Abstract
Importance
Nasal airway obstruction (NAO) is a common problem that affects patient quality of life. Surgical success for NAO correction is variable. Virtual surgery planning via computational fluid dynamics (CFD) has the potential to improve the success rates of NAO surgery.
Objective
To elicit surgeon feedback of a virtual surgery planning tool for NAO and to determine if this tool affects surgeon decision making.
Design, Setting, and Participants
For this cross-sectional study, 60-minute face-to-face interviews with board-certified otolaryngologists were conducted at a single academic otolaryngology department from September 16, 2016, through October 7, 2016. Virtual surgery methods were introduced, and surgeons were able to interact with the virtual surgery planning tool interface. Surgeons were provided with a patient case of NAO, and open feedback of the platform was obtained, with emphasis on surgical decision making.
Main Outcomes and Measures
Likert scale responses and qualitative feedback were collected for the virtual surgery planning tool and its influence on surgeon decision making.
Results
Our 9 study participants were all male, board-certified otolaryngologists with a mean (range) 15 (4-28) number of years in practice and a mean (range) number of nasal surgeries per month at 2.2 (0.0-6.0). When examined on a scale of 1 (not at all) to 5 (completely), surgeon mean (SD) score was 3.4 (0.5) for how realistic the virtual models were compared with actual surgery. On the same scale, when asked how much the virtual surgery planning tool changed surgeon decision making, mean (SD) score was 2.6 (1.6). On a scale of 1 (strongly disagree) to 7 (strongly agree), surgeon scores for perceived usefulness of the technology and attitude toward using it were 5.1 (1.1) and 5.7 (0.9), respectively.
Conclusions and Relevance
Our study shows positive surgeon experience with a virtual surgery planning tool for NAO based on CFD simulations. Surgeons felt that future applications and areas of study of the virtual surgery planning tool include its potential role for patient counseling, selecting appropriate surgical candidates, and identifying which anatomical structures should be targeted for surgical correction.
Level of Evidence
NA.
This cross-sectional study examines survey data from surgeons to determine whether virtual surgery planning via computational fluid dynamics for nasal airway obstruction procedures affects surgeon decision making.
Key Points
Question
What is surgeon feedback in regards to a virtual surgery planning tool using computational fluid dynamics (CFD) for nasal airway obstruction (NAO), and can this tool influence surgeon decision making?
Findings
In this cross-sectional study of 9 surgeons at a single academic institution, surgeons had overall positive feedback on the virtual surgery planning tool. Surgeon decision making was in some cases influenced by the tool, and potential applications of this tool in clinical care were examined.
Meaning
A virtual surgery planning tool for NAO using CFD was perceived positively by our pilot group of surgeons who see potential applications of this technology that would need further study.
Introduction
Nasal airway obstruction (NAO) is a common reason for referral to an otolaryngology or facial plastic surgery clinic, affects quality of life in all age groups, and has an estimated economic burden upwards of $5 billion annually. More than half of sinonasal procedures in 2006 were for septoplasty and/or turbinate surgery. Despite surgical correction for NAO, short-term studies report failure rates as high as 20% to 37%, with long-term studies reporting even higher failure over time. Treating patients with NAO is a challenge because there is a lack of reliable objective measures that correlate with patient symptoms. Currently, the decision to proceed with surgery for correction of NAO and the targets for anatomic correction are based on surgeon experience and intuition. Objective methods are needed to guide preoperative decision making to improve the success rates of NAO surgery.
Owing to the complex nature of the nasal airway, analysis with computational modeling tools can aid surgeons in decision making. Recent literature using 3-dimensional (3-D) nasal airway modeling and computational fluid dynamics (CFD) have been able to identify CFD-derived physiologic variables such as airflow and mucosal cooling that correlate with subjective nasal patency scores. These 3-D nasal airway models combined with CFD provide a promising future for use in virtual surgery planning where specific anatomic areas can be targeted to demonstrate changes to the CFD-derived physiologic variables.
Virtual reality training has been used in otolaryngology with most of the applications related to endoscopic surgery or temporal bone surgery. Virtual surgery is also used for plate design in mandibular reconstruction. However, there are very few virtual surgery planning applications within the field of otolaryngology. At present, there is no virtual surgery tool for patients with NAO.
Establishing this novel method will only be beneficial if the technology is accepted by the practicing surgeons who will actually use the technology. While health technologies have become more commonplace and have potential benefits, their value ultimately depends on health care providers perceiving the technology favorably, accepting it, and appropriately using it for patient care. Thus, the purpose of this study was 3-fold. Our first goal was to provide practicing otolaryngologists an opportunity to interact with a prototype CFD virtual surgery planning tool and elicit their feedback in regards to the tool’s usefulness and potential applications. Second, we wanted to determine if the planning tool would have any influence on surgeons’ choice of surgery for an actual case of a patient with NAO. The third goal was to obtain feedback from practicing surgeons on the prototype design of the technology interface, so that the technology interface can be refined to effectively communicate virtual surgery predictions to surgeons.
