Abstract
Objective: A variety of advanced biological therapies are available for the treatment of chronic wounds such as venous leg ulcers (VLUs), but real-world comparative effectiveness data that can help guide decisions around treatments are currently lacking.
Approach: This analysis was designed to compare the effectiveness of a bioengineered living cellular construct (BLCC) to a cryopreserved cadaveric skin allograft (CCSA) for the treatment of VLUs. Treatment records were collected from a large wound care-specific electronic medical record database on 717 patients (799 VLUs) receiving treatment at 177 wound care centers. Ulcers ≥28 days duration, between ≥1 and < 40 cm2 that closed ≤40% within the 28 days before treatment were included.
Results: Patient baseline demographics and wound characteristics were comparable between groups. The median time to wound closure was 52% faster with BLCC compared with CCSA (15 weeks vs. 31 weeks). In addition, the proportion of wounds healed were significantly higher for BLCC by 12 weeks (42% vs. 24%) and 24 weeks (65% vs. 41%) (p = 0.0002). Treatment with BLCC increased the probability of healing by 97% compared with CCSA (hazard ratio = 1.97 [95% confidence interval 1.39–2.79], p = 0.0002).
Innovation: This is the first real-world comparative effectiveness analysis to evaluate BLCC and CCSA for the treatment of VLUs.
Conclusion: Treatment with a bioengineered cellular technology significantly improved the incidence and speed of wound closure compared with a CCSA.
Keywords: : venous leg ulcer, bioengineered living cellular construct, cryopreserved cadaveric skin allograft, skin substitute, wound healing

Michael L. Sabolinski, MD
Introduction
Venous leg ulcers (VLUs), also referred to as venous stasis or venous insufficiency ulcers, are the most common type of chronic wound, accounting for 70% to 90% of lower-extremity ulcers.1,2 Approximately 600,000 individuals suffer from VLUs each year in the United States (US), and it has been estimated that 1% of adults will develop a VLU at some point in their lives. The average VLU can take 6 months to 1 year to heal, and half of patients will have unhealed wounds that extend beyond 1 year.3,4 Even following treatment and wound closure, a reported 72% of VLUs will recur within 5 years.4 VLUs substantially reduce patient quality of life. They are often painful, adversely affecting mobility and sleep.5–7 VLUs are directly associated with adverse events, serious adverse events, lost jobs, and lost time from work particularly in younger patients.6 It has been estimated that VLUs cause the loss of 2 million working days per year in the United States.8 VLUs cost US payers an estimated $14.9 billion per year for medical resource use and treatment costs.9
Compression therapy remains the standard of care for VLUs, but is associated with poor healing rates. Up to 50% of VLUs remain unhealed at 6 months, 20% at 2 years, and ∼8% at 5 years.10 Advanced treatment options, such as skin substitutes, can be effective adjunctive therapy to accelerate the wound healing process in nonhealing VLUs. The conditions for initiating advanced therapies in VLUs have not been definitely established. However, such therapies may be appropriate to use as part of the initial treatment regimen in patients with poor prognostic indicators, in particular, an ulcer size ≥10 cm2 and an ulcer that has been present ≥12 months.11,12 Data also suggest that advanced therapies should be considered in VLUs that have not reduced in surface area by at least 40% following 4 weeks of good standard wound care with compression therapy.13,14
One skin substitute technology is a bioengineered living cellular construct (BLCC, Apligraf; Organogenesis Inc., Canton, MA). BLCC is a bioengineered living cell therapy that contains human neonatal keratinocytes and fibroblasts in an extracellular matrix (ECM) of bovine, human collagen, and other ECM proteins. It is one of only three skin substitute products approved by the Food and Drug Administration (FDA) as a “wound treatment.” In the United States, wound treatments are approved via the FDA premarket approval process, which mandates the most rigorous FDA review procedures, including approval of at least one pivotal clinical trial to support its indication. BLCC is FDA-approved for the treatment of both diabetic foot ulcers and VLUs, and currently, it is the only skin substitute approved for the treatment of VLUs. In a large, randomized clinical trial (RCT), BLCC (in conjunction with compression therapy) significantly increased the rate of complete wound closure and reduced the median time to VLU closure, compared with compression therapy alone.15
Another skin substitute on the market is a cryopreserved cadaveric skin allograft (CCSA, TheraSkin; Soluble Systems, Newport News, VA). CCSA is a human skin allograft harvested from tissue donors within 24 h of death. It is processed with cleaning, hair removal, and meshing, and then exposed to antibiotics and reagents. The tissue is cryopreserved in an effort to retain the living cellular and extracellular components.16 CCSA is marketed under Section 361 of the Public Health Services (PHS) Act as Human Cells, Tissues, and Cellular and Tissue-based Products (361 HCT/Ps). Under this regulatory pathway, CCSA is considered a “wound covering” and adheres to the standards set forth for the tissue bank industry. Furthermore, clinical data establishing efficacy or safety are not required.
