Abstract
Significance: Chronic wounds are a major burden to patients and to healthcare systems worldwide. These wounds are difficult to heal and treatment is often lengthy and expensive. This has led to research efforts focussed on the wound environment attempting to understand the underlying pathological mechanisms of impaired wound healing. While some of this research has translated to advancements in wound therapies and implementation of new treatment options, chronic wounds remain a significant challenge to treat. Thus, identification of effective, low-cost, advanced wound therapies that enhance healing rates of these problematic wounds is still essential.
Recent Advances and Critical Issues: Xanthine oxidoreductase (XOR), a molybdoflavin enzyme, is emerging as an important source of reactive oxygen species (ROS) in various pathologies, including diabetes and chronic wounds. XOR has recently been shown to be upregulated in chronic wounds, stimulating the overproduction of ROS during dysfunctional wound healing. XOR-induced ROS can amplify and potentiate inflammation in the wound environment further delaying wound closure.
Future Directions: The detrimental role of XOR in impaired healing indicates it may be a therapeutic target. Targeted inhibition of XOR has been shown to reduce the expression and activity of this enzyme in diabetic wound models. In turn, this resulted in a significant decrease in ROS levels in the wound environment and improved wound healing. Therefore, repurposing existing XOR inhibitors that are approved for human use may be able to restore homeostasis at the wound site and enable damaged tissue to return to normal healing.
Keywords: : chronic wounds, xanthine dehydrogenase, xanthine oxidase, xanthine oxidoreductase, reactive oxygen species, inflammasome, uric acid

Melissa L. Fernandez, PhD
Scope and Significance
This review provides a brief overview of xanthine oxidoreductase (XOR), its structure and function. We then summarize the current evidence that suggests XOR may play an important role in impaired wound healing. In particular, we focus on the two products of XOR activity, reactive oxygen species (ROS) and uric acid, and how these factors may potentiate inflammation in nonhealing wounds. Finally, we discuss the benefits of repurposing common XOR inhibitors that are approved for human use as a treatment for patients with hard-to-heal wounds.
Translation Relevance
Chronic wounds are characterized by an amplified and prolonged inflammatory phase. XOR is thought to play a key role in chronic wounds by generating excessive amounts of ROS and uric acid. Together, these factors could stimulate inflammation and prevent proliferation, vascularization, and reepithelialization, thereby delaying the wound healing process. Inhibition of XOR therefore holds potential in preventing the overproduction of ROS and elevated levels of uric acid in the wound environment, restoring homeostasis, and improving wound healing.
Clinical Relevance
The management and treatment of chronic wounds is one of the biggest health issues today, adding a significant financial burden to sufferers and to already critically stretched healthcare budgets. An unmet clinical need exists for effective, low-cost, advanced wound care therapies to improve healing. The inhibition of XOR may offer a novel cost-effective approach to treat a subset of nonhealing wounds.
Background
Chronic wounds are a major burden worldwide
Chronic wounds are a significant and rapidly growing global health issue. The rising burden of diabetes, cardiovascular disease, and obesity on an epidemic scale, in combination with a progressively aging population, has led to the increasing incidence of these complex hard-to-heal wounds. The majority of chronic wounds present as leg ulcers with various etiologies, but common manifestations include diabetic foot ulcers and pressure ulcers. It is estimated that ∼1–2% of the population in developed countries will suffer from a chronic wound during their lifetime.1 Common outcomes for these wounds are long-term pain, loss of mobility, decreased quality of life, ongoing medical care and, frequently, limb amputation. Unfortunately, despite improvements in wound care and an increase in the variety of wound dressings and novel advanced wound therapies available, chronic wounds remain a challenge to treat, highlighting that there is still a need for new therapies to improve healing rates.
Inflammation is a key feature of chronic wounds
Over the last two decades, studies have demonstrated that the chronic wound environment is characterized by elevated levels of proteases such as matrix metalloproteinases (MMPs),2 reduced levels of protease inhibitors such as tissue inhibitors of metalloproteinases (TIMPs),3 and an abundance of inflammatory cells releasing excessive amounts of proinflammatory cytokines, the aforementioned proteolytic enzymes, and toxic free radicals.4 The combined effect of these factors results in accelerated degradation of the extracellular matrix5 and growth factors6 and an amplified inflammatory state.4,7 This leads to a decrease in cellular proliferation, inadequate vascularization, and the accumulation of necrotic tissue due to ischemia. This in turn encourages bacterial colonization and can perpetuate the inflammatory response, preventing wound repair.8 While significant progress has been made to understand the processes involved in impaired wound healing, more research is required to elucidate the underlying causes of dysregulation to develop targeted therapies to treat these problematic wounds.