Methods
Study Participants
The institutional review board at the Medical College of Wisconsin approved this study with informed consent obtained from each participant. In this pilot study, we aimed to collect feedback on a prototype virtual surgery dashboard from board-certified surgeons at our institution who have varying levels of experience with nasal surgery and included both surgeons who perform several cases per month and surgeons who perform a few cases per year.
Patient Case
An actual patient case was presented to surgeons and included a preoperative computed tomography (CT) scan for review (eFigure 1 in the Supplement). This patient case was part of a previous study and institutional review board approval and informed consent was obtained prior to enrollment. No patient identifiers were presented to surgeons. The patient described was a 27-year-old woman with long-standing right-sided nasal obstruction. Her preoperative Nasal Obstruction Symptom Evaluation (NOSE) score was 80. She had a C-shaped septal deformity with convexity on the right side causing obstruction on that side with contralateral inferior turbinate enlargement (eFigure 1 in the Supplement).
Virtual Surgery Models
A 3-D model of the nasal cavity anatomy was created based on the presurgery CT scan in Mimics (Materialise Inc) (magnetic resonance imaging can also be used to create the 3-D model, but in this study a CT scan was used). Virtual surgery models were created representing the following 7 possible surgical procedures: septoplasty alone, inferior turbinate reduction alone (left side only, right side only, or bilateral), and all possible combinations of septoplasty and inferior turbinate reduction(s) (eFigure 2 in the Supplement). Computational fluid dynamics simulations were run to quantify nasal airflow variables in the presurgery model and in all virtual surgery models using methods described in the Supplement.
Virtual Surgery Dashboard
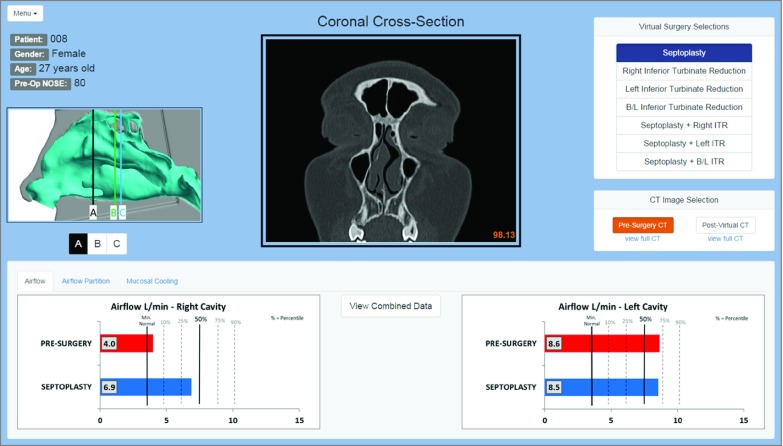
To provide surgeons with a technology interface, we created a virtual surgery dashboard as a web browser application using the NW.js platform (Figure 1). The dashboard allowed surgeons to select from the 7 different surgical procedures described above and view both the presurgery CT scan and simulated postsurgery CT scans for each virtual surgery scenario (eFigure 2 in the Supplement). Computational fluid dynamics measures of nasal airflow for each virtual surgery procedure were displayed both numerically and with bar plots (Figure 1). The following measures of nasal airflow were reported in the dashboard: unilateral airflow rate, airflow partition between nostrils, and unilateral surface area stimulated by mucosal cooling. These CFD variables were selected based on previous work suggesting that these 3 variables have the strongest correlation with subjective nasal patency. The dashboard included the normative range for each CFD variable based a cohort of 47 healthy subjects (Figure 1) (eFigure 3 in the Supplement).
Figure 1. Virtual Surgery Dashboard.
Seven virtual surgery scenarios are available to choose from at the top right of the dashboard. Computed tomography (CT) imaging and computational fluid dynamics variable predictions change with the different surgical selections. For each virtual surgery procedure, surgeons can view a presurgery CT or simulated postsurgery CT at 3 different coronal sections (A, B, C) whose locations are displayed in the box on the left. The full presurgery coronal CT as well as a full virtual surgery coronal CT are also available to view. Computational fluid dynamics variable predictions are listed at the bottom of the screen with separate tabs for airflow, airflow partition, and mucosal cooling. Both right and left nasal cavity values are shown, with graphs showing the presurgery value and the predicted value based on the specific virtual procedure. A combined graph that lists all 3 variables in the left and right cavities for all surgical combinations is also available for review (eFigure 3 in the Supplement) by clicking the button at the center of the screen.
