Abstract
Cancer progression often benefits from the selective conditions present in the tumour microenvironment, such as the presence of cancer-associated fibroblasts (CAFs), deregulated ECM deposition, expanded vascularisation and repression of the immune response. Generation of a hypoxic environment and activation of its main effector, hypoxia-inducible factor-1 (HIF-1), are common features of advanced cancers. In addition to the impact on tumour cell biology, the influence that hypoxia exerts on the surrounding cells represents a critical step in the tumorigenic process. Hypoxia indeed enables a number of events in the tumour microenvironment that lead to the expansion of aggressive clones from heterogeneous tumour cells and promote a lethal phenotype. In this article, we review the most relevant findings describing the influence of hypoxia and the contribution of HIF activation on the major components of the tumour microenvironment, and we summarise their role in cancer development and progression.
Hypoxia and hypoxia-inducible factors
The major components of the tumour microenvironment (TME) are blood vessels, lymphatic vessels, fibroblasts, immune cells and chemico-physical components such as the extracellular matrix (ECM)1. The functional and physical interaction of these elements with cancer cells determines clinical outcomes. During tumour development and progression, cancer and stromal cells often have restricted access to nutrients and oxygen. Most solid tumours indeed have regions permanently or transiently subjected to hypoxia because of aberrant vascularisation and a poor blood supply2. The hypoxic response is mainly ascribed to hypoxia-inducible factors (HIFs). HIF-dependent signalling can promote the adaptation and selection of both cancer and stromal cells to the surrounding conditions, thus promoting changes that favour cancer progression. The HIF family of transcription factors includes HIF1, HIF2 and HIF3. These factors all contain an oxygen-sensitive HIF-α subunit (HIF1-α, HIF2-α or HIF3-α, respectively), which dimerises with the constitutively expressed HIF1-β subunit3. HIF1-α and HIF2-α proteins are the best studied among HIF-α subunits. Each of these subunits contains two proline residues (HIF1-α: P402/P564 and HIF2-α: P405/P531), which are hydroxylated in the presence of oxygen by prolyl hydroxylase domain-containing proteins (PHDs). Hydroxylation of the proline residues promotes binding to von Hippel-Lindau tumour suppressor (pVHL), leading to HIF-α ubiquitination and degradation4,5. Another factor regulating HIF-α in an oxygen-dependent manner is factor inhibiting HIF1 (FIH1). Asparagine hydroxylation of HIF1-α (and to a lesser extent, of HIF2-α) driven by FIH1 impedes HIF1 interaction with its cofactors, histone acetylases p300 and CBP, and hence impairs HIF1 transcriptional activity6–8. The hypoxic tumour microenvironment (TME) is subjected to HIF-driven transcriptional responses in cancer and stromal cells. In addition, HIF activity switches the cell metabolism into glycolytic mode, increasing glucose consumption and pyruvate, lactate and H+ production. In this review article, we summarise and discuss the influence of hypoxia and HIFs on TME components and how this impacts cancer progression.
Cancer-associated fibroblasts (CAFs)
It is widely accepted that fibroblasts infiltrating tumour tissue acquire very different features from normal fibroblasts, leading to the definition of CAF. CAFs often represent the major component of tumour stroma, sometimes constituting up to the 80% of the entire tumour9. The population of CAFs can be quite heterogeneous, as several progenitor cell types can be reprogrammed into CAFs. Although most CAFs are considered to arise from resident fibroblasts, bone marrow cells, adipocytes, endothelial cells and epithelial cells can also turn into CAFs10–17.
Reciprocal paracrine signalling between murine cancer cells and fibroblasts was described by Olaso et al. Melanoma cells could induce proliferation and expression of CAF marker α-SMA in adjacent fibroblasts. These fibroblasts excessively produced glucosaminoglycans and MMP-2, promoting the migration of melanoma cells18. Following this initial study, the ability of CAFs to favour tumour progression was shown in a prostate cancer xenograft model when CAFs were co-injected with initiated (tumorigenic) prostatic epithelial cells and promoted their tumorigenic potential, in contrast to co-injection with normal fibroblasts19. A study by Bhomwick and Colleagues demonstrated that TGF-beta type II receptor deficiency in mouse fibroblasts led to increased HGF secretion and initiation of tumour formation in adjacent prostate and forestomach epithelium20, suggesting one possible mechanism of fibroblast transformation. Other examples of paracrine signalling that is deregulated by CAFs include the secretion of chemokine CXCL12 with subsequent tumour growth facilitation and the expression of intra-cellular and extracellular Ca2+-binding protein S100A4 with subsequent tumour progression and metastatic spread21,22. Except for paracrine signalling, the oncogenic functions of CAFs are partially mediated by altered ECM production. In a breast cancer study, ECM deposited by CAFs was organised differently (aligned) than ECM produced by normal fibroblasts and could influence premalignant human mammary epithelial cells, assigning them a mesenchymal phenotype and increasing their tumorigenic and metastatic potential. The mesenchymal phenotype transition in epithelial cells is dependent on the TGF-β-dependent Smad, Erk, Jun and Rho signalling pathways. As TGF-β is stored in the ECM before activation, the function of CAFs in that model could consist of increasing TGF-β availability as well as building an ECM framework with a metastasis-promoting spatial structure23–26. In addition to the direct effect of CAFs on cancer cells, they can promote angiogenesis via vascular endothelial growth factor-C (VEGF), CXCL12a and FGF-2 factor production and modulate the immune response by inducing macrophage infiltration and tumour-promoting cell polarisation, reducing T-cell infiltration and interfering with natural killer cell function27.
Hypoxia can influence both fibroblast reprogramming and tumour-promoting functions (Fig. 1). Oxygen deficiency influences paracrine signalling between cancer cells and fibroblasts. Hypoxia was shown to stimulate cytokine CXCL13 secretion by cancer-associated myofibroblasts in prostate cancer progression28. While CAFs secrete chemokine CXCL12, facilitating cancer growth22, hypoxia was shown to stimulate CXCR4 (CXCL12 receptor) expression in many cell types, therefore suggesting a feed-forward loop between cancer cells and CAFs29. Hypoxic cancer cells can secrete paracrine signalling molecules, which promote reprogramming of progenitor cells into CAFs30, and HIF1 was shown to regulate some of these signalling molecules, such as TGF-β, bFGF and PDGF-B31–33.
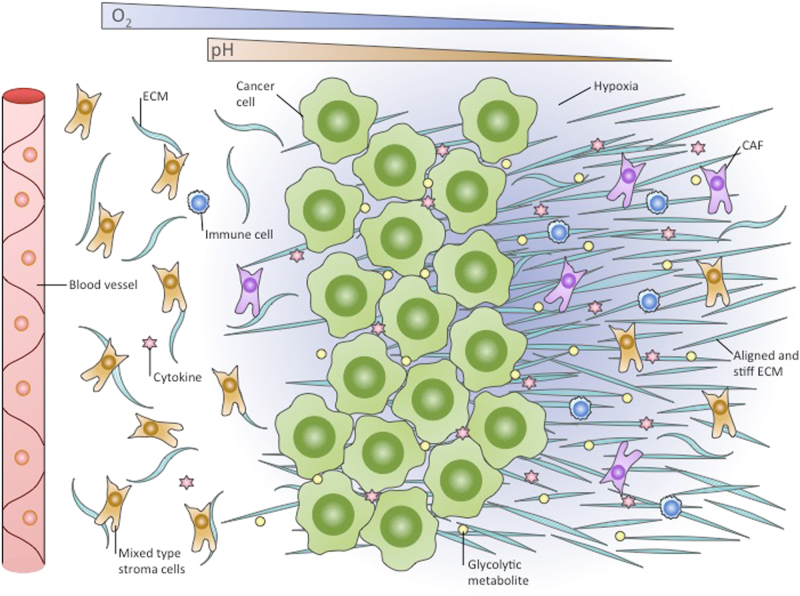
Fig. 1. Tumour stroma and extracellular matrix in hypoxia.
