Key Points
Question
Does idalopirdine, a selective 5-hydroxytryptamine-6 receptor antagonist, improve cognitive change in patients with mild to moderate Alzheimer disease when added to cholinesterase inhibitors?
Findings
In 3 randomized clinical trials that included a total of 2525 patients with Alzheimer disease treated with cholinesterase inhibitors, the added use of idalopirdine compared with placebo did not decrease cognitive loss over 24 weeks.
Meaning
The findings do not support the use of idalopirdine for the treatment of Alzheimer disease.
Abstract
Importance
New therapeutic approaches for Alzheimer disease (AD) are needed.
Objective
To assess whether idalopirdine, a selective 5-hydroxytryptamine-6 receptor antagonist, is effective for symptomatic treatment of mild to moderate AD.
Design, Setting, and Participants
Three randomized clinical trials that included 2525 patients aged 50 years or older with mild to moderate AD (study 1: n = 933 patients at 119 sites; study 2: n = 858 at 158 sites; and study 3: n = 734 at 126 sites). The 24-week studies were conducted from October 2013 to January 2017; final follow-up on January 12, 2017.
Interventions
Idalopirdine (10, 30, or 60 mg/d) or placebo added to cholinesterase inhibitor treatment (donepezil in studies 1 and 2; donepezil, rivastigmine, or galantamine in study 3).
Main Outcomes and Measures
Primary end point in all 3 studies: change in cognition total score (range, 0-70; a lower score indicates less impairment) from baseline to 24 weeks measured by the 11-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-Cog); key secondary end points: Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change Scale and 23-item Activities of Daily Living Inventory scores. Dose group efficacy required a significant benefit over placebo for the primary end point and 1 or more key secondary end points. Safety data and adverse event profiles were recorded.
Results
Among 2525 patients randomized in the 3 trials (mean age, 74 years; mean baseline ADAS-Cog total score, 26; between 62% and 65% of participants were women), 2254 (89%) completed the studies. In study 1, the mean change in ADAS-Cog total score between baseline and 24 weeks was 0.37 for the 60-mg dose of idalopirdine group, 0.61 for the 30-mg dose group, and 0.41 for the placebo group (adjusted mean difference vs placebo, 0.05 [95% CI, −0.88 to 0.98] for the 60-mg dose group and 0.33 [95% CI, −0.59 to 1.26] for the 30-mg dose group). In study 2, the mean change in ADAS-Cog total score between baseline and 24 weeks was 1.01 for the 30-mg dose of idalopirdine group, 0.53 for the 10-mg dose group, and 0.56 for the placebo group (adjusted mean difference vs placebo, 0.63 [95% CI, −0.38 to 1.65] for the 30-mg dose group; given the gated testing strategy and the null findings at the 30-mg dose, statistical comparison of the 10-mg dose was not performed). In study 3, the mean change in ADAS-Cog total score between baseline and 24 weeks was 0.38 for the 60-mg dose of idalopirdine group and 0.82 for the placebo group (adjusted mean difference vs placebo, −0.55 [95% CI, −1.45 to 0.36]). Treatment-emergent adverse events occurred in between 55.4% and 69.7% of participants in the idalopirdine groups vs between 56.7% and 61.4% of participants in the placebo groups.
Conclusions and Relevance
In patients with mild to moderate AD, the use of idalopirdine compared with placebo did not improve cognition over 24 weeks of treatment. These findings do not support the use of idalopirdine for the treatment of AD.
Trial Registration
clinicaltrials.gov Identifiers: NCT01955161, NCT02006641, and NCT02006654
This report describes findings from 3 randomized trials comparing the cognitive effects of 10, 30, or 60 mg/d of idalopirdine added to stable cholinesterase inhibitor treatment or placebo for symptomatic treatment of mild to moderate Alzheimer disease.
Introduction
Alzheimer disease, a complex disease involving multiple pathophysiological mechanisms, is increasing in prevalence and cost due to the aging population. Therapies with neurotransmitter-based mechanisms have the potential to affect multiple clinical domains in Alzheimer disease.
Two classes of medications are approved by the US Food and Drug Administration for the treatment of Alzheimer disease: cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) and the N-methyl-d-aspartate–receptor antagonist memantine. Higher doses of cholinesterase inhibitors may be needed in patients with advanced Alzheimer disease, but this strategy is limited by increased adverse events due to peripheral nervous system cholinergic effects (eg, nausea, vomiting, and diarrhea). Amplifying central nervous system cholinergic function without inducing peripheral nervous system cholinergic symptoms may therefore provide greater clinical benefits.
Evidence supports investigation of 5-hydroxytryptamine-6 (5-HT6) antagonism in Alzheimer disease, which may involve modulation of cholinergic, monoaminergic, and glutamatergic systems. The 5-HT6 receptors affect learning and memory and the 5-HT6 receptor antagonists have been shown to improve cognitive performance in animal models. Even though clinical trials did not support 5-HT6 antagonist monotherapy among patients with Alzheimer disease, 2 phase 2 trials suggested that administration of 5-HT6 antagonists added to cholinesterase inhibitor therapy may improve cognition in Alzheimer disease.
In one of the phase 2 studies, 90 mg/d of idalopirdine (30 mg taken 3 times per day) added to a stable dose of donepezil provided significant improvement in cognitive performance relative to donepezil monotherapy among patients with moderate Alzheimer disease dementia. Therefore, 3 randomized clinical trials (STARSHINE [study 1], STARBEAM [study 2], and STARBRIGHT [study 3]) were conducted to assess the efficacy of idalopirdine when added to donepezil or another cholinesterase inhibitor therapy for the treatment of mild to moderate Alzheimer disease.
Methods
Study Design and Patient Population
A phase 3 development program consisting of 3 randomized parallel-group, double-blind, fixed-dose, placebo-controlled 24-week studies was conducted. The phase 3 program also included a 28-week, open-label extension study (NCT02079246) for patients completing study 1 or study 2. The designs of the 3 studies are summarized in eFigure 1 in Supplement 1. All 3 studies were conducted in accordance with the principles of good clinical practice and the Declaration of Helsinki.
The trial protocol and the statistical analysis plan for study 1 appear in Supplement 2 and Supplement 3; study 2, Supplement 4 and Supplement 5; and study 3, Supplement 6 and Supplement 7. The local ethics committees approved all aspects of the trial design. Eligible patients or their legal representatives provided written informed consent before participating.
The 3 studies included patients (1) aged 50 years or older meeting the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association criteria for the diagnosis of probable Alzheimer disease, (2) with a Mini-Mental State Examination (MMSE) score at or between 12 and 22 at screening (range, 0-30; a lower score indicates higher impairment), and (3) taking a therapeutic and stable dose of a cholinesterase inhibitor for 4 months prior to screening. Patients were excluded if (1) taking memantine, (2) had an alternative cause of dementia, (3) had serious central nervous system or somatic disorders, (4) had clinically significant abnormalities (determined by laboratory testing), or (5) taking concomitant medications that would interfere with the safety and efficacy assessments.
