Abstract
Importance
Nivolumab plus ipilimumab (nivo + ipi) is a standard treatment of advanced melanoma. Two randomized trials describe high objective response rates by Response Evaluation Criteria in Solid Tumors. The trials assessed toxic effects using the Common Terminology Criteria for Adverse Events (CTCAE), which may underestimate incidence of clinically significant immune-related adverse events (AEs).
Objective
To describe detailed toxic effects and time to treatment failure of patients with melanoma treated with nivo + ipi in a prospective cohort.
Design, Setting, and Participants
A cohort of 64 adults with advanced or unresectable melanoma were examined at a single tertiary cancer and enrolled in an expanded access program of nivo + ipi conducted from December 2014 to January 2016.
Interventions
Intravenous nivolumab (1 mg/kg) and ipilimumab (3 mg/kg) administered every 3 weeks for up to 4 doses, followed by nivolumab (3 mg/kg) every 2 weeks or pembrolizumab (2 mg/kg) every 3 weeks until unacceptable toxic effects, disease progression, or complete response.
Main Outcomes and Measures
Clinically significant immune-related AEs were defined as CTCAE grade 2 or higher or any immune-related AEs requiring systemic steroids. Time to treatment failure was defined as the interval between initiating therapy and the earliest of clinical progression, new locally directed or systemic treatment other than anti–programmed cell death 1 protein (anti–PD-1) monotherapy, or death.
Results
Overall 64 adults with advanced or unresectable melanoma were enrolled (male to female ratio, 1:1; median [range] age, 56 [22-82] years); 25 patients (39%) received all 4 doses of nivo + ipi, and 31 patients (48%) received no maintenance anti–PD-1 therapy. Most who discontinued treatment (n = 31 [80%]) stopped because of toxic effects. Among those patients who were progression free at 12 weeks, time to treatment failure was similar between those who did or did not modify therapy for toxic effects. Fifty-eight patients (91%) had a clinically significant immune-related AE (median, 2/patient), and 46 patients (72%) required systemic steroids. Infliximab or mycophenolate was required in 16 patients (25%) for steroid-refractory immune-related AEs. Seven patients (11%) developed hyperglycemia, 32 patients (50%) had an emergency department visit, and 23 patients (36%) required a hospital admission related to an immune-related AE. Four of 31 patients (13%) who stopped combination therapy early for toxic effects developed a new, clinically significant immune-related AE more than 16 weeks after the last treatment.
Conclusions and Relevance
We observed a 91% incidence of clinically significant immune-related AEs leading to frequent emergency department visits, hospitalizations, and systemic immunosuppression. Immuno-oncology trials should routinely report these metrics. Most patients do not tolerate 4 doses of nivo + ipi; however, 4 doses may not be required for clinical benefit.
This study addressed toxic effects and time to treatment failure in patients with melanoma treated with nivolumab plus ipilimumab.
Key Points
Question
Do toxic effects of nivolumab plus ipilimumab impact efficacy in patients with melanoma?
Findings
In this single-center cohort of 64 patients with melanoma in an expanded access program of nivolumab plus ipilimumab, nearly three-fourths of patients required steroids, and over one-third of patients were hospitalized for an immune-related adverse event, some of which occurred months after discontinuation of therapy. Modifying therapy for toxic effects did not appear to impact efficacy.
Meaning
In this study, patients did not tolerate 4 doses, but fewer than 4 doses may provide clinical benefit.
Introduction
The combination of nivolumab and ipilimumab (nivo + ipi) has demonstrated impressive clinical efficacy in patients with advanced melanoma. In a phase 1 trial, the objective response rate for patients receiving the maximally tolerated dose was 53%; grade 3 to 4 adverse event rate was 53%. These results were confirmed in 2 randomized clinical trials with similar reported rates of grade 3 to 4 AEs. Discontinuation rates for toxic effects were 36% to 47% and objective response rates for these patients were similar to that of the overall cohort. However, in these trials, toxic effect data were only collected for 12 to 16 weeks after treatment discontinuation and were graded by the Common Terminology Criteria for Adverse Events (CTCAE), a system not designed for immunotherapy. Data regarding the use of steroids and/or additional steroid-sparing immunosuppressive agents were incomplete. Here we present a novel detailed report of immune-related adverse events (AEs) from nivo + ipi. We also propose time to treatment failure (TTF) as an efficacy metric for this therapeutic approach.