Unlike the majority of skin substitutes, which are acellular, both BLCC and CCSA are described as biologically active products. Both contain living cells and are thought to accelerate the wound healing process by generating growth factors and cytokines. They differ in that BLCC is bioengineered with neonatal cells whereas the cells in CCSA are from cadaveric donated tissue. As a bioengineered product, the cells in BLCC are well characterized for purity and potency, and manufactured to ensure lot-to-lot consistency. As a donated tissue product, CCSA contains the native cells from human skin but the properties of the tissue will be variable from donor-to-donor and depend on donor age, health, and location of harvested tissue.17
Clinical Problem Addressed
In today's healthcare environment, real-world studies are becoming more and more important to help guide decisions about treatments. Different from controlled clinical trials, real-world studies demonstrate the extent to which a treatment achieves its intended effect in the usual clinical setting. The purpose of the current study was to evaluate the comparative effectiveness in everyday practice of BLCC and CCSA for the treatment of VLUs, using data from an electronic medical record (EMR) database (WoundExpert; Net Health, Pittsburgh, PA) that is utilized by ∼90% of wound care facilities across the United States.18 EMR databases enable assessments of comparative effectiveness of therapeutics in real-world settings and allow for inclusion of larger patient populations studied over longer periods of observation than in RCTs.19,20
Materials and Methods
Study design
This study retrospectively analyzes the comparative effectiveness of BLCC and CCSA treatment of VLUs between January 1, 2014 and March 31, 2015 from 177 wound care facilities across the United States. The wounds included in the analysis had not received treatment with skin substitute before 2014. Treatment period started with the first use of BLCC or CCSA. The primary analyses were the incidence (proportion or percentage) of VLUs achieving wound closure by weeks 12 and 24 and the median time to wound closure in each of the two treatment groups. Length and width measurements were used to determine wound area in cm2. As patients with healed wounds do not always follow-up, the final visit denoting VLU closure may not have been recorded; thus, wound closure was defined as an ulcer achieving an area ≤0.25 cm2.
Patients
Eligible patients must have received at least one treatment of either BLCC or CCSA on a venous ulcer (partial or full thickness) with the location coded as ankle, lower leg, shin, pretibial, or calf. Baseline wound areas must have been ≥1 cm2 to <40 cm2, and the ulcer duration must have been longer than 28 days before first treatment with BLCC or CCSA. To ensure that VLU patients in the study had a history of being difficult to heal (refractory VLUs), wounds must have demonstrated a wound area reduction of no more than 40% at 28 days compared to baseline (before first treatment with BLCC or CCSA).
Patients were excluded if their VLUs had no baseline size measurements or follow-up area measurements. Patients were also excluded from the study if they received skin substitute treatment (Apligraf, Dermagraft, Oasis, Epifix, Primatrix, Theraskin, Grafix, Graftjacket) within 28 days or on the first day of BLCC or CCSA treatment. Censoring occurred for nonhealed wounds at their last visit with an area measurement, at the first visit where an alternate skin substitute product was applied, or at a visit >183 days after the previously recorded treatment with either BLCC or CCSA.
Data collection
Data were obtained from the WoundExpert EMR, which were de-identified and consistent with the terms and conditions of the Health Insurance Portability and Accountability Act of 1996 (HIPAA).
From January 1, 2014 to March 31, 2015 Net Health provided de-identified treatment records from 177 centers for any patient receiving at least one application of BLCC or CCSA.
Treatment records included patient demographics, including age (years ≤89 per HIPAA), race, and sex. Wound characteristics such as wound location, size, depth, and other treatments were also provided.