XOR—structure and function
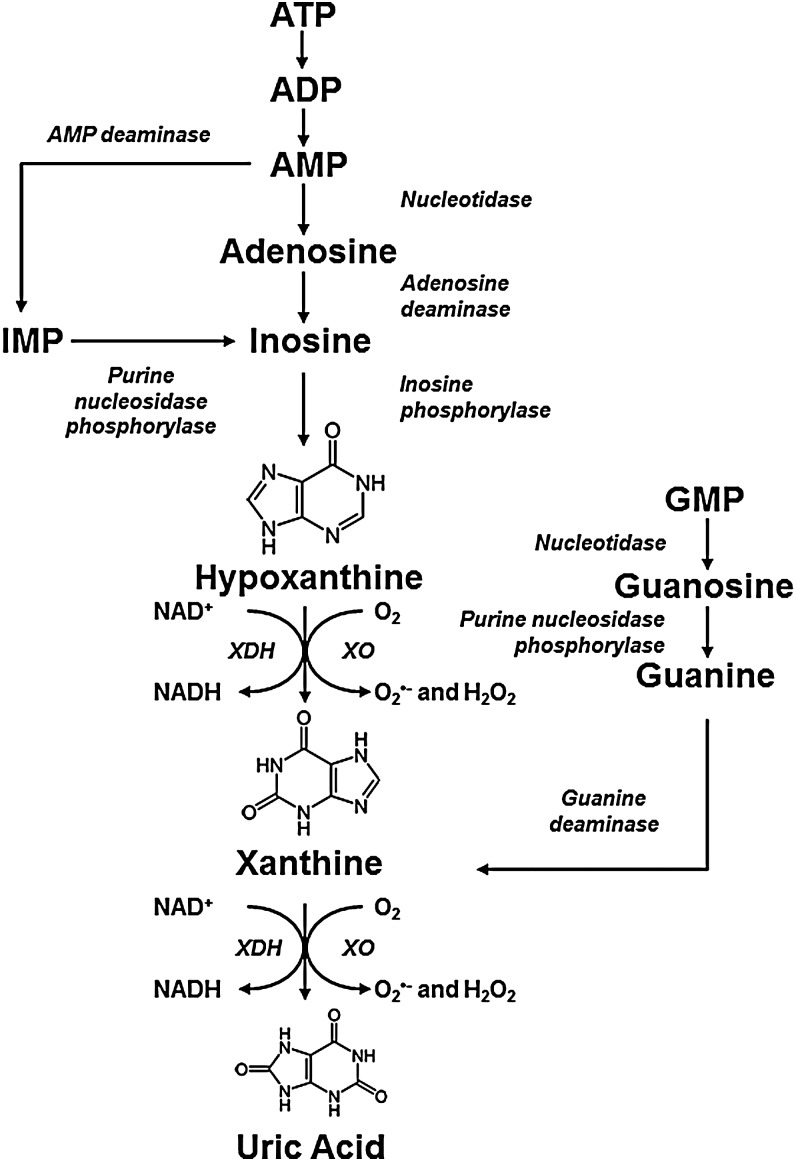
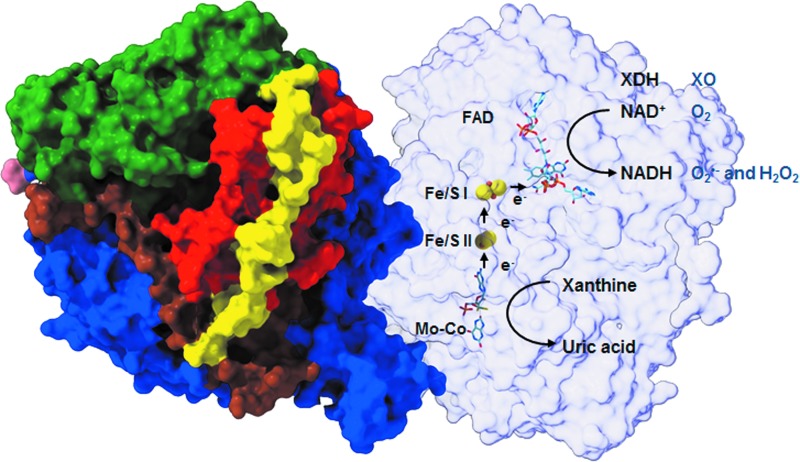
The chronic wound environment is considered to be highly oxidizing owing to the increased release of ROS. A large amount of ROS present in wounds are released by activated infiltrating neutrophils and macrophages undergoing “respiratory bursts” during phagocytosis.9 Another potential source of ROS in the wound environment is the enzyme XOR.10 XOR is a complex molybdoflavin enzyme that is well known for its role in catalyzing the two terminal reactions in the purine metabolic pathway, namely the conversion of hypoxanthine to xanthine and subsequently xanthine to uric acid using either NAD+ or O2 (Fig. 1).11 It is a homodimer with an approximate mass of 300 kDa, with each subunit consisting of an N-terminal 20 kDa domain containing two iron (Fe/S) centers, a central 40 kDa flavin adenine dinucleotide (FAD) domain and a C-terminal 85 kDa molybdopterin center (molybdenum cofactor; Mo-Co; Fig. 2).12,13 The Mo-Co is the site of purine oxidation, while NAD+ and O2 are reduced at the FAD.
FIG. 1.
The Purine Metabolic Pathway—ATP is sequentially degraded to hypoxanthine and xanthine, which are substrates of XOR. Guanine can also be converted to xanthine increasing substrate availability for XDH/XO. XOR exists in two interconvertible forms, the dehydrogenase form (XDH) that reduces NAD+, while the oxidase form (XO) of the enzyme reduces oxygen, concomitantly producing superoxide and hydrogen peroxide. The oxidase form of the enzyme is the predominant source of ROS production and is associated with disrupting homeostasis in inflammatory pathologies, including chronic wounds. Both forms of XOR catalyze the conversion of hypoxanthine to xanthine and finally xanthine to uric acid. In humans, uric acid is the terminal metabolite in the purine metabolic pathway and is found in elevated levels in wound fluid from chronic wounds. ADP, adenosine diphosphate; AMP, adenosine monophosphate; ATP, adenosine triphosphate; NADH, nicotinamide adenine dinucleotide reduced form; ROS, reactive oxygen species; XDH, xanthine dehydrogenase; XO, xanthine oxidase; XOR, xanthine oxidoreductase. (Reproduced from Pacher et al.11)
FIG. 2.