. A rapidly growing tumour leads to a reduction in the oxygen supply of the cancer and in tumour stromal cells that are far from the blood vessels. In hypoxia, these cells switch to glycolytic metabolism, which contributes to the acidification of the tumour microenvironment. Produced glycolytic metabolites such as lactate can be utilised by cancer cells and promote tumour growth. The hypoxic microenvironment is also enriched in diverse types of immune cells, and many of them are recruited from the circulation. Cytokine expression by tumour and stromal cells is altered by hypoxia. In particular, hypoxic cancer cells produce signalling molecules that promote the transformation of fibroblasts into CAFs. Together with cancer cells, in hypoxia, CAFs produce an ECM that is stiff and aligned, different from a normoxic ECM, and support cell migration. CAF cancer-associated fibroblasts, ECM extracellular matrix
It has been shown that the hypoxia-inducible factor-1 (HIF-1)α level is often upregulated in CAFs. In a model in which CAF formation is stimulated by TGF-β and PDGF treatment, the rate of aerobic glycolysis in primary CAFs was increased compared to that in normal fibroblasts, and this effect was associated with HIF-1α protein stabilisation34,35. The lactate produced by highly glycolytic CAFs can be consumed by adjacent cancer cells and lead to induced tumour growth, which suggests a negative outcome of HIF-1 upregulation in fibroblasts34. The role of the increase in HIF-1α levels during CAF formation is still poorly understood, that is, whether it is a driving force for reprogramming or a consequence. Chiavarina and colleagues observed that stable HIF-1α overexpression endowed fibroblasts with oncogenic functions, as they increased tumour growth after ectopic co-injection with breast cancer cells. The proposed mechanism included HIF-1α-promoted autophagy, mitophagy, and the production of lactate and recyclable nutrients, which could fuel cancer cells and provide them with building blocks36–39. However contradicting evidence supports a negative regulatory role for HIF-1a signalling in stromal fibroblast. Kim and Colleagues indeed showed that selective deletion of HIF-1a (or VEGFA) in fibroblast was enhancing tumour growth in murine mammary cancer models40. Additionally after 80 h of hypoxia normal fibroblasts were shown to produce a stiff, aligned matrix, and that this matrix supported the migration of breast cancer cells41. Confirming the abovementioned experiments hypoxia was inducing secretion of protumorigenic factors, such as hepatocyte growth factor (HGF), in human fibroblast cell line MRC5 due to HIF-1 activity. Conditioned media from hypoxic MRC5 could promote invasiveness of pancreatic cancer cell line PK842. Thus several studies have shown gain of oncogenic functions by fibroblasts in response to HIF-1 activation, and one can suggest that HIF-1 is able to drive fibroblast reprogramming to CAFs. Seeming controversially to the described studies, Madsen et al. suggest that long-term hypoxia dampens CAF function in a HIF-1-alfa-dependent way. These authors showed that 72 h of hypoxic treatment or 72 h of PHD2 silencing impeded the ability of head and neck CAFs and vulval CAFs to remodel ECM in vivo, which was accompanied by reduced expression of the activated fibroblast marker α-SMA. HIF-1α silencing in these conditions reverted the phenotype. In vivo treatment of breast cancer-bearing mice with PHD-inhibitor DMOG reduced tumour resilience and metastatic potential, while it had no effect on tumour size43. This controversy could arise from the fact that CAFs indeed are not identical to normal fibroblasts, originate from different cell types and can develop distinct response. It might also be possible that HIF-1 promotes some of the oncogenic functions of CAFs, such as increasing tumour growth, while inhibiting other oncogenic functions, i.e., metastasis promotion. HIF-2 is known to accumulate and mediate long-term hypoxia responses when HIF-1α is downregulated. Therefore, metastasis inhibition in long-term hypoxia and some other hypoxic effects of CAFs that are currently thought to be HIF-1 dependent indeed may be regulated by HIF-2. HIF-2 functions in CAFs have been poorly assessed and need further exploration. It is worth mentioning that after 80 h of hypoxia, normal fibroblasts produce a stiff, aligned matrix and that this matrix supported the migration of breast cancer cells41. Taken together, these studies also raise the possibility of different hypoxic responses in CAFs compared to normal fibroblasts.
Extracellular matrix
The ECM primarily consists of fibrillar proteins and proteoglycans, which together form a net that serves as a framework for most tissues44. Collagens are the dominant component of the ECM and account for approximately 90% of its mass45. The physical properties of tumour ECM differ from healthy tissue and continuously change46–49. In many cases, solid tumours are characterised by excessive deposition of ECM proteins (fibrosis)50–55, and especially by collagen deposition56–61. They are the main source for synthesis of ECM proteins, namely collagen, fibronectin and hyaluronan, and on the other hand CAFs are an important source for ECM-remodelling enzymes62. CAFs share several features with normal activated fibroblasts, including the ability to produce ECM components, which, on contrary of physiologic microenvironment, results in an abnormal ECM that supports tumour dissemination63. ECM and fibroblasts in TME are tightly reciprocally regulated. Modifications of ECM structure or composition induce cytoskeleton reorganisation and signalling cascades in CAFs, further regulating synthesis of ECM components and extracellular remodelling enzymes. Most of these changes in ECM are often supportive for formation of pro-tumorigenic microenvironment64. Besides CAFs, cancer cells themselves significantly contribute to ECM remodelling65. In breast cancer, localisation of fibrotic areas often coincides with localisation of hypoxic regions66,67. HIF1 was shown to directly influence ECM remodelling and promote fibrosis in kidney, liver and adipose tissue68–70. After hypoxic treatment, rats have increased mRNA levels of procollagens I, II, and IV71. Skin, heart and kidney fibroblasts contain elevated mRNA levels of procollagen I α1 chain when cultivated under hypoxic conditions72–74. In fibroblasts, fibulin-5 was shown to be transcriptionally induced by hypoxia or in a PHD inhibitor-induced hypoxic environment in a TGF-β/PI3K/Akt-dependent way, although the role of HIFs in that process was not assessed75. Thus, hypoxia may be able to potentiate ECM protein deposition in tumours in a HIF-dependent and HIF-independent manner (Fig. 1).
The metastatic potential of cancer cells depends on their interactions with the ECM. Cells receive mechanistic signals from the ECM by means of focal adhesions. Formation of focal adhesions requires the binding of ECM proteins with integrins and cell-surface ECM receptors and subsequent signal transduction through intermediate molecules to the cytoskeleton. Silencing of the ITGA5 gene encoding integrin α5, a subunit of fibronectin receptor α5β1, reduced breast cancer cell motility, migration and invasion capacity. These cells had decreased metastatic potential after orthotopic injection in mice76. The same study showed that both HIF-1 and HIF-2 could induce the transcription of integrin subunits α5 and β1. Integrin α6 was demonstrated to be a direct transcriptional target of HIF-1 and HIF-2 and to increase breast cancer cell invasion potential77. In addition to integrins, another fibronectin receptor, syndecan-4, is transcriptionally induced in hypoxia by an unknown mechanism78. Thus, hypoxia is implicated in the regulation of cell–ECM interactions, partially through HIF (Fig. 2). In addition to integrin expression, hypoxia regulates ECM proteins synthesis. HIFs can regulate collagen production at several stages (Fig. 2). Posttranslational modification of procollagen chains requires prolyl-4-hydroxylases and procollagen lysyl-hydroxylases. HIF-1 can induce the expression of prolyl-4-hydroxylase alfa-subunits P4HA1 and P4HA2 and procollagen lysyl-hydroxylases PLOD1 and PLOD2 in different cancer and non-cancer cell lines41,79–85. Expression of P4HA1, P4HA2, PLOD1 and PLOD2 is necessary for the production of stiff and aligned collagen fibrils. In this microenvironment, cancer cells were shown to take on an elongated, adhesive, motile phenotype and have an elevated capacity for invasion and migration41,81,83,84,86.
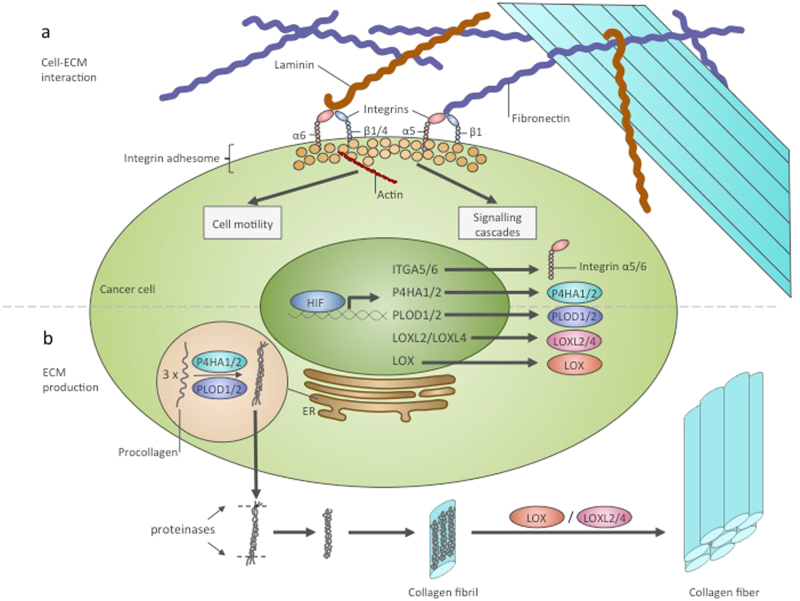
Fig. 2. HIF regulates interactions of cancer cells with ECM and ECM biosynthesis.
