Abstract
Importance
Patterns-of-failure studies suggest that in metastatic non–small-cell lung cancer (NSCLC) sites of gross disease at presentation are the first to progress when treated with chemotherapy. This knowledge has led to increased adoption of local ablative radiation therapy in patients with stage IV NSCLC, though prospective randomized evidence is limited.
Objective
To determine if intervening with noninvasive stereotactic ablative radiotherapy (SAbR) prior to maintenance chemotherapy in patients with non–progressive limited metastatic NSCLC after induction therapy led to significant improvements in progression-free survival (PFS).
Design, Setting, and Participants
This is a single-institution randomized phase 2 study of maintenance chemotherapy alone vs SAbR followed by maintenance chemotherapy for patients with limited metastatic NSCLC (primary plus up to 5 metastatic sites) whose tumors did not possess EGFR-targetable or ALK-targetable mutations but did achieve a partial response or stable disease after induction chemotherapy.
Interventions
Maintenance chemotherapy or SAbR to all sites of gross disease (including SAbR or hypofractionated radiation to the primary) followed by maintenance chemotherapy.
Main Outcomes and Measures
The primary end point was PFS; secondary end points included toxic effects, local and distant tumor control, patterns of failure, and overall survival.
Results
A total of 29 patients (9 women and 20 men) were enrolled; 14 patients (median [range] age, 63.5 [51.0-78.0] years) were allocated to the SAbR-plus-maintenance chemotherapy arm, and 15 patients (median [range] age, 70.0 [51.0-79.0] years) were allocated to the maintenance chemotherapy–alone arm. The trial was stopped to accrual early after an interim analysis found a significant improvement in PFS in the SAbR-plus-maintenance chemotherapy arm of 9.7 months vs 3.5 months in the maintenance chemotherapy–alone arm (P = .01). Toxic effects were similar in both arms. There were no in-field failures with fewer overall recurrences in the SAbR arm while those patients receiving maintenance therapy alone had progression at existing sites of disease and distantly.
Conclusions and Relevance
Consolidative SAbR prior to maintenance chemotherapy appeared beneficial, nearly tripling PFS in patients with limited metastatic NSCLC compared with maintenance chemotherapy alone, with no difference in toxic effects. The irradiation prevented local failures in original disease, the most likely sites of first recurrence. Furthermore, PFS for patients with limited metastatic disease appeared similar to those patients with a greater metastatic burden, further arguing for the potential benefits of local therapy in limited metastatic settings.
Trial Registration
clinicaltrials.gov Identifier: NCT02045446
This phase 2 randomized clinical trial examines whether intervening with noninvasive stereotactic ablative radiotherapy prior to maintenance chemotherapy in patients with nonprogressive limited metastatic non–small-cell lung cancer after induction therapy led to significant improvements in progression-free survival.
Key Points
Question
Does consolidative radiotherapy (primarily stereotactic ablative radiotherapy) increase progression-free survival in patients with limited metastatic non–small-cell lung cancer (NSCLC)?
Findings
This single-institution randomized phase 2 trial found a statistically significant improvement in progression-free survival from 3.5 to 9.7 months with the addition of consolidative radiotherapy to maintenance chemotherapy for patients with limited metastatic NSCLC.
Meaning
Based on the findings of this study among others, the use of consolidative radiation therapy after induction systemic therapy is being evaluated in the phase 3 setting with overall survival as primary end point for patients with limited metastatic NSCLC.
Introduction
Approximately 60% of patients with non–small-cell lung cancer (NSCLC) present with stage IV disease, but following standard first-line chemotherapy, the introduction of maintenance chemotherapy has led to limited gains in progression-free survival (PFS) and overall survival (OS). Progression in advanced disease most frequently occurs in original sites of gross disease, and by intervening with stereotactic ablative radiotherapy (SAbR) for localized disease, there is the potential for improvement in PFS and OS compared with maintenance chemotherapy alone.
A phase 2 single-arm prospective study demonstrated improved PFS and OS in patients with metastatic NSCLC with oligoprogression in the second-line (and beyond) setting who had been treated with SAbR plus erlotinib when compared with historical rates of survival from systemic therapy only. Though in the salvage setting, the addition of SAbR shifted failures from original sites of gross disease to new distant sites, albeit after a long disease-free interval without an increase in toxic effects. In another single-arm phase 2 study that added hypofractionated and SAbR-like radiation up front to primary disease and metastases in the oligometastatic NSCLC setting with induction chemotherapy, median OS was approximately 23 months. Multiple meta-analyses, single-institution observational studies, retrospective experiences, and limited single-arm prospective trials suggest that local therapy in the limited metastatic disease state for NSCLC may augment survival. Finally, a recent phase 2 randomized study including patients with limited metastatic NSCLC, with or without targetable mutation positive disease (EGFR and/or ALK), directed patients toward receiving local therapy (hypofractionated radiation, SAbR, chemoradiation, surgery) with or without maintenance chemotherapy or observation vs maintenance chemotherapy or observation alone.