The Structure of Bovine XOR—The 3D model illustrates bovine XDH, the best available structure and analogous to the human form of XOR. It exists as a homodimer of ∼150 kDa subunits with the three major domains and two connecting loops. The domains are an N-terminal 20 kDa domain containing two iron (Fe/S) centers (red), a central 40 kDa FAD domain (green) and a C-terminal 85 kDa molybdopterin center (molybdenum cofactor; Mo-Co) (blue). The loop connecting the Fe/S domain with the FAD domain is shown in yellow and the one connecting the FAD domain with the Mo-Co is depicted in brown. The Mo-Co is the site of xanthine and hypoxanthine oxidation, during which electrons that are transferred to molybdenum are then transferred to FAD via the two Fe/S centers. The electron acceptors, NAD+ and O2, are subsequently reduced at the FAD site. FAD, flavin adenine dinucleotide. (Adapted from Enroth et al.12)
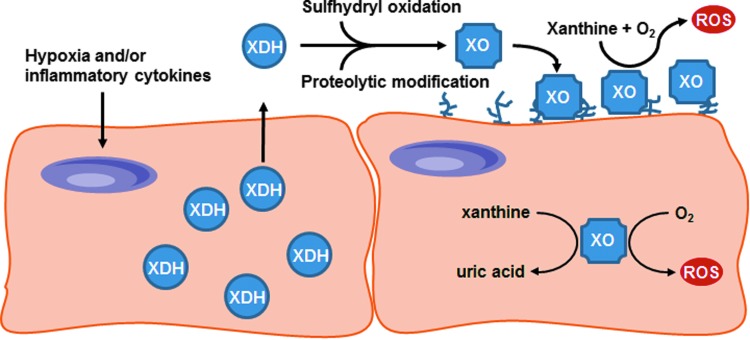
XOR exists in two interconvertible forms, xanthine dehydrogenase (XDH) and xanthine oxidase (XO). Under normal physiological conditions, XOR is predominantly present in the dehydrogenase form and uses NAD+ as its preferred electron acceptor to yield nicotinamide adenine dinucleotide (NADH reduced form). Hypoxia, among other conditions, can lead to the conversion of XDH to the oxidase form, either by reversible sulfhydryl modification or by irreversible proteolytic cleavage.12,14 Unlike the dehydrogenase form, XO is unable to bind NAD+ as these modifications obstruct the binding site of the FAD region.12 Instead, XO consumes molecular oxygen to catalyze the conversion of hypoxanthine to xanthine and finally to uric acid, with concomitant production of superoxide (O2•−) and hydrogen peroxide (H2O2)11,15 (Fig. 1). Distinguishing between the two forms of XOR is challenging due to the lack of antibodies that are specific for the dehydrogenase and oxidase forms. However, it is widely acknowledged that the oxidase form of the enzyme is the predominant source of ROS production and is most frequently associated with disrupting homeostasis in inflammatory disease states.11,16
The potential role of XO in chronic wound healing
XOR has been shown to play a role in normal wound healing in an excisional murine wound model particularly during angiogenesis and keratinocyte proliferation.17 These processes within the wound healing cascade are likely mediated by XO-derived ROS. At physiological levels, ROS are known to shield the body against infectious agents and participate in numerous biological signaling pathways.18 In contrast, XO expression and activity are significantly increased in tissue extracts from diabetic murine wounds when compared to wild-type/acute wounds.10 This upregulation of XO activity has been shown to lead to an overproduction of free radicals in diabetic wound tissues, further prolonging wound closure.10 In vitro studies have shown that XO-derived ROS can also mediate the release of interleukin-1β (IL-1β),19 a potent proinflammatory cytokine found to be elevated in wound fluid and tissue samples from chronic venous leg ulcers.20,21 Elevated levels of IL-1β can increase recruitment of immune cells to the site of injury further potentiating inflammation.22 Finally, uric acid, the terminal metabolite of XO activity, has been shown to be elevated in wound fluids from chronic venous leg ulcers.23 Elevated XO activity can lead to sustained production of uric acid, which at high concentrations can crystallize into monosodium urate (MSU). MSU crystals can trigger an inflammatory response stimulating the production of proinflammatory cytokines,19 further perpetuating inflammation.
With this in mind, the following review examines the expression of XOR in normal and wounded skin and the role of XO in the chronic wound environment. In particular, we outline how excessive XO activity may play an important role in prolonging inflammation and delaying wound closure in a subset of chronic wounds. Finally, we discuss the relevance of specifically targeting XOR overactivity in the wound environment with common XOR inhibitors repurposed for the treatment of patients with hard-to-heal wounds.
Discussion of Findings and Relevant Literature
XOR expression is upregulated on wounding
XOR is widely expressed in a range of organs, with the highest activity and expression found in the liver and intestines.22 More recently, it has also been detected in normal intact murine skin, largely in the thin epidermal layer.17 On wounding, XOR expression is upregulated at the wound edge in comparison to the surrounding intact tissue and the wound area. XOR immunoreactivity was also observed in cells in the peri-wound dermis and subcutaneous tissue; these were morphologically consistent with neutrophils. Immunofluorescence analysis of wounds 14 days after wound closure showed that the neoepidermal layer still expressed high levels of XOR and to a lesser extent the dermis also exhibited high levels of XOR.17
Other cell types in the wound environment, such as macrophages and endothelial cells, have also been shown to express XOR in vitro. Increased intracellular XO activity was detected in bone marrow-derived macrophages stimulated with octacalcium phosphate (OCP).19 Furthermore, assessment of the relative contribution of XO and XDH activities demonstrated that stimulation of bone marrow-derived macrophages with OCP crystals or alum increased the percentage of XO activity in these cells. Similarly, when exposed to hypoxic conditions, cultured endothelial cells exhibited increased XO expression, enhanced XO activity, and the extracellular release of XO.24,25 XO released into the circulation can bind to negatively charged glycosaminoglycans (GAGs) on the luminal surface of vascular endothelial cells.26,27 The binding and sequestration of XO by GAGs increases local XO concentration and subsequent ROS production, thereby disrupting homeostasis by inducing oxidative stress and causing local tissue damage (Fig. 3). Taken together, these observations indicate that XOR is highly expressed in cutaneous wounds throughout the healing process, with the majority of the expression confined to the keratinocytes and early inflammatory infiltrating cells.17
FIG. 3.