. a Regulation of cell–ECM interactions by HIF. HIF was shown to transcriptionally induce ITGA5 and ITGA6 genes encoding integrins α5 and α6. Each integrin α subunit together with a β subunit forms a specific ECM receptor. Integrin α5β1 binds fibronectin and integrin α6β1, or α6β4 binds integrin. In the cell, integrins bind with a multi-component complex named the integrin adhesome. Some proteins of this complex can be involved in signalling cascades, and others interact with the cytoskeleton. As a result of interactions with the ECM, cells undergo alteration of their signalling networks and their motility. b HIF contributes to collagen production. P4HA1, P4HA2, PLOD1, PLOD2, LOX, LOXL2 and LOXL4 are transcriptional targets of HIF that are involved in collagen posttranslational modification. P4HA1/2 and PLOD1/2 catalyse the first step of procollagen molecule modification, which occurs in the ER and allows the formation of the triple-stranded procollagen molecule. Triple-stranded procollagens are exported from the cell and into the extracellular space, where they are modified by proteinases and assembled in collagen fibrils. Subsequently, LOX, LOXL2 and LOXL4 catalyse the crosslinking of collagen fibrils and the formation of a functional collagen fibre. ER endoplasmic reticulum
Another point in the collagen deposition process that is regulated by HIFs is the deamination of secreted collagen fibrils on lysine and hydroxylysine residues by lysyl-oxidases. This deamination is required for fibril crosslinking and collagen fibre formation87. HIF-1 was shown to induce the expression of lysyl-oxidases LOX88, LOXL289 and LOXL490. The LOX family is intimately linked to the metastatic process via several aspects, involving ECM remodelling and potentially ECM-independent EMT regulation91–93. The role of LOX expression can be crucial, as lung metastases in a breast cancer model were shown to be dependent on LOX-driven fibrosis94. LOX family proteins can mediate several oncogenic HIF-1 functions. LOX knockdown prevented focal adhesion formation and reduced cell motility, abrogating HIF-1-dependent cell migration and invasion95. In another study, the transformation of cancer cells to an invasive mesenchymal phenotype was dependent on HIF-1-driven LOX and LOXL2 expression89. Hypoxic LOX, LOXL2, and LOXL4 secretion by breast cancer cells results in collagen remodelling in lungs, which allows the recruitment of bone-marrow derived cells (BMDCs) to the area. ECM remodelling performed by LOX and BMDC-derived matrix metalloproteinases favours the formation of pre-metastatic niches, showing that the oncogenic ECM remodelling and tumour cell recruitment driven by hypoxia can also have long-distance effects88,90,96.
Simultaneous with collagen synthesis, hypoxia promotes the degradation of extracellular matrix. HIF1 is known to induce the expression of matrix metalloproteinases MMP297, MMP998 and MMP1599 and the expression of urokinase receptor uPAR97. HIF-2 can increase MMP14 levels100. Hypoxia-driven expression of these MMPs can promote invasion and correlates with poor patient prognosis97,99,101. Downregulation of tissue inhibitors of metalloproteinases TIMP2 and TIMP3 by hypoxia represents an additional level of hypoxic control over ECM remodelling98,102. The simultaneous increases in ECM biosynthesis and degradation, which are driven by HIFs, and the hypoxic regulation of focal adhesion formation creates a mechanism for the metastatic spread of cancer cells.
Blood vessels
Vascularisation is one of the main outcomes of HIF signalling. HIF-1α and HIF-2α inactivation led to developmental lethality in mice, which was linked to defects in blood vessel formation103,104. A connection between excessive vascularisation and cancer was shown in several models. Redundant vessel formation is considered a feature of cancer, and it promotes cancer progression. Some therapeutic approaches aiming to suppress vascularisation have been developed105–108. New vessel development was shown to be important for the transition from hyperplasia to neoplasia109. Although vessels transport oxygen, they can be affected by hypoxia. The two major components of blood vessels are endothelial cells and pericytes. Those endothelial cells that reside at the end of growing capillaries and direct their branching are located far from functional vessels, and they can become hypoxic and thus develop a hypoxic cellular response110. HIF-1α and HIF-2α presence in endothelial cells differentially affects vascularisation. While HIF-1α deletion reduces tumour vascularisation and tumour growth, HIF2-α deletion, on the contrary, is able to augment angiogenesis with the formation of a more disorganised vascular system and more hypoxic tumours111–113.
Tumours become hypoxic when they grow too large in size apparently because the blood supply is insufficient. At this point, tumour growth decelerates, and HIF1 promotes the secretion of factors inducing vascularisation from tumour and stromal cells; for example, VEGF influences endothelial cells, pericytes and BMDC to induce vessel growth114–116. The ECM produced by cancer cells cultured under hypoxic conditions was also shown to support angiogenic growth117,118. HIF-induced neovascularization attempts a compensation of the oxygen deficiency in the tumour tissue. But the rate of uncontrollable proliferation of cancer cells exceeds the speed of organised capillary net formation. Indeed sometimes new blood vessels are able to transiently restore the oxygenation, and in this case cancer tissues have interchanging hypoxic areas with a combination of both acute and chronic hypoxic regions. Fluctuations in red cell flux in tumour microvessels can lead to transient hypoxia and reoxygenation in tumour parenchyma119. This uncontrolled activation of hypoxia signalling in tumour mass often results in an aberrant, disorganised vascularisation that fails to compensate oxygen deficiency.
It is noteworthy that endothelial cells play an important role in cancer cell migration, as they are the major structural component of blood vessels and serve as a barrier in extravasation and intravasation processes120. HIF1-α depletion in endothelial cells was shown to suppress the migration of tumour cells through endothelial cells, but HIF2-α depletion was shown to stimulate metastatic spread. These opposite effects of HIF1-α and HIF2-α on vessel formation and metastasis can be explained by the ability of these factors to differentially regulate the nitric oxide level, which regulates endothelial cell function121.
Pericytes are cells embedded in basement membrane of blood microvessels and cover the endothelial cells. They regulate angiogenesis, but also participate to other functions, such as formation of blood-brain barrier. Hypoxic stress of brain pericytes leads to their migration out of the blood vessels, but at the same time HIF-1-signalling leads to VEGF level upregulation and vascularisation stimulation122. During the process of kidney fibrosis detachment of pericytes from the capillaries in response to VEGF and PDGF is followed by their transformation to mesenchymal fibroblasts, actively producing ECM123. As both of these factors can be produced in epithelial cells due to HIF-1 signalling, presumably hypoxia in TME could also lead to detachment of pericytes and their subsequent transformation, but this formal experimental evidence in support of this assumption are currently missing.
Lymphatic vessels
In addition to dissemination in the blood circulation, cancer cells can disseminate through lymphatic vessels. Lymphatic vessel density is increased in breast cancer tissues and correlates with positive lymph node metastases and worsened prognosis124. An increased HIF1-α level in primary malignant neoplasias is tightly associated with the density of the surrounding tumour lymphatic vessels and with breast cancer patient mortality125,126. In oesophageal cancer, HIF1-α levels correlate with the colonisation of lymph nodes127. Different studies suggest a direct contribution of HIF1-α in the regulation of lymphangiogenesis128,129. In particular, HIF1-α was proven to induce lymphatic metastases through the activation of different growth factors, including VEGF-c a and PDGF-B33. Expression of VEGF-c, one of the major lymphangiogenesis-driving factors, was shown to correlate with HIF1-α expression130; however, it remains unclear whether VEGF-c induction requires HIF1-α, as the data are controversial131,132. HIF1 contribution to lymphatic vessel formation can be mediated by VEGF-a133. In contrast, there is evidence for the opposite role of HIF2-α in lymphangiogenesis, as HIF2-α knockdown increased lymphatic vessel formation in vivo in a xenograft model134. The influence of hypoxia on lymphatic vessel formation is a poorly studied topic that needs further investigation, as lymphatic vessel formation contributes to metastatic tumour spread.
Immune cells
Adaptive immune cells can potentially inhibit tumour growth by the recognition of tumour-specific antigens on the surface of cancer cells and the elimination of those cells. Innate immune cells can promote the antitumour activity of infiltrating lymphocytes and lead to significant tumour regression. HIFs have been shown to be tightly connected with the inflammatory processes135,136, and hypoxia can directly or indirectly influence the function of almost all immune cell types, thereby influencing tumour development137.
Innate tumour immunity
Myeloid cell-specific PHD2 depletion was shown to suppress tumour growth and metastases, underlining the importance of oxygen sensitivity for myeloid cells138. A hypoxic TME is known to promote neutrophil engagement in tumours by regulation of their adherence to epithelial cells139,140. HIF1-α and HIF2-α were separately shown to increase the survival and function of neutrophils141–144. The outcome of this hypoxic effect is still unknown, as cancer-associated neutrophils can induce both tumour suppression and tumour progression145.
An increase in cancer-associated macrophage density correlates with poor patient prognosis in different types of cancer146,147. Macrophage polarisation plays a significant role in tumorigenesis. Indeed, macrophages polarised to the M1 type (classical activation) counteract cancer progression and metastases, while M2-polarised macrophages (alternative activation) can promote it148. Hypoxia induces tumour cells to secrete chemoattractants, such as Sema3A, EMAPII, ET-1 and ET-2, promoting the chemotaxis of macrophages from the circulation149–151. HIF1-α was shown to be necessary for macrophage maturation, function141 and glycolytic reprogramming and when associated with HIF1-α-induced PDK1 activity, it increases the migratory capacity of macrophages, representing a possible mechanism of macrophage infiltration regulation by HIF1-α152. In addition, hypoxia determines macrophage polarisation through the induction of M2 polarisation-related genes153 and promotes lactic-acid-induced M2 polarisation154. In vivo experiments show that HIF1-α and HIF2-α are both crucial for macrophage infiltration and immune suppression in tumours, as their separate ablation led to reduced tumour growth155,156.
