Abstract
Despite the high efficacy and safety of arsenic trioxide (ATO) in treating acute promyelocytic leukemia (APL) and eradicating APL leukemia-initiating cells (LICs), the mechanism underlying its selective cytotoxicity remains elusive. We have recently demonstrated that APL cells undergo a novel cell death program, termed ETosis, through autophagy. However, the role of ETosis in ATO-induced APL LIC eradication remains unclear. For this study, we evaluated the effects of ATO on ETosis and the contributions of drug-induced ETosis to APL LIC eradication. In NB4 cells, ATO primarily increased ETosis at moderate concentrations (0.5–0.75 μM) and stimulated apoptosis at higher doses (1.0–2.0 μM). Furthermore, ATO induced ETosis through mammalian target of rapamycin (mTOR)-dependent autophagy, which was partially regulated by reactive oxygen species. Additionally, rapamycin-enhanced ATO-induced ETosis in NB4 cells and APL cells from newly diagnosed and relapsed patients. In contrast, rapamycin had no effect on apoptosis in these cells. We also noted that PML/RARA oncoprotein was effectively cleared with this combination. Intriguingly, activation of autophagy with rapamycin-enhanced APL LIC eradication clearance by ATO in vitro and in a xenograft APL model, while inhibition of autophagy spared clonogenic cells. Our current results show that ATO exerts antileukemic effects at least partially through ETosis and targets LICs primarily through ETosis. Addition of drugs that target the ETotic pathway could be a promising therapeutic strategy to further eradicate LICs and reduce relapse.
Introduction
Acute promyelocytic leukemia (APL) is a hematological malignancy driven by a t(15;17) chromosomal translocation that generates the promyelocytic leukemia-retinoic acid receptor (PML/RARα) fusion gene1,2. The prognosis for patients with APL has been revolutionized by the use of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), both of which target PML/RARα for degradation3,4. Recently, benefits from ATO-including therapy in APL have sparked new interest in ATO. For example, patients receiving ATO plus ATRA induction therapy experienced fewer relapses and faster complete remission compared to patients receiving standard ATRA chemotherapy5–8. ATO induces high rates of complete hematologic remission (CR) and molecular remission (CMR) followed by a long relapse-free survival9. Despite the remarkable improvement in treatment outcomes in APL, refractory and relapse remain clinically significant problems10. Thus, further understanding of the antileukemic mechanisms of ATO when treating newly diagnosed APL and/or relapse is urgently needed.
It is known that treatment by standard chemotherapy reagents induces apoptosis while ATRA results in differentiation3. However, APL relapse occurs because leukemia-initiating cells (LICs) remain untouched by conventional chemotherapy and even ATRA-monotherapy11,12, in contrast to ATO therapy, which implies that neither apoptosis or differentiation induction is sufficient to eradicate LICs. It is attractive to speculate whether another uncovered LIC death program exists, which can be induced by ATO. Autophagy contributes to arsenic-induced PML/RARα degradation13, which is responsible for LIC loss in APL cells14,15, and it is also widely proposed to account for arsenic-induced cell death16–18. However, these studies did not fully address the questions of whether or how autophagy leads to LIC death by ATO.
First described as an alternative route of bacterial killing in 2004, the formation of neutrophil extracellular traps (NETs) (ETs) is a process of cell death distinct from apoptosis, which has since been referred to as NETosis19–21. Formed mainly by immune cells, ETs can also be released by human leukemia cells when exposed to microorganisms, reactive oxygen species (ROS) or tunicamycin22,23. Studies from our laboratory have shown that APL cells from patients can also undergo this novel cell death process, producing ETs through autophagy24,25, that has been linked to the mechanisms of ATO. More interestingly, ATRA promotes ETosis leading to procoagulant promyelocytic extracellular chromatin25. However, little is known about its response to ATO treatment or the role of ETosis in leukemia cell eradication.
In this study, we characterized the concentration-dependent effects of ATO exposure on ETosis in APL cells. We also continued our previous study by investigating the upstream mammalian target of rapamycin (mTOR)-mediated autophagy pathway and the role of ROS production in this process. Finally, we explored the role of ETosis in APL LIC loss, helping identify a novel pathway to target LICs and further prevent relapse in APL patients following ATO administration.
Results
ATO induces ETosis and apoptosis in NB4 cells in a dose-dependent manner
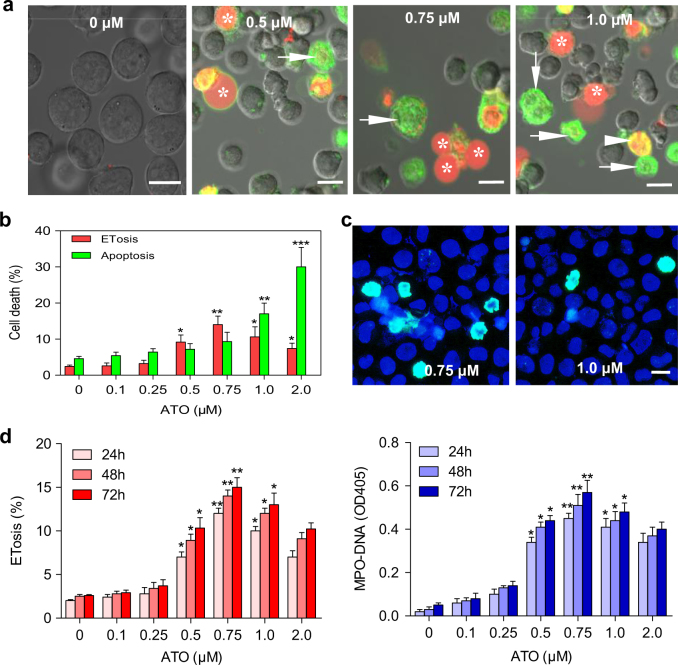
To distinguish the effect of ATO on ETosis and apoptosis, lactadherin and propidium iodide (PI) were used to stain NB4 cells24,25. In ETotic cells, the chromatin expands while the cytoplasmic membrane remains intact. PI staining can be observed in the absence of lactadherin membrane staining (green) or visible membrane blebbing. Cells undergoing ETosis could be seen releasing a single swelling bubble that stained with PI24,25. To investigate the effect of varying concentrations of ATO on ETosis in cultured NB4 cells, an APL cell line, cells were treated with 0, 0.1, 0.25, 0.5, 0.75, 1.0, or 2.0 μM ATO for different time points. When cultured for 48 h, concentrations of ATO over 0.5 μM caused a significant increase in the number of ETotic cells (Fig. 1a, b). When NB4 cells were treated with ATO at 1.0 μM or higher concentrations, both ETotic and apoptotic cells were visible (Fig. 1a). Using immunofluorescence, we identified that promyelocytic ET backbone as DNA-histone (Fig. 1c). ETosis% counted by DAPI/anti-histone-3 and lactadherin/PI staining were comparable (Supplementary Fig. S1)24. PI staining was used to define the morphological changes leading to ETosis. Evaluating the changes in nuclear size and shape, we identified four morphologies (Supplementary Fig. S2): (i) round, (ii) budding, (iii) spread nuclei, and (iv) extracellular DNA. Because DNA material ultimately became cell-free, MPO–DNA complexes could be detected in the supernatant and used as a marker of ETosis26. Our results showed that ETosis dramatically increased at 0.5 μM, peaked at 0.75 μM, and decreased at higher ATO concentrations (Fig. 1d).