The Proposed Mechanism of Action of Circulating XOR—Hypoxia and proinflammatory cytokines induce XDH expression in tissues and vascular endothelial cells. XDH is released into the circulation where it is rapidly converted to XO and sequestered by negatively charged GAGs on the surface of vascular endothelial cells. The binding and sequestration of XO by GAGs concentrates local XO-mediated ROS production, which can disrupt homeostasis. GAGs, glycosaminoglycans. (Adapted from Cantu-Medellin and Kelley 2013.42)
XO activity is elevated in chronic wounds in comparison to acute wounds
Chronic wounds result from disruptions of the normal wound healing process. Unlike acute wounds, chronic wounds remain stalled in the inflammatory phase, exhibiting elevated levels of inflammatory cells, proinflammatory cytokines, and proteases.2,7,20,21 Proinflammatory cytokines, in particular, have been reported to stimulate the expression and activity of XOR in various tissues.28 However, until recently, there have been no studies that examined XO activity in acute or chronic wounds. Weinstein et al.10 used a diabetic murine wound model to evaluate XO expression and activity in intact and wounded tissue. The study demonstrated that there were no differences in XO activity and expression in intact skin of both wild-type and diabetic mice. Wounding resulted in a threefold increase in XO activity in wild-type mice compared to intact wild-type skin. In contrast, wounded diabetic mice exhibited a sevenfold increase in XO activity in the wound environment in comparison to intact diabetic skin. Interestingly, comparison of the two wound types demonstrated that XO activity in the diabetic wound environment was approximately double that detected in the wild-type wound environment. In addition, a corresponding increase was observed at the mRNA level in diabetic wounds compared to wild-type wounds.10 These findings are consistent with research from our laboratory which found that XOR is upregulated in wound fluids from severe, nonhealing venous leg ulcers in contrast to less severe, healing wounds.23 Collectively, these results suggest that XO activity is increased following wounding but is significantly elevated in chronic wounds compared to acute wounds.
Elevated XO activity results in overproduction of ROS in the wound environment
Given that XO is a known source of free radicals, Weinstein et al.10 further examined if elevated XO activity may be responsible for the overproduction of ROS in the chronic wound environment. Using a DNA modification marker of oxidative stress and ROS levels, the authors confirmed that the increase in XO activity was accompanied by an overproduction of ROS in the diabetic wound site. The levels of ROS measured in diabetic wound tissue were significantly increased in comparison to intact diabetic skin and nondiabetic wound tissue. Moreover, selective inhibition of XO using siRNA led to a significant reduction in ROS levels, indicating that a significant proportion of ROS in the diabetic wound environment is generated by overactive XO at the wound site.10
The chronic wound environment is highly oxidizing owing to the release of significant amounts of free radicals. Overproduction of free radicals can have deleterious effects in the wound environment, including lipid peroxidation and protein modification detected in wound fluids from chronic wounds.29,30 Post-translational modifications such as these alter the structure and function of proteins, increase their susceptibility to proteolysis, and frequently result in tissue damage.29 A wound environment rich in oxidants can also lead to the activation of redox-sensitive transcription factors, such as nuclear factor-κB (NF-κB) and activator protein-1 (AP-1).31–34 In vitro studies of redox regulation indicate that activation of these transcription factors upregulates the expression of various genes involved in the inflammatory response, leading to the increased production of proinflammatory cytokines and MMPs.35,36 Indeed, stimulation by various proinflammatory cytokines, such as interferon (IFN), tumor necrosis factor-α (TNF-α), and ILs, has also been shown to induce XOR expression and enzyme activity.28 Thus, the evidence indicates that elevated XO stimulates the overproduction of ROS at the wound site, which in turn could disturb the redox homeostasis and further amplify inflammation.
MSU crystals could further stimulate the inflammatory response in wounds
Uric acid is the other important by-product of the XO-catalyzed purine metabolism pathway (Fig. 1). The production of uric acid is associated with the release of free radicals, which in excess can cause cellular damage and further amplify inflammation as described earlier. Nonetheless, uric acid itself can trigger an immune response.37 Using a transgenic uricase mouse model, Kono et al.37 demonstrated that neutrophil infiltration and myeloperoxidase production were reduced in these transgenic mice in response to cell death caused by hepatotoxicity. However, this reduced inflammatory response was not replicated in uric acid-depleted mice stimulated with silica crystals, zymosan, and lipopolysaccharide (LPS).37 This suggests that these substances can trigger inflammation on their own, whereas the innate response to cell death is driven by uric acid. Therefore, uric acid released on cell death acts as an alarm to trigger inflammation.
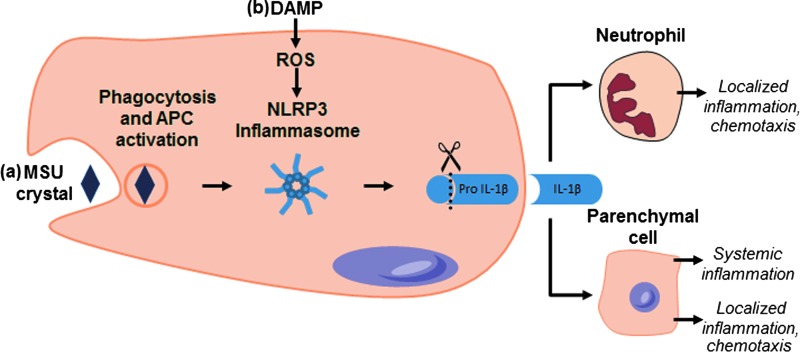
High concentrations of uric acid alone are incapable of inducing an inflammatory response in vitro.19 This can only occur if MSU crystals are formed after large amounts of uric acid are released from dying cells. MSU crystals induce inflammation via the activation of the NLRP3 inflammasome leading to the secretion of active IL-1β (Fig. 4a).38 Indeed, bone marrow-derived macrophages exposed to MSU crystals secrete high levels of IL-1β, which is reduced on exposure to uricase.19 Secreted IL-1β as a result of inflammasome activation can potentially lead to increased recruitment of neutrophils and other immune cells to the site, again amplifying inflammation39 (Fig. 4). Elevated levels of uric acid have been detected in wound fluid from venous leg ulcers with relative concentrations correlated with wound chronicity.23 XOR is also present in elevated levels in wound fluids obtained from these chronic wounds. Taken together, these results suggest that elevated XO activity leads to the overproduction of uric acid in the chronic wound environment. However, we have been unable to confirm the presence of urate crystals in the wound environment due to incompatibilities associated with sample collection and processing techniques. Similarly, in vitro studies have been unsuccessful in detecting urate crystal formation within dying cells or in cell culture supernatants.19 Therefore, we hypothesize that the sustained production together with the accumulation of high levels of uric acid leads to the precipitation of urate crystals in the wound environment. MSU crystals can then activate the inflammasome leading to the secretion of IL-1β, which serves as a potent signal of inflammation in the wound environment.