Randomization, Intervention, and Blinding
The 3 studies used a parallel-group, fixed-dose design to explore the dose-response relationship for idalopirdine among patients receiving stable treatment with donepezil in studies 1 and 2 and any cholinesterase inhibitor (donepezil, rivastigmine, or galantamine) in study 3. Patents received 10 mg, 30 mg, or 60 mg of idalopirdine or an identical-appearing placebo (Figure 1, Figure 2, and Figure 3). Randomization and blinding were applied via an interactive voice response system to minimize risk of bias in the evaluation of the clinical effects of idalopirdine.
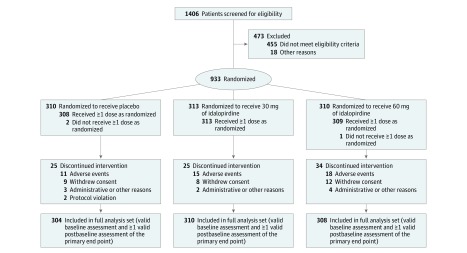
Figure 1. Patient Enrollment and Flow in Study 1 (Placebo Compared With 30 mg or 60 mg of Idalopirdine).
All patients were taking stable doses of donepezil.
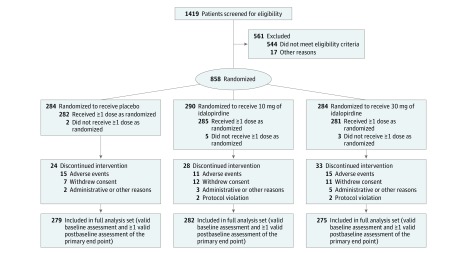
Figure 2. Patient Enrollment and Flow in Study 2 (Placebo Compared With 10 mg or 30 mg of Idalopirdine).
All patients were taking stable doses of donepezil.
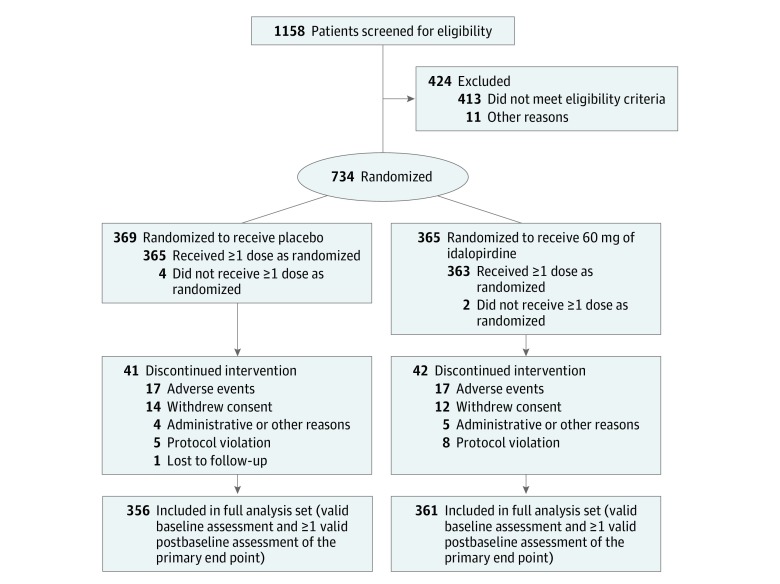
Figure 3. Patient Enrollment and Flow in Study 3 (Placebo Compared With 60 mg of Idalopirdine).
All patients were taking stable doses of donepezil, rivastigmine, or galantamine.
In all 3 studies, symmetric randomization to the groups was stratified by MMSE score stratum. In study 3, randomization was also stratified for base therapy (cholinesterase inhibitor therapy). Block randomization with block sizes of 3 (for study 1 and study 2) and 4 (for study 3) was used and restricted such that the first 2 patients at each site would always get different treatments. In study 3, Latin squares were used to balance treatments, strata, and sites. At least 50% of the patients were to be enrolled from the 12 to 18 MMSE score stratum (further details appear in the eMethods section in Supplement 1). Compliance was assessed via pill count at each visit.
During screening, patients were monitored by Lundbeck medical staff to assess eligibility prior to randomization. External quality oversight methods (including central review of scale administration) were used to try to achieve consistent and accurate ratings throughout the study for the 11-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-Cog), the 23-item Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory (ADCS-ADL23), the Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change Scale (ADCS-CGIC), and the MMSE.
All study staff (including raters who performed the ADAS-Cog, ADCS-ADL23, ADCS-CGIC, and MMSE assessments) were blind to patient randomization (ie, drug group assignment). The ADCS-CGIC rater was blind to any study assessment after baseline.
Outcomes
The primary and key secondary end points were identical across the 3 studies. The primary end point was change in cognition total score measured by the 11-item ADAS-Cog (range, 0-70; a lower score indicates less impairment) from baseline to 24 weeks. Key secondary end points were changes from baseline to 24 weeks in activities of daily living (function) measured by the ADCS-ADL23 (score range, 0-78; a higher score indicates less impairment) and overall clinical response (global outcome) measured by the ADCS-CGIC.
At the baseline visit, the ADCS-CGIC was used to assess the severity of illness and has a rating from 1 (normal, not at all ill) to 7 (among the most extremely ill patients). At subsequent visits, the ADCS-CGIC was used to assess global change from baseline and has a rating from 1 (very much improved) to 7 (very much worse), with a rating of 4 indicating no change. These end points were assessed at baseline and at weeks 4, 12, and 24. The eMethods section in Supplement 1 contains a list of other prespecified end points. A safety follow-up visit occurred at week 28. For patients who withdrew from the studies, efforts were made to collect selected efficacy assessments after study drug discontinuation.
Safety Data and Adverse Events
Safety data were collected via measurement of vital signs and weight, through physical and neurological examination, with clinical safety laboratory tests, by using electrocardiographic parameters, and via assessment of suicidality (using the Columbia-Suicide Severity Rating Scale). Qualified personnel coded adverse events using the lowest level term according to the Medical Dictionary for Regulatory Activities (version 19.0). According to the law of Hy, a potentially severe liver injury was defined as an alanine aminotransferase level or an aspartate aminotransferase level greater than 3 times the upper limit of normal in combination with a bilirubin level greater than 2 times the upper limit of normal. All criteria were measured as maximal values observed during the 3 studies; the criteria did not need to occur at the same time.
Statistical Analysis
In all 3 studies, the criterion for showing efficacy of a dose required demonstration of a significant positive effect on the ADAS-Cog followed by (in a gated procedure) a significant positive effect on either the ADCS-ADL23 or the ADCS-CGIC. A significant effect meant that the adjusted mean difference for the idalopirdine dose vs placebo was statistically significant at a significance level of .05 using 2-sided testing. Due to the multiple end points and multiple active dose groups (10 mg, 30 mg, and 60 mg), multiple testing procedures were used to control the overall type I error in each study (details appear in the eMethods section in Supplement 1). For the testing strategies used to determine the positive or negative outcome of the trials, the direction of the effects on the end points was considered to ensure that a significantly deleterious treatment effect would not contribute to a positive outcome. All reported P values are 2-sided.