Because several surgical scenarios predicted airflow variables within the normal range, a criterion was needed to select the CFD-recommended surgery. We reasoned that performing less surgery would have lower morbidity, require less time in the operating room (OR), and reduce costs. We also estimated that unilateral inferior turbinate reduction alone would require less time in the OR than septoplasty alone (approximately 15 minutes vs 30 minutes, respectively). Thus, the CFD-recommended surgery was defined as the procedure that normalized all airflow variables close to the 50th percentile, while minimizing surgical time. Based on these criteria, our virtual surgery models and CFD simulations predicted that septoplasty would be the “best” procedure for our patient case.
Questionnaire and Interview
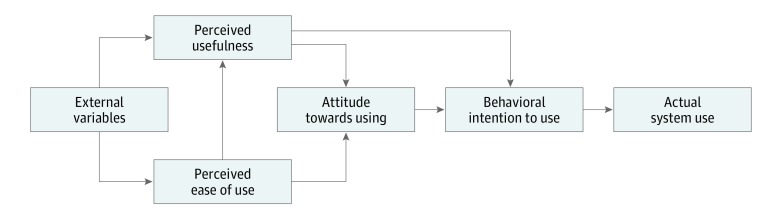
Our survey questionnaire was developed based on the Technology Acceptance Model (Figure 2). This model was developed in the 1980s to understand why workers were not using information technology available to them. To increase use of a technology, the technology must first be accepted, and the factors that affect its acceptance must be evaluated. Based on this model (Figure 2), external factors affect both perceived usefulness and perceived ease of use, which, in turn, affect attitude toward use. An improved attitude toward use will lead to behavioral intention to use. Once the technology has been accepted, this will then lead to actual use.
Figure 2. The Technology Acceptance Model.
Diagram of the interplay among factors influencing use of a new technology, adapted from Holden and Karsh.
Surgeons participated in a 60-minute face-to-face interview with the same member of our research team (D.L.V.) who was the only person who had access to surgeon identifiers. This information was blinded from Drs Rhee and Pawar, who are cofaculty of these surgeons at our institution. Surgeons were provided with a brief presentation on CFD technology and a summary of previous studies on the correlation of CFD variables with subjective nasal patency. Then, we explained to surgeons how the virtual surgery models were created and the 7 possible surgical combinations. The patient case was presented to surgeons, and they were given the opportunity to rank their surgical choices for this patient among the 7 possible options. The virtual surgery dashboard was then presented. After surgeons were given time to review the virtual surgery predictions, surgeons were asked to reevaluate their rank of surgical choices. Surgeon feedback was recorded throughout this interview process via a structured questionnaire.
Data Analysis
This pilot study focused on obtaining feedback from practicing surgeons to refine the virtual surgery dashboard. Based on the Technology Acceptance Model, our questionnaire asked open-ended questions and Likert scale questions, as is standard in Technology Acceptance Model research. The Cronbach α, a measure of internal consistency, was calculated where applicable (Cronbach α > 0.70 indicates internal consistency). Based on methods of qualitative data analysis, we used both inductive and deductive approaches to identify strengths and weaknesses of the technology and also to identify general concepts and overall categories from the specific feedback. Our conventional approach evaluated text data to establish categories focusing solely on manifest content. Quantitative data, including the Likert scale data, were collected where applicable and were represented by descriptive analysis.
Results
Demographics
Our 9 study participants were all male, board-certified otolaryngologists at our academic institution, and 7 white and 2 Asian. Mean (SD) years in practice was 15 (8) years with a range of 4 to 28 years. Mean (SD) nasal surgeries performed in a month (septoplasty, inferior turbinate reduction, nasal valve repair, and functional rhinoplasty) was 2.2 (2.4) with range of 0 to 6 surgeries a month (with some surgeons performing only a few a year).
Acceptability of Virtual Surgery Models
When surgeons were asked how well does the virtual surgery model replicate what could be performed in the operating room, on a scale of 1 (not at all) to 5 (completely), the mean (SD) score was 3.4 (0.5). In regards to how accurately the virtual surgery models replicate what would be a realistic outcome after healing on the same scale, the mean (SD) score was also 3.4 (0.5) (eTable 1 in the Supplement). Strengths and weaknesses of the model noted by the surgeons are listed in Table 1 and Table 2, respectively. There were many different opinions regarding the accuracy of the virtual inferior turbinate reduction model.