FIG. 4.
Proposed XOR-Mediated Inflammasome Activation Pathways—(a) XOR-catalyzed production of uric acid can lead to hyperuricemia and precipitation of urate crystals. These crystals are phagocytosed, resulting in the activation of the NLRP3 inflammasome. The activation of NLRP3 by either pathway results in the activation of caspase-1, which cleaves proIL-1β to its active form. Secreted IL-1β can act as an attractant for neutrophils and other immune cells and can lead to the upregulation of other proinflammatory cytokines. This positive feedback mechanism of IL-1β can sustain inflammation in chronic wounds and impair wound healing. (b) XOR-mediated production of ROS triggers IL-1β secreted on stimulation with crystal DAMPS, damage associated molecular patterns; IL-1β, interleukin 1β. (Adapted from Shi et al.39)
XOR is involved in crystal-induced IL-1β secretion
A common and well-documented feature of chronic wounds is a sustained inflammatory response with continual infiltration of macrophages secreting elevated levels of proinflammatory cytokines.7,20,21 IL-1β is a potent mediator of inflammation and is elevated in wound fluids and tissues from chronic venous leg ulcers.20,21 It elicits an inflammatory response by signaling through its receptor (IL-1R), attracting neutrophils and macrophages to the site of injury. Ligation of IL-1R has been shown to induce the production and secretion of other proinflammatory cytokines, such as IL-6, thus perpetuating inflammation.40 Therefore, it is likely that IL-1β is part of a positive feedback system that sustains inflammation in chronic wounds and contributes to impaired wound healing (Fig. 4). Cultured murine bone marrow-derived macrophages elicit elevated IL-1β secretion on stimulation with OCP crystals; this has been shown to be accompanied by a rapid increase in intracellular uric acid levels and XO activity.19 This correlation was further validated with the use of XOR-specific inhibitors and siRNA, both of which impair IL-1β secretion in macrophages before and after stimulation with MSU, alum, and OCP crystals. Furthermore, inhibition of XOR also reduced secreted caspase-1 and production of ROS in crystal-induced macrophages in vitro.19 These authors also demonstrated that the trigger for IL-1β secretion on inflammasome activation is XO-derived ROS, suggesting that XO plays a role in caspase-1 processing, which in turn leads to IL-1β secretion in macrophages stimulated with crystals (Fig. 4b).
Alternative emerging mechanisms of XOR
While studies suggest that elevated XO plays a role in dysfunctional wound healing, there is also evidence in the literature that demonstrates that XOR may play an important part in normal wound healing.17 Using a murine excisional wound healing model, Madigan et al.17 demonstrated that inhibition of XOR with dietary tungsten significantly delayed wound closure and reduced wound angiogenesis and keratinocyte proliferation. Similarly, when applied topically, allopurinol, a common XOR inhibitor, reduced ROS production and significantly delayed wound closure.17 However, it is important to note that this study used a normal wound healing model to examine acute wound healing responses, which are often severely dysregulated in chronic wounds. It is therefore unlikely that levels of XO expression and activity observed this acute model can be applied to chronic wounds. In fact, the authors themselves state that XOR may be upregulated in impaired wound healing and targeting XOR under these circumstances may be beneficial for patients with hard-to-heal wounds. This was substantiated by Weinstein et al.10 who demonstrated that XO activity is elevated in diabetic murine wounds compared to wild-type wounds and that inhibition of XO improved wound closure.10
The capacity of XO to generate nitric oxide radical (NO) is another pathway that is attracting increasing attention. There is evidence to suggest that XOR can function as a nitrite reductase, reducing nitrite (NO2−) to beneficial •NO under anoxic conditions.41 This nitrite reductase activity of XOR could possibly be harnessed to increase •NO production in wounds through NO2− supplementation, however, results from studies to date have been conflicting. In vitro supplementation of nitrate has been shown to significantly increase keratinocyte and endothelial cell proliferation.17 These beneficial effects were significantly reduced when XOR was inhibited using allopurinol, suggesting that nitrite acts via XOR to promote proliferation in these cells.17 On the contrary, supplementation and depletion of dietary nitrate failed to alter wound healing rates in murine excisional wound healing models.17 Currently, there are no knockout models that can be used to provide insights into this XO pathway. Homozygous and heterozygous XDH knockout mice exhibit premature death making in vivo experimentation difficult, hindering progress in this area.42,43
Inhibition of XOR as a treatment for chronic wounds
As highlighted in this review, XOR-induced ROS production has the potential to amplify and potentiate inflammation in the wound environment resulting in delayed wound closure. This suggests that XOR might be a good therapeutic target; the inhibition of this enzyme is therefore a potential novel approach to treat nonhealing wounds. Targeted inhibition of XOR has already been shown to have beneficial effects both in vitro and in vivo.17,19 As noted earlier, the treatment of diabetic wounds in mice with XDH siRNA was found to reduce wound damage caused by ROS and improved wound closure.10 In addition, the inhibition of XOR in macrophages stimulated in vitro with crystalline activators also exhibit reduced inflammation indicated by the reduced secretion of caspase-1 and IL-1β.19 Thus, a therapeutic approach that attenuates XOR activity directly is also likely to decrease the amount of uric acid released in situ, and prevent the precipitation of proinflammatory MSU crystals in the wound site. Taken together, these studies suggest that the targeted inhibition of XOR might help to restore homeostasis at the wound site and that processes which lead to tissue damage might return to normal healing.