Methods
Patients
This nivo + ipi Expanded Access Program (CheckMate 218) was sponsored by Bristol-Myers Squibb and approved by the Memorial Sloan Kettering Cancer Center institutional review board. Patients age 18 years and older with advanced or unresectable melanoma were eligible; no prior checkpoint blockade was permitted. Written informed consent was obtained for all patients.
Procedures
Nivolumab (1 mg/kg) and ipilimumab (3 mg/kg) were administered every 3 weeks for 4 doses followed by either nivolumab (3 mg/kg) every 2 weeks or off-protocol pembrolizumab (2 mg/kg) every 3 weeks until unacceptable toxic effects, disease progression, or complete response.
Outcomes
Patients were followed for TTF, defined as the interval between first nivo + ipi infusion to the earliest date of clinical progression, new locally directed treatment, new non–PD-1 (programmed cell death protein 1)-based systemic treatment, or death. Adverse events were graded using CTCAE v4.0. Clinically significant immune-related AEs were defined as grade 2 or higher or grade 1 events requiring systemic steroids. Treatment modification was defined as receiving fewer than 4 nivo + ipi doses or omitting PD-1 monotherapy due to immune-related AEs.
Statistical Analysis
The TTF was estimated using Kaplan-Meier approaches. The comparison of TTF between those who did and did not undergo treatment modification for toxic effects was landmarked at 12 weeks. Adverse events, steroid and other immunosuppressive medication administration, and emergency department visits and hospital admissions were summarized using descriptive statistics.
Results
Patient Characteristics
Sixty-four patients were enrolled and treated. The median age at start of treatment was 56 (range 22-82). The male to female ratio was 1:1. The majority of patients (n = 47 [73%]) had cutaneous melanoma and all had an Eastern Cooperative Oncology Group performance status of 0 or 1. Half of patients had M1c disease (metastases to all non–lung visceral sites or distant metastases to any site combined with an elevated serum lactate dehydrogenase [LDH] level); 20 patients (31%) had elevated LDH levels.
Treatment and Toxic Effects
Minimum follow-up after the last nivo + ipi treatment among living patients was 6.7 months. Twenty-five patients (39%) received 4 doses of nivo + ipi; 18 patients went on to receive maintenance nivolumab. Of the remaining 39 patients, 11 (17%) received 3 doses, 20 (31%) received 2 doses, and 8 (13%) received 1 dose. Fewer than 4 doses were given because of toxic effects (n = 31 [80%]), progression (n = 7 [11%]), or death due another cause (n = 1 [2%]). Overall, 31 patients (48%) received no maintenance anti–PD-1 therapy. Among the patients who received fewer than 4 nivo + ipi doses due to toxic effects, 17 (54%) received anti–PD-1 monotherapy (median [range] doses, 4 [1-16]).
Fifty-eight of 64 patients (91%) had 1 or more clinically significant immune-related AE (median [range], 2 [0-7]); 38 of 64 patients (59%) had a grade 3 to 4 immune-related AE. The most common grade 3 to 4 immune-related AEs included diarrhea (44%) and endocrinopathies (42%) (Table). Notably, 11% developed hyperglycemia. Neurologic events included autoimmune meningitis (n = 2) and myasthenia gravis (n = 1). Four of 31 patients (13%) who stopped combination therapy early for toxic effects developed a new, clinically significant immune-related AE more than 16 weeks after the discontinuation date (grade 2 sarcoidosis, hyperglycemia, hypophysitis, and alopecia; range, 22-33 weeks postdose).