Table 1. Surgeon Feedback on the Strengths of the Computational Fluid Dynamics Models, the Virtual Surgery Dashboard, and the Influence on Surgical Decision Making.
| Topic Strengthsa | Category | Surgeon Statements |
|---|---|---|
| Model acceptability | Accuracy of septoplasty | “The septoplasty is not too idealized.” |
| Model influence on surgical decision making | Determining need for turbinate reduction | “If you can get by with the septoplasty, that is great because the turbinates come back often.” |
| Objective data | “It helps me be more objective in my surgical decision making.” | |
| Ease of use | Organized | “…intuitive very quickly.” |
| Visual | “…having both the graphs and the numbers.” | |
| Perceived usefulness | Patient counseling | “…the undecided patient and the patient who I am unsure how much benefit they will get from surgery.” |
| Quantification of surgical outcomes | “…an ability to track surgical outcomes.” | |
| Targeted surgery | “…great uses for the technology, addresses certain areas of the septum to give the most benefit.” | |
| Attitude toward using | Objective measure | “…adds an element of objectivity that correlates well with the subjective symptoms.” |
| Well-studied method | “This isn’t out of the blue; this has been studied and is well verified.” |
Topics correspond to the concepts in the Technology Acceptance Model (Figure 2) targeted by the questionnaire. Surgeon feedback to open-ended questions was reviewed and representative categories from these statements were created based on deductive qualitative methods.
Table 2. Surgeon Feedback on the Weaknesses of the Computational Fluid Dynamics Models, the Virtual Surgery Dashboard, and the Influence on Surgical Decision Making.
| Topic Weaknessesa | Category | Surgeon Statements |
|---|---|---|
| Model acceptability | Accuracy of turbinate reduction model | “I would also do a turbinate outfracture.” |
| Unpredictability of healing | “Turbinates come back and won’t stay like that.” | |
| Model influence on surgical decision making | Criterion to select best surgical procedure | “The additional procedures I would do would be to maximize the benefit…the maximal benefit in 1 procedure.” |
| Ease of use | Additional information wanted | “I would like a patient case for review.” “I want to see time estimates for surgery.” |
| Limitations on presentation of data | “I would like to modify the model in real-time.” “I would like the bilateral information.” |
|
| Perceived usefulness | CT scan | “I rarely use preoperative CT.” “…costs of a CT scan…” “…radiation exposure…” |
| Time | “Time required to create the post–virtual surgery models…” “Time involved to go through the models…” |
|
| Attitude toward using | Unfamiliar technology | “I can’t be absolutely certain until I have personally used this and confirmed the data…” |
| The art of medicine | “Apprehensive confidence based on my ability to replicate what is done on the computer…gives more information like how a CT or sleep study gives you more information, but doesn’t make your decision.” |
Abbreviation: CT, computed tomography.
Topics correspond to the concepts in the Technology Acceptance Model (Figure 2) targeted by the questionnaire. Surgeon feedback to open-ended questions was reviewed and representative categories from these statements were created based on inductive qualitative methods.
Virtual Surgery Influence on Surgeon’s Decision
When asked to rank which surgical procedure the surgeon was most likely to perform based on the case presentation alone, 6 chose septoplasty with bilateral inferior turbinate reduction, 2 chose septoplasty with left inferior turbinate reduction, and 1 chose septoplasty alone. When asked if surgeons would reconsider their initial surgical choice after being presented with the virtual surgery prediction that septoplasty alone was the “best” option, on a scale of 1 (not at all) to 5 (completely), mean (SD) score was 2.6 (1.6) with 4 surgeons who stated “not at all;” 2,“somewhat;” 2, “quite a lot;” and 1, “completely” (eTable 1 in the Supplement). Surgeons who had some consideration for changing their rankings mentioned the need for objective data to inform surgical decisions as a motivation to reconsider (Table 1). Contrary to our assumption that minimizing OR time was an important criterion to select the “best surgery,” some surgeons felt that the additional time, cost, and risk of a turbinate reduction was minimal and hence stated that they would still perform turbinate reduction to maximize the benefit of surgery (Table 2).
Ease of Use of the Virtual Surgery Dashboard
As the interface between surgeons and the CFD-based virtual surgery technology, our virtual surgery dashboard represents the ease of use portion of the Technology Acceptance Model with the strengths listed in Table 1 and the weaknesses listed in Table 2. The dashboard strengths include the organization and visual aspect of the presentation. Weaknesses include the inability to modify the models in real time and the desire for additional information.
Perceived Usefulness and Attitude Toward Using
Surgeons were asked Likert-scale questions (scale 1 [strongly disagree]-7 [strongly agree]) regarding the perceived usefulness of virtual surgery with CFD and their attitude toward using the technology. Mean (SD) for the 4 questions on perceived usefulness was 5.1 (1.1) with a Cronbach α of 0.9 (eTable 2 in the Supplement). Qualitative feedback from the questions regarding the perceived usefulness revealed categories that the CFD virtual surgery technology would provide a better tool for patient counseling, help make objective decisions, and select the surgical procedure with greatest benefit (Table 1). The major disadvantages of the technology were the time involved to both create the models and use them, the radiation exposure for the CT scan, and costs involved (Table 2).