XOR has been pharmacologically targeted and found to lower urate levels in patients with gout and hyperuricemia.11,16 Various XOR inhibitors are therefore already approved for human use, making them suitable for reformulation and repurposing to inhibit XOR in chronic wounds. Allopurinol is the most commonly prescribed XOR inhibitor used to treat gout and hyperuricemia. It is a noncompetitive inhibitor and reacts with XOR to yield oxypurinol (alloxanthine), which in turn binds effectively and irreversibly to XOR preventing further activity.11,16 It is important to recognize that conversion of allopurinol to oxypurinol requires enzyme turnover, and hence, some ROS are produced before inhibition is accomplished. There are also some known adverse side effects of allopurinol when administeredorally, including gastrointestinal intolerance, hypersensitivity reactions, and skin rashes.11 Nonetheless, allopurinol offers a number of advantages for reformulation as a wound therapy: it is cheap, off-patent, and can be readily monitored using its breakdown product oxypurinol. It has been formulated previously for a number of applications, including topical, intravenous and oral administration, as a gel, tablet, powdered form, and mouthwash.44–48 Unlike systemic hyperuricemia, which is managed with oral therapy, chronic wounds present a unique opportunity to directly target the disease site enabling a “site-specific” topical delivery approach. Topical application offers a number of other advantages, including ease of application, sustained high local drug concentrations, reduced risks of adverse drug interactions, and direct access to the disease site. We propose that incorporating powdered allopurinol into a hydrogel will ensure a sustained drug release delivery mechanism. The active ingredient, in this case allopurinol, will gradually be released as the soluble fraction and adsorbed into the wound site.
XOR inhibitors have been used broadly to treat other XOR-related pathologies. Studies conducted in humans and animal models have reported positive results with the use of allopurinol in the treatment of corneal alkali burns49; in the prevention and treatment of chemotherapy tumor lysis syndrome48; radiation-induced mucositis and dermatitis45; and leishmaniasis.46 In each of these clinical studies, the exact mechanism of action of allopurinol was not determined. However, clinical observations demonstrated that treatment with allopurinol alleviated symptoms or facilitated return to tissue homeostasis. The treatment of chronic venous leg ulcers with a combination of allopurinol and compression therapy is known to stimulate wound healing.47 Crushed allopurinol tablets were administered daily for 7 days and thereafter once per week until the end of the study at 12 weeks. Allopurinol significantly stimulated wound healing when compared to compression therapy alone; 50% of ulcers healed in 4 weeks and 93% of ulcers healed within 12 weeks.47 The authors of this study suggest that oxygen-derived free radicals are implicated in venous ulceration and delayed wound closure, and that treatment with free radical scavengers such as allopurinol stimulated wound healing. While these authors demonstrated that topical allopurinol improved wound healing, the amount of allopurinol used in the study is unclear. The mechanism of action and supporting biochemical data were not reported.47 Therefore, a more rigorous clinical trial is required to acquire the critical data that is required to assess the clinical efficacy and long- term safety profile of topical XOR inhibitors as new options for the management of chronic wounds. Moreover, chronic wounds are clinically heterogeneous and multifaceted; hence, given the complexity of chronic wounds, it is unlikely that chronic inflammation associated with elevated XOR activity is the sole underlying cause of all hard-to-heal wounds.
Hyperuricemia, may develop as a consequence of elevated XOR activity. Therefore, measures of uric acid in wound fluid and tissues offer potential as a biomarker to identify hard-to-heal wounds with elevated XOR activity. Such a diagnostic tool may, for example, enable clinicians to select patients who would benefit from therapies directed at dampening wound inflammation, for example those that inhibit XOR. Uric acid could also be used as a prognostic marker to monitor the effectiveness of treatment with XOR inhibitor and response in individual patients. Developing a diagnostic test for this purpose should be straightforward. Uric acid is already measured in biological samples, including plasma and urine, using established assays. Sophisticated sensor-based technologies are also in development and may be suitable for incorporation into various dressing materials to measure uric acid at the wound site.50 The development of simple and convenient diagnostic tests could therefore be a major step to assist in the prognosis and management of chronic wounds and potentially also guide therapy.
Summary
In summary, XO activity is significantly elevated in chronic wounds, which results in an overproduction of ROS disrupting homeostasis and impairing wound healing. In common with increased ROS, levels of uric acid can also increase as a consequence of elevated XOR activity. The release of large amounts to uric acid in the wound environment can potentially crystallize to MSU, triggering inflammasome activation and IL-1β secretion. XO has also been shown to mediate IL-1β secretion on inflammasome activation in cells stimulated with crystals. Elevated levels of proinflammatory cytokines, in particular IL-1β, can further amplify inflammation through the recruitment of inflammatory cells. These immune cells can secrete proinflammatory cytokines, proteases, and release ROS into the wound environment amplifying the inflammatory state. Despite these deleterious effects, XOR-mediated nitrite conversion to nitric oxide is emerging as a beneficial pathway. However, existing experimental data is conflicting and more research is required to elucidate the regulation of XOR and its interactions with other cellular pathways during normal and impaired wound healing. Nevertheless, targeting XOR in hard-to-heal wounds could reduce uric acid and ROS production enabling damaged tissue to return to normal healing.