Another noteworthy innate immune cell is the myeloid-derived suppressor cell (MDSC). These cells originate from bone marrow cells, and their numbers are significantly increased in cancer. Their main feature is the ability to suppress the activity of other immune cells and therefore suppress the antitumour immune response157. The role of hypoxia in MDSC regulation has been considered mostly oncogenic. Hypoxia-treated MDSCs show activation of HIF-signalling and of those HIF targets that enhance MDSC function158. Hypoxia can also augment MDSC function through a mechanism partially dependent on HIF: by miR-210 regulation and ArgI expression158,159. However, some studies suggest that MDSCs are able to gain immunostimulatory properties160. Liu and Colleagues described MDSCs’ differentiation into the tumour-suppressing M1 subtype after SIRT1 induction, and this differentiation occurred as a result of mTOR/HIF-1α-dependent glycolytic reprogramming161. This observation suggests that HIF1 may also endow MDSCs with tumour-suppressive functions and that the role of HIFs in MDSC regulation needs to be further investigated.
Adaptive tumour immunity
Although T-cells are able to infiltrate into the tumour, anticancer immunity is often limited due to characteristics of the TME and a hypoxic environment162. Increased glycolysis in the tumour, which is partially mediated by HIF activity, is a reason for so-called “metabolic competition” between cancer cells and T-cells. Lack of nutrients suppresses T-cell function and the antitumour response163–165. Hypoxia induces the differentiation of non-specific CD4+ T-cells into regulatory T-cells (CD4 + CD25HighFOXP3+) or T-helpers (Тн17), and expression of transcriptional factors FOXP3 and RORγt, which are crucial for differentiation, is regulated by HIF-1166–168. While regulatory T-cells have immunosuppressive functions, the contribution of Тн17 cells to the immune response is unclear169. In addition, under hypoxic conditions, cancer cells and macrophages synthesise chemokines and cytokines, which attract regulatory T-cells from the circulation and repress the antitumour response of other T-cells170–172. Regulatory T-cells in hypoxia produce extracellular adenosine, which represses effector T-cell function173,174. Another mechanism of effector T-cell suppression involves a hypoxia-dependent increase in lysyl oxidase secretion, which causes the formation of premetastatic niches. MDSCs can migrate into those niches and suppress the anti-tumour response of T-killers95,175,176.
HIF1 is involved in the regulation of T-cell immune checkpoints (Fig. 3). In cancer cells, macrophages, dendritic cells and MDSCs, HIF1-α directly induces PD-L1 expression177–179. Binding of PD-L1 expressed on the cell surface to the PD-1 receptor on T-cells leads to their dysfunction, and hence, this mechanism is a target for different pharmaceutical approaches, such as the development of anti-PD-L1 antibodies and PD-1/PD-L1 interaction inhibitors180,181. Another checkpoint regulated by hypoxia is the CTLA-4 receptor, which is upregulated on CD8+ T-cells in hypoxia potentially via HIF1182. Binding of CTLA-4 on T-cells to ligands CD80 and CD86 on the surface of antigen-presenting cells results in effector T-cell inhibition and regulatory T-cell activation183. As with PD-1, anti-cancer treatments using CTLA-4 blocking antibodies have been developed. CTLA-4 and PD-1/PD-L1-targeted therapies showed positive responses in clinical trials for several types of cancer184–186. However, the outcome of the therapies is dependent on many parameters, including the frequency of tumour-infiltrating lymphocytes, which inversely correlates with the glycolytic rate and HIF1-α expression187,188.
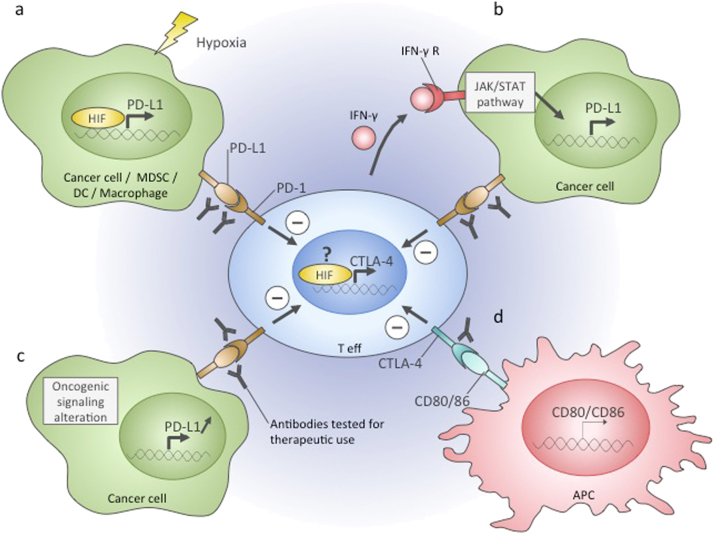
Fig. 3. Immune checkpoints in the tumour microenvironment.
. Effector T cells infiltrating the tumour can become repressed due to the activation of immune checkpoints. The targeting of PD-1 and CTLA-4 checkpoint pathways with specific antibodies is a promising therapeutic approach. In the tumour microenvironment, these pathways can be activated by the following mechanisms. a PD-1 receptor binding to its ligand PD-L1 leads to effector T-cell repression. PD-L1 can be expressed on the surface of cancer cells, MDSCs, DCs, and macrophages, and in these cells, it is directly transactivated by HIF in hypoxia. b Binding of interferon γ secreted by active effector T cells to its receptor on cancer cells results in activation of PD-L1 gene expression and subsequent T-cell repression. c PD-L1 gene expression can be constantly upregulated in cancer cells because of oncogenic mutations and signalling alteration, which leads to effector T cell repression upon interaction with this type of tumour cell. d Antigen-presenting cells express CD80 and CD86 ligands. Upon CD80/86 binding to CTLA-4 receptor on T cells, they become functionally repressed. Hypoxia was shown to induce CTLA-4 expression in T-cells, which potentially could contribute to their repression in the hypoxic tumour microenvironment. Teff effector T-cell, MDSC myeloid-derived suppressor cell, DC dendritic cell, IFNγ interferon gamma, IFN-γ R interferon gamma receptor, APC antigen-presenting cell
In contrast to the described effector T-cell suppression by hypoxia, some data indicate that HIF function is important for the activity of effector cytotoxic T-cells. It was shown by Doedens and colleagues in 2013 that elevated HIF-1 and HIF-2 support the function of cytotoxic CD8+ T-cells182. In agreement with these findings, a recent study by Tyrakis et al. has suggested a role for HIF1-α in CD8+ T-cell proliferation, differentiation and antitumour activity through the regulation of L-2HG189. Apart from cytotoxic T-cells, HIF-1 was also shown to contribute to natural killer cell priming and activation via regulation of the glycolytic rate190, thus demonstrating a stimulatory role in immune activation.
Conclusions
The role of TME in cancer progression is currently attracting impressive interest in the field. Hypoxia is a condition that often occurs at late stages of cancer, and even before that, HIFs can be upregulated due to environmental acidification and the presence of glycolytic metabolites191–193. The HIF-mediated hypoxic impact on TME in most cases is mediated by transcriptional activity of HIFs, secretion of signalling molecules by cancer cells and tumour stromal cells, and metabolic changes associated with the switch from oxygen-dependent catabolism to glycolysis. Non-tumorous cells in the TME are all affected by hypoxia and HIFs (Table 1). Often, hypoxia leads to their dysregulation in a way that supports cancer growth: fibroblasts can be transformed into tumour-prone CAFs, ECM remodelling supports metastases, vascularisation process facilitates cancer progression, and antitumour immune function becomes generally repressed. Nevertheless, the hypoxic response can also be detrimental for tumorigenesis (Table 1). This finding can be partially explained by the differences in HIF-1 and HIF-2 stability and function, as acute hypoxic response is mainly mediated by HIF1 activity, and chronic hypoxia by HIF2194. Dual roles can also arise from the presence of HIF interactors, among which the p53 family and MDM2 can play important roles, as they can affect HIF stability and function195,196. In this context, the interplay between HIF and the p53 family can influence a wide range of cellular processes specifically associated with complexity of p53 family members in controlling cell death197–201, metabolism202–207, reproduction208,209, and development210–213.
Table 1.