Fig. 1. ATO induces ETosis and apoptosis in NB4 cells in a dose-dependent manner.
a NB4 cells were incubated in 0, 0.1, 0.5, 0.75, 1.0, or 2.0 μM ATO for 48 h and then stained with lactadherin (green) and PI (red). Nuclei bubbled, lost shape, expanded, and filled most of the cytoplasm, enclosed by a thin layer of membrane in ETotic cells (*). In contrast, cells undergoing early apoptosis showed diffuse rim staining by lactadherin but no PI staining (arrow). Late apoptotic cells were stained brightly with lactadherin (green) showing typical condensed nuclei (arrowhead). b Cells undergoing ETosis or apoptosis were counted and analyzed as described in methods (n = 5). c Immunofluorescence analysis of ET components. After incubation with 0.75 μM (left) or 1.0 μM (right) ATO for 48 h, NB4 cells were stained with DAPI (blue) and anti-histone-3 (green). d Quantification of the percentage of ETotic cells and the concentration of MPO–DNA complexes levels in the supernatant showed dose-dependent and time-dependent fluctuation (n = 5). All values are mean ± SD. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. 0 (PBS) μM. Bars represent 15 μm in a, c
Moderate concentrations of ATO mainly promotes ETotic cell death in NB4 cells
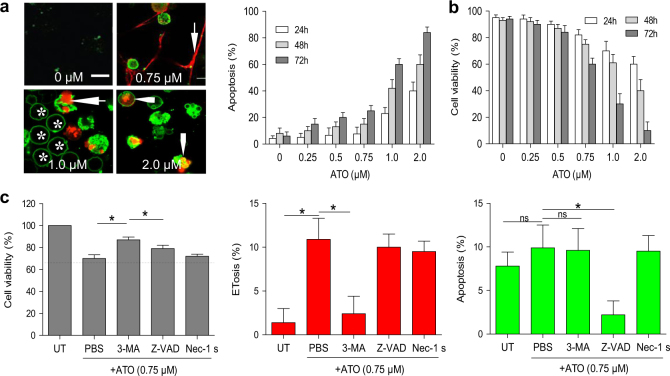
To better understand the mechanism behind ATO-induced cell death, we dual stained cells with lactadherin and PI (Fig. 2a). Apoptosis was analyzed by flow cytometry, and confirmed by frequency of cells that were TUNEL-positive. Surprisingly, the results showed that the percentage of apoptotic cells (lactadherin+ and PI+) had a marked increase only at concentrations over 1.0 μM (Fig. 2a), while the viability of NB4 cells dramatically decreased at 0.75 μM ATO (Fig. 2b). Thus, 0.75 μM ATO treatment probably induced non-apoptotic cell death. To confirm the role of ETosis in non-apoptotic cell death induced by 0.75 μM ATO, NB4 cells were preincubated with various cell death inhibitors. We found that 3-methyladenine (3-MA) significantly increased the number of viable cells reduced by ATO, while other cell death inhibitors had few effects. Moreover, 3-MA only decreased ETosis induced by ATO while having limited effects on apoptosis (Fig. 2c). Taken together, these results suggested that the majority of NB4 cell death induced by 0.75 μM ATO was ETosis.
Fig. 2. ATO induces ETotic cell death in NB4 cells.
a NB4 cells were treated with 0 (PBS), 0.25, 0.5, 0.75, 1.0, or 2.0 μM ATO for 48 h and then stained with lactadherin (green) and PI (red). Confocal microscopy images showed that untreated NB4 cells showed little staining with either lactadherin or PI. The nuclei of ET-releasing cells spread into the extracellular space (arrow). NB4 cells undergoing early apoptosis showed ring-like staining by lactadherin but no PI staining (*). Cells in late apoptosis had nuclei that were condensed and staining by both lactadherin and PI (arrowhead). Apoptosis was determined by lactadherin/PI staining for 15 min at room temperature analyzed by flow cytometry. The data represents mean ± SD of three independent experiments done in duplicate. b Cell viability was detected by trypan blue exclusion. The data show mean ± SD of three independent experiments done in duplicate (n = 3). c NB4 cells were preincubated with corresponding cell death inhibitors (Z-VAD, Nec-1, 3-MA) for 1 h, and treated with 0.75 μM ATO for 48 h. The cell viability in untreated group was counted as 100%. Apoptosis was analyzed by flow cytometry and ETosis were counted as described in methods (n = 5). All values are mean ± SD. *P < 0.05, ns = not significant. Bars represent 10 μm in a
ATO increase mTOR signaling-dependent autophagy that mediated ETosis
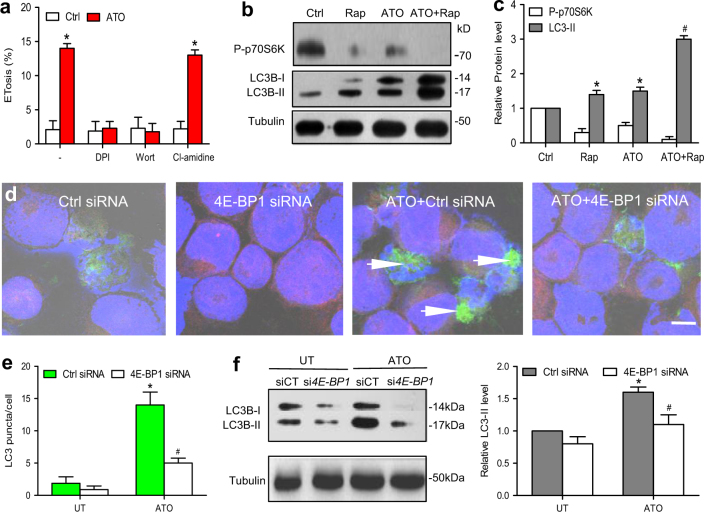
The ETotic process involves interplay between autophagy, the cytoskeleton, superoxide production, and histone citrullination21,27. To further unravel the molecular mechanism by which ATO primes cells for ETosis, we investigated possible signaling pathways preceding ETosis. Diphenyleneiodonium chloride (DPI) and wortmannin but not Cl-amidine were able to block ATO-triggered ETosis (Fig. 3a). When nutrient deprivation is used to induce autophagy, mTOR-mediated phosphorylation of p70 ribosomal S6 kinase (p70S6K) is prevented. Therefore, a reduced level of phosphorylated p70S6K (P-p70S6K) can be used as an indicator of mTOR-dependent autophagy28. ATO-treated NB4 cells exhibited increased levels of LC3-II and decreased levels of P-p70S6K by immunoblotting (Fig. 3b, c), suggesting that ATO activated autophagy via the mTOR pathway. Moreover, siRNA-mediated inhibition of the negative downstream effector of mTOR pathway, translational repressor 4E-BP1 (Supplementary Fig. S3), reversed the effects of ATO on LC3-positive structures (autophagosomes) (Fig. 3d, e), LC3 expression (measured by western blot) and ETosis (Supplementary Fig. S4), confirming ATO-induced ETosis was dependent on mTOR-mediated autophagy.