Statistical analysis
Descriptive data are expressed as mean (standard deviation) and median for continuous variables and n (%) for categorical variables. The level of p < 0.05 was used to determine statistical significance. Baseline characteristics were compared using two-sample t-tests for continuous variables and two-tailed Fisher's exact tests for difference in proportions between treatments. Missing covariates were imputed with the mean value of the treatment group. The primary analyses comparing incidence of and median time to wound closure were determined by Kaplan–Meier analysis with a two-tailed log-rank test. The last observation was carried forward for missing data. Cox proportional hazards regression analysis was used to estimate the percentage of VLUs with closure at weeks 12 and 24 and median time to heal. The frequency of wounds closed at weeks 12 and 24 and the median time to wound closure, hazard ratio with 95% confidence interval, and p-value were estimated from the Cox model with terms for treatment, baseline wound area, baseline wound duration, baseline wound depth, and patient age at first treatment.
Results
All VLUs that received their first treatment with BLCC or CCSA between January 1, 2014 and March 31, 2015 at all participating Net Health centers were eligible for inclusion. A total of 688 wounds (608 patients) treated with BLCC and 111 wounds (109 patients) treated with CCSA met the eligibility requirements for inclusion in the analysis.
Baseline patient demographic characteristics are shown in Table 1 and baseline wound characteristics in Table 2. Treatment groups were similar with respect to age, gender, and body mass index. Mean age was 69 to 70 years. Women represented a little more than half of the population, and the average body mass index was 33 kg/m2. There were statistically significant differences in the average number of wounds per patient, with BLCC-treated patients having more wounds per patient (mean 1.13 vs. 1.02 wounds per patient; p < 0.001). In addition, 11.3% of patients treated with BLCC had multiple wounds compared with 1.8% of patients treated with CCSA (p = 0.001). Most patients had a single wound. There were no significant differences between treatment groups for baseline wound area, depth, duration, and location (Table 2). At the first treatment application, the mean wound area was 10.2 and 9.5 cm2 in the BLCC and CCSA treatment groups, respectively. The median wound duration was 4.1 and 6 months in the BLCC and CCSA treatment groups, respectively.
Table 1.
Baseline Patient Characteristics
| Patient characteristic | BLCC (n = 608) | CCSA (n = 109) | pa |
|---|---|---|---|
| Age (years), n | 608 | 109 | |
| Mean ± SD | 69.0 ± 14.3 | 70.1 ± 13.3 | 0.453 |
| Median | 70.0 | 72.0 | |
| Gender, n (%) | |||
| Female | 319 (53.1) | 59 (54.6) | 0.834 |
| Male | 282 (46.9) | 49 (45.4) | |
| Body mass index (kg/m2), n | 461 | 89 | |
| Mean ± SD | 33.1 ± 10.7 | 31.8 ± 11.2 | 0.329 |
| Median | 31.0 | 30 | |
| Number of wounds per patient, n | 608 | 109 | |
| Mean ± SD | 1.13 ± 0.39 | 1.02 ± 0.14 | <0.001 |
| Median | 1.0 | 1.0 | |
| Single/multiple wounds per patient, n (%) | |||
| Single wound | 539 (88.7) | 107 (98.2) | 0.001 |
| Multiple wounds | 69 (11.3) | 2 (1.8) | |
For continuous variables, the p-value is from a two-sided, two sample t-test, testing for a difference in means between treatments. For categorical variables, the p-value is from a two-sided Fisher's exact test testing for a difference in proportions between treatments.
BLCC, bioengineered living cellular construct; CCSA, cryopreserved cadaveric skin allograft; SD, standard deviation.
Table 2.