Stereotactic ablative radiotherapy, unlike surgical metastectomy, is noninvasive with limited toxic effects, allowing rapid reinitiation of NSCLC chemotherapy. An increasing number of sites in the body are amenable to SAbR’s 5 or fewer treatments. Recent global surveys revealed that SAbR is being used off protocol for many patients with metastatic NSCLC for consolidation or salvage despite an absence of higher-level data. Based on previous experiences, we sought to conduct a randomized phase 2 study to determine if consolidative radiation—primarily SAbR—could work in concert with maintenance chemotherapy to improve PFS over maintenance chemotherapy alone for patients with limited metastatic NSCLC.
Methods
Study Design
This single-institution study was a 2-arm randomized phase 2 trial comparing SAbR-plus-maintenance chemotherapy with maintenance chemotherapy alone in the setting of limited metastatic NSCLC. The study was approved by the University of Texas Southwestern Medical Center’s institutional review board. Written informed consent was obtained for every study participant. Patients were assessed within 21 to 42 days following completion of first-line chemotherapy with repeat diagnostics including computed tomography (CT) and/or positron emission tomography (PET)-CT. Patients with limited metastatic disease amenable to SAbR were randomized via permuted block to either maintenance chemotherapy alone or SAbR followed by maintenance chemotherapy. The trial protocol is available in Supplement 1.
Patients
Patients were eligible if they were 18 years or older, had a Karnofsky Performance Status score of 70 or better, and had biopsy-proven metastatic NSCLC. Patients must have received 4 to 6 cycles of first-line platinum-based chemotherapy, achieving stable disease or a partial response on imaging by RECIST (Response Evaluation Criteria In Solid Tumors). Those receiving first-line targeted therapy for EGFR-positive and/or ALK-positive NSCLC were excluded. Patients were allowed to have up to 6 sites of extracranial disease (including primary) with no more than 3 sites in the liver or lung identified by diagnostic CT, PET-CT, or magnetic resonance imaging prior to enrollment. Individuals were ineligible if previously irradiated primary disease progressed within 3 months of that treatment. Patients with untreated and/or uncontrolled brain metastases or disease involving the gastrointestinal tract and skin were ineligible.
Study Medications
Choices of first-line and maintenance chemotherapy were determined by the medical oncology team. Maintenance chemotherapy included erlotinib, pemetrexed, docetaxel, gemcitabine, or bevacizumab. Maintenance chemotherapy was given until disease progression, intolerable toxic effects, or death and was initiated within 1 week of randomization to the maintenance-only arm and within 1 week after all radiation on the consolidative arm unless otherwise indicated.
Radiation Technique
All primary sites of disease and identified metastases were treated with external beam radiation. Stereotactic ablative radiotherapy began within 1 to 2 weeks after randomization to the radiation arm. Planning CT simulation, immobilization, and target volume delineation have been previously described. Patients were simulated with CT guidance using 2- to 3-mm slice thicknesses. Fluoroscopy was used to assess extent of tumor motion with validated techniques permitted for motion control. For thoracic and/or upper abdominal lesions, 4-dimensional CT data were used to create internal target volumes. For each treated site, a gross tumor volume and/or internal target volume was created with appropriate expansion up to 5 mm for creation of a planning target volume. For hypofractionated treatment of lung primary, an internal target volume was expanded 5 mm for both clinical target volume and sequential planning target volume expansion.
Accepted single-fraction cumulative SAbR doses included 21 to 27 Gy (with minor acceptable deviation of ≥16 Gy but <21 Gy per fraction [to convert to rad, multiply by 100]). Accepted 3-fraction cumulative SAbR doses included 26.5 to 33.0 Gy (with minor acceptable deviation of ≥24.5 Gy but <26.5 Gy). Accepted 5-fraction cumulative SAbR doses included 30.0 to 37.5 Gy (with minor acceptable deviation of ≥28 Gy but <30 Gy). If the treating radiation oncologist believed that the SAbR fractionation schemes for primary disease did not allow normal tissue constraints to be met, an alternative acceptable prescription was 45 Gy in 15 fractions. All permissible treatment schemas had biologically similar tumor doses per the Universal Survival Model (α = 0.33 Gy-1; Do = 1.25 Gy; Dq = 1.8 Gy). The Universal Survival Model weds concepts in radiation biology to account for the ablative effects of stereotactic radiation in predicting for tumor-kill characteristics. If there were multiple lesions in close proximity, effort was made to treat them with the same dose and fractionation; if that was not possible, all were converted to similar dose fractionations using the Universal Survival Model and previously reported cumulative normal tissue constraints were used. In the situation in which 2 lesions in close proximity were treated separately, they were treated on separate days to allow interfraction normal tissue repair. Standard dosimetric radiation quality assurance was performed on all cases by the principal investigator (P.I.). Stereotactic ablative radiotherapy treatments were delivered with at least 18 hours between each fraction with no more than 2 sites treated per day.