Influence of hypoxia on TME components
| TME component | Influence of hypoxic environment | Cancer suppression (+)/promotion (−) |
|---|---|---|
| CAF progenitors | Recruitment, activation and transformation into CAFs | − |
| CAFs | Secretion of cytokines, promoting tumour growth | − |
| Deactivation by chronic hypoxia | + | |
| ECM | Increase of ECM deposition (fibrosis) and remodelling | − |
| Increase of interaction with cancer cells | − | |
| Blood vessels | Increase in vascularisation by HIF-1 | − |
| Decrease in vascularisation by HIF-2 | + | |
| Lymphatic vessels | Increase in lymphangiogenesis by HIF-1 | − |
| Decrease in lymphangiogenesis by HIF-2 | ? | |
| Neutrophils | Recruitment to tumour | ? |
| Increase in survival and function | ? | |
| Macrophage | Maturation and functioning are dependent on HIF1 | ? |
| Recruitment from bloodflow | − | |
| Increase in migratory capacity | − | |
| M2 polarisation | − | |
| MDSCs | Enhancement of immune-suppressive function | − |
| Polarisation to M1 type, driven by SIRT1 | + | |
| Non-specific CD4+ cells | Differentiation into regulatory T-cells and T-helpers | −/? |
| Regulatory T-cells | Recruitment from bloodflow | − |
| Enhancement of immune suppressive function | − | |
| Effector T-cells | Immune checkpoints activation | − |
| Repression due to “metabolic competition” for nutrients | − | |
| CD8+ T-cells | Support in proliferation, differentiation and activation | + |
Considering the global impact of HIF on cancer cells and the TME, therapies targeting HIF activity, such as the usage of small molecules preventing the interactions of the HIF-α and HIF1-β subunits214, can be beneficial for some groups of patients.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J. Cell. Sci. 2012;125:5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 2.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 3.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 5.Ohh M, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat. Cell. Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 6.Koivunen P, Hirsila M, Gunzler V, Kivirikko KI, Myllyharju J. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J. Biol. Chem. 2004;279:9899–9904. doi: 10.1074/jbc.M312254200. [DOI] [PubMed] [Google Scholar]
- 7.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes. Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandl M, Lieberum MK, Depping R. A HIF-1alpha-driven feed-forward loop augments HIF signalling in Hep3B cells by upregulation of ARNT. Cell. Death Dis. 2016;7:e2284. doi: 10.1038/cddis.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olive KP, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baroni S, et al. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell. Death Dis. 2016;7:e2312. doi: 10.1038/cddis.2016.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bochet L, et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73:5657–5668. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- 12.Lecomte J, et al. Bone marrow-derived myofibroblasts are the providers of pro-invasive matrix metalloproteinase 13 in primary tumor. Neoplasia. 2012;14:943–951. doi: 10.1593/neo.121092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald LT, et al. Hematopoietic stem cell-derived cancer-associated fibroblasts are novel contributors to the pro-tumorigenic microenvironment. Neoplasia. 2015;17:434–448. doi: 10.1016/j.neo.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quante M, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J. Cell. Biochem. 2007;101:830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang X, et al. Stromal miR-200s contribute to breast cancer cell invasion through CAF activation and ECM remodeling. Cell. Death. Differ. 2016;23:132–145. doi: 10.1038/cdd.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 18.Olaso E, et al. Tumor-dependent activation of rodent hepatic stellate cells during experimental melanoma metastasis. Hepatology. 1997;26:634–642. doi: 10.1002/hep.510260315. [DOI] [PubMed] [Google Scholar]
- 19.Olumi AF, et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhowmick NA, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 21.Grum-Schwensen B, et al. Suppression of tumor development and metastasis formation in mice lacking the S100A4(mts1) gene. Cancer Res. 2005;65:3772–3780. doi: 10.1158/0008-5472.CAN-04-4510. [DOI] [PubMed] [Google Scholar]
- 22.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 23.Dumont N, et al. Breast fibroblasts modulate early dissemination, tumorigenesis, and metastasis through alteration of extracellular matrix characteristics. Neoplasia. 2013;15:249–262. doi: 10.1593/neo.121950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fornetti J, et al. Mammary epithelial cell phagocytosis downstream of TGF-beta3 is characterized by adherens junction reorganization. Cell. Death. Differ. 2016;23:185–196. doi: 10.1038/cdd.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghavami S, et al. Autophagy is a regulator of TGF-beta1-induced fibrogenesis in primary human atrial myofibroblasts. Cell. Death Dis. 2015;6:e1696. doi: 10.1038/cddis.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, et al. Upregulation of MiR-205 under hypoxia promotes epithelial-mesenchymal transition by targeting ASPP2. Cell. Death Dis. 2016;7:e2517. doi: 10.1038/cddis.2016.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp. Cell. Res. 2010;316:1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 28.Ammirante M, Shalapour S, Kang Y, Jamieson CA, Karin M. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc. Natl. Acad. Sci. USA. 2014;111:14776–14781. doi: 10.1073/pnas.1416498111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schioppa T, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J. Exp. Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat. Rev. Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caniggia I, et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J. Clin. Invest. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/S1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 33.Schito L, et al. Hypoxia-inducible factor 1-dependent expression of platelet-derived growth factor B promotes lymphatic metastasis of hypoxic breast cancer cells. Proc. Natl. Acad. Sci. USA. 2012;109:E2707–2716. doi: 10.1073/pnas.1214019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiaschi T, et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012;72:5130–5140. doi: 10.1158/0008-5472.CAN-12-1949. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, et al. Metabolic reprogramming of cancer-associated fibroblasts by IDH3alpha downregulation. Cell. Rep. 2015;10:1335–1348. doi: 10.1016/j.celrep.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Chiavarina B, et al. HIF1-alpha functions as a tumor promoter in cancer associated fibroblasts, and as a tumor suppressor in breast cancer cells: Autophagy drives compartment-specific oncogenesis. Cell. Cycle. 2010;9:3534–3551. doi: 10.4161/cc.9.17.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jawhari S, Ratinaud MH, Verdier M. Glioblastoma, hypoxia and autophagy: a survival-prone ‘menage-a-trois’. Cell. Death Dis. 2016;7:e2434. doi: 10.1038/cddis.2016.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi Y, et al. PTEN induces apoptosis and cavitation via HIF-2-dependent Bnip3 upregulation during epithelial lumen formation. Cell. Death. Differ. 2015;22:875–884. doi: 10.1038/cdd.2014.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sukumaran P, Sun Y, Vyas M, Singh BB. TRPC1-mediated Ca(2)(+) entry is essential for the regulation of hypoxia and nutrient depletion-dependent autophagy. Cell. Death Dis. 2015;6:e1674. doi: 10.1038/cddis.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JW, et al. Loss of fibroblast HIF-1alpha accelerates tumorigenesis. Cancer Res. 2012;72:3187–3195. doi: 10.1158/0008-5472.CAN-12-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilkes DM, Bajpai S, Chaturvedi P, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J. Biol. Chem. 2013;288:10819–10829. doi: 10.1074/jbc.M112.442939. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Ide T, et al. Tumor-stromal cell interaction under hypoxia increases the invasiveness of pancreatic cancer cells through the hepatocyte growth factor/c-Met pathway. Int. J. Cancer. 2006;119:2750–2759. doi: 10.1002/ijc.22178. [DOI] [PubMed] [Google Scholar]
- 43.Madsen CD, et al. Hypoxia and loss of PHD2 inactivate stromal fibroblasts to decrease tumour stiffness and metastasis. Embo. Rep. 2015;16:1394–1408. doi: 10.15252/embr.201540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J. Cell. Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Rest M, Garrone R. Collagen family of proteins. Faseb J. 1991;5:2814–2823. [PubMed] [Google Scholar]
- 46.Chen SZ, et al. The miR-181d-regulated metalloproteinase Adamts1 enzymatically impairs adipogenesis via ECM remodeling. Cell. Death Differ. 2016;23:1778–1791. doi: 10.1038/cdd.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clarijs R, Ruiter DJ, De Waal RM. Pathophysiological implications of stroma pattern formation in uveal melanoma. J. Cell. Physiol. 2003;194:267–271. doi: 10.1002/jcp.10214. [DOI] [PubMed] [Google Scholar]
- 48.Oh SY, Lee SJ, Jung YH, Lee HJ, Han HJ. Arachidonic acid promotes skin wound healing through induction of human MSC migration by MT3-MMP-mediated fibronectin degradation. Cell. Death Dis. 2015;6:e1750. doi: 10.1038/cddis.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Kempen LC, Ruiter DJ, van Muijen GN, Coussens LM. The tumor microenvironment: a critical determinant of neoplastic evolution. Eur. J. Cell. Biol. 2003;82:539–548. doi: 10.1078/0171-9335-00346. [DOI] [PubMed] [Google Scholar]