Fig. 3. ATO induce autophagy through the mTOR pathway.
a NB4 cells were preincubated with the indicated inhibitors (pretreatment with DPI (10 μM, 4 h), wortmannin (1 mg/ml) for 30 min, Cl-amidine (200 μM)) before treatment with ATO (0.75 μM, 48 h) to induce ETosis. b, c NB4 cells were treated with vehicle or rapamycin (10 nM) in the presence or absence of ATO (0.75 μM) for 48 h. Cell lysates were examined by western blot by the use of anti-P-p70S6K, anti-LC3, or anti-tubulin antibodies. Quantification of the data shown in panel c (n = 3). *P < 0.05 vs. control. d NB4 cells were stained with DAPI (blue) and anti-LC3 (green). Immunofluorescence images showed inhibition of the negative downstream effector of mTOR pathway by knocking down 4E-BP1 reduced the aggregation of LC3 (green). e The LC3 puncta per cell in ATO-treated NB4 cells measured by immunofluorescence (n = 3). f The levels of LC3B-I and LC3B-II were detected by western blotting (n = 3). All values are mean ± SD. *P < 0.05 vs. untreated group, #P < 0.05 vs. control e, f. Bars represent 20 μm in d. DPI diphenyleneiodonium chloride, Wort Wortmannin, Rap rapamycin (mTOR inhibitor), PAD4 peptidylarginine deiminase 4, UT untreated group, Ctrl, control
Rapamycin enhances the autophagy-dependent ETosis but not autophagy-independent apoptosis in response to ATO
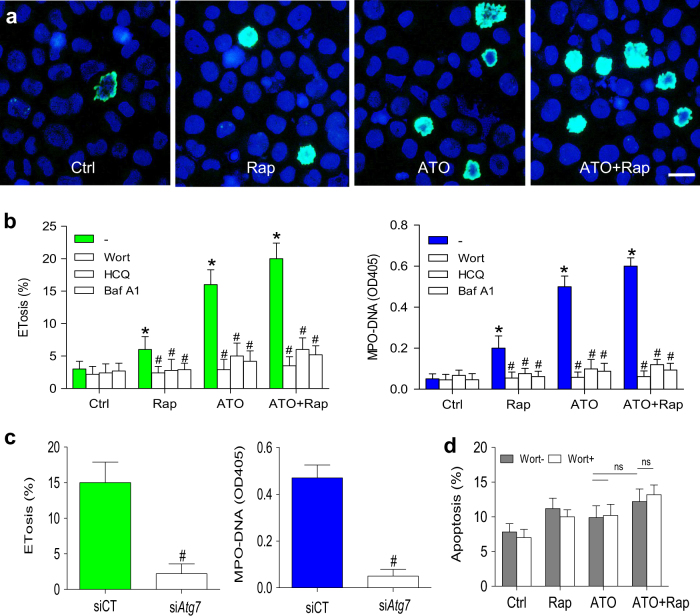
Because induction of autophagy increase ETosis24 and the aforementioned experiments demonstrated a role of mTOR in ATO-induced autophagy, we also examined whether downregulation of mTOR activity by rapamycin would affect ETosis in NB4 cells. We found that rapamycin alone was capable of inducing a modest increase ETosis and that this drug potentiated ATO-mediated ETosis (Fig. 4a, b). However, the same combination treatment (ATO + rapamycin) of NB4 cells along with inhibition of autophagy with various autophagy inhibitors (wortmannin, hydroxychloroquine (HCQ) and bafilomycin A1 (Baf A1)) resulted in a significant decrease in ETosis (Fig. 4b). Furthermore, we knocked down autophagy-related gene Atg7 using small-interfering RNA (siRNA) to confirm the role of autophagy in ATO-induced ETosis in APL. As shown in our previous work24, inhibition of autophagy nearly abolished autophagy as the markedly decreased conversion of LC3B-I to LC3B-II. Knocking down of Atg7 resulted in significantly reduced ATO-induced ETosis and MPO–DNA complexes (Fig. 4c). Interestingly, rapamycin did not increase ATO-induced apoptosis, which was not affected by autophagy inhibition with wortmannin (Fig. 4d).
Fig. 4. Rapamycin enhances the effects of ATO on autophagy-dependent ETosis.
a, b NB4 cells were cultured in rapamycin (10 nM) or/and ATO (0.75 μM) for 48 h and treated with vehicle or with various autophagy inhibitors (wortmannin, HCQ and Baf A1). a NB4 cells were stained with DAPI (blue) and anti-histone-3 (green). Immunostaining images showed ATO-induced ETosis was enhanced by concomitant addition of rapamycin. b Quantification of the percentage of ETotic cells and corresponding MPO–DNA complexes concentrations for the different treatments (n = 5). c NB4 cells were transiently transfected with Atg7 siRNA (siAtg7) at a concentration of 100 nM and scrambled siRNA (scr) was used as a negative control (siCT). Seventy-two hours after transfection, cells were treated with ATO (0.75 μM) for 48 h. The percentage of ETosis and the concentration of MPO–DNA complexes were then measured (n = 5). d Apoptosis was analyzed by flow cytometry (n = 5). All values are mean ± SD. *P < 0.05 vs. control, #P < 0.05 vs. Wort-, ns = not significant. Bars represent 20 μm in a. UT untreated group, Rap rapamycin, Wort wortmannin
The role of ROS production during ETosis
We monitored intracellular ROS production in NB4 cells to investigate its role in ETosis. The results showed that intracellular ROS was increased in NB4 cells activated by ATO, and remained at baseline if inhibited by NADPH oxidase (Nox) with DPI (Supplementary Fig. S5). Inhibition of autophagy with wortmannin or activation of autophagy with rapamycin did not alter basal ROS levels or ATO-induced ROS production (Supplementary Fig. S6). We then assessed whether ROS was essential for ETosis. NB4 cells were stained with DAPI and anti-histone-3 to identify ETotic cells. While ATO notably increased histone staining, the addition of DPI abrogated ETosis (Supplementary Fig. S5). Overall, these results demonstrated that Nox-dependent ROS production plays a key role as an early event of ATO-induced ETosis, independent of autophagy.
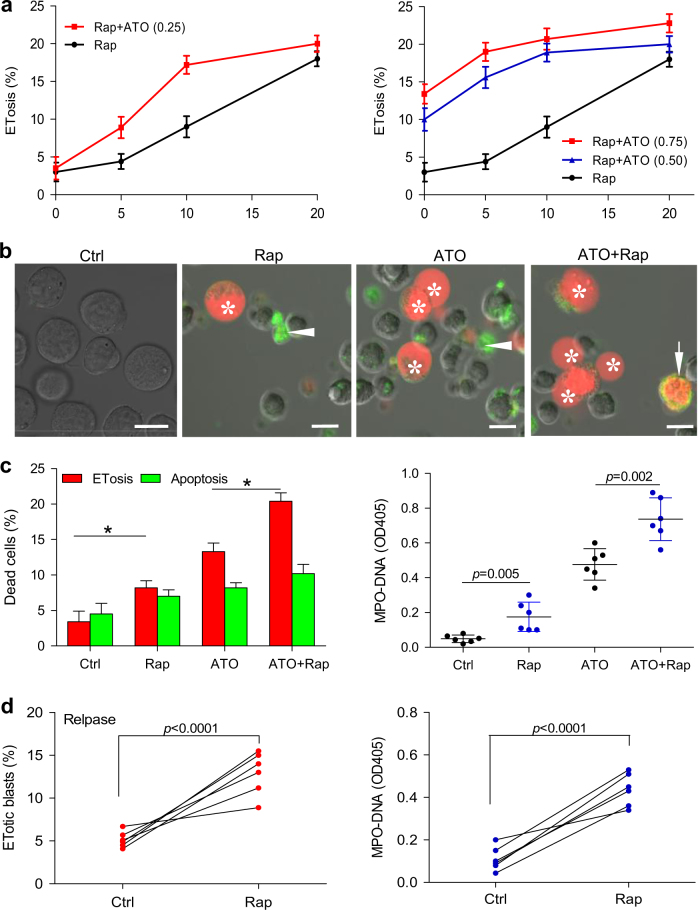
Rapamycin enhances ATO-induced ETosis in NB4 cell lines and APL cells from patients at diagnose and after relapse
For the evaluation of the combinated effects on ETosis, NB4 cell line was first treated with a fixed non-toxic concentration of ATO (0.25 μM, 48 h) and graded concentrations of rapamycin. The drug combination resulted in synergistic effects at all combination data points. When cells were treated with toxic concentration of ATO (0.5 μM and 0.75 μM, 48 h) and graded concentrations of rapamycin, lower doses of rapamycin resulted in synergistic effects while higher doses of rapamycin (10 and 20 nM) suggested additive effects (Fig. 5a).