Future Directions
Targeting the XOR enzymatic pathway to reduce uric acid and ROS generation in situ would appear to be beneficial for chronic wounds. A range of existing XOR inhibitors could be reformulated and trialed as novel wound therapies. However, currently, no suitable animal models of chronic wounds that also include neuropathy or vascular insufficiency exist, necessitating direct human clinical trials to determine the safety and efficacy of these treatments. While challenging, the outcome of these studies could deliver diagnostic and companion assays and novel therapeutics enabling individualized treatment for patients with chronic wounds.
Take-Home Messages.
• XOR expression and activity are upregulated in the chronic wound environment.
• XOR may be responsible for the overproduction of ROS resulting in oxidative stress at the wound site and prolong wound closure.
• XOR may play an important role in sustaining inflammation in chronic wounds by regulating IL-1β secretion on inflammasome activation.
• Sustained XOR-mediated uric acid production could precipitate to form urate crystals that can further stimulate the inflammatory system.
• XOR inhibition could potentially reduce excess uric acid and ROS generation, thus restoring the wound bed environment to a healing phenotype.
Abbreviations and Acronyms
- H2O2
hydrogen peroxide
- O2
oxygen
- O2•−
superoxide
- NO2−
nitrite
- •NO
nitric oxide
- AP-1
activator protein-1
- ATP
adenosine triphosphate
- DNA
deoxyribonucleic acid
- FAD
flavin adenine dinucleotide
- GAGs
glycosaminoglycans
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- MMPs
matrix metalloproteinases
- Mo-Co
molybdenum cofactor
- MSU
monosodium urate
- NAD
nicotinamide adenine dinucleotide
- NADH
nicotinamide adenine dinucleotide reduced form
- NF-κβ
nuclear factor-κB
- OCP
octacalcium phosphate
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- TIMPs
tissue inhibitors of metalloproteinases
- TNF-α
tumor necrosis factor-α
- XDH
xanthine dehydrogenase
- XO
xanthine oxidase
- XOR
xanthine oxidoreductase
Acknowledgments and Funding Sources
No funding was obtained for the writing of this review.
Author Disclosure and Ghostwriting
The authors have no conflict of interest to declare in relation to this work. No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Melissa L. Fernandez, PhD, is a Senior Research Fellow within the Tissue Technologies research group at the Institute of Medical Biology, Agency for Science, Technology and Research (A*STAR). Her research is focused on identifying the underlying causes of inflammation in chronic wounds, in particular venous leg ulcers. Dario Stupar, PhD, is a Research Fellow within the Tissue Technology research group at the Institute of Medical Biology, A*STAR. His research is focused on identifying factors in wound fluid that sustain inflammation. Tristan Croll, PhD, is a Research Fellow in structural biology in the Read Laboratory at Cambridge Institute for Medical Research, Cambridge, United Kingdom. David Leavesley, PhD, is a Senior Principal Investigator and coleader of the Tissue Technology research group at the Institute of Medical Biology, A*STAR. His interests are in the interactions of cells with their microenvironment. Zee Upton, PhD, is a biochemist. She is Research Director and coleader of the Tissue Technology research group at the Institute of Medical Biology, A*STAR, focused on adopting interdisciplinary approaches to improve wound and tissue repair.
References
- 1.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol 2008;158:951–961 [DOI] [PubMed] [Google Scholar]
- 3.Vaalamo M, Leivo T, Saarialho-Kere U. Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, -2, -3, and -4) in normal and aberrant wound healing. Hum Pathol 1999;30:795–802 [DOI] [PubMed] [Google Scholar]
- 4.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 2007;127:514–525 [DOI] [PubMed] [Google Scholar]
- 5.Grinnell F, Ho CH, Wysocki A. Degradation of fibronectin and vitronectin in chronic wound fluid: analysis by cell blotting, immunoblotting, and cell adhesion assays. J Invest Dermatol 1992;98:410–416 [DOI] [PubMed] [Google Scholar]
- 6.Lauer G, Sollberg S, Cole M, et al. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol 2000;115:12–18 [DOI] [PubMed] [Google Scholar]
- 7.Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol 1998;111:850–857 [DOI] [PubMed] [Google Scholar]
- 8.Stadelmann WK, Digenis AG, Tobin GR. Impediments to wound healing. Am J Surg 1998;176(2A Suppl):39S-47S [DOI] [PubMed] [Google Scholar]
- 9.Schafer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res 2008;58:165–171 [DOI] [PubMed] [Google Scholar]
- 10.Weinstein AL, Lalezarzadeh FD, Soares MA, Saadeh PB, Ceradini DJ. Normalizing dysfunctional purine metabolism accelerates diabetic wound healing. Wound Repair Regen 2015;23:14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 2006;58:87–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enroth C, Eger BT, Okamoto K, Nishino T, Nishino T, Pai EF. Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: structure-based mechanism of conversion. Proc Natl Acad Sci U S A 2000;97:10723–10728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hille R. The mononuclear molybdenum enzymes. Chem Rev 1996;96:2757–2816 [DOI] [PubMed] [Google Scholar]
- 14.Nishino T. The conversion of xanthine dehydrogenase to xanthine oxidase and the role of the enzyme in reperfusion injury. J Biochem 1994;116:1–6 [DOI] [PubMed] [Google Scholar]
- 15.Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med 2002;33:774–797 [DOI] [PubMed] [Google Scholar]
- 16.Borges F, Fernandes E, Roleira F. Progress towards the discovery of xanthine oxidase inhibitors. Curr Med Chem 2002;9:195–217 [DOI] [PubMed] [Google Scholar]