Follow-Up
All patients were evaluated for study end points with follow-up at 2-to-3-month intervals with history and physical examination, imaging including CT of the chest and/or abdomen, and laboratory testing including complete blood cell counts and/or comprehensive metabolic panels as needed. All evaluations of disease response used RECIST (v1.1) criteria. Time to the development of new lesions, progression of existing lesions, or death, whichever came first, represented the primary end point of PFS; OS was defined as time to death from any cause.
Local failure (in-field and marginal) was defined as progressive consolidation on CT within 1 cm of the SAbR treatment site and not consistent with benign radiation-induced changes (also known as progressive enlargement as defined in Radiation Therapy Oncology Group [RTOG] SAbR trials). As per RTOG guidelines, if such changes were not diagnostic of tumor recurrence, PET-CT or directed biopsy was required to determine local failure. In cases where local failure was determined by serial imaging alone, failure was scored from the first radiographic appearance of the abnormality.
Statistical Analysis
The primary end point of the trial was PFS. Secondary end points included in-field local control and out-of-field disease progression, safety, and OS. The sample size was calculated with the 2-sided significance level of 0.1 and 80% statistical power using a 2-sample log-rank test. We predicted the patients randomly assigned to the SAbR-plus-maintenance chemotherapy arm and maintenance chemotherapy–alone arm to have median PFS of 10 months and 4 months, respectively, which translated to a hazard ratio of 0.400. Estimates for PFS were based on data from randomized phase 3 trials, meta-analyses, retrospective experiences, and single-arm prospective trials. The total sample size of 36 patients, 18 per arm, was estimated to achieve the desired statistical power. Progression-free survival was estimated using the Kaplan-Meier method from time of treatment start to progression or death, and OS was calculated in the same manner as PFS. The log-rank test was used to test for difference in PFS between the 2 treatment arms. The Cox proportional hazard model was used to determine hazard ratios and confidence intervals. All testing was performed at the .05 significance level.
All safety measures were reported using descriptive statistics (mean, median, SD, proportions, and 95% CIs). There was an early stopping rule for unexpected toxic effects. If at any point during the study more than one-sixth of patients treated to date experienced treatment-related grade 4 to 5 toxic effects of any kind study enrollment would be suspended. As this study was halted as the result of an unplanned interim analysis, repeated significance testing of continuous sequential boundaries was performed.
Results
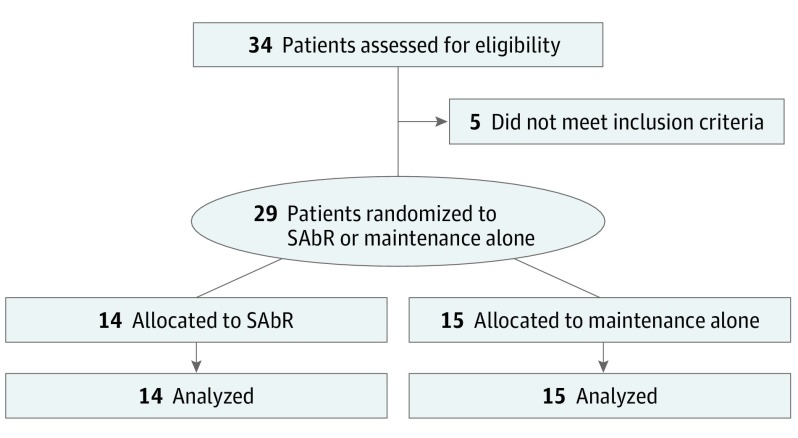
After a parallel trial evaluating local therapy for limited metastatic NSCLC closed early to accrual owing to a significant benefit for the consolidative local therapy arm, our institutional Data Safety and Monitoring Committee (DSMC) recommended we conduct an unplanned interim analysis, at which time more than 80% of target patient accrual had been achieved (29 of 36 patients). On completion and presentation of our interim analysis, a DSMC recommendation was made to close the trial early to further accrual because the PFS improvement would not have changed significance statistically if the final patients were accrued. Twenty-nine patients were enrolled on the study between April 2014 and July 2016. Median (range) follow-up was 9.6 (2.4-30.2) months. Comprehensive demographics and trial schema are included in Table 1 and Figure 1. A total of 11 patients, 6 from the SAbR arm and 5 from the maintenance chemotherapy–alone arm, had brain metastases treated prior to enrollment (eTable 1 in Supplement 2).