Baseline Wound Characteristics
| Wound characteristic | BLCC (n = 688) | CCSA (n = 111) | pa |
|---|---|---|---|
| Wound area (cm2), n | 688 | 111 | |
| Mean ± SD | 10.2 ± 9.3 | 9.5 ± 8.8 | 0.418 |
| Median | 6.9 | 6.3 | |
| Wound depth (mm), n | 681 | 107 | |
| Mean ± SD | 1.8 ± 1.1 | 2.0 ± 1.3 | 0.165 |
| Median | 2.0 | 2.0 | |
| Wound duration (months)b, n | 588 | 101 | |
| Mean ± SD | 13.3 ± 37.6 | 16.6 ± 32.8 | 0.406 |
| Median | 4.13 | 6 | |
| Wound location, n (%) | |||
| Ankle | 153 (22.2) | 32 (28.8) | 0.370 |
| Lower leg | 419 (60.9) | 63 (56.8) | |
| Shin | 3 (0.4) | 0 (0.0) | |
| Calf | 33 (4.8) | 2 (1.8) | |
| Pretibial | 80 (11.6) | 14 (12.6) | |
| Lateral/medial, n (%) | |||
| Lateral | 226 (49.8) | 36 (50.0) | 1.000 |
| Medial | 228 (50.2) | 36 (50.0) | |
For continuous variables, the p-value is from a two-sided, two sample t-test, testing for a difference in means between treatments. For categorical variables, the p-value is from a two-sided Fisher's exact test testing for a difference in proportions between treatments.
Wound duration was reported in days and converted to months (30 days = 1 month).
Treatment characteristics are shown in Table 3. The average number of treatment applications received was similar between treatments, with a mean 2.8 treatment applications in both groups. However, the percentage of patients treated with a single application was significantly lower in the BLCC-treatment group compared with the CCSA treatment group (p = 0.006). For patients receiving multiple applications, the median interval between applications was significantly longer with CCSA (21 days vs. 14 days; p = 0.001). There was no significant difference in the number of debridements at or within 28 days before initial treatment application. Treatment with hyperbaric oxygen (HBO) or negative-pressure wound therapy (NPWT) before or concurrent with BLCC or CCSA treatment was uncommon and comparable between treatment groups.
Table 3.
Treatment Characteristics
| Treatment characteristic | BLCC (n = 688) | CCSA (n = 111) | pa |
|---|---|---|---|
| Number of treatment applications, n | 688 | 111 | |
| Mean ± SD | 2.8 ± 1.8 | 2.8 ± 2.3 | 0.936 |
| Median | 2.0 | 2.0 | |
| Single/multiple applications, n (% patients) | |||
| Single | 172 (25.0) | 42 (37.8) | 0.006 |
| Multiple | 516 (75.0) | 69 (62.2) | |
| Interval between applications (days), n | 516 | 69 | |
| Mean ± SD | 18.4 ± 14.2 | 24.3 ± 13.2 | 0.001 |
| Median | 14.0 | 21.0 | |
| Number of debridements at or within 28 days before day 0b, n | 490 | 95 | |
| Mean ± SD | 2.5 ± 1.2 | 2.7 ± 1.1 | 0.096 |
| Median | 2.0 | 3.0 | |
| Other treatments, n (% patients) | |||
| Hyperbaric oxygen therapy | |||
| Day −28 to <day 0 | 6 (0.9) | 2 (1.8) | 0.308 |
| Day 0 to last follow-up visit | 12 (1.7) | 3 (2.7) | 0.451 |
| Negative-pressure wound therapy, n (%) | |||
| Day −28 to <day 0 | 11 (1.6) | 3 (2.7) | 0.427 |
| Day 0 to last follow-up visit | 15 (2.2) | 1 (0.9) | 0.712 |
For continuous variables, the p-value is from a two-sided, two sample t-test, testing for a difference in means between treatments. For categorical variables, the p-value is from a two-sided Fisher's exact test testing for a difference in proportions between treatments.
Day 0 defined as first application visit.