Fig. 5. Effects of ATO and rapamycin on apoptosis and ETosis in NB4 cells and patient APL cells.
a ETosis induced by low doses of ATO (0.25, 0.5, 0.75 μM) and increasing concentrations of rapamycin (0, 5, 10, and 20 nM) was evaluated at 48 h in NB4 cells. Values are expressed as mean ± SD (n = 6). b, c APL cells from newly diagnosed APL patients were treated with vehicle or rapamycin (10 nM) in the presence or absence of ATO (0.75 μM) for 48 h. b APL cells were stained with lactadherin (green) and PI (red) and imaged with confocal microscopy. ETotic cells showed nuclei (stained with PI) that had lost shape and expanded to fill most of the cytoplasm, but did not stain with lactadherin (*). In contrast, cells undergoing early apoptosis showed diffuse rim staining by lactadherin but no PI staining (arrowhead). Late apoptotic cells had condensed nuclei and were stained by both lactadherin and PI (arrow). c Cells undergoing ETosis or apoptosis were counted as described in methods (n = 6). MPO–DNA complexes levels were also measured (n = 6), *P < 0.05. d Primary APL cells from patients at relapse were treated with vehicle or rapamycin (10 nM) in the presence of ATO (0.75 μM, 48 h). The percentage of ET-formation blasts and MPO–DNA complexes in the supernatant were assayed in duplicate wells (n = 6). Bars represent 15 μm in a. Rap rapamycin
Since resistance is the major puzzle in APL therapy, we investigated whether this combination (ATO + rapamycin) is effective on ATRA-resistant and ATO-resistant cell lines (NB4-R4 and NB4 ATO-R). Increasing doses of rapamycin were tested, starting at 5 nM and scaling up to 20 nM. Marked increase of ETosis was initially detected NB4-R4 cells using 10 nM rapamycin, whereas 20 nM rapamycin induced a significant increase in ETosis not only in NB4-R4 but also in NB4 ATO-R cells. In NB4 ATO-R cell line, less sensitive to rapamycin as single agent, the combination of 1 μM ATO with rapamycin (20 nM) resulted in a remarkable increase in the percentage of ETotic cells, as shown by lactadherin/PI staining after 48 h (Supplementary Fig. S7).
To confirm the effect of rapamycin and ATO on ETosis, primary APL blasts from newly diagnosed APL patients (patient 1–6 in Table 1) were incubated with rapamycin (10 nM) in the presence or absence of ATO. We found that rapamycin alone was capable of inducing a modest increase (from 3.4 to 8.2%) in ETosis and that this drug potentiated ATO-mediated ETosis (from 13.3 to 20.4%) (Fig. 5b, c), suggesting that the effects of ATO and rapamycin are additive. Furthermore, addition of the mTOR inhibitor rapamycin strongly enhanced the percentage of ATO-mediated ETosis (paired P-value < 0.0001, n = 6) in APL cells taken from patients after relapse (patient 7–12 in Table 1) (Fig. 5d). Measurements of MPO–DNA complexes in the supernatant were used to indirectly quantify ETs. We found that the increase in ETotic APL cells was paralleled by an elevated abundance of MPO–DNA complexes in the supernatant (Fig. 5b, c).
Table 1.
APL patients’ characteristics and results of ATO therapy
| No. | Sex/age | Diagnosis | Blasts (BM%) | WBC (×109/l) | Plts (×109/l) | Hb (g/l) | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | F/44 | M3/bcr1 | 80 | 5.1 | 43 | 62 | HCR |
| 2 | F/45 | M3/bcr1 | 84 | 10.4 | 27 | 57 | HCR |
| 3 | M/34 | M3/bcr3 | 78 | 4.5 | 37 | 77 | HCR |
| 4 | M/38 | M3/bcr1 | 88 | 11.2 | 33 | 68 | HCR |
| 5 | F/17 | M3/bcr3 | 88 | 28.2 | 26 | 90 | HCR |
| 6 | F/44 | M3/bcr1 | 85 | 18.2 | 45 | 67 | HCR |
| 7 | F/42 | M3/bcr1 | 95 | 25.7 | 31 | 77 | PR |
| 8 | M/41 | M3/bcr1 | 92 | 26.1 | 22 | 60 | PR |
| 9 | M/45 | M3/bcr1 | 87 | 14.2 | 14 | 77 | HCR |
| 10 | F/34 | M3/bcr1 | 90 | 32.5 | 12 | 79 | HCR |
| 11 | M/53 | M3/bcr3 | 92 | 20.4 | 77 | 58 | PR |
| 12 | F/29 | M3/bcr1 | 91 | 24.4 | 34 | 86 | HCR |
| 13 | M/39 | M3/bcr3 | 94 | 6.2 | 37 | 66 | HCR |
| 14 | M/35 | M3/bcr3 | 94 | 26.2 | 33 | 76 | HCR |
| 15 | M/62 | M3/bcr1 | 80 | 4.5 | 55 | 76 | HCR |
| 16 | M/23 | M3/bcr1 | 89 | 4.1 | 12 | 62 | HCR |
| 17 | M/33 | M3/bcr1 | 82 | 20.2 | 13 | 61 | ED |
| 18 | M/23 | M3/bcr3 | 81 | 18.9 | 19 | 62 | HCR |
| 19 | F/39 | M3/bcr3 | 83 | 17.7 | 12 | 68 | HCR |
| 20 | F/41 | M3/bcr1 | 87 | 12.8 | 32 | 55 | PR |
| Ref. range | 0–0.4 | 4–10 | 100–300 | 110–170 | HCR | ||
The main clinical and laboratory features of 20 newly diagnosed APL patients and results of ATO therapy during induction therapy
Blasts promyelocytes + blasts, PB peripheral blood, WBC white blood cell, Plts platelets, Hb hemoglobin, bcr breakpoint cluster region (bcr1 = intron 6, bcr3 = intron 3), HCR complete hematologic remission, PR partial remission, ED early death
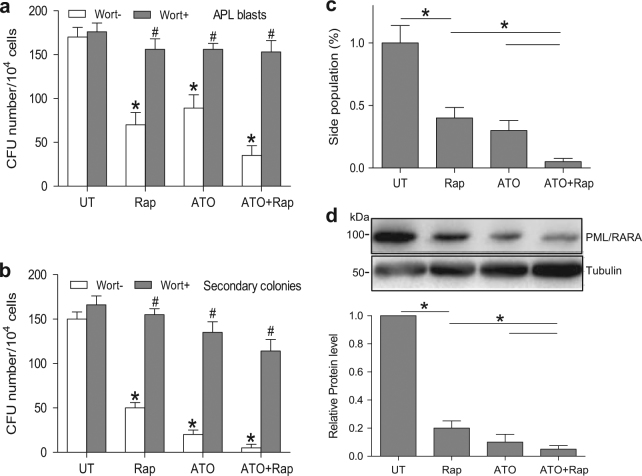
Combined rapamycin and ATO treatment synergistically decreases LIC production
Primary APL blasts were isolated from APL patients (patient 13–20 in Table 1) and cultured in methylcellulose, a semisolid medium, in the presence of rapamycin, ATO, or both. ATO resulted in a striking decrease in colony-forming units when in combination with rapamycin compared with either drug alone (Fig. 6a). Furthermore, when cultured for an additional week, treated colonies had a reduction in the number of secondary colonies (Fig. 6b). Thus, rapamycin and ATO synergistically diminished the clonogenicity of APL cells. Meanwhile, inhibition of ETosis with wortmannin blocked the effects of both ATO and rapamycin (Fig. 6a, b). To confirm our results obtained from primary APL cells, we investigated the effect of ATO-induced ETosis on the side population of NB4 cells, a model of APL-derived LICs. We observed that rapamycin exhibits synergism with ATO (0.75 μM, 48 h) for efficient side population clearance (Fig. 6c) and PML/RARα degradation in NB4 cells (Fig. 6d).