Based on the testing strategies used to control for multiplicity in the individual studies, sample sizes were determined to achieve 90% power for studies 1 and 3 and 80% power for study 2. For the power calculations, the assumed treatment differences and correlation of the end points were based on the outcome of a phase 2 study. The assumed difference in ADAS-Cog total score of −2 points was similar to the effects produced by cholinesterase inhibitors of −2.02 to −2.73 points in the midrange of the ADAS-Cog.
In each study, efficacy analyses were performed on the full analysis set, which was defined as all randomized patients who took at least 1 dose of investigational medicinal product and who had a valid baseline assessment and at least 1 valid postbaseline assessment of the primary end point. Changes from baseline in the ADAS-Cog, ADCS-ADL23, and ADCS-CGIC were analyzed using a restricted maximum likelihood–based mixed model for repeated-measures approach. The model included terms to adjust for the effects of the MMSE strata and, in study 3 only, cholinesterase inhibitor therapy in addition to the effects of country and baseline scores. The effect of the idalopirdine doses was estimated as a mean difference vs placebo at week 24 using least-squares means for the treatment × visit interaction effect in the mixed model.
Sensitivity analyses that adjusted for the study site effects instead of country effects were done to evaluate the potential effects of site on the outcomes. Sensitivity analyses using a pattern-mixture model were done to evaluate the suitability of the missing at random assumption imposed by the mixed model. In these analyses, monotone missing values in patients withdrawn from treatment were imputed using multiple imputation based on the placebo group.
The statistical analyses were performed using SAS version 9.4 (SAS Institute Inc). Additional details appear in the eMethods section in Supplement 1.
Results
Among 2525 patients (mean age, 74 years; mean baseline ADAS-Cog total score, 26; between 62% and 65% of participants were women), 2254 (89%) completed the studies. The 3 studies were conducted in 34 countries worldwide from October 2013 to January 2017 (study 1: 933 patients at 119 sites in 16 countries; study 2: 858 patients at 158 sites in 18 countries; study 3: 734 patients at 126 sites in 16 countries). The final follow-up date was January 12, 2017.
Patient demographic and baseline clinical characteristics are presented in Table 1, Table 2, and Table 3. Patients had been diagnosed with Alzheimer disease a median of 1.4 to 1.9 years prior to enrollment, and had a mean duration of background cholinesterase inhibitor treatment of between 0.9 to 1.2 years. Patients had a mean MMSE score at baseline of approximately 18. The percentage of patients screening positive as carriers for at least 1 APOE ε4 allele ranged from 55.6% to 60.2% (study 1: 59.2%; study 2: 60.2%; study 3: 55.6%). The baseline patient demographics and clinical characteristics were not different between the idalopirdine and placebo groups across studies.
Table 1. Patient Demographics and Clinical Characteristics for 922 Patients in Study 1.
| Characteristics | Placebo (n = 304) | Idalopirdine | |
|---|---|---|---|
| 30 mg (n = 310) | 60 mg (n = 308) | ||
| Age, mean (SD) [range], y | 73.7 (8.0) [50-94] | 74.1 (8.8) [50-99] | 73.7 (8.6) [50-93] |
| Women, No. (%) | 194 (63.8) | 206 (66.5) | 201 (65.3) |
| Time since diagnosis of Alzheimer disease, median (IQR), y | 1.7 (0.9-3.1) | 1.4 (0.8-2.6) | 1.4 (0.7-2.7) |
| Time since current cholinesterase inhibitor treatment initiated, median (IQR), y | 1.1 (0.6-2.0) | 1.0 (0.6-1.7) | 0.9 (0.6-1.8) |
| APOE ε4 carrier, No. (%)a | 184 (61.3) | 178 (59.3) | 172 (57.0) |
| Mini-Mental State Examination scoreb | |||
| Mean (SD) [range] | 17.4 (2.9) [12-22] | 17.2 (3.1) [12-22] | 17.4 (2.9) [12-22] |
| Score stratum, No. (%) | |||
| 12-18 | 178 (58.6) | 181 (58.4) | 184 (59.7) |
| 19-22 | 126 (41.4) | 129 (41.6) | 124 (40.3) |
| 11-Item cognitive subscale of the Alzheimer’s Disease Assessment Scale total score, mean (SD)c | 25.8 (8.5) | 26.7 (8.2) | 26.3 (8.0) |
| Alzheimer’s Disease Cooperative Study score, mean (SD) | |||
| Clinical Global Impression of Change Scaled | 3.8 (0.8) | 3.9 (0.7) | 3.8 (0.7) |
| 23-Item Activities of Daily Living Inventorye | 56.2 (13.0) | 55.8 (13.3) | 56.6 (12.8) |
| Neuropsychiatric Inventory score, mean (SD)f | 10.8 (11.9) | 10.8 (13.0) | 10.0 (10.8) |
| Geographic region, No. (%) | |||
| North America | 56 (18.4) | 57 (18.4) | 56 (18.2) |
| Western Europe | 72 (23.7) | 81 (26.1) | 76 (24.7) |
| Eastern Europe | 106 (34.9) | 98 (31.6) | 104 (33.8) |
| South or Latin America | 52 (17.1) | 55 (17.7) | 52 (16.9) |
| Asia or other | 18 (5.9) | 19 (6.1) | 20 (6.5) |
Abbreviation: IQR, interquartile range.
Twenty patients were not assessed (4 in the placebo group, 10 in the 30 mg of idalopirdine group, and 6 in the 60 mg of idalopirdine group).
Range, 0 to 30; a higher score indicates less impairment.
Range, 0 to 70; a lower score indicates less impairment.
Range, 1 (normal, not at all ill) to 7 (among the most extremely ill patients).
Range, 0 to 78; a higher score indicates less impairment.
Range, 0 to 144; a higher score indicates higher neuropsychiatric burden.
Table 2. Patient Demographics and Clinical Characteristics for 836 Patients in Study 2.