Table 1. Comprehensive Demographics of 29 Patients.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| SAbR Plus Maintenance | Maintenance Only | ||
| Sex | .70 | ||
| Male | 9 (64.3) | 11 (73.3) | |
| Female | 5 (35.7) | 4 (26.7) | |
| Median (range) age, y | 63.5 (51.0-78.0) | 70.0 (51.0-79.0) | .13 |
| Histology | .61 | ||
| Squamous | 1 (7.1) | 3 (20.0) | |
| Nonsquamous | 13 (92.9) | 12 (80.0) | |
| Sites of disease prior to induction chemotherapy, median No. (range) | 3 (2-6) | 2 (2-5) | .58 |
| Previously treated brain metastases | .61 | ||
| Yes | 6 (42.9) | 5 (33.3) | |
| No | 8 (57.1) | 10 (66.7) | |
| Induction chemotherapy, median cycles (range) | 4.5 (4-6) | 4 (4-6) | .31 |
Abbreviation: SAbR, stereotactic ablative radiotherapy.
Figure 1. CONSORT Diagram.
SAbR indicates stereotactic ablative radiotherapy.
The most commonly used induction chemotherapy regimen was carboplatin plus pemetrexed (48%) (eTable 1 in Supplement 2). The most common maintenance chemotherapy was pemetrexed monotherapy (62%). Every patient had all residual disease sites treated with SAbR as intended on that arm of the trial. A total of 31 lesions were treated in 14 patients. Intrathoracic sites were the most common locations of treatment for SAbR (Table 2). There were no unacceptable deviations from protocol with regard to radiation therapy delivery and no grade 5 toxic effects attributable to either arm of the trial. There were 2 instances of grade 3 toxic effects and 1 instance of grade 4 toxic effects probably or definitely attributable to treatment on the maintenance alone arm and 4 grade 3 toxicities of similar attribution on the SAbR-plus-maintenance arm (eTable 2 in Supplement 2). The median (range) number of maintenance chemotherapy cycles was 3 (1-15) for the maintenance-only group and 5 (3-19) for the SAbR group; individual patient treatment information is included in Tables 2 and 3. There were no delays in the start of maintenance chemotherapy in the SAbR arm owing to local therapy.
Table 2. Residual Sites of Disease, Radiation Details, and Chemotherapy Details for All Patients in the Trial.
| Patient Identification No. | Treatment Site | Dose (rad) | Fractions | Maintenance | Cycles, No. | Progressiona | Local Failure |
|---|---|---|---|---|---|---|---|
| 1 | Right upper lobe | 2000 | 1 | Docetaxel | 11 | Yes | No |
| Right hilum | 3000 | 5 | |||||
| Right adrenal | 3000 | 5 | |||||
| 2 | Right adrenal | 3000 | 5 | Bevacizumab | 4 | Yes | No |
| Left adrenal | 3000 | 5 | |||||
| 3 | Left lung | 2000 | 1 | Gemcitabine | 4 | No | No |
| Left lung | 3300 | 3 | |||||
| 4 | Right lung, overlapping sites, plus mediastinum | 4500 | 15 | Erlotinib | 6 | Nob | No |
| Nasopharynx | 3750 | 5 | |||||
| 5 | Left lower lobe | 2000 | 1 | Pemetrexed | 4 | Nob | No |
| Mediastinum | 4500 | 15 | |||||
| 6 | Mediastinum | 4500 | 15 | Bevacizumab | 19 | Yes | No |
| Right upper lobe | 2000 | 1 | |||||
| Right axilla | 3000 | 5 | |||||
| 7 | Liver | 3300 | 3 | Pemetrexed | 9 | No | No |
| Left lung | 4500 | 15 | |||||
| 8 | Liver | 3300 | 3 | Pemetrexed | 11 | No | No |
| Left lung | 4500 | 15 | |||||
| 9 | Left upper lobe | 1800 | 1 | Pemetrexed | 4 | Nob | No |
| Mediastinum | 4500 | 15 | |||||
| 10 | Right lower lobe | 4500 | 15 | Bevacizumab | 6 | Yes | No |
| T1 | 2400 | 1 | |||||
| T10 | 2460c | 3 | |||||
| Left axilla | 2400 | 1 | |||||
| 11 | Right upper lobe | 2100 | 1 | Pemetrexed | 6 | No | No |
| Right middle lobe | 2100 | 1 | |||||
| Right lower lobe | 2100 | 1 | |||||
| 12 | Right upper lobe | 2100 | 1 | Pemetrexed | 8 | No | No |
| Mediastinum | 3000 | 5 | |||||
| 13 | Right upper lobe plus mediastinum | 4500 | 15 | Pemetrexed | 4 | No | No |
| 14 | Right upper lobe | 2000 | 1 | Pemetrexed | 3 | No | No |
| 15 | Right upper lobe | NR | Pemetrexed | 3 | No | No | |
| 16 | Right upper lobe | NR | Bevazicumab | 6 | Yes | Yesd | |
| Mediastinum | |||||||
| 17 | Left upper lobe | NR | Pemetrexed | 6 | Yes | Yes | |
| Left hilum | |||||||
| 18 | Right upper lobe | NR | Gemcitabine | 3 | Yes | Yes | |
| 19 | Right upper lobe | NR | Pemetrexed | 15 | Yes | No | |
| 20 | Left lung | NR | Pemetrexed | 9 | No | No | |
| Mediastinum | |||||||
| 21 | Left upper lobe | NR | Pemetrexed | 2 | Yes | No | |
| Left hilum | |||||||
| Mediastinum | |||||||
| 22 | Right lower lobe | NR | Gemcitabine | 3 | Yes | Yesd | |
| Mediastinum | |||||||
| 23 | Right lower lobe | NR | Pemetrexed | 8 | Yes | Yes | |
| Lingula | |||||||
| Left lower lobe | |||||||
| 24 | Right lower lobe | NR | Pemetrexed | 7 | Yes | No | |
| 25 | Right upper lobe | NR | Docetaxel | 4 | No | No | |
| Right hilum | |||||||
| Left lower lobe | |||||||
| 26 | Right lower lobe | NR | Pemetrexed | 2 | Yes | Yesd | |
| Mediastinum | |||||||
| 27 | Right Upper Lobe | NR | Pemetrexed | 2 | Yes | Yes | |
| Mediastinum | |||||||
| Left lower lobe | |||||||
| 28 | Left upper lobe | NR | Pemetrexed | 1 | Nob | No | |
| Left hilum | |||||||
| Rib | |||||||
| 29 | Right upper lobe | NR | Pemetrexed | 1 | No | No | |
| Right axilla | |||||||
Abbreviations: NR, no radiation; SAbR, stereotactic ablative radiotherapy.