Table. Adverse Events During Nivo + Ipi and PD-1 Monotherapy.
| Immune-Related Adverse Events | No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Nivo + Ipi | Anti–PD-1 Monotherapy | Total Across Nivo + Ipi and PD-1 Monotherapy | ||||||
| Grade | Immune-Related AEs Associated With Nivo + Ipi Dose Delays | Grade | Total | Immune-Related AEs Requiring Steroid Treatment | ||||
| 1 | 2 | 3 or 4 | 2 | 3 or 4 | ||||
| Asymptomatic lipase/amylase | 0 | 4 (6) | 17 (27) | 11 of 21 (52) | 0 | 7 (11) | 28 (44) | 6 (9) |
| Diarrhea/colitis | 3 (5) | 9 (14) | 13 (20) | 20 of 25 (80) | 3 (5) | 0 | 28 (42) | 20 (31) |
| Endocrinopathy | 1 (2) | 12 (19) | 5 (8) | 4 of 18 (22) | 6 (9) | 3 (5) | 27 (42) | 10 (16) |
| Hypothyroidism | 1 (2) | 8 (13) | 0 | 2 of 9 (22) | 3 (5) | 0 | 12 (19) | 1 (2) |
| Hypophysitis | 0 | 4 (6) | 2 (3) | 2 of 6 (33) | 2 (3) | 0 | 8 (13) | 8 (13) |
| Hyperglycemia | 0 | 0 | 3 (5) | 0 of 3 (0) | 1 (2) | 3 (5) | 7 (11) | 1 (2) |
| Rash/pruritus | 1 (2) | 12 (19) | 5 (8) | 11 of 18 (61) | 2 (3) | 0 | 19 (30) | 10 (16) |
| Transaminitis | 1 (2) | 6 (9) | 10 (16) | 15 of 17 (88) | 1 (2) | 1 (2) | 19 (30) | 17 (27) |
| Fatigue | 0 | 11 (17) | 0 | 2 of 11 (18) | 0 | 0 | 11 (17) | 1 (2) |
| Pneumonitis | 0 | 6 (9) | 1 (2) | 7 of 7 (100) | 1 (2) | 0 | 8 (13) | 8 (13) |
| Pancreatitis | 0 | 0 | 1 (2) | 1 of 1 (100) | 2 (3) | 1 (2) | 4 (6) | 4 (6) |
Abbreviations: AEs, adverse events; nivo + ipi, nivolumab plus ipilimumab; PD-1, programmed cell death protein 1.
Forty-six of 64 patients (72%) required systemic steroids, 14 (22%) required infliximab for steroid-refractory diarrhea, and 2 (3%) required mycophenolate for steroid-refractory transaminitis. Forty patients (63%) had at least 1 emergency department visit (range, 0-5); 32 patients (50%) had an immune-related AE–related emergency department visit. Thirty patients (48%) had at least 1 admission to the hospital (range, 0-4); 23 patients (36%) had an immune-related AE–related admission
Time to Treatment Failure
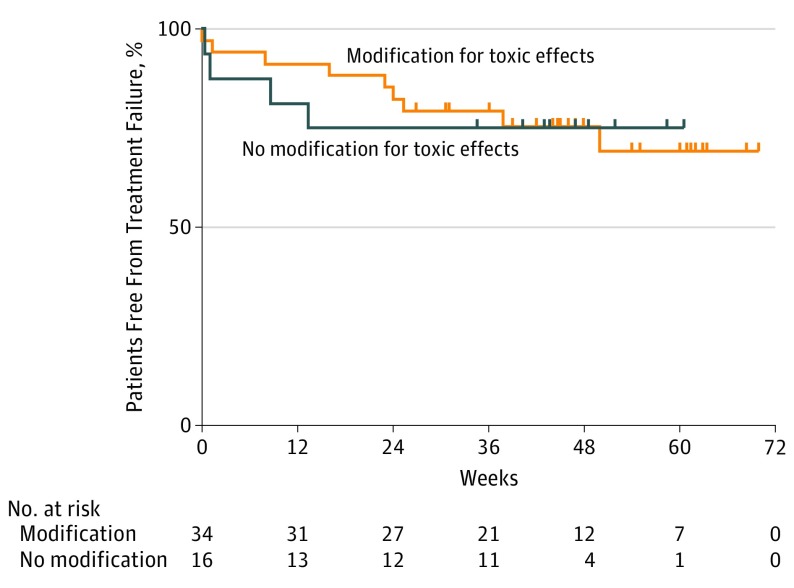
After a median follow up of 14 months, there were 27 treatment failures (42%); 3 required surgery or radiation only. In the group of 50 patients who were free of treatment failure at 12 weeks, there did not appear to be a difference in TTF between those who underwent treatment modification due to toxic effects (n = 34) and those who did not modify treatment for toxic effects (Figure). In each group, the estimated 1-year treatment failure rate was 25%.