| Characteristics | Placebo (n = 279) | Idalopirdine | |
|---|---|---|---|
| 10 mg (n = 282) | 30 mg (n = 275) | ||
| Age, mean (SD) [range], y | 73.5 (8.2) [52-94] | 74.9 (8.1) [54-94] | 74.9 (8.0) [52-96] |
| Women, No. (%) | 179 (64.2) | 170 (60.3) | 166 (60.4) |
| Time since diagnosis of Alzheimer disease, median (IQR), y | 1.6 (0.9-2.9) | 1.8 (0.9-3.2) | 1.4 (0.8-2.9) |
| Time since current cholinesterase inhibitor treatment initiated, median (IQR), y | 1.2 (0.7-2.5) | 1.2 (0.7-2.4) | 1.1 (0.6-2.2) |
| APOE ε4 carrier, No. (%)a | 156 (57.4) | 168 (60.4) | 170 (63.0) |
| Mini-Mental State Examination scoreb | |||
| Mean (SD) [range] | 17.6 (2.9) [12-22] | 17.4 (3.0) [12-22] | 17.5 (3.0) [12-22] |
| Score stratum, No. (%) | |||
| 12-18 | 161 (57.7) | 174 (61.7) | 162 (58.9) |
| 19-22 | 118 (42.3) | 108 (38.3) | 113 (41.1) |
| 11-Item cognitive subscale of the Alzheimer’s Disease Assessment Scale total score, mean (SD)c | 25.7 (8.0) | 25.9 (7.5) | 25.3 (7.9) |
| Alzheimer’s Disease Cooperative Study score, mean (SD) | |||
| Clinical Global Impression of Change Scaled | 3.7 (0.7) | 3.7 (0.7) | 3.7 (0.7) |
| 23-Item Activities of Daily Living Inventorye | 57.6 (11.9) | 56.6 (12.0) | 55.3 (12.9) |
| Neuropsychiatric Inventory score, mean (SD)f | 10.4 (11.5) | 9.5 (10.0) | 11.0 (13.6) |
| Geographic region, No. (%) | |||
| North America | 64 (22.9) | 67 (23.8) | 60 (21.8) |
| Western Europe | 69 (24.7) | 71 (25.2) | 67 (24.4) |
| Eastern Europe | 88 (31.5) | 81 (28.7) | 89 (32.4) |
| South or Latin America | 36 (12.9) | 39 (13.8) | 34 (12.4) |
| Asia or other | 22 (7.9) | 24 (8.5) | 25 (9.1) |
Abbreviation: IQR, interquartile range.
Sixteen patients were not assessed (7 in the placebo group, 4 in the 10 mg of idalopirdine group, and 5 in the 30 mg of idalopirdine group).
Range, 0 to 30; a higher score indicates less impairment.
Range, 0 to 70; a lower score indicates less impairment.
Range, 1 (normal, not at all ill) to 7 (among the most extremely ill patients).
Range, 0 to 78; a higher score indicates less impairment.
Range, 0 to 144; a higher score indicates higher neuropsychiatric burden.
Table 3. Patient Demographics and Clinical Characteristics for 717 Patients in Study 3.
| Characteristics | Placebo (n = 356) | 60 mg of Idalopirdine (n = 361) |
|---|---|---|
| Age, mean (SD) [range], y | 74.2 (8.0) [51-92] | 73.7 (8.5) [53-93] |
| Women, No. (%) | 226 (63.5) | 226 (62.6) |
| Time since diagnosis of Alzheimer disease, median (IQR), y | 1.9 (0.9-3.3) | 1.6 (0.9-2.9) |
| Time since current cholinesterase inhibitor treatment initiated, median (IQR), y | 1.1 (0.7-2.1) | 1.0 (0.6-1.9) |
| APOE ε4 carrier, No. (%)a | 200 (58.5) | 182 (52.8) |
| Mini-Mental State Examination scoreb | ||
| Mean (SD) [range] | 17.5 (3.0) [12-22] | 17.2 (2.9) [12-22] |
| Score stratum, No. (%) | ||
| 12-18 | 212 (59.6) | 234 (64.8) |
| 19-22 | 144 (40.4) | 127 (35.2) |
| Current cholinesterase inhibitor treatment at time of study enrollment, No. (%) | ||
| Donepezil | 224 (62.9) | 230 (63.7) |
| Rivastigmine | 106 (29.8) | 103 (28.5) |
| Galantamine | 26 (7.3) | 28 (7.8) |
| 11-Item cognitive subscale of the Alzheimer’s Disease Assessment Scale total score, mean (SD)c | 25.9 (8.5) | 26.2 (8.2) |
| Alzheimer’s Disease Cooperative Study score, mean (SD) | ||
| Clinical Global Impression of Change Scaled | 3.9 (0.8) | 3.9 (0.7) |
| 23-Item Activities of Daily Living Inventorye | 53.9 (14.7) | 54.0 (13.3) |
| Neuropsychiatric Inventory score, mean (SD)f | 9.0 (10.5) | 9.5 (11.1) |
| Geographic region, No. (%) | ||
| North America | 40 (11.2) | 39 (10.8) |
| Western Europe | 117 (32.9) | 121 (33.5) |
| Eastern Europe | 76 (21.3) | 77 (21.3) |
| South or Latin America | 47 (13.2) | 44 (12.2) |
| Asia or other | 76 (21.3) | 80 (22.2) |
Abbreviation: IQR, interquartile range.
Thirty patients were not assessed (14 in the placebo group and 16 in the 60 mg of idalopirdine group).
Range, 0 to 30; a higher score indicates less impairment.
Range, 0 to 70; a lower score indicates less impairment.
Range, 1 (normal, not at all ill) to 7 (among the most extremely ill patients).
Range, 0 to 78; a higher score indicates less impairment.
Range, 0 to 144; a higher score indicates higher neuropsychiatric burden.
The patient withdrawal patterns were similar among the treatment groups; the proportion of total withdrawals was 9.7% for the 10-mg dose of idalopirdine, 9.7% for the 30-mg dose, 11.3% for the 60-mg dose, and 9.3% for placebo. In the full analysis sets, the proportion of missing postbaseline ADAS-Cog assessments (due to withdrawal, missed visits, or invalid assessment) was 3.7% in study 1 (Figure 1), 3.8% in study 2 (Figure 2), and 4.7% in study 3 (Figure 3).
Efficacy Outcomes
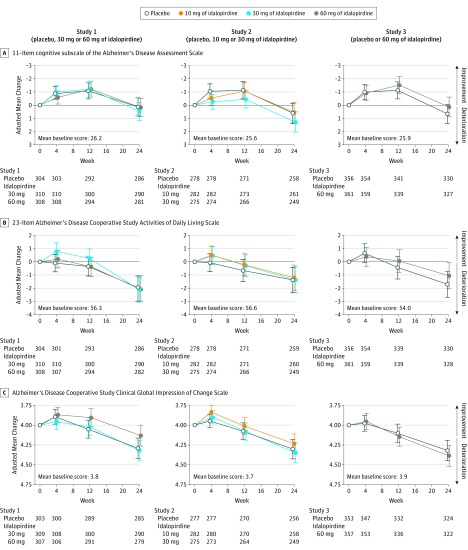
At week 24, there were no significant differences in the change in cognitive performance as measured by the ADAS-Cog total score in the idalopirdine groups compared with placebo (Table 4 and Figure 4); therefore, none of the doses of idalopirdine met the criterion for efficacy in any of the studies.