- 50.Artinian V, Kvale PA. Cancer and interstitial lung disease. Curr. Opin. Pulm. Med. 2004;10:425–434. doi: 10.1097/00063198-200409000-00017. [DOI] [PubMed] [Google Scholar]
- 51.Bissell DM. Chronic liver injury, TGF-beta, and cancer. Exp. Mol. Med. 2001;33:179–190. doi: 10.1038/emm.2001.31. [DOI] [PubMed] [Google Scholar]
- 52.Boyd NF, Jensen HM, Cooke G, Han HL. Relationship between mammographic and histological risk factors for breast cancer. J. Natl. Cancer Inst. 1992;84:1170–1179. doi: 10.1093/jnci/84.15.1170. [DOI] [PubMed] [Google Scholar]
- 53.Boyd NF, et al. Mammographic densities and the prevalence and incidence of histological types of benign breast disease. Reference Pathologists of the Canadian National Breast Screening Study. Eur. J. Cancer Prev. 2000;9:15–24. doi: 10.1097/00008469-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Boyd NF, et al. Heritability of mammographic density, a risk factor for breast cancer. N. Engl. J. Med. 2002;347:886–894. doi: 10.1056/NEJMoa013390. [DOI] [PubMed] [Google Scholar]
- 55.Zhao J, Du F, Shen G, Zheng F, Xu B. The role of hypoxia-inducible factor-2 in digestive system cancers. Cell. Death Dis. 2015;6:e1600. doi: 10.1038/cddis.2014.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coussens LM, et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes. Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gould VE, Koukoulis GK, Virtanen I. Extracellular matrix proteins and their receptors in the normal, hyperplastic and neoplastic breast. Cell. Differ. Dev. 1990;32:409–416. doi: 10.1016/0922-3371(90)90057-4. [DOI] [PubMed] [Google Scholar]
- 58.Huijbers IJ, et al. A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS. One. 2010;5:e9808. doi: 10.1371/journal.pone.0009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kauppila S, Stenback F, Risteli J, Jukkola A, Risteli L. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J. Pathol. 1998;186:262–268. doi: 10.1002/(SICI)1096-9896(1998110)186:3<262::AID-PATH191>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 60.Shapiro FD, Eyre DR. Collagen polymorphism in extracellular matrix of human osteosarcoma. J. Natl. Cancer Inst. 1982;69:1009–1016. [PubMed] [Google Scholar]
- 61.Zhu GG, et al. Immunohistochemical study of type I collagen and type I pN-collagen in benign and malignant ovarian neoplasms. Cancer. 1995;75:1010–1017. doi: 10.1002/1097-0142(19950215)75:4<1010::AID-CNCR2820750417>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 62.Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am. J. Cancer Res. 2011;1:482–497. [PMC free article] [PubMed] [Google Scholar]
- 63.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 64.Alexander J, Cukierman E. Stromal dynamic reciprocity in cancer: intricacies of fibroblastic-ECM interactions. Curr. Opin. Cell. Biol. 2016;42:80–93. doi: 10.1016/j.ceb.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ungefroren H, Sebens S, Seidl D, Lehnert H, Hass R. Interaction of tumor cells with the microenvironment. Cell. Commun. Signal. 2011;9:18. doi: 10.1186/1478-811X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colpaert CG, et al. The presence of a fibrotic focus in invasive breast carcinoma correlates with the expression of carbonic anhydrase IX and is a marker of hypoxia and poor prognosis. Breast Cancer Res. Treat. 2003;81:137–147. doi: 10.1023/A:1025702330207. [DOI] [PubMed] [Google Scholar]
- 67.Trastour C, et al. HIF-1alpha and CA IX staining in invasive breast carcinomas: prognosis and treatment outcome. Int. J. Cancer. 2007;120:1451–1458. doi: 10.1002/ijc.22436. [DOI] [PubMed] [Google Scholar]
- 68.Halberg N, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Higgins DF, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moon JO, Welch TP, Gonzalez FJ, Copple BL. Reduced liver fibrosis in hypoxia-inducible factor-1alpha-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G582–592. doi: 10.1152/ajpgi.90368.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berg JT, Breen EC, Fu Z, Mathieu-Costello O, West JB. Alveolar hypoxia increases gene expression of extracellular matrix proteins and platelet-derived growth factor-B in lung parenchyma. Am. J. Respir. Crit. Care Med. 1998;158:1920–1928. doi: 10.1164/ajrccm.158.6.9804076. [DOI] [PubMed] [Google Scholar]
- 72.Falanga V, et al. Low oxygen tension increases mRNA levels of alpha 1 (I) procollagen in human dermal fibroblasts. J. Cell. Physiol. 1993;157:408–412. doi: 10.1002/jcp.1041570225. [DOI] [PubMed] [Google Scholar]
- 73.Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000;58:2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- 74.Tamamori M, Ito H, Hiroe M, Marumo F, Hata RI. Stimulation of collagen synthesis in rat cardiac fibroblasts by exposure to hypoxic culture conditions and suppression of the effect by natriuretic peptides. Cell. Biol. Int. 1997;21:175–180. doi: 10.1006/cbir.1997.0130. [DOI] [PubMed] [Google Scholar]
- 75.Topalovski M, Hagopian M, Wang M, Brekken RA. Hypoxia and transforming growth factor beta cooperate to induce fibulin-5 expression in pancreatic cancer. J. Biol. Chem. 2016;291:22244–22252. doi: 10.1074/jbc.M116.730945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ju JA, et al. Hypoxia selectively enhances integrin receptor expression to promote metastasis. Mol Cancer Res. 2017;15:723–734. doi: 10.1158/1541-7786.MCR-16-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brooks DL, et al. ITGA6 is directly regulated by hypoxia-inducible factors and enriches for cancer stem cell activity and invasion in metastatic breast cancer models. Mol. Cancer. 2016;15:26. doi: 10.1186/s12943-016-0510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koike T, et al. Hypoxia induces adhesion molecules on cancer cells: a missing link between Warburg effect and induction of selectin-ligand carbohydrates. Proc. Natl. Acad. Sci. USA. 2004;101:8132–8137. doi: 10.1073/pnas.0402088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aro E, et al. Hypoxia-inducible factor-1 (HIF-1) but not HIF-2 is essential for hypoxic induction of collagen prolyl 4-hydroxylases in primary newborn mouse epiphyseal growth plate chondrocytes. J. Biol. Chem. 2012;287:37134–37144. doi: 10.1074/jbc.M112.352872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bentovim L, Amarilio R, Zelzer E. HIF1alpha is a central regulator of collagen hydroxylation and secretion under hypoxia during bone development. Development. 2012;139:4473–4483. doi: 10.1242/dev.083881. [DOI] [PubMed] [Google Scholar]
- 81.Eisinger-Mathason TS, et al. Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 2013;3:1190–1205. doi: 10.1158/2159-8290.CD-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Elvidge GP, et al. Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1alpha, HIF-2alpha, and other pathways. J. Biol. Chem. 2006;281:15215–15226. doi: 10.1074/jbc.M511408200. [DOI] [PubMed] [Google Scholar]
- 83.Gilkes DM, et al. Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol. Cancer Res. 2013;11:456–466. doi: 10.1158/1541-7786.MCR-12-0629. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Gilkes DM, et al. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res. 2013;73:3285–3296. doi: 10.1158/0008-5472.CAN-12-3963. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Hofbauer KH, et al. Oxygen tension regulates the expression of a group of procollagen hydroxylases. Eur. J. Biochem. 2003;270:4515–4522. doi: 10.1046/j.1432-1033.2003.03846.x. [DOI] [PubMed] [Google Scholar]
- 86.Xiong G, Deng L, Zhu J, Rychahou PG, Xu R. Prolyl-4-hydroxylase alpha subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC Cancer. 2014;14:1. doi: 10.1186/1471-2407-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gordon MK, Hahn RA. Collagens. Cell. Tissue Res. 2010;339:247–257. doi: 10.1007/s00441-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Erler JT, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schietke R, et al. The lysyl oxidases LOX and LOXL2 are necessary and sufficient to repress E-cadherin in hypoxia: insights into cellular transformation processes mediated by HIF-1. J. Biol. Chem. 2010;285:6658–6669. doi: 10.1074/jbc.M109.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wong CC, et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc. Natl. Acad. Sci. USA. 2011;108:16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nat. Rev. Cancer. 2012;12:540–552. doi: 10.1038/nrc3319. [DOI] [PubMed] [Google Scholar]
- 92.Li X, et al. Negative feedback loop between p66Shc and ZEB1 regulates fibrotic EMT response in lung cancer cells. Cell. Death Dis. 2015;6:e1708. doi: 10.1038/cddis.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yun-Ju Huang R, Yo-Yan Huang T. A new dimension in drug discovery: reversing epithelial-mesenchymal transition (EMT) Cell. Death Dis. 2016;7:e2417. doi: 10.1038/cddis.2016.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cox TR, et al. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73:1721–1732. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 96.Wong CC, et al. Inhibitors of hypoxia-inducible factor 1 block breast cancer metastatic niche formation and lung metastasis. J. Mol. Med. 2012;90:803–815. doi: 10.1007/s00109-011-0855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krishnamachary B, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138–1143. [PubMed] [Google Scholar]