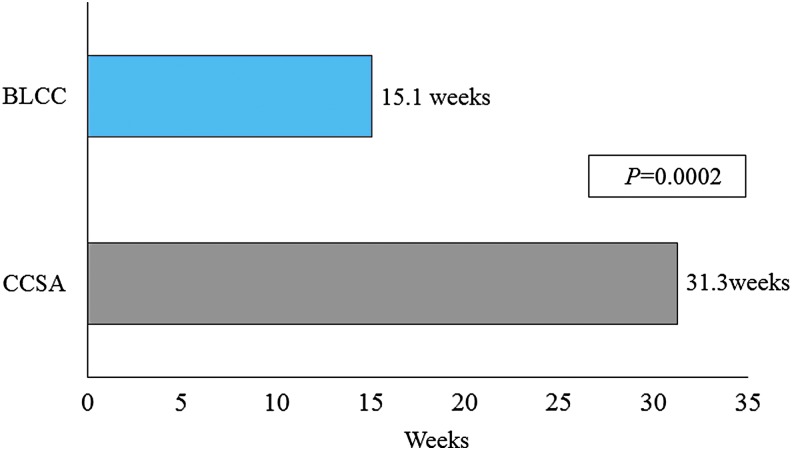
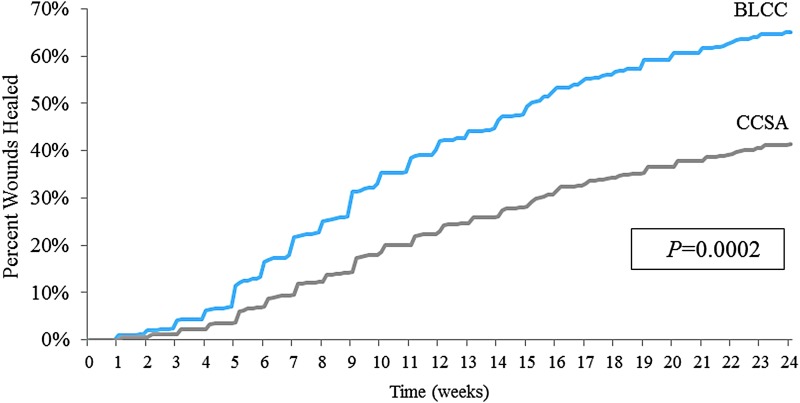
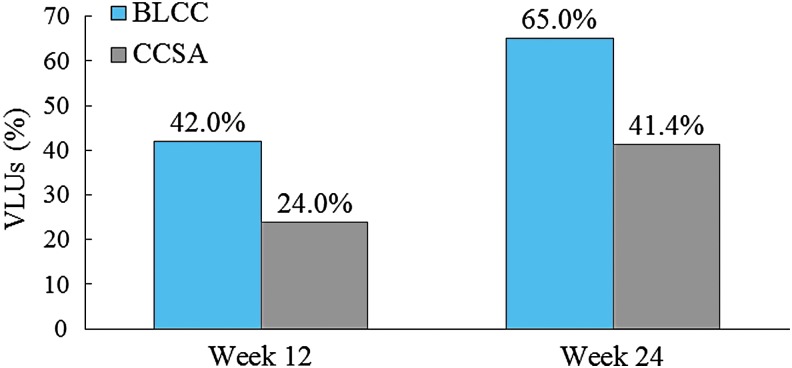
Cox proportional hazards regression analysis that adjusted for ulcer area, duration, depth, and patient age at first treatment showed that BLCC treatment significantly improved the median time to VLU wound closure by 52%, achieving the endpoint 16.2 weeks sooner than CCSA-treated patients (15.1 weeks for BLCC vs. 31.3 weeks for CCSA; p = 0.0002) (Fig. 1). The estimated incidence of wound closure for BLCC compared with CCSA was significantly improved by week 12 (42.0% vs. 24.0%) and week 24 (65.0% vs. 41.4%) (Figs. 2 and 3). BLCC treatment approximately doubled (97% increase) the probability of healing compared with CCSA (hazard ratio = 1.97 [95% confidence interval 1.39–2.79], p = 0.0002).
Figure 1.
Estimated median time to wound closure from a Cox regression model with terms for treatment, baseline wound area, baseline wound duration, baseline wound depth, and patient age at first treatment. BLCC, bioengineered living cellular construct; CCSA, cryopreserved cadaveric skin allograft.
Figure 2.
Estimated incidence of wound closure from a Cox regression model with terms for treatment, baseline wound area, baseline wound duration, baseline wound depth, and patient age at first treatment.
Figure 3.
Estimated percent VLUs achieving closure from a Cox regression model with terms for treatment, baseline wound area, baseline wound duration, baseline wound depth, and patient age at first treatment. VLUs, venous leg ulcers.
Discussion
To our knowledge, this is the first comparative effectiveness analysis to evaluate BLCC and CCSA for the treatment of VLUs. Notably, the VLUs in this analysis were refractory “hard-to-heal” VLUs. Wounds that closed >40% within the 28 days before treatment were excluded. This is consistent with current clinical practice where the use of this 4-week prognostic indicator has become widely adopted to help identify patients who may benefit from advanced wound care therapy, including skin substitutes. In our analysis, we found that use of BLCC closed significantly more VLUs in significantly less time compared with CCSA. Furthermore, BLCC nearly doubled the probability of healing compared with CCSA.