Fig. 6. ETosis contributes to LICs loss in APL in vitro.
a Primary APL cells isolated from patients were cultured in methylcellulose for a week with rapamycin (10 nM) or ATO (0.75 μM) alone or in combination and in the presence or absence of wortmannin (1 mg/ml). Number of colonies is shown (n = 8). b After 1 week of treatment, cells were harvested, washed and cultured in semisolid medium in the absence of any drug (n = 8). Number of secondary colonies is shown. *P < 0.05 vs. UT, #P < 0.05 vs. wortmannin (-). c, d NB4 cells treated with rapamycin (10 nM) and/or ATO (0.75 μM) for 48 h. c NB4 side population was defined by exclusion of Hoechst 33342 in the absence or presence of verapamil. The percentage of cells representing the side population was measured. d Lysates from NB4 cells were analyzed by immunoblotting by the use of antibodies against anti-PML/RARA, and tubulin was used as a loading control (n = 5), *P < 0.05. Wort, wortmannin
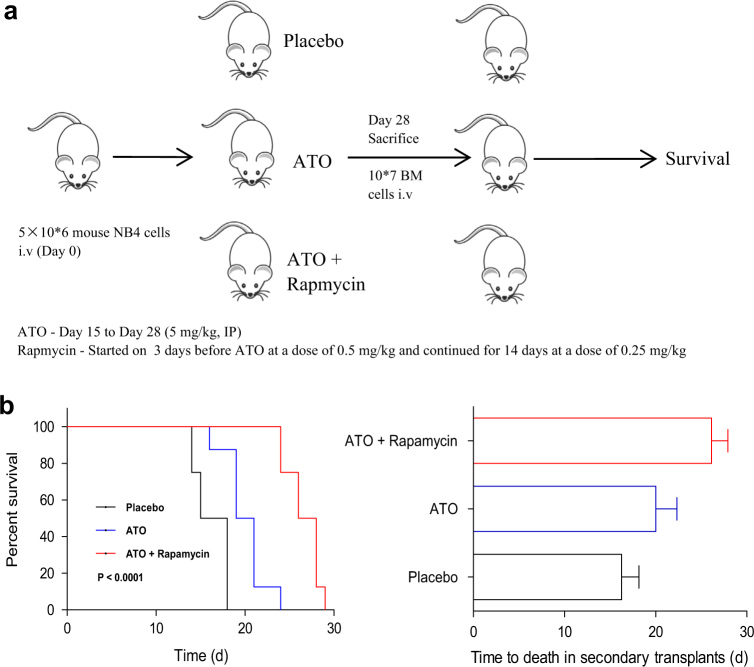
Combination of rapamycin and ATO reduces leukemic burden and LICs in an APL model
After identifying the effect of ATO-induced ETosis on the side population of NB4 cells, we next confirmed the combination’s effect on LICs in a xenograft APL model using NB4 cell line. The combination therapy showed a significant reduction in the tumor burden on day 28 compared with mice treated with either agent alone, as evidenced by reduction in spleen size, decreased PML-RARA copy numbers and decreased bone marrow blasts analyzed by flow cytometry and immunohistochemistry (hematoxylin and eosin staining (data not show)). We then performed secondary transplantation of the bone marrow cells of leukemic mice harvested on day 28 after treatment (Fig. 7a). We observed that ATO alone or ATO + rapamycin produced significantly longer survival of secondary recipients. Mice receiving bone marrow treated with ATO + rapamycin had a median survival time of 27 days compared to 16.5 days with placebo or 20 days with ATO alone (Fig. 7b). Altogether, these data suggest that this combination is able to significantly decrease the LIC compartment in a mouse model of APL.
Fig. 7. ETosis contributes to LICs loss in in an APL model.
a Schematic representation of secondary transplantation experiment (not treated post transplantation). Mice were then observed up to their death. b The survival curve and median survival time (days) of the secondary transplantation of bone marrow cells from the three animal treatment groups. Statistical significance was calculated using log-rank test and nonparametric, unpaired, two-tailed, Mann–Whitney test. BM bone marrow, IP intraperitoneal injection, i.v. intravenous injection. Values are mean ± SD (n = 8). UT untreated group
Discussion
This is the first study to show that ATO significantly increases the amount of ETosis in APL cells. ATO has dose-dependent antileukemic effects on NB4 cells, preferentially inducing ETosis at moderate concentration (0.5–0.75 μM) and switching to apoptosis at relatively high doses (1.0–2.0 μM). Further investigation indicated that ATO-induced ETosis requires both mTOR-mediated autophagy and Nox-dependent ROS production. More importantly, we found that rapamycin promoted ATO-induced ETosis leading to decreased LIC activity, suggesting that ETosis facilitates LIC loss in APL cells. These results further clarify the cellular and molecular mechanisms of ATO treatment. Our discovery suggests that therapy-triggered ETosis could be used to target LICs and, therefore, reduce relapse risk in patients.
We have recently found that ATRA promotes a novel cell death process, named ETosis, in APL cells24,25. Since ATO exerts dose-dependent cellular effects16,17,29, we investigated its dosage effect on ETosis. In concordance with the finding that low doses of ATO cause only minimal cell death29, ETotic cell death started and peaked at median doses of ATO. ATO at high concentrations triggered the apoptosis pathway29, which is thought to block the ETosis pathway20. As expected, we observed that ETosis decreased at higher concentrations. We performed all our experiments in the presence APL serum containing cytokines, which mimicked the conditions at inflammatory sites as a two-hit model to favor ET formation30. It is not surprising, however, that ATO can induce this novel form of cell death. Indeed, previous reports have shown that ATO is a potent inducer of apoptotic and non-apoptotic cell death16,17. While our work strongly supports a role for ATO in priming ETosis, the fluctuating levels of circulating APL-derived ETs in the plasma of hospitalized patients treated with ATO need to be explored. An assay of patient plasma for MPO–DNA complexes, a marker of ETosis, could potentially be used to predict drug sensitivity and/or risk of relapse.