Table 4. Changes From Baseline in Primary End Point in the Full Analysis Setsa.
| Mean ADAS-Cog Total Scoreb | Change From Baseline (95% CI)c |
Mean ADAS-Cog Total Scoreb | Change From Baseline (95% CI)c |
Mean ADAS-Cog Total Scoreb | Change From Baseline (95% CI)c |
Between-Group Difference in Change, Least-Squares Mean (95% CI)d |
P Valuee |
Between-Group Difference in Change, Least-Squares Mean (95% CI)d |
P Valuee |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 24 |
Baseline | Week 24 |
Baseline | Week 24 |
||||||||
| Study 1 | Placebo (n = 304) | Idalopirdine (30 mg; n = 310) | Idalopirdine (60 mg; n = 308) | Idalopirdine (30 mg) vs Placebo | Idalopirdine (60 mg) vs Placebo | ||||||||
| 25.8 | 26.2 | 0.41 (−0.28 to 1.11) | 26.7 | 27.1 | 0.61 (−0.09 to 1.32) | 26.3 | 26.4 | 0.37 (−0.32 to 1.06) | 0.33 (−0.59 to 1.26) | .96 | 0.05 (−0.88 to 0.98) | >.99 | |
| Study 2 | Placebo (n = 278) | Idalopirdine (10 mg; n = 282) | Idalopirdine (30 mg; n = 275) | Idalopirdine (10 mg) vs Placebo | Idalopirdine (30 mg) vs Placebo | ||||||||
| 25.7 | 26.1 | 0.56 (−0.18 to 1.30) | 25.9 | 26.3 | 0.53 (−0.22 to 1.27) | 25.3 | 26.2 | 1.01 (0.26 to 1.76) | −0.09 (−1.10 to 0.92)f | 0.63 (−0.38 to 1.65) | .22 | ||
| Study 3 | Placebo (n = 356) | Idalopirdine (60 mg; n = 361) | Idalopirdine (60 mg) vs Placebo | ||||||||||
| 25.9 | 26.6 | 0.82 (0.13 to 1.51) | 26.2 | 26.4 | 0.38 (−0.25 to 1.01) | −0.55 (−1.45 to 0.36) | .24 | ||||||
Abbreviation: ADAS-Cog, 11-item cognitive subscale of the Alzheimer’s Disease Assessment Scale.
Includes patients who received at least 1 dose of treatment and had a valid baseline assessment and at least 1 valid postbaseline assessment of the primary end point.
Range, 0 to 70; a lower score indicates less impairment.
Indicates the observed mean change (ie, only for patients with baseline and week 24 assessments), so it does not match the difference between baseline and week 24 scores.
Indicates the difference in change from baseline to 24 weeks between the placebo and idalopirdine groups. A negative value indicates a treatment effect in favor of idalopirdine.
Adjusted P values that are computed as the smallest significance levels at which one can reject the associated tests under the prespecified test hierarchies.
Between-group statistical comparison was not performed due to the null findings of the 30-mg dose of idalopirdine vs placebo and the prespecified gated testing strategy that allowed statistical testing of the 10-mg dose of idalopirdine vs placebo only if findings with the 30-mg dose were statistically significant.
Figure 4. Primary and Key Secondary Efficacy Results in the 3 Studies.
Error bars indicate 95% CIs. The primary end point was change in cognition total score (range, 0 to 70; a lower score indicates less impairment) from baseline to 24 weeks as assessed by the 11-item cognitive subscale of the Alzheimer’s Disease Assessment Scale. Key secondary end points were changes in activities of daily living (function) measured by the 23-item Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory (total score range, 0-78; higher scores indicate less impairment) and overall clinical response (global outcome) measured by the Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change Scale from baseline to 24 weeks (rated from 1 [normal, not at all ill] to 7 [among the most extremely ill patients] at baseline; at subsequent visits, rated from 1 [very much improved] to 7 [very much worse]; 4 indicates no change). These end points were rated at baseline and at weeks 4, 12, and 24 (completion or withdrawal from the study). The targeted effect sizes for idalopirdine treatment was a difference of −2 points vs placebo for the cognitive subscale of the Alzheimer’s Disease Assessment Scale, a difference of 2 points vs placebo for the 23-item Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory, and a difference of −0.25 points vs placebo for the Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change Scale.
In study 1, the mean baseline ADAS-Cog total score was 26.3 for the 60-mg dose of idalopirdine group, 26.7 for the 30-mg dose group, and 25.8 for the placebo group. The mean change in ADAS-Cog total score between baseline and 24 weeks was 0.37 for the 60-mg dose of idalopirdine group, 0.61 for the 30-mg dose group, and 0.41 for the placebo group. Compared with placebo, the adjusted mean difference in the change in ADAS-Cog total score between baseline and 24 weeks was 0.05 (95% CI, −0.88 to 0.98) for the 60-mg dose of idalopirdine group and 0.33 (95% CI, −0.59 to 1.26) for the 30-mg dose group.
In study 2, the mean baseline ADAS-Cog total score was 25.3 for the 30-mg dose of idalopirdine group, 25.9 for the 10-mg dose group, and 25.7 for the placebo group. The mean change in ADAS-Cog total score between baseline and 24 weeks was 1.01 for the 30-mg dose of idalopirdine group, 0.53 for the 10-mg dose group, and 0.56 for the placebo group. Compared with placebo, the adjusted mean difference in the change in ADAS-Cog total score between baseline and 24 weeks was 0.63 (95% CI, −0.38 to 1.65) for the 30-mg dose of idalopirdine group and −0.09 (95% CI, −1.10 to 0.92) for the 10-mg dose group. Given the gated testing strategy for statistical significance and the null findings at the 30-mg dose of idalopirdine, statistical comparison of the 10-mg dose vs placebo was not performed.
In study 3, the mean baseline ADAS-Cog total score was 26.2 for the 60-mg dose of idalopirdine group and 25.9 for the placebo group. The mean change in ADAS-Cog total score between baseline and 24 weeks was 0.38 for the 60-mg dose of idalopirdine group and 0.82 for the placebo group. Compared with placebo, the adjusted mean difference in the change in ADAS-Cog total score between baseline and 24 weeks was −0.55 (95% CI, −1.45 to 0.36) for the 60-mg dose of idalopirdine group.
As a consequence of the prespecified gated testing strategy for statistical significance and the nonsignificant findings for the primary end point, findings for the key secondary end points were considered nonsignificant de facto; therefore, no P values are reported for the key secondary end points. Similarly, the nonsignificant effects with the 30-mg dose of idalopirdine in study 2 implied nonsignificant effects with the 10-mg dose in that study.
The baseline and final values for the key secondary end points appear in Figure 4 and the within-group changes from baseline are reported in eTable 1 in Supplement 1. Individual patient-level data for the primary and key secondary end points are presented in eFigure 2 in Supplement 1. In all treatment groups and across all 3 studies, mean treatment adherence was greater than 98% and overall completion rates were high (89%-91%).
Adverse Events
Adverse event data from studies 1, 2, and 3 are presented in Table 5, Table 6, and Table 7, including treatment-emergent adverse events reported by 3% or greater of the patients in any treatment group, the events leading to study withdrawal, serious adverse events, and cumulative increased levels of liver enzymes.