Overall, surgeons had a positive attitude toward using virtual surgery planning for NAO, as demonstrated by a mean (SD) score of 5.7 (0.9) for the 3 questions probing their attitude toward using the technology (eTable 2 in the Supplement). The Cronbach α of 0.8 revealed internal consistency for questions in the “Attitude Towards Using” category. Specific surgeon feedback is listed in Table 1 and Table 2.
Applications of Virtual Surgery Technology
Examination of feedback from surgeons in regards to future applications of this virtual surgery technology led to 4 broad categories: patient counseling, determining appropriate surgical candidates, determining the anatomic site to target when operating, and other uses of this technology in otolaryngology (Table 3).
Table 3. Surgeon Feedback on Future Applications of Virtual Surgery Based on Computational Fluid Dynamics Simulations.
| Categorya | Specific Surgeon Feedback |
|---|---|
| Patient counseling | “…the undecided patient and the patient I am unsure how much benefit they will get from surgery.” “…the patient with symptoms out of proportion to their physical exam or radiographic findings.” “…benefit in the patient who you treat medically, and they say they have not improved…you can show them that they have improved.” |
| Determining appropriate surgical candidates | “…beneficial with patients who are higher surgical risk…and could predict if worth the risk to go to the operating room.” “…can show patients that surgery will not get them much benefit over their medical treatment.” |
| Anatomic site to target when operating |
“A septoplasty may have more benefit than just a turbinate…determining if turbinate [surgery] actually help.” “You could see the situations where doing the turbinate reduction whether bilateral or unilateral would be beneficial.” |
| Other applications in otolaryngology |
“Very helpful in preadolescent cleft patients with nasal obstruction because they have unique anatomic obstructions.” “…useful for a fixed obstruction, such as subglottic stenosis or tracheal stenosis, in order to know how wide to open to improve airflow.” |
Summary of the categories for future uses of virtual surgery.
Discussion
Virtual surgery planning based on CFD simulations of nasal airflow has the potential to improve surgical outcomes for NAO patients. However, this technology needs to be accepted and implemented by practicing surgeons to have a real-world effect in health care. To our knowledge, this study represents the first time that practicing surgeons have had an opportunity to interact with a prototype CFD virtual surgery tool for NAO with the goal of soliciting open feedback and discussion to refine the virtual surgery dashboard. Qualitative research is a well-established method in other areas of medicine but with relatively few studies within the field of otolaryngology. We used many of the concepts and ideas of qualitative research to gain important insights that could not have been obtained with numeric data alone.
Only 1 of the 9 surgeons selected septoplasty alone (the CFD-predicted “best surgical procedure”) for this patient before being presented with the virtual surgery predictions. The CFD simulations predicted slightly better values in regards to airflow, airflow partition, and mucosal cooling for septoplasty plus any combination of turbinate reduction as compared with septoplasty alone. However, the “best surgical procedure” was chosen based also on decreased operative time and thus decreased risk and cost. While 4 surgeons chose “not at all” when asked if the CFD predictions would make them reconsider their original decision, all 4 felt validated in their choices because CFD predicted a greater change in airflow variables with septoplasty combined with inferior turbinate reduction.
Some of the most interesting feedback was based on questions related to strengths of this technology and situations where this technology would be most helpful. Review of answers to these questions led to the 4 broad categories listed in Table 3. This technology could be a useful tool for patient counseling, especially in the scenario where surgery is not likely to improve patient outcomes. As stated in Table 3, the use of virtual surgery with CFD could provide an objective measure for surgeons to counsel patients with “symptoms out of proportion to their physical examination.” The use of virtual surgery with CFD could also be beneficial when deciding whether or not to operate on a patient with NAO. This tool would be useful in situations where the benefit of surgery may be minimal compared with medical management, or the benefit may be small compared with surgical risk. An example of this would be patients who are anticoagulated or who may have other comorbidities that put them at a higher surgical risk. Virtual surgery with CFD could also help predict which surgical procedure or anatomic targets may give patients the most benefit, which would be very beneficial if you could get acceptable results with the less morbid procedure of turbinate reduction alone vs also needing to perform a septoplasty. Furthermore, the virtual surgery tool could be used to test different versions of a surgical procedure (ie, targeting different areas of the septum or performing different variations of turbinectomy techniques).