Four patients had progression during treatment, but none within the radiated field for the SAbR-plus-maintenance chemotherapy arm while 10 patients had progression in the maintenance chemotherapy–alone arm, many at sites of residual gross disease after chemotherapy.
Died with no evidence of progression.
Due to normal tissue constraints, the treating physician chose to use 3 fractions with the lowest variation acceptable dose.
Evidence of local and distant failure.
Table 3. Patterns of Failure by Treatment Assignmenta.
| Site of Progression | SAbR Plus Maintenance, No. | Maintenance, No. |
|---|---|---|
| Brain | 1 | 4 |
| Liver | 2 | 0 |
| Lung | 0 | 8 |
| Bone | 1 | 1 |
| Pancreas | 1 | 0 |
| In-field | 0 | 7 |
Abbreviation: SAbR, stereotactic ablative radiotherapy.
Patients treated with SAbR had no failure within the treated field.
At the time of analysis, 10 of 15 patients had progression in the maintenance chemotherapy–alone arm. Of those 10 patients, 7 had progression at the original sites of gross tumor (Table 2). In the SAbR arm, 4 of 14 had progression, but none of who were within the radiated field (Table 2). Patterns of failure are described in Table 3, demonstrating no local radiation failures compared with 7 local failures in the maintenance chemotherapy–alone arm at sites of original gross disease.
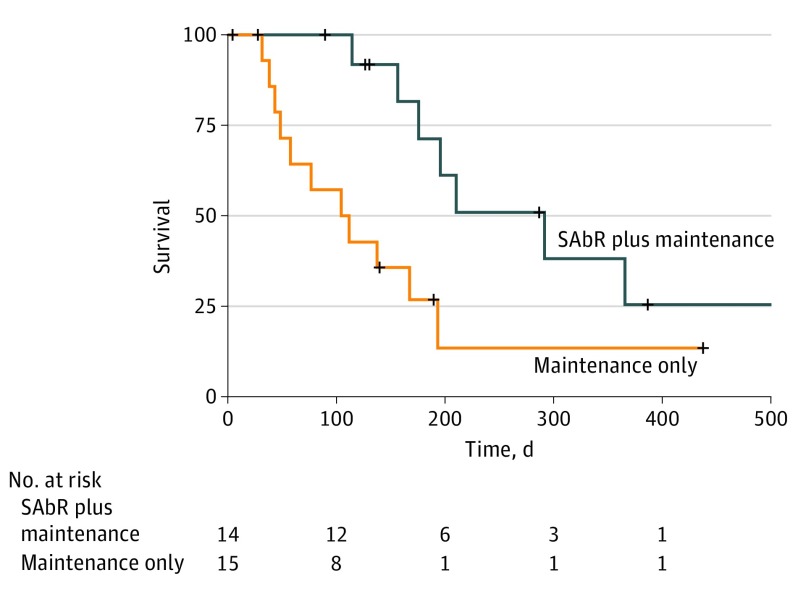
Median PFS was 3.5 months for the maintenance chemotherapy–alone arm and 9.7 months for the SAbR-plus-maintenance chemotherapy arm (hazard ratio, 0.304; 95% CI, 0.113-0.815; P = .01) (Figure 2). Accounting for the unplanned interim analysis, the nominal significance level was greater than 0.01 which still yielded a statistically significant result. A total of 2 patients (13%) in the maintenance chemotherapy–alone arm crossed over to SAbR at oligoprogression. For the majority of patients who did not crossover to receive SAbR on this arm, OS was nearly 1 year. With crossover, the median OS in the maintenance chemotherapy–alone arm was 17 months. Median OS was not reached for the SAbR-plus-maintenance chemotherapy arm, though the study was not powered to show a statistical difference in survival. When PFS and OS were analyzed by presence (vs absence) of treated brain metastases prior to enrollment or number of extracranial metastatic lesions at enrollment (≤2 vs >2), no statistical difference in survival was noted between groups (eTable 3 in Supplement 2).