Table 5. Adverse Events in Study 1.
| No. (%) of Patientsa | |||
|---|---|---|---|
| Placebo (n = 308) |
Idalopirdine | ||
| 30 mg (n = 313) | 60 mg (n = 309) | ||
| Women | 198 (64.3) | 208 (66.5) | 201 (65.0) |
| Patient-years of exposure | 133 | 136 | 133 |
| Deaths | 1 (0.3) | 1 (0.3) | 3 (1.0) |
| Treatment-emergent adverse eventsb | |||
| Total | 189 (61.4) | 193 (61.7) | 203 (65.7) |
| Seriousc | 12 (3.9) | 18 (5.8) | 20 (6.5) |
| Leading to study withdrawal | 11 (3.6) | 16 (5.1) | 22 (7.1) |
| Common typesd | |||
| Fall | 15 (4.9) | 16 (5.1) | 19 (6.1) |
| Accidental overdosee | 27 (8.8) | 27 (8.6) | 16 (5.2) |
| γ-Glutamyltransferase increasedf | 2 (0.6) | 17 (5.4) | 15 (4.9) |
| Alanine aminotransferase increasedf | 3 (1.0) | 7 (2.2) | 10 (3.2) |
| Nasopharyngitis | 10 (3.2) | 13 (4.2) | 15 (4.9) |
| Headache | 11 (3.6) | 5 (1.6) | 12 (3.9) |
| Diarrhea | 10 (3.2) | 10 (3.2) | 11 (3.6) |
| Urinary tract infection | 5 (1.6) | 7 (2.2) | 11 (3.6) |
| Nausea | 5 (1.6) | 11 (3.5) | 5 (1.6) |
| Cumulative increased levels of liver enzymes, No./total (%)g | 4/304 (1.3) | 4/310 (1.3) | 3/308 (1.0) |
| Suicidal ideation, No./total (%)h | 12/304 (3.9) | 4/310 (1.3) | 8/308 (2.6) |
| Suicidal behavior, No./totalh | 0/304 | 0/310 | 0/308 |
Unless otherwise indicated. Includes patients who received at least 1 dose of treatment.
Defined as an adverse event that starts after the first dose of idalopirdine and prior to the last protocol-specified contact with the patient.
Defined as any adverse event that results in death; is life-threatening (refers to an event in which the patient was at risk of death at the time of the event but does not refer to an event that hypothetically might have caused death had it been more severe); requires inpatient hospitalization or prolongation of existing hospitalization; results in persistent or significant disability or incapacity; is a congenital anomaly or birth defect; and is medically important (refers to an event that may not be immediately life-threatening or result in death or hospitalization, but may jeopardize the patient or may require an intervention to prevent any of these other defined events).
Incidence of 3% or greater; Medical Dictionary for Regulatory Activities (MedDRA; version 19.0) preferred terms.
MedDRA term that includes any instance of dosing outside specified regimen for study-specific treatment.
Defined by MedDRA as any increase found to be clinically significant.
Based on single maximal alanine aminotransferase or aspartate aminotransferase level measured as 3 times greater than the upper limit of normal for each patient.
Occurred after study entry.
Table 6. Adverse Events in Study 2.
| No. (%) of Patientsa | |||
|---|---|---|---|
| Placebo (n = 282) |
Idalopirdine | ||
| 10 mg (n = 285) | 30 mg (n = 281) | ||
| Women | 181 (64.2) | 172 (60.4) | 169 (60.1) |
| Patient-years of exposure | 124 | 125 | 120 |
| Deaths | 1 (0.4) | 0 | 1 (0.4) |
| Treatment-emergent adverse eventsb | |||
| Total | 160 (56.7) | 158 (55.4) | 171 (60.9) |
| Seriousc | 12 (4.3) | 13 (4.6) | 15 (5.3) |
| Leading to study withdrawal | 15 (5.3) | 12 (4.2) | 16 (5.7) |
| Common typesd | |||
| Fall | 8 (2.8) | 12 (4.2) | 13 (4.6) |
| Accidental overdosee | 33 (11.7) | 26 (9.1) | 31 (11.0) |
| γ-Glutamyltransferase increasedf | 4 (1.4) | 5 (1.8) | 12 (4.3) |
| Anxiety | 3 (1.1) | 3 (1.1) | 9 (3.2) |
| Headache | 12 (4.3) | 12 (4.2) | 5 (1.8) |
| Diarrhea | 4 (1.4) | 9 (3.2) | 5 (1.8) |
| Urinary tract infection | 10 (3.5) | 8 (2.8) | 7 (2.5) |
| Cumulative increased levels of liver enzymes, No./total (%)g | 0/279 | 2/284 (0.7) | 7/277 (2.5) |
| Suicidal ideation, No./total (%)h | 7/279 (2.5) | 6/284 (2.1) | 8/275 (2.9) |
| Suicidal behavior, No./totalh | 0/279 | 0/284 | 0/275 |
Unless otherwise indicated. Includes patients who received at least 1 dose of treatment.
Defined as an adverse event that starts after the first dose of idalopirdine and prior to the last protocol-specified contact with the patient.
Defined as any adverse event that results in death; is life-threatening (refers to an event in which the patient was at risk of death at the time of the event but does not refer to an event that hypothetically might have caused death had it been more severe); requires inpatient hospitalization or prolongation of existing hospitalization; results in persistent or significant disability or incapacity; is a congenital anomaly or birth defect; and is medically important (refers to an event that may not be immediately life-threatening or result in death or hospitalization, but may jeopardize the patient or may require an intervention to prevent any of these other defined events).
Incidence of 3% or greater; Medical Dictionary for Regulatory Activities (MedDRA; version 19.0) preferred terms.
MedDRA term that includes any instance of dosing outside specified regimen for study-specific treatment.
Defined by MedDRA as any increase found to be clinically significant.
Based on single maximal alanine aminotransferase or aspartate aminotransferase level measured as 3 times greater than the upper limit of normal for each patient.
Occurred after study entry.
Table 7. Adverse Events in Study 3.
| No. (%) of Patientsa | ||
|---|---|---|
| Placebo (n = 365) | 60 mg of Idalopirdine (n = 363) | |
| Women | 234 (64.1) | 228 (62.8) |
| Patient-years of exposure | 155 | 154 |
| Deaths | 1 (0.3) | 0 |
| Treatment-emergent adverse eventsb | ||
| Total | 220 (60.3) | 253 (69.7) |
| Seriousc | 22 (6.0) | 28 (7.7) |
| Leading to study withdrawal | 18 (4.9) | 21 (5.8) |
| Common typesd | ||
| Fall | 22 (6.0) | 22 (6.1) |
| Accidental overdosee | 43 (11.8) | 40 (11.0) |
| γ-Glutamyltransferase increasedf | 2 (0.5) | 22 (6.1) |
| Aspartate aminotransferase increasedf | 3 (0.8) | 19 (5.2) |
| Alanine aminotransferase increasedf | 3 (0.8) | 16 (4.4) |
| Nasopharyngitis | 12 (3.3) | 15 (4.1) |
| Headache | 6 (1.6) | 12 (3.3) |
| Diarrhea | 7 (1.9) | 12 (3.3) |
| Urinary tract infection | 10 (2.7) | 11 (3.0) |
| Nausea | 9 (2.5) | 16 (4.4) |
| Dizziness | 10 (2.7) | 12 (3.3) |
| Vomiting | 3 (0.8) | 15 (4.1) |
| Weight lossg | 8 (2.2) | 12 (3.3) |
| Cumulative increased levels of liver enzymes, No./total (%)h | 1/359 (0.3) | 12/361 (3.3) |
| Suicidal ideation, No./total (%)i | 9/358 (2.5) | 5/361 (1.4) |
| Suicidal behavior, No./totali | 0/358 | 0/361 |
Unless otherwise indicated. Includes patients who received at least 1 dose of treatment.