Limitations
Our study is limited by the fact that this was a pilot study, conducted within a single academic institution with surgeons who have varying levels of experience with surgery for nasal airway obstruction. Also the surgeons at our institution with the most experience and most referrals for nasal airway obstruction (J.S.R. and S.S.P.) are members of our research team and were not interviewed for the study. Also, surgery for NAO is not limited to septoplasty and inferior turbinate reduction, but our virtual surgery dashboard did not include procedures for addressing the lateral nasal wall or for functional rhinoplasty. In addition, turbinate surgery is performed in a variety of ways and surgeons interviewed felt that this variability was not well reflected in the technique chosen for virtual inferior turbinate reduction (for example, it did not replicate turbinate outfracture). Finally, given our small sample size and focus on qualitative feedback, our study was not designed to produce quantitative conclusions based on a large sample size.
Conclusions
Our study is the first that we know of to introduce virtual surgery planning with CFD to surgeons in a real patient scenario and to evaluate if this virtual model had any effect on surgeon decision making. We focused on ease of use, perceived usefulness, and attitude toward using based on the Technology Acceptance Model (Figure 2) as a means to identify how to best implement this technology with practicing surgeons. Incorporation of the feedback obtained in this pilot study will allow for further refinement of this technology as a possible tool for surgeon decision making for patients with NAO. Future studies should focus on creating a more efficient dashboard as the surgeon-technology interface, including the ability to modify the nasal geometry in real time and update the corresponding airflow variables, so that the virtual surgery tool can be implemented in clinical practice.
eAppendix. Creation of Three-Dimensional Models and Virtual Surgery and CFD Simulations.
eTable 1. Surgeon feedback on acceptability of virtual surgery models.
eTable 2. Surgeon feedback on perceived usefulness and attitude towards using.
eFigure 1. Pre-surgery coronal CT of NAO patient.
eFigure 2. Coronal CT images illustrating the pre-surgery nasal anatomy and the seven different virtual surgery scenarios.
eFigure 3. Graph used on the dashboard to summarize the CFD results for all virtual surgery scenarios.
References
- 1.Teti VP, Akdagli S, Most SP. Cost-effectiveness of corticosteroid nasal spray vs surgical therapy in patients with severe to extreme anatomical nasal obstruction. JAMA Facial Plast Surg. 2016;18(3):165-170. [DOI] [PubMed] [Google Scholar]
- 2.Rhee JS, Book DT, Burzynski M, Smith TL. Quality of life assessment in nasal airway obstruction. Laryngoscope. 2003;113(7):1118-1122. [DOI] [PubMed] [Google Scholar]
- 3.Kimmelman CP. The problem of nasal obstruction. Otolaryngol Clin North Am. 1989;22(2):253-264. [PubMed] [Google Scholar]
- 4.Bhattacharyya N. Ambulatory sinus and nasal surgery in the United States. Laryngoscope. 2010;120(3):635-638. [DOI] [PubMed] [Google Scholar]
- 5.Dommerby H, Rasmussen OR, Rosborg J. Long-term results of septoplastic operations. ORL J Otorhinolaryngol Relat Spec. 1985;47(3):151-157. [DOI] [PubMed] [Google Scholar]
- 6.Fjermedal O, Saunte C, Pedersen S. Septoplasty and/or submucous resection? J Laryngol Otol. 1988;102(9):796-798. [DOI] [PubMed] [Google Scholar]
- 7.Samad I, Stevens HE, Maloney A. The efficacy of nasal septal surgery. J Otolaryngol. 1992;21(2):88-91. [PubMed] [Google Scholar]
- 8.Illum P. Septoplasty and compensatory inferior turbinate hypertrophy. Eur Arch Otorhinolaryngol. 1997;254(suppl 1):S89-S92. [DOI] [PubMed] [Google Scholar]
- 9.André RF, D’Souza AR, Kunst HP, Vuyk HD. Sub-alar batten grafts as treatment for nasal valve incompetence. Rhinology. 2006;44(2):118-122. [PubMed] [Google Scholar]
- 10.Konstantinidis I, Triaridis S, Triaridis A, Karagiannidis K, Kontzoglou G. Long term results following nasal septal surgery. Auris Nasus Larynx. 2005;32(4):369-374. [DOI] [PubMed] [Google Scholar]
- 11.Sundh C, Sunnergren O. Long-term symptom relief after septoplasty. Eur Arch Otorhinolaryngol. 2015;272(10):2871-2875. [DOI] [PubMed] [Google Scholar]
- 12.Ho WK, Yuen APW, Tang KC, Wei WI, Lam PKY. Time course in the relief of nasal blockage after septal and turbinate surgery. Arch Otolaryngol Head Neck Surg. 2004;130(3):324-328. [DOI] [PubMed] [Google Scholar]
- 13.Jessen M, Ivarsson A, Malm L. Nasal airway resistance and symptoms after functional septoplasty. Clin Otolaryngol Allied Sci. 1989;14(3):231-234. [DOI] [PubMed] [Google Scholar]
- 14.Dinis PB, Haider H. Septoplasty: long-term evaluation of results. Am J Otolaryngol. 2002;23(2):85-90. [DOI] [PubMed] [Google Scholar]
- 15.Lam DJ, James KT, Weaver EM. Comparison of anatomic, physiological, and subjective measures of the nasal airway. Am J Rhinol. 2006;20(5):463-470. [DOI] [PubMed] [Google Scholar]
- 16.André RF, Vuyk HD, Ahmed A, Graamans K, Nolst Trenité GJ. Correlation between subjective and objective evaluation of the nasal airway. Clin Otolaryngol. 2009;34(6):518-525. [DOI] [PubMed] [Google Scholar]