- 98.Choi JY, Jang YS, Min SY, Song JY. Overexpression of MMP-9 and HIF-1alpha in breast cancer cells under hypoxic conditions. J. Breast Cancer. 2011;14:88–95. doi: 10.4048/jbc.2011.14.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu SK, et al. Overexpression of membrane-type 2 matrix metalloproteinase induced by hypoxia-inducible factor-1alpha in pancreatic cancer: Implications for tumor progression and prognosis. Mol. Clin. Oncol. 2014;2:973–981. doi: 10.3892/mco.2014.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Petrella BL, Lohi J, Brinckerhoff CE. Identification of membrane type-1 matrix metalloproteinase as a target of hypoxia-inducible factor-2 alpha in von Hippel-Lindau renal cell carcinoma. Oncogene. 2005;24:1043–1052. doi: 10.1038/sj.onc.1208305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Munoz-Najar UM, Neurath KM, Vumbaca F, Claffey KP. Hypoxia stimulates breast carcinoma cell invasion through MT1-MMP and MMP-2 activation. Oncogene. 2006;25:2379–2392. doi: 10.1038/sj.onc.1209273. [DOI] [PubMed] [Google Scholar]
- 102.Kai AK, et al. Down-regulation of TIMP2 by HIF-1alpha/miR-210/HIF-3alpha regulatory feedback circuit enhances cancer metastasis in hepatocellular carcinoma. Hepatology. 2016;64:473–487. doi: 10.1002/hep.28577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iyer NV, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes. Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peng J, Zhang L, Drysdale L, Fong GH. The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proc. Natl. Acad. Sci. USA. 2000;97:8386–8391. doi: 10.1073/pnas.140087397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 106.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 108.Watson EC, Whitehead L, Adams RH, Dewson G, Coultas L. Endothelial cell survival during angiogenesis requires the pro-survival protein MCL1. Cell. Death. Differ. 2016;23:1371–1379. doi: 10.1038/cdd.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 110.Coulon C, et al. From vessel sprouting to normalization: role of the prolyl hydroxylase domain protein/hypoxia-inducible factor oxygen-sensing machinery. Arterioscler. Thromb. Vasc. Biol. 2010;30:2331–2336. doi: 10.1161/ATVBAHA.110.214106. [DOI] [PubMed] [Google Scholar]
- 111.Skuli N, et al. Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood. 2009;114:469–477. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Skuli N, et al. Endothelial HIF-2alpha regulates murine pathological angiogenesis and revascularization processes. J. Clin. Invest. 2012;122:1427–1443. doi: 10.1172/JCI57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tang N, et al. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 114.Du R, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fernandez-Alonso R, et al. p73 is required for endothelial cell differentiation, migration and the formation of vascular networks regulating VEGF and TGFbeta signaling. Cell. Death. Differ. 2015;22:1287–1299. doi: 10.1038/cdd.2014.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nauta TD, et al. Identification of HIF-2alpha-regulated genes that play a role in human microvascular endothelial sprouting during prolonged hypoxia in vitro. Angiogenesis. 2017;20:39–54. doi: 10.1007/s10456-016-9527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hielscher, A., Qiu, C., Porterfield, J., Smith, Q. & Gerecht, S. Hypoxia affects the structure of breast cancer cell-derived matrix to support angiogenic responses of endothelial cells. J. Carcinog. Mutagen. (Suppl 13), 005 (2013). [DOI] [PMC free article] [PubMed]
- 118.Wang L, et al. Glycation of vitronectin inhibits VEGF-induced angiogenesis by uncoupling VEGF receptor-2-alphavbeta3 integrin cross-talk. Cell. Death Dis. 2015;6:e1796. doi: 10.1038/cddis.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brurberg KG, Graff BA, Olsen DR, Rofstad EK. Tumor-line specific pO(2) fluctuations in human melanoma xenografts. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:403–409. doi: 10.1016/j.ijrobp.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 120.Franses JW, Baker AB, Chitalia VC, Edelman ER. Stromal endothelial cells directly influence cancer progression. Sci. Transl. Med. 2011;3:66ra65. doi: 10.1126/scitranslmed.3001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Branco-Price C, et al. Endothelial cell HIF-1alpha and HIF-2alpha differentially regulate metastatic success. Cancer Cell. 2012;21:52–65. doi: 10.1016/j.ccr.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 2016;19:771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kawakami T, Mimura I, Shoji K, Tanaka T, Nangaku M. Hypoxia and fibrosis in chronic kidney disease: crossing at pericytes. Kidney Int. Suppl. 2014;4:107–112. doi: 10.1038/kisup.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mohammed RA, Ellis IO, Elsheikh S, Paish EC, Martin SG. Lymphatic and angiogenic characteristics in breast cancer: morphometric analysis and prognostic implications. Breast Cancer Res. Treat. 2009;113:261–273. doi: 10.1007/s10549-008-9936-1. [DOI] [PubMed] [Google Scholar]
- 125.Bos R, et al. Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97:1573–1581. doi: 10.1002/cncr.11246. [DOI] [PubMed] [Google Scholar]
- 126.Schoppmann SF, et al. Hypoxia inducible factor-1alpha correlates with VEGF-C expression and lymphangiogenesis in breast cancer. Breast Cancer Res. Treat. 2006;99:135–141. doi: 10.1007/s10549-006-9190-3. [DOI] [PubMed] [Google Scholar]
- 127.Kurokawa T, et al. Overexpression of hypoxia-inducible-factor 1alpha(HIF-1alpha) in oesophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Br. J. Cancer. 2003;89:1042–1047. doi: 10.1038/sj.bjc.6601186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ji RC. Hypoxia and lymphangiogenesis in tumor microenvironment and metastasis. Cancer Lett. 2014;346:6–16. doi: 10.1016/j.canlet.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 129.Zampell JC, et al. HIF-1alpha coordinates lymphangiogenesis during wound healing and in response to inflammation. FASEB J. 2012;26:1027–1039. doi: 10.1096/fj.11-195321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liang X, et al. Hypoxia inducible factor-alpha expression correlates with vascular endothelial growth factor-C expression and lymphangiogenesis/angiogenesis in oral squamous cell carcinoma. Anticancer. Res. 2008;28:1659–1666. [PubMed] [Google Scholar]
- 131.min Y, et al. C/EBP-delta regulates VEGF-C autocrine signaling in lymphangiogenesis and metastasis of lung cancer through HIF-1alpha. Oncogene. 2011;30:4901–4909. doi: 10.1038/onc.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morfoisse F, et al. Hypoxia induces VEGF-C expression in metastatic tumor cells via a HIF-1alpha-independent translation-mediated mechanism. Cell. Rep. 2014;6:155–167. doi: 10.1016/j.celrep.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 133.Hirakawa S, et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Geis T, et al. HIF-2alpha attenuates lymphangiogenesis by up-regulating IGFBP1 in hepatocellular carcinoma. Biol. Cell. 2015;107:175–188. doi: 10.1111/boc.201400079. [DOI] [PubMed] [Google Scholar]
- 135.Botta C, et al. The route to solve the interplay between inflammation, angiogenesis and anti-cancer immune response. Cell. Death Dis. 2016;7:e2299. doi: 10.1038/cddis.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Triner D, Shah YM. Hypoxia-inducible factors: a central link between inflammation and cancer. J. Clin. Invest. 2016;126:3689–3698. doi: 10.1172/JCI84430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat. Rev. Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 138.Mamlouk S, et al. Loss of prolyl hydroxylase-2 in myeloid cells and T-lymphocytes impairs tumor development. Int. J. Cancer. 2014;134:849–858. doi: 10.1002/ijc.28409. [DOI] [PubMed] [Google Scholar]
- 139.Bose T, Cieslar-Pobuda A, Wiechec E. Role of ion channels in regulating Ca(2)(+) homeostasis during the interplay between immune and cancer cells. Cell. Death Dis. 2015;6:e1648. doi: 10.1038/cddis.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yoshida N, et al. Anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Am. J. Physiol. 1992;262:H1891–1898. doi: 10.1152/ajpheart.1992.262.6.H1891. [DOI] [PubMed] [Google Scholar]
- 141.Cramer T, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/S0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thompson AA, et al. Hypoxia-inducible factor 2alpha regulates key neutrophil functions in humans, mice, and zebrafish. Blood. 2014;123:366–376. doi: 10.1182/blood-2013-05-500207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Walmsley SR, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J. Exp. Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wendland K, Thielke M, Meisel A, Mergenthaler P. Intrinsic hypoxia sensitivity of the cytomegalovirus promoter. Cell. Death Dis. 2015;6:e1905. doi: 10.1038/cddis.2015.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 146.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J. Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 147.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rolny C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 149.Casazza A, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 150.Matschurat S, et al. Regulation of EMAP II by hypoxia. Am. J. Pathol. 2003;162:93–103. doi: 10.1016/S0002-9440(10)63801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 152.Semba H, et al. HIF-1alpha-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat. Commun. 2016;7:11635. doi: 10.1038/ncomms11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Laoui D, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014;74:24–30. doi: 10.1158/0008-5472.CAN-13-1196. [DOI] [PubMed] [Google Scholar]