Defined as an adverse event that starts after the first dose of idalopirdine and prior to the last protocol-specified contact with the patient.
Defined as any adverse event that results in death; is life-threatening (refers to an event in which the patient was at risk of death at the time of the event but does not refer to an event that hypothetically might have caused death had it been more severe); requires inpatient hospitalization or prolongation of existing hospitalization; results in persistent or significant disability or incapacity; is a congenital anomaly or birth defect; and is medically important (refers to an event that may not be immediately life-threatening or result in death or hospitalization, but may jeopardize the patient or may require an intervention to prevent any of these other defined events).
Incidence of 3% or greater; Medical Dictionary for Regulatory Activities (MedDRA; version 19.0) preferred terms.
MedDRA term that includes any instance of dosing outside specified regimen for study-specific treatment.
Defined by MedDRA as any increase found to be clinically significant.
Defined by MedDRA as any weight loss found to be clinically significant.
Based on single maximal alanine aminotransferase or aspartate aminotransferase level measured as 3 times greater than the upper limit of normal for each patient.
Occurred after study entry.
The most common treatment-emergent adverse event across treatment groups was accidental overdose (idalopirdine treatment groups: 5.2%-11.0%; placebo: 8.8%-11.8%) followed by falls (idalopirdine treatment groups: 4.2%-6.1%; placebo: 2.8%-6.0%). Cholinergic treatment-emergent adverse events, including vomiting and nausea, were noted at a numerically higher rate in the idalopirdine treatment groups (vomiting: 1.8%-4.1%; nausea: 1.6%-4.4%) compared with the placebo groups (vomiting: 0.7%-1.3%; nausea: 1.1%-2.5%). The proportion of study withdrawals due to treatment-emergent adverse events was 4.2% for the 10-mg dose of idalopirdine group, 5.1%-5.7% for the 30-mg dose groups, 5.8%-7.1% for the 60-mg dose groups, and 3.6%-5.3% for the placebo groups.
The incidence of an elevated alanine aminotransferase level or an aspartate aminotransferase level greater than 3 times the upper limit of normal were higher in the 3 idalopirdine dose groups (0.7%-3.3%) compared with the placebo groups (0%-1.3%) across studies. No patient fulfilled the criteria of the law of Hy (indicative of hepatic injury).
The frequency of serious adverse events was low across system organ classes. A numerically higher percentage of patients in the 60-mg dose of idalopirdine groups (6.5%-7.7%) and in the 30-mg dose groups (5.3%-5.8%) experienced serious adverse events compared with the 10-mg dose group (4.6%) and the placebo groups (3.9%-6.0%). No specific category of serious adverse events explained the overall difference and an association with idalopirdine treatment was not established.
A total of 3 deaths occurred among patients receiving placebo (n = 955) and 5 deaths occurred among those receiving idalopirdine (2 in the 30-mg dose groups [n = 594] and 3 in the 60-mg dose groups [n = 672]) across the studies. Examination of reported causes of death revealed no indication of a treatment-related pattern. There were no notable differences in suicidal ideation or suicidal behavior among the treatment groups in any study.
Sensitivity Analyses
The study results for the primary outcome remained unchanged in the sensitivity analyses using multiple imputation for patients withdrawn from the study based on values from the placebo group (eTable 2 in Supplement 1). Sensitivity analyses adjusting for site yielded results consistent with the main analysis that adjusted for country.
Discussion
In these 3 randomized double-blind, placebo-controlled trials conducted in 2525 patients with mild to moderate Alzheimer disease in 34 countries, 6 months of idalopirdine treatment added to background cholinesterase inhibitor therapy did not improve cognition or mitigate the decline in symptoms measured by performance on the ADAS-Cog, ADCS-ADL23, or ADCS-CGIC.
The hierarchical statistical testing strategy for each study dictated that (regardless of observations on key secondary outcomes, which measured domains other than cognition) failure to meet significance on the primary outcome equated to a null study. The findings for the key secondary outcomes measuring daily function (ADCS-ADL23) and global clinical outcome (ADCS-CGIC) appeared congruent with a null effect. The results on 3 different dementia domains provide strong support for the absence of efficacy.
This study showed no efficacy in contrast to a phase 2 study that supported the potential efficacy of adding idalopirdine to a cholinesterase inhibitor. There were several major differences between the phase 2 study and the phase 3 program. These differences were in (1) dose (90 mg/d in phase 2 vs 10, 30, and 60 mg/d in phase 3) and schedule (30 mg taken 3 times per day in phase 2 vs 10, 30, and 60 mg/d in phase 3), (2) sample population (the phase 3 program broadened the range of cognitive impairment [MMSE score stratum of 12-22 vs 12-19 in the phase 2 study]), (3) scope of the phase 3 program, which was a multinational program conducted at hundreds of sites, and (4) allowable type of background cholinesterase inhibitor use (galantamine and rivastigmine also were allowed in study 3).
A different schedule and lower daily dose of idalopirdine were used in the phase 3 program compared with the phase 2 study. The results of an in vivo positron emission tomography receptor occupancy study conducted among healthy participants taking idalopirdine, which became available after completion of the phase 2 study, were used to guide dose and schedule selection in the phase 3 studies, as suggested by the US Food and Drug Administration. A high 5-HT6 receptor occupancy (>80%) was achieved at doses of 30 and 60 mg/d of idalopirdine, which was maintained for 24 hours, supporting the use of once daily dosing. Although there is an approximate 40% decrease in 5-HT6 receptor expression among patients with Alzheimer disease compared with healthy individuals, there are also potential limitations in translating occupancy data from healthy individuals to those with a diseased brain.
These 3 studies have several strengths. Each study tested multiple doses; used appropriate instruments for stage and purpose to assess multiple dementia domains and adverse events; had standardized training and monitoring of study site staff; used central monitoring of data and assessment methods; and had high completion rates. The demographics of the study patients were also representative of Alzheimer disease populations (eg, approximate mean age of 74 years; 65% were women; and 55%-60% were APOE ε4 carriers). Several characteristics support the external validity of this program, including the geographic, ethnic, and cultural diversity of the patients; inclusion of a broad range of cognitive scores (MMSE scores of 12-22); and relatively liberal inclusion criteria (eg, age ≥50 years with no upper limit, no central reading of brain magnetic resonance imaging or computed tomography, no requirement for Alzheimer disease profile positivity with amyloid positron emission tomography or cerebrospinal fluid profile). This phase 3 program also allowed concomitant use of any cholinesterase inhibitor (not just donepezil) as background treatment in study 3.