The process of ETosis is distinct from apoptosis, in that there is no caspase activation or phosphatidylserine (PS) exposure before cell death, and the morphological characteristics of the two forms of active cell death are different24,25. Beyond apoptosis, ATO has at least a partial tendency to favor ETosis which results in lower overall PS exposure on APL cells. PS exposure is a major mechanism through which APL cells support procoagulant activity31. Therefore, our results explain the mechanism by which ATO treatment results in rapid correction of patient coagulopathy preceding disappearance of the circulating leukemic promyelocytes32. Thus, this alternative cell death process may represent a therapeutic target in the treatment of resistant cases and attenuating apoptosis-associated complications. NET-like structures are also generated in other myeloid cell lines23, and ATO is known to exert anticancer effects on other hematopoietic tumor cells17,18. Further studies are needed to determined whether ATO induces ETosis in other hematological malignancies.
ATO induces autophagic cell death in several types of tumor cells16,17, and degrades PML/RARα protein via mTOR pathway-mediated autophagy in APL cells13. In this study, we have identified the pivotal role for the mTOR pathway in ETosis via regulation of autophagy. From a novel cell death perspective, our data explains the results obtained by Altman et al.33 that pharmacological inhibition of mTOR enhances the suppressive effects of ATO on leukemic progenitor colony formation. Beyond the prior reports, we found that mTOR-mediated signals regulated the ETotic response to ATO implying that mTOR could be a potential pathway to suppress LIC activity. Although the role of the PI3K-Akt-mTOR pathway in NET formation is contradictory, reports of fMLP-induced NETosis are consistent with our results showing that this pathway inhibits autophagy34. Our results reveal a new facet of this pathway, suggesting that ETosis might be directly linked to, or be a variant of, the autophagic cell death pathway. This preclinical data suggests the need for a clinical investigation of ATO in combination with other agents interfering with mTOR-mediated autophagy. Combined therapy could contribute to the killing of APL LICs through the induction of ETosis and thereby improve remission rates.
Oxidative production is one of the proposed mechanisms by which ATO affects cellular behavior. NB4 cells exposed to ATO show increased Nox-derived ROS production35–37, which shapes a critical component of NETosis27. In accordance with other studies, our results show that ATO enhanced ET formation via a Nox-dependent mechanism, which is supported by the finding that Nox is involved in ATO-induced cytotoxicity35. Evidence has emerged that autophagy and activated Nox are both required in ETosis where they work together to inactivate caspases and prevent cells from entering apoptosis26,38,39. This explains our observation that blocking either autophagy or ROS generation decreases ETosis in NB4 cells. Based on the current findings, it seems that ROS regulate autophagy that mediates ATO-induced ETosis40.
Since ATO-based therapy is strongly correlated with APL LIC eradication12,41, we tested whether ATO-induced ETosis plays a role in LIC loss. Rapamycin as a classic autophagy inducer augmented ATO-induced cytotoxicity in NB4 cells42. Since LICs remain untouched by apoptosis-inducing conventional chemotherapy11,43 and rapamycin did not promotes apoptosis by ATO, it is convincing that activation of the ETotic rather than apoptotic cell death pathway contributes to rapamycin-enhanced LIC loss by ATO. In addition, we confirmed the role of ETosis in ATO-induced APL LIC loss through inhibition experiments. Therefore, rapamycin potentiates ATO-induced LIC loss in patient APL cells and stem cell population clearance in NB4 cells at least partially through ETosis. Based on these encouraging in vitro results, we further validated the efficacy of the combination of rapamycin and ATO in a transplantable mouse model of APL.
At the molecular level, a previous study suggested that autophagy contributes to ATO-induced degradation of the PML/RARα13, whose degradation is necessary for LIC eradication14,43. By monitoring APL LIC activity-linked oncoprotein PML/RARα, we observed that enhanced ETosis significantly correlated with oncoprotein degradation. Thus, it seems that autophagic degradation of PML/RARA leads LICs to ETosis. This study goes beyond the prior reports, correlating the cellular fate of LICs with ETotic cell death. Besides loss of self-renewal, differentiation and apoptosis, our finding adds ETosis to the potential cellular mechanisms of LIC clearance, though there is not yet direct evidence of ETotic LICs.
Since prompt treatment with ATO has been the most important step in treating and preventing relapse, future therapeutic strategies could focus on combined application of rapamycin or autophagy inducer with ATO to eradicate LICs and further decrease risk of relapse, especially in high-risk APL patients. In brief, our study uncovers the role of ETosis in LIC eradication, representing a novel mechanism for ATO anticancer activity. Thus, enhancing ATO-induced ETosis could be a potential strategy to eradicate LICs.
Materials and methods
See Supplementary files for a full description of materials and methods (available on the Cell Death and Disease Web site).
Reagents
ATO, rapamycin (200 μM stock in EtOH), 5′-(4-Fluorosulfonylbenzoyl) adenosine hydrochloride (wortmannin), 3-MA (stock 100 mM in EBSS), HCQ and Baf A1 (stock 200 μM EtOH) were from Sigma-Aldrich (St Louis, MO). ATO stock solutions were made at the concentration of 1 mM with phosphate-buffered saline (PBS) and diluted to working concentrations before use.
Patients
Twenty newly diagnosed APL patients admitted to the First and Second Affiliated Hospital of Harbin Medical University from May 2014–February 2016 agreed to participate in the study after informed consent. The diagnosis was based on clinical data, morphology, cytochemistry, immunology, cytogenetics, and molecular pathology testing or alternatively confirmation of the presence of the t(15;17) (PML-RARα) fusion gene31. Compassionate use of ATO was initiated in all the 20 previously untreated APL patients, and this treatment was very well tolerated. This study was approved by Ethics Committee of Harbin Medical University and conducted in accordance with the Declaration of Helsinki.
Cell lines and primary cells
Human APL NB4 cell line and ATRA-resistant cell line, NB4-R4, were gifts from Dr. James O’kelly (Los Angeles, CA). While the ATO-resistant cell line, NB4-ATO-R, was established by culturing in increasing concentrations of ATO, with initial concentration of 200 nM, until the cell line survived a concentration of 1 μM. The resistant cell line was then maintained in complete RPMI 1640 medium containing 1 μM ATO. NB4 cells transiently transfected with Atg7 siRNA were obtained in the same way as our previous work24. Bone marrow samples from APL patients were collected before intravenous ATO (0.1 mg/kg per day) treatment and at hematological relapse after obtaining a written informed consent. APL cells were harvested from the bone marrow of 14 newly diagnosed APL patients for further experiment. APL cells from the BM of another six APL patients at hematological relapse were treated with vehicle or rapamycin (10 nM) in ATO (0.75 μM) for 48 h.
Cell culture
Freshly isolated APL blasts were obtained from bone marrow specimens by centrifugation through Ficoll-Hypaque and were used for experiments immediately. These cells and NB4 cells (5 × 105/ml) were resuspended in complete RPMI 1640 medium supplemented with 10% FBS, 10% APL serum, 2 mM L-glutamine and 1% penicillin–streptomycin solution at 37 °C in a 5% CO2 humidified atmosphere.