In clinical research, RCTs provide the least biased estimates of efficacy and are important to demonstrate that treatment works. RCTs are needed to obtain regulatory approval, and as such, often compare products being developed with placebo or conventional care rather than another available active treatment. However, results from RCTs may not always correspond to what is seen in real-world practice, where the treatment is applied to a broader range of patients and where there are often significant variations in clinical practice.21 Effectiveness trials are designed to evaluate the actual usefulness of the treatment in routine clinical practice. In other words, efficacy answers the question, “Can it work?” and effectiveness answers the question, “Does it work?” when the product is used in real-world settings without the controls of a clinical trial. The evidence is generally considered strong when results are consistent between efficacy and effectiveness trials.22,23
We found the effectiveness results for BLCC in the current analysis to be supportive of the efficacy results from its pivotal trial in VLUs.15 The pivotal trial for BLCC was a large, prospective, multicenter, randomized controlled trial. A total of 293 patients were randomized to BLCC plus compression therapy or compression therapy alone (control). By week 24, Falanga et al. reported that 63% of VLUs treated with BLCC healed (Note: data not reported at 12 weeks).15 This is similar to the current analysis, which found that 65% of VLUs healed at 24 weeks with BLCC treatment. To date, there has not been a RCT conducted with CCSA to establish efficacy or safety in the treatment of VLUs; as a Section 361 HCT/P, FDA premarket review of clinical data is not required. A retrospective chart review of CCSA-treated wounds by Landsman et al. found that 61% of VLUs were healed by 12 weeks and 75% by 20 weeks with CCSA plus standard care therapy.16 These results reflect a single center's experience with CCSA and were not limited to only hard-to-heal VLUs (those demonstrating <40% closure in the prior 28 days), which may, in part, explain the higher rates of closure compared with those reported in the current analysis.
This is the second comparative effectiveness analysis of BLCC with another skin substitute for the treatment of VLUs, using the WoundExpert EMR database. In the first analysis, Marston et al. compared BLCC with porcine small intestine submucosa (SIS; Oasis, Healthpoint, Fort Worth, TX), an acellular wound dressing.24 This was a larger study that included data from nearly 1,500 patients with about 1,800 refractory VLUs collected over a 3-year period. The frequency of VLU closure was significantly higher with BLCC compared with SIS (p = 0.01). At 12 weeks, it was 31% with BLCC versus 26% with SIS; and at 24 weeks, it was 50% versus 41%, respectively. The wound closure rates reported by Marston et al. are somewhat lower than those observed in the current analysis, which may potentially be explained by the larger baseline wound size eligible for inclusion in the analysis (VLUs between 1 and 150 cm2 in Marston et al. compared to 1 and 40 cm2 in the current analysis). The BLCC-treated VLUs in the Marston et al. analysis were larger (mean wound size 16.2 cm2 vs. 10.2 cm2 in current analysis) and of longer average duration (17.0 months vs. 13.3 months), which suggests a more severe, recalcitrant cohort.
The Net Health database did not include costs related to wound care and outcomes; however, VLU-related treatment costs are directly related to time to achieve complete wound closure. Rice et al. showed that patients with VLUs used significantly more medical resources and incurred more costs during a 12-month follow-up period compared with matched non-VLU patients.9 In the Medicare population, patients with VLUs had 77.8% more hospitalization days, 50% more emergency department visits, 27% more outpatient office visits, and 60% more days of home healthcare. Total healthcare costs were $537 per week higher for patients with nonhealed VLUs compared with those whose ulcers healed. In the current analysis, the significant difference in time to healing for VLUs suggests that BLCC may provide significant cost savings compared with CCSA. The median time to wound closure was 15.1 weeks with BLCC versus 31.3 with CCSA weeks—a 16.2 week difference. The greater proportion of patients achieving VLU closure also support a cost savings benefit with BLCC versus CCSA.