Placing the results of this study in the broader context of the 5-HT6 antagonism adjunctive to cholinesterase inhibitor therapy mechanism of action suggests a lack of efficacy for this approach in the treatment of Alzheimer disease. In the phase 3 MINDSET study, intepirdine (another 5-HT6 antagonist) was administered at a dose of 35 mg daily (added to background donepezil therapy) in patients with mild to moderate Alzheimer disease and it failed to meet cognitive and functional efficacy outcomes. The negative results from the phase 3 MINDSET study run counter to the phase 2 results with intepirdine that had suggested a potential benefit in Alzheimer disease.
Limitations
The 3 studies have limitations. There was no requirement for evidence of Alzheimer disease biomarker positivity for inclusion, which may have allowed some patients to be included without having Alzheimer disease pathology. In addition, background therapy with memantine was not allowed, which may limit extrapolation of the results to patients with Alzheimer disease with similar MMSE scores (ie, MMSE scores of 12-22) who are taking background monotherapy with memantine, or, more commonly, who are taking background combination therapy with a cholinesterase inhibitor and memantine.
Conclusions
In patients with mild to moderate Alzheimer disease, the use of idalopirdine compared with placebo did not improve cognition over 24 weeks of treatment. These findings do not support the use of idalopirdine for the treatment of Alzheimer disease.
eFigure 1. Idalopirdine phase III development program
eFigure 2. Patient-level baseline to week 24 changes across efficacy primary and key-secondary endpoints
eMethods. Additional assessments and statistical methodology
eTable 1. Observed change from baseline in key secondary endpoints (full analysis set)
eTable 2. Sensitivity analysis of primary endpoint using multiple imputation from the placebo group (full analysis set)
Trial protocol for study 1
Statistical analysis plan for study 1
Trial protocol for study 2
Statistical analysis plan for study 2
Trial protocol for study 3
Statistical analysis plan for study 3
References
- 1.Alzheimer’s Association 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12(4):459-509. [DOI] [PubMed] [Google Scholar]
- 2.Schneider LS. A critical review of cholinesterase inhibitors as a treatment modality in Alzheimer’s disease. Dialogues Clin Neurosci. 2000;2(2):111-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farlow MR, Salloway S, Tariot PN, et al. . Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer’s disease: a 24-week, randomized, double-blind study. Clin Ther. 2010;32(7):1234-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodríguez JJ, Noristani HN, Verkhratsky A. The serotonergic system in ageing and Alzheimer’s disease. Prog Neurobiol. 2012;99(1):15-41. [DOI] [PubMed] [Google Scholar]
- 5.Upton N, Chuang TT, Hunter AJ, Virley DJ. 5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer’s disease. Neurotherapeutics. 2008;5(3):458-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramírez MJ. 5-HT6 receptors and Alzheimer’s disease. Alzheimers Res Ther. 2013;5(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geldenhuys WJ, Van der Schyf CJ. Role of serotonin in Alzheimer’s disease: a new therapeutic target? CNS Drugs. 2011;25(9):765-781. [DOI] [PubMed] [Google Scholar]
- 8.Maher-Edwards G, Zvartau-Hind M, Hunter AJ, et al. . Double-blind, controlled phase II study of a 5-HT6 receptor antagonist, SB-742457, in Alzheimer’s disease. Curr Alzheimer Res. 2010;7(5):374-385. [DOI] [PubMed] [Google Scholar]
- 9.Maher-Edwards G, Dixon R, Hunter J, et al. . SB-742457 and donepezil in Alzheimer disease: a randomized, placebo-controlled study. Int J Geriatr Psychiatry. 2011;26(5):536-544. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson D, Windfeld K, Colding-Jørgensen E. Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer’s disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2014;13(11):1092-1099. [DOI] [PubMed] [Google Scholar]
- 11.Maher-Edwards G, Watson C, Ascher J, et al. . Two randomized controlled trials of SB742457 in mild-to-moderate Alzheimer’s disease. Alzheimers Dement. 2015;1(1):23-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services; US Food and Drug Administration ICH harmonised tripartite guideline E6: guideline for good clinical practice. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073122.pdf. Accessed October 31, 2016.
- 13.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. [DOI] [PubMed] [Google Scholar]
- 15.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141(11):1356-1364. [DOI] [PubMed] [Google Scholar]
- 16.Galasko D, Bennett D, Sano M, et al. . An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S33-S39. [PubMed] [Google Scholar]
- 17.Schneider LS, Olin JT, Doody RS, et al. . Validity and reliability of the Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S22-S32. [DOI] [PubMed] [Google Scholar]
- 18.Posner K, Brown GK, Stanley B, et al. . The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services; US Food and Drug Administration; Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research Guidance for industry: drug-induced liver injury: premarketing clinical evaluation. https://www.fda.gov/downloads/Guidances/UCM174090.pdf. Accessed December 7, 2017.
- 20.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayele BT, Lipkovich I, Molenberghs G, Mallinckrodt CH. A multiple-imputation-based approach to sensitivity analyses and effectiveness assessments in longitudinal clinical trials. J Biopharm Stat. 2014;24(2):211-228. [DOI] [PubMed] [Google Scholar]
- 22.Bretz F, Maurer W, Brannath W, Posch M. A graphical approach to sequentially rejective multiple test procedures. Stat Med. 2009;28(4):586-604. [DOI] [PubMed] [Google Scholar]
- 23.US Department of Health and Human Services; US Food and Drug Administration; Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research Guidance for industry: exposure-response relationships—study design, data analysis, and regulatory applications. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm072109.pdf. Accessed December 7, 2017.
- 24.Schmidt E, Kuwabara H, Areberg J, et al. . A clinical positron emission tomography (PET) study investigating occupancy at the 5-HT6 receptor after multiple oral doses of LU AE58054 to healthy men. Alzheimers Dement. 2014;10(4):P925. [Google Scholar]
- 25.Lorke DE, Lu G, Cho E, Yew DT. Serotonin 5-HT2A and 5-HT6 receptors in the prefrontal cortex of Alzheimer and normal aging patients. BMC Neurosci. 2006;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Axovant Sciences Inc Axovant announces negative topline results of intepirdine phase 3 MINDSET trial in Alzheimer's disease. http://investors.axovant.com/news-releases/news-release-details/axovant-announces-negative-topline-results-intepirdine-phase-3. Accessed November 28, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Idalopirdine phase III development program
eFigure 2. Patient-level baseline to week 24 changes across efficacy primary and key-secondary endpoints
eMethods. Additional assessments and statistical methodology
eTable 1. Observed change from baseline in key secondary endpoints (full analysis set)
eTable 2. Sensitivity analysis of primary endpoint using multiple imputation from the placebo group (full analysis set)
Trial protocol for study 1
Statistical analysis plan for study 1
Trial protocol for study 2
Statistical analysis plan for study 2
Trial protocol for study 3
Statistical analysis plan for study 3