Confocal microscopy
To characterize cell death, smears of APL or NB4 cells (5 × 105) were incubated with DAPI (100 ng/ml)44 or the indicated concentration of PI and FITC-labeled lactadherin45. Cells were washed to remove unbound proteins and analyzed immediately on Zeiss LSM 510 Meta confocal microscope (Carl Zeiss Jena GmbH, Jena, Germany). The samples were excited with the 488 nm emission line of a krypton-argon laser, and the emission wavelength overlap was restricted with narrow bandpass filters.
Determination of ETosis and apoptosis
Cells undergoing ETosis were characterized by rounded morphology, PI staining, and the presence of nuclear content diffused throughout the cell24,25. When cells were stained with DAPI/anti-histone-3, ETosis was identified by histone-3-stained cells24. When cells were stained with lactadherin and PI, cells in early apoptosis showed diffuse rim staining by lactadherin but no PI staining. Late apoptosis was characterized by condensed nuclei and stained by both lactadherin and PI. 45 Apoptosis with PI staining was defined by the presence of membrane blebbing and nuclei fragmentation. Cells were counted from six random fields in triplicate wells for each condition and expressed as percentage of total number of cells in the field46.
Quantification of ETosis
To quantify ETs in cell culture supernatant, a capture ELISA to measure myeloperoxidase (MPO) associated with DNA was performed as described previously26,47. For the capture antibody, Mouse MPO ELISA kit (Hycult biotech, HK210–01) was used according to the manufacturer’s directions. A peroxidase-labeled anti-DNA mAb (component No.2, Cell Death ELISAPLUS, Roche; Cat. No: 11774424001) was used.
Cell apoptosis assay
For the apoptosis assay, cells were washed with PBS, resuspended in binding buffer from BD Biosciences and stained with Lactadherin-FITC and PI for 15 min. Fluorescence was measured with a flow cytometer, and the membrane integrity of the cells was simultaneously assessed by the PI exclusion method. Apoptosis was determined using the Lactadherin-FITC apoptosis detection kit (BD Pharmingen, San Diego, USA), according to the manufacturer’s instructions. Prepared cells were analyzed with FACScan flow cytometer and CELLQuest software (Becton Dickinson, Franklin Lakes, USA). The TUNEL (terminal deoxynucleotidyl transferasemediated deoxyuridine triphosphate nick end labeling) assay was performed using the in situ cell death detection kit (Roche, Philadelphia, USA), according to the manufacturer’s instructions.
Inhibition assays
For autophagy inhibition assays, cells were pretreated with the autophagy inhibitors wortmannin (1 mg/ml) for 30 min24, 3-MA (5 mM) during the last 24 h of the incubation, HCQ (20 μM) or Baf A1 (100 nM) during the last 12 h of the incubation13,48. Cells were pretreated with DPI (10 μM, 4 h) to inhibit NADPH oxidase, and cultured with peptidylarginine deiminase 4 (PAD4) inhibitor Cl-amidine (200 μM) to block histone citrullination, necrostain-1 (Nec-1 s) (30 μM) to inhibit necroptosis, or z-VAD-FMK (25 μM) to inhibit apoptosis.
Assays of primary hematopoietic progenitors from APL patients
To evaluate drug effects on LIC activity in APL cells, APL cells were cultured (10,000 cells per well) in the methylcellulose medium, supplemented with recombinant cytokines (GF M3434, StemCell Technologies) in the presence or absence of the drugs. Rapamycin (10 nM) was used alone or in combination with ATO (0.75 μM). After a week of treatment, colonies were counted and cells were harvested, washed and, when specified, plated in the methylcellulose medium for a further week without drugs.
Side population of NB4 cells
In cell lines, the stem cell population is contained in the so-called side population determined by staining exclusion in the presence or absence of ABC transporter inhibitors such as verapamil49,50.
Secondary transplantation experiments
In all, 5 × 106 NB4 cells were injected into 6–8-weeks-old SCID mice followed by treatment with ATO with or without rapamycin or no drug treatment (placebo) from day 15–day 28. On day 28, the mice were sacrificed and 1 × 107 bone marrow cells were transplanted into a new batch of 6–8-weeks-old mice (no treatment post-transplant) and followed up to their death. Details of methodology used for “experimental animals”, “in vivo xenograft model”, and “secondary transplantation experimental design” are provided in “Supplemental Methods”.
Statistical analysis
The results are expressed as mean ± standard deviation (SD), and all statistical analyses were performed using Student’s t-test (two-tailed, unpaired). A P-value of 0.05 or less was considered significant. In cell viability assays, the data are normalized to control cells and expressed as the percentage of live cells relative to total cells.
Electronic supplementary material
Acknowledgements
We thank Yanming Xue for the sample colle ction, and Jiangtian Tian, Ji Li, Hulun Li for excellent technical assistance. We thank James O’Kelly (Los Angeles, CA, USA) for providing NB4 cells. This work was supported by grants from the National Science Foundation of China (81470301, 81670128, 61575058).
Authors' contributions
T.L. designed the research, performed experiments, analyzed results, made the figures and wrote the paper; J.S. obtained funding, designed the study, performed some experiments, analyzed results, made the figures, and revised the manuscript; J.Z. provided partial funding support; Y.Z., H.M., R.M., L.W., X.Y. performed some experiments; H.C., V.A.N., S.F., Y.B., J.K., J.W., S.H., Y.B., analyzed data and revised the manuscript.
Competing interests
The authors declare that they have no competing financial interests.
Footnotes
Edited by M. Diederich
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article 10.1038/s41419-017-0018-3) contains supplementary material.
Contributor Information
Shaoshan Hu, Phone: +13946172292, Email: shaoshanhu@126.com.
Jin Zhou, Phone: +13946172292, Email: jinzhou1956@163.com.
Jialan Shi, Phone: +1 857 203 5914, Email: Jialan_shi@hms.harvard.edu.