Figure. Time to Treatment Failure in Patients Who Modified vs Did Not Modify Therapy for Toxic Effects.
Time to treatment failure, landmarked at 12 weeks, in patients who stopped combination or programmed cell death protein 1 maintenance for toxic effects (n = 34) and those who did not (n = 16). This did not vary significantly (P = .95, log-rank). Week 0 in this landmark analysis corresponds to day 84 of therapy, and all patients who experienced disease progression prior to day 84 are excluded.
Discussion
Our extensive experience using combination checkpoint blockade led us to believe the AEs reported in clinical trials did not capture the full extent of the clinically relevant toxic effects. Trials uniformly emphasize CTCAE grade 3 to 4 events even though grade 2 events are often serious. Also, trials often miss late toxic effects and do not capture emergency department visits unless they result in admission. In these 64 patients, we offer a more detailed analysis of the potential toxic effects of nivo + ipi, as well as TTF stratified by treatment modification for toxic effects. Most patients (91%) had a clinically significant immune-related AE compared with the 59% with a grade 3 to 4 immune-related AE using typical reporting methods of published trials. Our expanded definition includes other immune-related AEs like grade 2 pneumonitis, hepatitis, and diarrhea that often required steroid therapy or treatment delay. Overall, 72% of our patients required at least 1 course of oral steroids, and 25% of patients required additional immunosuppression for steroid-refractory symptoms.
Importantly, approximately 10% of patients who discontinued therapy for toxic effects developed new late-onset immune-related AEs which would not be captured in randomized trials and thus may be underreported. For example, 11% of patients were diagnosed with insulin-dependent hyperglycemia, which appears higher than the 0.8% incidence previously described for PD-1 monotherapy. Future clinical trials using checkpoint inhibitors should consider increasing follow-up time to capture delayed toxic effects and standardizing the reporting of types, timing, and duration of immunosuppressive therapy.
Novel toxic effect signals were seen. Half of patients had at least 1 emergency department visit, and over one-third had at least 1 inpatient admission related to an immune-related AE. These numbers may be higher than those in clinical trials owing to a sicker expanded access program patient population or our individual center’s experience with this regimen. Other practitioners might direct patients to the emergency department more or less frequently.
Among patients who did not progress prior to 12 weeks, there was no apparent difference in TTF between patients who had to discontinue nivo + ipi due to toxic effects and those who did not. To our knowledge, this is the first landmarked analysis of the impact of toxic effects on efficacy with nivo + ipi in melanoma. Patients can be reassured that if significant toxic effects arise, further doses of combination therapy can be delayed or omitted without decreasing the likelihood of benefit. Moreover, because half of these patients received no maintenance anti–PD-1 therapy, the need for maintenance anti–PD-1 therapy is unclear and should be assessed in a future randomized study.
Conclusions
We believe that these data will assist clinicians in conducting an informed risk-benefit discussion with patients regarding treatment options. These data suggest that CTCAE, while useful for standardizing AE reporting, may not appropriately reflect immune-related AE severity, duration, or timing. There is also likely insufficient reporting of immunosuppressive use to manage immune-related AEs. Investigators should consider this carefully when designing future immuno-oncology trials.
References
- 1.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17(11):1558-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Updated results from a phase III trial of nivolumab (NIVO) combined with ipilimumab (IPI) in treatment-naive patients (pts) with advanced melanoma (MEL) (CheckMate 067). J Clin Oncol 33. 2016;(18_suppl). doi: 10.1200/jco.2015.33.18_suppl.lba1 [DOI] [Google Scholar]
- 6.Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190-209. [DOI] [PubMed] [Google Scholar]