- 154.Colegio OR, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Doedens AL, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Imtiyaz HZ, et al. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J. Clin. Invest. 2010;120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Corzo CA, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Noman MZ, et al. Tumor-promoting effects of myeloid-derived suppressor cells are potentiated by hypoxia-induced expression of miR-210. Cancer Res. 2015;75:3771–3787. doi: 10.1158/0008-5472.CAN-15-0405. [DOI] [PubMed] [Google Scholar]
- 160.Pastula A, Marcinkiewicz J. Myeloid-derived suppressor cells: a double-edged sword? Int. J. Exp. Pathol. 2011;92:73–78. doi: 10.1111/j.1365-2613.2010.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu G, et al. SIRT1 limits the function and fate of myeloid-derived suppressor cells in tumors by orchestrating HIF-1alpha-dependent glycolysis. Cancer Res. 2014;74:727–737. doi: 10.1158/0008-5472.CAN-13-2584. [DOI] [PubMed] [Google Scholar]
- 162.Le QT, et al. Galectin-1: a link between tumor hypoxia and tumor immune privilege. J. Clin. Oncol. 2005;23:8932–8941. doi: 10.1200/JCO.2005.02.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chang CH, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ho PC, et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee HJ, et al. Glycerol-3-phosphate acyltransferase-1 upregulation by O-GlcNAcylation of Sp1 protects against hypoxia-induced mouse embryonic stem cell apoptosis via mTOR activation. Cell. Death Dis. 2016;7:e2158. doi: 10.1038/cddis.2015.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. Eur. J. Immunol. 2008;38:2412–2418. doi: 10.1002/eji.200838318. [DOI] [PubMed] [Google Scholar]
- 167.Clambey ET, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc. Natl. Acad. Sci. USA. 2012;109:E2784–2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bailey SR, et al. Th17 cells in cancer: the ultimate identity crisis. Front. Immunol. 2014;5:276. doi: 10.3389/fimmu.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Facciabene A, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 171.Viguier M, et al. Foxp3 expressing CD4 + CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J. Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 172.Zhao X, et al. A novel differentiation pathway from CD4(+) T cells to CD4(-) T cells for maintaining immune system homeostasis. Cell. Death Dis. 2016;7:e2193. doi: 10.1038/cddis.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ohta A, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl. Acad. Sci. USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Synnestvedt K, et al. Ecto-5’-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 2002;110:993–1002. doi: 10.1172/JCI0215337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cox TR, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106–110. doi: 10.1038/nature14492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 176.Sceneay J, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906–3911. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- 177.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 178.Noman MZ, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang SC, et al. PD-1 and Tim-3 pathways are associated with regulatory CD8+ T-cell function in decidua and maintenance of normal pregnancy. Cell. Death Dis. 2015;6:e1738. doi: 10.1038/cddis.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chinai JM, et al. New immunotherapies targeting the PD-1 pathway. Trends Pharmacol. Sci. 2015;36:587–595. doi: 10.1016/j.tips.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yamazaki T, et al. The oncolytic peptide LTX-315 overcomes resistance of cancers to immunotherapy with CTLA4 checkpoint blockade. Cell. Death. Differ. 2016;23:1004–1015. doi: 10.1038/cdd.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Doedens AL, et al. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat. Immunol. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin. Cancer Res. 2013;19:5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 185.Shaabani N, et al. CD169+ macrophages regulate PD-L1 expression via type I interferon and thereby prevent severe immunopathology after LCMV infection. Cell. Death Dis. 2016;7:e2446. doi: 10.1038/cddis.2016.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wolchok JD, Chan TA. Cancer: antitumour immunity gets a boost. Nature. 2014;515:496–498. doi: 10.1038/515496a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dominguez C, Tsang KY, Palena C. Short-term EGFR blockade enhances immune-mediated cytotoxicity of EGFR mutant lung cancer cells: rationale for combination therapies. Cell. Death Dis. 2016;7:e2380. doi: 10.1038/cddis.2016.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ottensmeier CH, et al. Upregulated glucose metabolism correlates inversely with CD8+T-cell infiltration and survival in squamous cell carcinoma. Cancer Res. 2016;76:4136–4148. doi: 10.1158/0008-5472.CAN-15-3121. [DOI] [PubMed] [Google Scholar]
- 189.Tyrakis PA, et al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature. 2016;540:236–241. doi: 10.1038/nature20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Velasquez SY, et al. Short term hypoxia synergizes with interleukin 15 priming in driving glycolytic gene transcription and supports human natural killer cell activities. J. Biol. Chem. 2016;291:12960–12977. doi: 10.1074/jbc.M116.721753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Filatova A, et al. Acidosis acts through HSP90 in a PHD/VHL-Independent manner to promote HIF function and stem cell maintenance in glioma. Cancer Res. 2016;76:5845–5856. doi: 10.1158/0008-5472.CAN-15-2630. [DOI] [PubMed] [Google Scholar]
- 192.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J. Biol. Chem. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 193.Mekhail K, Gunaratnam L, Bonicalzi ME, Lee S. HIF activation by pH-dependent nucleolar sequestration of VHL. Nat. Cell. Biol. 2004;6:642–647. doi: 10.1038/ncb1144. [DOI] [PubMed] [Google Scholar]
- 194.Koh MY, Lemos R, Jr, Liu X, Powis G. The hypoxia-associated factor switches cells from HIF-1alpha- to HIF-2alpha-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res. 2011;71:4015–4027. doi: 10.1158/0008-5472.CAN-10-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Amelio I, Melino G. The p53 family and the hypoxia-inducible factors (HIFs): determinants of cancer progression. Trends Biochem. Sci. 2015;40:425–434. doi: 10.1016/j.tibs.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 196.Charni M, Aloni-Grinstein R, Molchadsky A, Rotter V. p53 on the crossroad between regeneration and cancer. Cell. Death. Differ. 2017;24:8–14. doi: 10.1038/cdd.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Belle JI, et al. Repression of p53-target gene Bbc3/PUMA by MYSM1 is essential for the survival of hematopoietic multipotent progenitors and contributes to stem cell maintenance. Cell. Death. Differ. 2016;23:759–775. doi: 10.1038/cdd.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Charni M, et al. Novel p53 target genes secreted by the liver are involved in non-cell-autonomous regulation. Cell. Death. Differ. 2016;23:509–520. doi: 10.1038/cdd.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Desantis A, et al. Che-1 modulates the decision between cell cycle arrest and apoptosis by its binding top53. Cell. Death Dis. 2015;6:e1764. doi: 10.1038/cddis.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat. Rev. Mol. Cell. Biol. 2015;16:393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- 201.Rufini A, et al. p73 in Cancer. Genes. & Cancer. 2011;2:491–502. doi: 10.1177/1947601911408890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Agostini M. et al. (2017). p73 Regulates primary cortical neuron metabolism: a global metabolic profile. Mol. Neurobiol. [DOI] [PubMed]
- 203.Amelio I, et al. TAp73 promotes anabolism. Oncotarget. 2014;5:12820–12934. doi: 10.18632/oncotarget.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Amelio I, Cutruzzola F, Antonov A, Agostini M, Melino G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 2014;39:191–198. doi: 10.1016/j.tibs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Amelio I, et al. p73 regulates serine biosynthesis in cancer. Oncogene. 2014;33:5039–5046. doi: 10.1038/onc.2013.456. [DOI] [PubMed] [Google Scholar]
- 206.Candi E, et al. Metabolic pathways regulated by p63. Biochem. Biophys. Res. Commun. 2017;482:440–444. doi: 10.1016/j.bbrc.2016.10.094. [DOI] [PubMed] [Google Scholar]
- 207.Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer. 2016;16:650–662. doi: 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- 208.Inoue S, et al. TAp73 is required for spermatogenesis and the maintenance of male fertility. Proc. Natl. Acad. Sci. USA. 2014;111:1843–1848. doi: 10.1073/pnas.1323416111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Levine AJ, Tomasini R, McKeon FD, Mak TW, Melino G. The p53 family: guardians of maternal reproduction. Nat. Rev. Mol. Cell. Biol. 2011;12:259–265. doi: 10.1038/nrm3086. [DOI] [PubMed] [Google Scholar]
- 210.Candi E, Amelio I, Agostini M, Melino G. MicroRNAs and p63 in epithelial stemness. Cell. Death. Differ. 2015;22:12–21. doi: 10.1038/cdd.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lena AM, et al. Skn-1a/Oct-11 and DeltaNp63alpha exert antagonizing effects on human keratin expression. Biochem. Biophys. Res. Commun. 2010;401:568–573. doi: 10.1016/j.bbrc.2010.09.102. [DOI] [PubMed] [Google Scholar]
- 212.Niklison-Chirou MV, et al. How Does p73 Cause Neuronal Defects? Mol. Neurobiol. 2016;53:4509–4520. doi: 10.1007/s12035-015-9381-1. [DOI] [PubMed] [Google Scholar]
- 213.Vanbokhoven H, Melino G, Candi E, Declercq W. p63, a story of mice and men. J. Invest. Dermatol. 2011;131:1196–1207. doi: 10.1038/jid.2011.84. [DOI] [PubMed] [Google Scholar]
- 214.Semenza GL. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]