- 17.Zhao K, Blacker K, Luo Y, Bryant B, Jiang J. Perceiving nasal patency through mucosal cooling rather than air temperature or nasal resistance. PLoS One. 2011;6(10):e24618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardcastle PF, White A, Prescott RJ. Clinical or rhinometric assessment of the nasal airway—which is better? Clin Otolaryngol Allied Sci. 1988;13(5):381-385. [DOI] [PubMed] [Google Scholar]
- 19.Hardcastle PF, White A, Prescott RJ. Clinical and rhinometric assessment of the nasal airway—do they measure the same entity? Clin Otolaryngol Allied Sci. 1988;13(3):185-191. [DOI] [PubMed] [Google Scholar]
- 20.Thulesius HL, Cervin A, Jessen M. Can we always trust rhinomanometry? Rhinology. 2011;49(1):46-52. [DOI] [PubMed] [Google Scholar]
- 21.Bailey RS, Casey KP, Pawar SS, Garcia GJM. Nasal mucosal temperature and its potential correlation to subjective nasal patency in healthy individuals. JAMA Facial Plast Surg. 2017;19(1):46-52. doi: 10.1001/jamafacial.2016.1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roblin DG, Eccles R. What, if any, is the value of septal surgery? Clin Otolaryngol Allied Sci. 2002;27(2):77-80. [DOI] [PubMed] [Google Scholar]
- 23.Kimbell JS, Frank DO, Laud P, Garcia GJ, Rhee JS. Changes in nasal airflow and heat transfer correlate with symptom improvement after surgery for nasal obstruction. J Biomech. 2013;46(15):2634-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan CD, Garcia GJ, Frank-Ito DO, Kimbell JS, Rhee JS. Perception of better nasal patency correlates with increased mucosal cooling after surgery for nasal obstruction. Otolaryngol Head Neck Surg. 2014;150(1):139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaberino C, Rhee JS, Garcia GJ. Estimates of nasal airflow at the nasal cycle mid-point improve the correlation between objective and subjective measures of nasal patency. Respir Physiol Neurobiol. 2017;238:23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casey KP, Borojeni AA, Koenig LJ, Rhee JS, Garcia GJ. Correlation between subjective nasal patency and intranasal airflow distribution. Otolaryngol Head Neck Surg. 2017;156(4):741-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piromchai P, Avery A, Laopaiboon M, Kennedy G, O’Leary S. Virtual reality training for improving the skills needed for performing surgery of the ear, nose or throat. Cochrane Database Syst Rev. 2015;(9):CD010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirsch DL, Garfein ES, Christensen AM, Weimer KA, Saddeh PB, Levine JP. Use of computer-aided design and computer-aided manufacturing to produce orthognathically ideal surgical outcomes. J Oral Maxillofac Surg. 2009;67(10):2115-2122. [DOI] [PubMed] [Google Scholar]
- 29.Zweifel DF, Simon C, Hoarau R, Pasche P, Broome M. Are virtual planning and guided surgery for head and neck reconstruction economically viable? J Oral Maxillofac Surg. 2015;73(1):170-175. [DOI] [PubMed] [Google Scholar]
- 30.Holden RJ. Physicians’ beliefs about using EMR and CPOE: in pursuit of a contextualized understanding of health IT use behavior. Int J Med Inform. 2010;79(2):71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel VL, Kannampallil TG. Human factors and health information technology. Yearb Med Inform. 2014;9:58-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130(2):157-163. [DOI] [PubMed] [Google Scholar]
- 33.Rhee JS, Sullivan CD, Frank DO, Kimbell JS, Garcia GJ. A systematic review of patient-reported nasal obstruction scores. JAMA Facial Plast Surg. 2014;16(3):219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia GJ, Bailie N, Martins DA, Kimbell JS. Atrophic rhinitis: a CFD study of air conditioning in the nasal cavity. J Appl Physiol (1985). 2007;103(3):1082-1092. [DOI] [PubMed] [Google Scholar]
- 35.Borojeni ATA, Frank-Ito DO, Kimbell JS, Rhee JS, Garcia GJM. Creation of an idealized nasopharynx geometry for accurate computational fluid dynamics simulations of nasal airflow in patient-specific models lacking the nasopharynx anatomy. Int J Numer Methods Biomed Eng. 2017;33:e2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holden RJ, Karsh B-T. The technology acceptance model: its past and its future in health care. J Biomed Inform. 2010;43(1):159-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis FD, Bagozzi RP, Warshaw PR. User acceptance of computer technology. Manage Sci. 1989;35:982-1003. [Google Scholar]
- 38.Nunnally JC, Bernstein IH. Psychometric theory. 3rd ed New York:McGraw-Hill;1994. [Google Scholar]
- 39.Elliott R, Fischer CT, Rennie DL. Evolving guidelines for publication of qualitative research studies in psychology and related fields. Br J Clin Psychol. 1999;38(Pt 3):215-229. [DOI] [PubMed] [Google Scholar]
- 40.Creswell JW, Klassen AC, Plano Clark VL, Smith KC; for the Office of Behavioral and Social Sciences Research. Best practices for mixed methods research in the health sciences. National Institutes of Health. 2011. https://obssr.od.nih.gov/wp-content/uploads/2016/01/Best_Practices_for_Mixed_Methods_Research_acknowledgement.pdf. Accessed August 17, 2017.
