. World J Urol. 2015;33(11):1753-1761. [DOI] [PubMed] [Google Scholar]
- 23.Baumann BC, He J, Hwang WT, et al. Validating a local failure risk stratification for use in prospective studies of adjuvant radiation therapy for bladder cancer. Int J Radiat Oncol Biol Phys. 2016;95(2):703-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy AV, Pariser JJ, Pearce SM, et al. Patterns of failure after radical cystectomy for pT3-4 bladder cancer: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2016;94(5):1031-1039. [DOI] [PubMed] [Google Scholar]
- 25.Baumann BC, Noa K, Wileyto EP, et al. Adjuvant radiation therapy for bladder cancer: a dosimetric comparison of techniques. Med Dosim. 2015;40(4):372-377. [DOI] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology NCCN Guidelines: bladder cancer. Version 2. http://www.nccn.org. Published 2016. Accessed October 1, 2016.
- 27.Froehner M, Novotny V, Wirth MP, Brookman-May S, Aziz A, May M. External validation of a model to predict locoregional failure after radical cystectomy. Cancer. 2014;120(22):3584. [DOI] [PubMed] [Google Scholar]
- 28.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology NCCN Guidelines: bladder cancer. Version 2. http://www.nccn.org. Published 2017. Accessed April 1, 2017.
- 29.James ND, Hussain SA, Hall E, et al. ; BC2001 Investigators . Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366(16):1477-1488. [DOI] [PubMed] [Google Scholar]
- 30.Darby S, McGale P, Correa C, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGale P, Taylor C, Correa C, et al. ; EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) . Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. [published correction appears in Lancet. 2014;384(9957):1848]. Lancet. 2014;383(9935):2127-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Gijn W, Marijnen CA, Nagtegaal ID, et al. ; Dutch Colorectal Cancer Group . Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12(6):575-582. [DOI] [PubMed] [Google Scholar]
- 33.Kunos C, Simpkins F, Gibbons H, Tian C, Homesley H. Radiation therapy compared with pelvic node resection for node-positive vulvar cancer: a randomized controlled trial. Obstet Gynecol. 2009;114(3):537-546. [DOI] [PubMed] [Google Scholar]
- 34.Christodouleas JP, Hwang WT, Baumann BC. Adjuvant radiation for locally advanced bladder cancer? a question worth asking. Int J Radiat Oncol Biol Phys. 2016;94(5):1040-1042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Schematic of the Clinical Trial Design
eFigure 2. Local-Regional Recurrence Free Survival for Chemo+RT vs Chemotherapy Alone for Patients With Urothelial Carcinoma
eTable 1. Univariate Analysis of Factors Predictive of Local Recurrence-Free Survival (LRFS)
eTable 2. Univariate Analysis of Factors Predictive of Disease-Free Survival (DFS)
eTable 3. Univariate Analysis of Factors Predictive of Distant Metastasis-Free Survival (DMFS)
eTable 4. Univariate Analysis of Factors Predictive of Overall Survival (OS)
eTable 5. Summary Comparison of LRFS, DFS, DMFS, and OS Between the Two Arms (Chemotherapy plus RT vs Chemotherapy Alone)