- 17.Madigan MC, McEnaney RM, Shukla AJ, et al. Xanthine oxidoreductase function contributes to normal wound healing. Mol Med 2015;21:313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sen CK, Roy S. Redox signals in wound healing. Biochim Biophys Acta 2008;1780:1348–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ives A, Nomura J, Martinon F, et al. Xanthine oxidoreductase regulates macrophage IL1beta secretion upon NLRP3 inflammasome activation. Nat Commun 2015;6:6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J Vasc Surg 2009;49:1013–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen 2000;8:13–25 [DOI] [PubMed] [Google Scholar]
- 22.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009;27:519–550 [DOI] [PubMed] [Google Scholar]
- 23.Fernandez ML, Upton Z, Edwards H, Finlayson K, Shooter GK. Elevated uric acid correlates with wound severity. Int Wound J 2012;9:139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelley EE, Hock T, Khoo NK, et al. Moderate hypoxia induces xanthine oxidoreductase activity in arterial endothelial cells. Free Radic Biol Med 2006;40:952–959 [DOI] [PubMed] [Google Scholar]
- 25.Partridge CA, Blumenstock FA, Malik AB. Pulmonary microvascular endothelial cells constitutively release xanthine oxidase. Arch Biochem Biophys 1992;294:184–187 [DOI] [PubMed] [Google Scholar]
- 26.Adachi T, Fukushima T, Usami Y, Hirano K. Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial-cell surface. Biochem J 1993;289 (Pt 2):523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radi R, Rubbo H, Bush K, Freeman BA. Xanthine oxidase binding to glycosaminoglycans: kinetics and superoxide dismutase interactions of immobilized xanthine oxidase-heparin complexes. Arch Biochem Biophys 1997;339:125–135 [DOI] [PubMed] [Google Scholar]
- 28.Page S, Powell D, Benboubetra M, et al. Xanthine oxidoreductase in human mammary epithelial cells: activation in response to inflammatory cytokines. Biochim Biophys Acta 1998;1381:191–202 [DOI] [PubMed] [Google Scholar]
- 29.Moseley R, Hilton JR, Waddington RJ, Harding KG, Stephens P, Thomas DW. Comparison of oxidative stress biomarker profiles between acute and chronic wound environments. Wound Repair Regen 2004;12:419–429 [DOI] [PubMed] [Google Scholar]
- 30.Yeoh-Ellerton S, Stacey MC. Iron and 8-isoprostane levels in acute and chronic wounds. J Invest Dermatol 2003;121:918–925 [DOI] [PubMed] [Google Scholar]
- 31.Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition 1996;12:274–277 [DOI] [PubMed] [Google Scholar]
- 32.Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. Embo J 1993;12:2005–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schreck R, Baeuerle PA. A role for oxygen radicals as second messengers. Trends Cell Biol 1991;1:39–42 [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, Oberley LW. Redox regulation of transcriptional activators. Free Radic Biol Med 1996;21:335–348 [DOI] [PubMed] [Google Scholar]
- 35.Halliwell B, Gutteridge J. Free Radicals in Biology and Medicine, 3rd ed. New York: Oxford University Press Inc., 1999 [Google Scholar]
- 36.Wenk J, Brenneisen P, Wlaschek M, et al. Stable overexpression of manganese superoxide dismutase in mitochondria identifies hydrogen peroxide as a major oxidant in the AP-1-mediated induction of matrix-degrading metalloprotease-1. J Biol Chem 1999;274:25869–25876 [DOI] [PubMed] [Google Scholar]
- 37.Kono H, Chen CJ, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest 2010;120:1939–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006;440:237–241 [DOI] [PubMed] [Google Scholar]
- 39.Shi Y, Mucsi AD, Ng G. Monosodium urate crystals in inflammation and immunity. Immunol Rev 2010;233:203–217 [DOI] [PubMed] [Google Scholar]
- 40.Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol 2007;147:227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Godber BLJ, Doe JJ, Sapkota GP, et al. Reduction of nitrite to nitric oxide catalysed by xanthine oxidoreductase. J Biol Chem 2000; 275:7757–7763 [DOI] [PubMed] [Google Scholar]
- 42.Cantu-Medellin N, Kelley EE. Xanthine oxidoreductase-catalyzed reactive species generation: a process in critical need of reevaluation. Redox Biol 2013;1:353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohtsubo T, Rovira II, Starost MF, Liu C, Finkel T. Xanthine oxidoreductase is an endogenous regulator of cyclooxygenase-2. Circ Res 2004;95:1118–1124 [DOI] [PubMed] [Google Scholar]
- 44.Elzawawy A. Treatment of 5-fluorouracil-induced stomatitis by allopurinol mouthwashes. Oncology 1991;48:282–284 [DOI] [PubMed] [Google Scholar]
- 45.Kitagawa J, Nasu M, Okumura H, et al. Allopurinol gel mitigates radiation-induced mucositis and dermatitis. J Radiat Res (Tokyo) 2008;49:49–54 [DOI] [PubMed] [Google Scholar]
- 46.Martinez S, Gonzalez M, Vernaza ME. Treatment of cutaneous leishmaniasis with allopurinol and stibogluconate. Clin Infect Dis 1997;24:165–169 [DOI] [PubMed] [Google Scholar]
- 47.Salim AS. The role of oxygen-derived free radicals in the management of venous (varicose) ulceration: a new approach. World J Surg 1991;15:264–269 [DOI] [PubMed] [Google Scholar]
- 48.Smalley RV, Guaspari A, Haase-Statz S, Anderson SA, Cederberg D, Hohneker JA. Allopurinol: intravenous use for prevention and treatment of hyperuricemia. J Clin Oncol 2000;18:1758–1763 [DOI] [PubMed] [Google Scholar]
- 49.Sekundo W, Augustin AJ, Strempel I. Topical allopurinol or corticosteroids and acetylcysteine in the early treatment of experimental corneal alkali burns: a pilot study. Eur J Ophthalmol 2002;12:366–372 [DOI] [PubMed] [Google Scholar]
- 50.Kassal P, Kim J, Kumar R, et al. Smart bandage with wireless connectivity for uric acid biosensing as an indicator of wound status. Electrochem Commun 2015;56:6–10 [Google Scholar]