- 41.Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. [DOI] [PubMed] [Google Scholar]
- 42.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288. [DOI] [PubMed] [Google Scholar]
- 43.Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24(2):105-112. [DOI] [PubMed] [Google Scholar]
- 44.Chen XB, Lee HP, Chong VF, Wang Y. Assessment of septal deviation effects on nasal air flow. Laryngoscope. 2009;119(9):1730-1736. [DOI] [PubMed] [Google Scholar]
- 45.Chen XB, Leong SC, Lee HP, Chong VF, Wang DY. Aerodynamic effects of inferior turbinate surgery on nasal airflow. Rhinology. 2010;48(4):394-400. [DOI] [PubMed] [Google Scholar]
- 46.Na Y, Chung KS, Chung SK, Kim SK. Effects of single-sided inferior turbinectomy on nasal function and airflow characteristics. Respir Physiol Neurobiol. 2012;180(2-3):289-297. [DOI] [PubMed] [Google Scholar]
- 47.Frank-Ito DO, Kimbell JS, Laud P, Garcia GJ, Rhee JS. Predicting postsurgery nasal physiology with computational modeling. Otolaryngol Head Neck Surg. 2014;151(5):751-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhee JS, Pawar SS, Garcia GJ, Kimbell JS. Toward personalized nasal surgery using computational fluid dynamics. Arch Facial Plast Surg. 2011;13(5):305-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hariri BM, Rhee JS, Garcia GJ. Identifying patients who may benefit from inferior turbinate reduction using computer simulations. Laryngoscope. 2015;125(12):2635-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia GJ, Rhee JS, Senior BA, Kimbell JS. Septal deviation and nasal resistance: an investigation using virtual surgery and computational fluid dynamics. Am J Rhinol Allergy. 2010;24(1):e46-e53. [DOI] [PubMed] [Google Scholar]
- 51.Bunne M. Qualitative research methods in otorhinolaryngology. Int J Pediatr Otorhinolaryngol. 1999;51(1):1-10. [DOI] [PubMed] [Google Scholar]
- 52.Gorodzinsky AY, Hong P, Chorney JM. Parental knowledge in pediatric otolaryngology surgical consultations. Int J Pediatr Otorhinolaryngol. 2015;79(7):1135-1139. [DOI] [PubMed] [Google Scholar]
- 53.Hudson A, Macdonald M, Blake K. Packing and problematic feeding behaviors in CHARGE syndrome. Int J Pediatr Otorhinolaryngol. 2016;82:107-115. [DOI] [PubMed] [Google Scholar]
- 54.Cole S, Arnold M, Sanderson A, Cupp C. Pregnancy during otolaryngology residency. Am Surg. 2009;75(5):411-415. [PubMed] [Google Scholar]
- 55.Tsang GF, McKnight CL, Kim LM, Lee JM. Exploring the psychological morbidity of waiting for sinus surgery using a mixed methods approach. J Otolaryngol Head Neck Surg. 2016;45(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Creation of Three-Dimensional Models and Virtual Surgery and CFD Simulations.
eTable 1. Surgeon feedback on acceptability of virtual surgery models.
eTable 2. Surgeon feedback on perceived usefulness and attitude towards using.
eFigure 1. Pre-surgery coronal CT of NAO patient.
eFigure 2. Coronal CT images illustrating the pre-surgery nasal anatomy and the seven different virtual surgery scenarios.
eFigure 3. Graph used on the dashboard to summarize the CFD results for all virtual surgery scenarios.