Figure 2. Analysis of Progression-Free Survival.
Log-rank testing reveals a statistically significant benefit in progression-free survival for SAbR-plus-maintenance chemotherapy (hazard ratio, 0.304; 95% CI, 0.113-0.815; P = .01). SAbR indicates stereotactic ablative radiotherapy.
Discussion
Limited metastatic disease has been described as an intermediate state between locally advanced and widely metastatic disease, which may offer a window for the use of local therapies. The use of local therapy at oligometastatic sites has increased survival in patients with colorectal cancer and sarcoma. However, NSCLC may have a narrower therapeutic window given that 45% of patients with locally advanced disease develop metastases within 2 years, and patients with metastatic disease have an OS of less than 1 year when treated with cytotoxic regimens. Until recently, only retrospective series and limited nonrandomized institutional experiences have suggested benefit from SAbR for patients with limited metastatic NSCLC. Single-arm phase 2 studies incorporating surgery or radiotherapy then showed the benefits of local therapy for synchronous metastatic NSCLC. In salvage or oligoprogressive NSCLC settings, SAbR appeared to change failure patterns from local to distant sites.
We initiated this randomized phase 2 study to determine the benefit of adding SAbR to standard maintenance chemotherapy in improving PFS in patients with limited metastatic NSCLC. We felt it important to add SAbR in consolidation rather than at oligoprogression primarily to follow the tenets of the Norton-Simon hypothesis. By intervening as early as possible, we hoped to identify patients with limited metastatic disease who would benefit from local therapy that would not delay maintenance therapy but prevent, delay, or shift failures anatomically.
An unplanned interim analysis recommended by our institutional DSMC before completion of accrual was conducted because a similar study being performed at other institutions in parallel to our effort showed a tripling in PFS with addition of local therapy (radiation and/or surgery) also in a population of patients with limited metastatic NSCLC. In our interim analysis, performed after more than 80% accrual, we also identified a near tripling of PFS in favor of the SAbR arm. The DSMC felt that closure of the study was appropriate given the significant benefit noted across multiple studies and statistical likelihood that adding the remaining patients would not have changed the study outcomes, thereby informing our actions.
Large trials using standard maintenance chemotherapy after first-line chemotherapy for the broad population of patients with metastatic NSCLC provides a PFS between 2 and 4 months with a median OS of 6 to 9 months. With the addition of SAbR to maintenance chemotherapy for patients with limited metastatic NSCLC, the median PFS increased from 3.5 months to 9.7 months. Notably, the PFS for patients with limited metastatic disease in this trial is similar to PFS reported from trials of maintenance chemotherapy for patients with greater burdens of metastatic disease. Nearly all patients who received maintenance chemotherapy alone had progression at known sites of original disease, whereas SAbR prevented any failures in original sites of gross disease. The clear and dramatic shift in patterns of failure suggests that SAbR effectively augments systemic therapy in controlling gross disease, shifting subsequent focus to prevention and control of distant sites of disease. This protection against local recurrence appears to have promoted the longer PFS periods and may ultimately be shown to increase OS. A more ideal companion systemic therapy, tailored by predictive assays, that reduces distant recurrence rates with greater efficiency may work even more synergistically with local radiation to improve survival.
Our study is unique in a number of ways when compared with similar, recently published findings: (1) only radiation, primarily SAbR, was used as local therapy with hypofractionation for primary disease as needed, with no patients undergoing surgery; (2) all disease in patients on the consolidative local therapy arm of our study were treated with similar biological doses of radiation, permitting comparisons of local control parameters; (3) there were no patients included with tumors with targetable mutations; (4) local therapy and maintenance chemotherapy were delivered sequentially for the entire cohort; and (5) all patients received some maintenance therapy. These characteristics supported a uniformity and consistency of our patient’s disease and their respective treatments on study.
Limitations
While we have shown a statistically significant increase in PFS with the addition of SAbR to standard maintenance chemotherapy, a statistically significant OS benefit was not immediately obvious. This may reflect both the design of the trial, which was not powered to detect an OS difference, as well as the administration of crossover SAbR postprogression in 13% of patients in the maintenance chemotherapy–alone arm. There were limitations to our study including: (1) a single center of accrual; (2) the lack of inclusion of emerging systemic therapies such as checkpoint inhibitors; (3) the small sample size; and (4) use of multiple induction chemotherapy regimens introducing potential heterogeneity. Future use of immunotherapy with SAbR may offer greater synergy in controlling local disease while reducing distant disease through direct and abscopal effects.