References
- 1.Goddard AD, Borrow J, Freemont P, Solomon E. Characterization of a zinc finger gene disrupted by the t(15;17) in acute apromyelocytic leukemia. Science. 1991;254:1371–1374. doi: 10.1126/science.1720570. [DOI] [PubMed] [Google Scholar]
- 2.Korf K, et al. The PML domain of PML-RARα blocks senescence to promote leukemia. Proc. Natl Acad. Sci. USA. 2014;111:12133–12138. doi: 10.1073/pnas.1412944111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 4.Nasr R, Lallemand-Breitenbach V, Zhu J, Guillemin MC, de Thé H. Therapy-induced PML/RARα proteolysis and acute promyelocytic leukemia cure. Clin. Cancer Res. 2009;15:6321–6326. doi: 10.1158/1078-0432.CCR-09-0209. [DOI] [PubMed] [Google Scholar]
- 5.Ganesan S, et al. Rationale and efficacy of proteasome inhibitor combined with arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia. 2016;30:2169–2178. doi: 10.1038/leu.2016.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soignet SL, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N. Engl. J. Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 7.Lo-Coco F, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N. Engl. J. Med. 2013;369:111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 8.Watts JM, Tallman MS. Acute promyelocytic leukemia: what is the new standard of care? Blood Rev. 2014;28:205–212. doi: 10.1016/j.blre.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Platzbecker U, et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 trial. J. Clin. Oncol. 2017;35:605–612. doi: 10.1200/JCO.2016.67.1982. [DOI] [PubMed] [Google Scholar]
- 10.Lengfelder E, et al. Arsenic trioxide-based therapy of relapsed acute promyelocytic leukemia: registry results from the European LeukemiaNet. Leukemia. 2015;29:1084–1091. doi: 10.1038/leu.2015.12. [DOI] [PubMed] [Google Scholar]
- 11.Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Zheng X, et al. Arsenic but not all-trans retinoic acid overcomes the aberrant stem cell capacity of PML/RARα-positive leukemic stem cells. Haematologica. 2007;92:323–331. doi: 10.3324/haematol.10541. [DOI] [PubMed] [Google Scholar]
- 13.Isakson P, Bjoras M, Boe SO, Simonsen A. Autophagy contributes to therapy-induced degradation of the PML/RARA oncoprotein. Blood. 2010;116:2324–2331. doi: 10.1182/blood-2010-01-261040. [DOI] [PubMed] [Google Scholar]
- 14.Nasr R, et al. Eradication of acute promyelocytic leukemia-initiating cells through PML-RARα degradation. Nat. Med. 2008;14:1333–1342. doi: 10.1038/nm.1891. [DOI] [PubMed] [Google Scholar]
- 15.Kogan SC. Curing APL: differentiation or destruction? Cancer Cell. 2009;15:7–8. doi: 10.1016/j.ccr.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63:2103–2108. [PubMed] [Google Scholar]
- 17.Qian W, Liu J, Jin J, Ni W, Xu W. Arsenic trioxide induces not only apoptosis but also autophagic cell death in leukemia cell lines via upregulation of Beclin-1. Leuk. Res. 2007;31:329–339. doi: 10.1016/j.leukres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Goussetis DJ, et al. Autophagy is a critical mechanism for the induction of the antileukemic effects of arsenic trioxide. J. Biol. Chem. 2010;285:29989–29997. doi: 10.1074/jbc.M109.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs TA, et al. Novel cell death program leads to neutrophil extracellular traps. J. Cell. Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai J, Mulay SR, Nakazawa D, Anders HJ. Matters of life and death. How neutrophils die or survive along NET release and is “NETosis”=necroptosis? Cell. Mol. Life Sci. 2016;73:2211–2219. doi: 10.1007/s00018-016-2195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldmann O, Medina E. The expanding world of extracellular traps: not only neutrophils but much more. Front. Immunol. 2012;3:420. doi: 10.3389/fimmu.2012.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakayama T, et al. Nuclear extrusion precedes discharge of genomic DNA fibers during tunicamycin-induced neutrophil extracellular trap-osis (NETosis)-like cell death in cultured human leukemia cells. Cell Biol. Int. 2016;40:597–602. doi: 10.1002/cbin.10594. [DOI] [PubMed] [Google Scholar]
- 24.Ma R, et al. Extracellular DNA traps released by acute promyelocytic leukemia cells through autophagy. Cell Death Dis. 2016;7:e2283. doi: 10.1038/cddis.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao M, et al. Promyelocytic extracellular chromatin exacerbates coagulation and fibrinolysis in acute promyelocytic leukemia. Blood. 2017;129:1855–1864. doi: 10.1182/blood-2016-09-739334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kessenbrock K, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remijsen Q, et al. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011;21:290–304. doi: 10.1038/cr.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang YY, et al. Nutrient-dependent regulation of autophagy through the target of rapamycin pathway. Biochem. Soc. Trans. 2009;37:232–236. doi: 10.1042/BST0370232. [DOI] [PubMed] [Google Scholar]
- 29.Chen GQ, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 30.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J, et al. Phosphatidylserine exposure and procoagulant activity in acute promyelocytic leukemia. J. Thromb. Haemost. 2010;8:773–782. doi: 10.1111/j.1538-7836.2010.03763.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhu J, et al. Tissue factors on acute promyelocytic leukemia and endothelial cells are differently regulated by retinoic acid, arsenic trioxide and chemotherapeutic agents. Leukemia. 1999;13:1062–1070. doi: 10.1038/sj.leu.2401448. [DOI] [PubMed] [Google Scholar]
- 33.Altman JK, et al. Regulatory effects of mammalian target of rapamycin-mediated signals in the generation of arsenic trioxide responses. J. Biol. Chem. 2008;283:1992–2001. doi: 10.1074/jbc.M705227200. [DOI] [PubMed] [Google Scholar]
- 34.Itakura A, McCarty OJ. Pivotal role for the mTOR pathway in the formation of neutrophil extracellular traps via regulation of autophagy. Am. J. Physiol. Cell Physiol. 2013;305:C348–C354. doi: 10.1152/ajpcell.00108.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chou WC, et al. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc. Natl Acad. Sci. USA. 2004;101:4579–4583. doi: 10.1073/pnas.0306687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jie W, Lingna L, Hui C, Guiying S, Jing Y. NADPH oxidase-derived reactive oxygen species are responsible for the high susceptibility to arsenic cytotoxicity in acute promyelocytic leukemia cells. Leuk. Res. 2008;32:429–436. doi: 10.1016/j.leukres.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Jeanne M, et al. PML-RARA oxidation and arsenic binding initiate the antileukemia response of As2O3. Cancer Cell. 2010;18:88–98. doi: 10.1016/j.ccr.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Fadeel B, Ahlin A, Henter JI, Orrenius S, Hampton MB. Involvement of caspases in neutrophil apoptosis: regulation by reactive oxygen species. Blood. 1998;92:4808–4818. [PubMed] [Google Scholar]
- 39.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–975. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 40.Huang J, Lam GY, Brumell JH. Autophagy signaling through reactive oxygen species. Antioxid. Redox Signal. 2011;14:2215–2231. doi: 10.1089/ars.2010.3554. [DOI] [PubMed] [Google Scholar]
- 41.Ito K, et al. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453:1072–1078. doi: 10.1038/nature07016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren Y, Xie Y, Chai L, Wang S, Cheng M. Autophagy modification augmented the treatment effects initiated by arsenic trioxide in NB4 cells. Med. Oncol. 2011;28:231–236. doi: 10.1007/s12032-010-9430-6. [DOI] [PubMed] [Google Scholar]
- 43.Werner B, et al. Dynamics of leukemia stem-like cell extinction in acute promyelocytic leukemia. Cancer Res. 2014;74:5386–5396. doi: 10.1158/0008-5472.CAN-14-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen G, Zhang DC, Fuchs TA, Wagner DD, Frenette PS. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood. 2014;123:3818–3827. doi: 10.1182/blood-2013-10-529982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi J, et al. Lactadherin detects early phosphatidylserine exposure on immortalized leukemia cells undergoing programmed cell death. Cytometry A. 2006;69:1193–1201. doi: 10.1002/cyto.a.20345. [DOI] [PubMed] [Google Scholar]
- 46.Demers M, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl Acad. Sci. USA. 2012;109:13076–13081. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoo DG, Floyd M, Winn M, Moskowitz SM, Rada B. NET formation induced by pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase-DNA and neutrophil elastase-DNA complexes. Immunol. Lett. 2014;160:186–194. doi: 10.1016/j.imlet.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Folkerts H, et al. Inhibition of autophagy as a treatment strategy for p53 wild-type acute myeloid leukemia. Cell Death Dis. 2017;8:e2927. doi: 10.1038/cddis.2017.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirschmann-Jax C, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc. Natl Acad. Sci. USA. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc. Natl Acad. Sci. USA. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.