Conclusions
This randomized phase 2 study measuring radiation’s consolidative contributions showed a near tripling of PFS with equivalent toxic effects and is suggestive of a benefit of SAbR for limited metastatic NSCLC. It is promising that a phase 3 study, based on this trial design, has been activated by NRG Oncology (NRG LU 002 [NCT03137771]) to answer the benefit of local therapy on OS. SARON, another NSCLC specific phase 3 randomized trial with similar arms (chemotherapy with or without local therapy) has opened in the United Kingdom as well (NCT02417662). Though an improvement of PFS was hypothesized and therefore expected, the more critical question will be if an extension of PFS will translate into OS increases. This study was not large enough to mine subsets of clinical features to identify predictors of survival benefit, another postulated advantage of the phase 3 trial. Several studies are currently enrolling in multiple disease sites which will further elucidate the role of local therapy for limited metastatic disease (NCT01446744, NCT02228356, NCT02581670, NCT02805530). Despite the approval of immunotherapy in the first-line setting, there will still be a large percentage of patients with metastatic NSCLC who will receive cytotoxic chemotherapy as part of their treatment. Therefore, the findings of this study will continue to be very relevant to patients with NSCLC.
Trial Protocol.
eTable 1. Analysis of Factors Predicting Benefit for Consolidation
eTable 2. Comprehensive Toxicity Assessment
eTable 3. Analysis of Factors Predicting Benefit for Consolidation
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. [DOI] [PubMed] [Google Scholar]
- 2.Socinski MA, Evans T, Gettinger S, et al. . Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5)(suppl):e341S-e368S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodowicz T, Ciuleanu T, Crawford J, et al. ; Central European Cooperative Oncology Group (CECOG) . Third CECOG consensus on the systemic treatment of non-small-cell lung cancer. Ann Oncol. 2012;23(5):1223-1229. [DOI] [PubMed] [Google Scholar]
- 4.Brodowicz T, Krzakowski M, Zwitter M, et al. ; Central European Cooperative Oncology Group CECOG . Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer. 2006;52(2):155-163. [DOI] [PubMed] [Google Scholar]
- 5.Paz-Ares LG, de Marinis F, Dediu M, et al. . PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31(23):2895-2902. [DOI] [PubMed] [Google Scholar]
- 6.Ramalingam S, Belani C. Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist. 2008;13(suppl 1):5-13. [DOI] [PubMed] [Google Scholar]
- 7.Behera M, Owonikoko TK, Chen Z, et al. . Single agent maintenance therapy for advanced stage non-small cell lung cancer: a meta-analysis. Lung Cancer. 2012;77(2):331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. ; SATURN investigators . Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11(6):521-529. [DOI] [PubMed] [Google Scholar]
- 9.Ciuleanu T, Brodowicz T, Zielinski C, et al. . Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374(9699):1432-1440. [DOI] [PubMed] [Google Scholar]
- 10.Fidias PM, Dakhil SR, Lyss AP, et al. . Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27(4):591-598. [DOI] [PubMed] [Google Scholar]
- 11.Gerber DE. Maintenance therapy for advanced lung cancer: who, what, and when? J Clin Oncol. 2013;31(24):2983-2990. [DOI] [PubMed] [Google Scholar]
- 12.Paz-Ares L, de Marinis F, Dediu M, et al. . Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13(3):247-255. [DOI] [PubMed] [Google Scholar]
- 13.Rusthoven KE, Hammerman SF, Kavanagh BD, Birtwhistle MJ, Stares M, Camidge DR. Is there a role for consolidative stereotactic body radiation therapy following first-line systemic therapy for metastatic lung cancer? A patterns-of-failure analysis. Acta Oncol. 2009;48(4):578-583. [DOI] [PubMed] [Google Scholar]
- 14.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8(6):378-382. [DOI] [PubMed] [Google Scholar]
- 15.Iyengar P, Kavanagh BD, Wardak Z, et al. . Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol. 2014;32(34):3824-3830. [DOI] [PubMed] [Google Scholar]
- 16.Collen C, Christian N, Schallier D, et al. . Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann Oncol. 2014;25(10):1954-1959. [DOI] [PubMed] [Google Scholar]
- 17.Ashworth AB, Senan S, Palma DA, et al. . An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer. 2014;15(5):346-355. [DOI] [PubMed] [Google Scholar]
- 18.Cheruvu P, Metcalfe SK, Metcalfe J, Chen Y, Okunieff P, Milano MT. Comparison of outcomes in patients with stage III versus limited stage IV non-small cell lung cancer. Radiat Oncol. 2011;6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber DE, Dahlberg SE, Sandler AB, et al. . Baseline tumour measurements predict survival in advanced non-small cell lung cancer. Br J Cancer. 2013;109(6):1476-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasselle MD, Haraf DJ, Rusthoven KE, et al. . Hypofractionated image-guided radiation therapy for patients with limited volume metastatic non-small cell lung cancer. J Thorac Oncol. 2012;7(2):376-381. [DOI] [PubMed] [Google Scholar]
- 21.Mehta N, Mauer AM, Hellman S, et al. . Analysis of further disease progression in metastatic non-small cell lung cancer: implications for locoregional treatment. Int J Oncol. 2004;25(6):1677-1683. [PubMed] [Google Scholar]
- 22.Milano MT, Katz AW, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83(3):878-886. [DOI] [PubMed] [Google Scholar]
- 23.Rusthoven KE, Kavanagh BD, Burri SH, et al. . Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27(10):1579-1584. [DOI] [PubMed] [Google Scholar]
- 24.Rusthoven KE, Kavanagh BD, Cardenes H, et al. . Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27(10):1572-1578. [DOI] [PubMed] [Google Scholar]
- 25.Salama JK, Hasselle MD, Chmura SJ, et al. . Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer. 2012;118(11):2962-2970. [DOI] [PubMed] [Google Scholar]
- 26.Xanthopoulos EP, Handorf E, Simone CB II, et al. . Definitive dose thoracic radiation therapy in oligometastatic non-small cell lung cancer: A hypothesis-generating study. Pract Radiat Oncol. 2015;5(4):e355-e363. [DOI] [PubMed] [Google Scholar]
- 27.De Ruysscher D, Wanders R, van Baardwijk A, et al. . Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol. 2012;7(10):1547-1555. [DOI] [PubMed] [Google Scholar]
- 28.Gomez DR, Blumenschein GR Jr, Lee JJ, et al. . Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(12):1672-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potters L, Kavanagh B, Galvin JM, et al. ; American Society for Therapeutic Radiology and Oncology; American College of Radiology . American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76(2):326-332. [DOI] [PubMed] [Google Scholar]
- 30.Timmerman R, Paulus R, Galvin J, et al. . Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer JJ, Foster RD, Lev-Cohain N, et al. . A phase I dose-escalation trial of single-fraction stereotactic radiation therapy for liver metastases. Ann Surg Oncol. 2016;23(1):218-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis SL, Porceddu S, Nakamura N, et al. . Definitive stereotactic body radiotherapy (sbrt) for extracranial oligometastases: an international survey of >1000 radiation oncologists. Am J Clin Oncol. 2015. [DOI] [PubMed] [Google Scholar]
- 33.Park C, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70(3):847-852. [DOI] [PubMed] [Google Scholar]
- 34.Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18(4):215-222. [DOI] [PubMed] [Google Scholar]
- 35.Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 36.Geller NL, Pocock SJ. Interim analyses in randomized clinical trials: ramifications and guidelines for practitioners. Biometrics. 1987;43(1):213-223. [PubMed] [Google Scholar]
- 37.Armitage P, McPherson CK, Rowe BC. Repeated significance tests on accumulating data. J Royal Stat Soc Series A (General). 1969;132(2):235-244. [Google Scholar]
- 38.McPherson CK, Armitage P. Repeated significance tests on accumulating data when the null hypothesis is not true. J Royal Stat Soc Series A (General). 1971;134(1):15-25. [Google Scholar]
- 39.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8-10. [DOI] [PubMed] [Google Scholar]
- 40.Choti MA, Sitzmann JV, Tiburi MF, et al. . Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235(6):759-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minagawa M, Makuuchi M, Torzilli G, et al. . Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231(4):487-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kager L, Zoubek A, Pötschger U, et al. ; Cooperative German-Austrian-Swiss Osteosarcoma Study Group . Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21(10):2011-2018. [DOI] [PubMed] [Google Scholar]
- 43.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pastorino U, Buyse M, Friedel G, et al. ; International Registry of Lung Metastases . Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113(1):37-49. [DOI] [PubMed] [Google Scholar]
- 45.Strong VE, D’Angelica M, Tang L, et al. . Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol. 2007;14(12):3392-3400. [DOI] [PubMed] [Google Scholar]
- 46.Bradley JD, Paulus R, Komaki R, et al. . Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136(1):260-271. [DOI] [PubMed] [Google Scholar]
- 48.Downey RJ, Ng KK, Kris MG, et al. . A phase II trial of chemotherapy and surgery for non-small cell lung cancer patients with a synchronous solitary metastasis. Lung Cancer. 2002;38(2):193-197. [DOI] [PubMed] [Google Scholar]
- 49.Norton L, Simon R. Tumor size, sensitivity to therapy, and design of treatment schedules. Cancer Treat Rep. 1977;61(7):1307-1317. [PubMed] [Google Scholar]
- 50.Postow MA, Callahan MK, Barker CA, et al. . Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaverdian N, Lisberg AE, Bornazyan K, et al. . Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eTable 1. Analysis of Factors Predicting Benefit for Consolidation
eTable 2. Comprehensive Toxicity Assessment
eTable 3. Analysis of Factors Predicting Benefit for Consolidation