There are several limitations to this retrospective study. The use of EMR databases to collect data may introduce some reporting differences between or within centers. Information made available from all participating centers may not reflect uniform standards of patient assessments and standardization of general wound care practices. Completion of baseline demographic information fields such as medical history, prior surgical interventions, or concomitant medications may vary across wound care facilities. Specific information regarding the types of dressings other than the primary wound contact materials may not be entered into EMR wound databases. Brands of compression therapy products in this study were often recorded in “free text” fields making analyses of these data problematic. Also, the reporting of safety-related outcomes, adverse events, or ulcer recurrence was difficult as this information was not uniformly coded or captured. Finally, as with all retrospective studies, the possibility of selection bias may exist with imbalances between groups in risk factors resulting. However, given the number of wounds and centers providing information for the analyses, it is less likely that a uniform bias was present affecting study results. In addition, statistical analyses are reported in this study that adjusted for significant, potential risk factors known to affect wound healing.
In general, comparative effectiveness research (CER) studies are best used to accurately report effectiveness of specific treatments in one defined patient population. Subgroup analyses become problematic for a variety of reasons that may include smaller sample sizes, incomplete reporting, and missing data. Importantly, clinical interpretations of subgroup analyses in CER should be viewed with caution given potentially confounding variables not accounted for in the databases studied. RCTs typically control for or eliminate key, potentially confounding variables. This CER determined wound outcomes under whatever real-world conditions BLCC and CCSA were used at the 177 participating centers. Statistically and clinically meaningful conclusions for many important questions such as the effects of potential immune responses to CCSA, multiple applications of study treatments, interval of time between use of study treatments, and adjunctive therapies, including HBO and NPWT, are best evaluated in well-controlled RCTs.
In conclusion, this real-world comparative effectiveness study demonstrates that treatment with BLCC significantly improves the incidence and decreases the time to wound closure compared with CCSA. These results are similar to and further support the efficacy data from the pivotal RCT for BLCC in the treatment of VLUs.
Innovation
This is the first real-world comparative effectiveness analysis to evaluate BLCC and CCSA for the treatment of VLUs. Data were obtained from a wound care-specific EMR database (WoundExpert; Net Health) that is utilized by ∼90% of wound care facilities across the United States and which allowed for the inclusion of a large patient population. Our data show a significantly greater rate and speed of wound closure with BLCC compared with CCSA. This finding has both clinical and economic implications.
Abbreviations and Acronyms
- BLCC
bioengineered living cellular construct
- CCSA
cryopreserved cadaveric skin allograft
- CER
comparative effectiveness research
- ECM
extracellular matrix
- EMR
electronic medical record
- FDA
Food and Drug Administration
- HBO
hyperbaric oxygen
- HCT/P
Human Cells, Tissues, and Cellular and Tissue-based Product
- HIPAA
Health Insurance Portability and Accountability Act of 1996
- NPWT
negative-pressure wound therapy
- RCT
randomized controlled trial
- SIS
small intestine submucosa
- VLU
venous leg ulcer
Acknowledgments and Funding Sources
The authors thank Biostatistical Consulting, Inc. for statistical analyses. De-identified patient data released to Organogenesis, Inc. were consistent with the terms and conditions of Net Health's client contracts and the requirements of the Health Insurance Portability and Accountability Act of 1996 (HIPAA). Net Health was not involved in the analysis, interpretation, or reporting of the data. This study was funded by Organogenesis, Inc.
Key Findings.
• Treatment with a bioengineered living cellular construct (BLCC) significantly improves the incidence and speed of VLU closure compared with a cryopreserved cadaveric skin allograft (CCSA).
• The effectiveness results for BLCC in the current analysis were supportive of the efficacy results from its pivotal trial in VLUs.
• BLCC nearly doubled the probability of healing compared with CCSA, which suggests a greater clinical benefit as well as a potential cost savings benefit.
Author Disclosure and Ghostwriting
T.T. serves as a consultant for Organogenesis, Inc. M.L.S. serves as a consultant for Organogenesis, Inc, AOBiome, Neumedicines, and Allergan. N.B.P. is an employee of Organogenesis. M.S. is a former employee of Organogenesis, Inc. The authors thank Tina Lin for writing assistance and editorial support in article preparation.
About the Authors
Terry Treadwell, MD, FACS, a vascular and general surgeon in Montgomery, Alabama, founded the Institute for Advanced Wound Care and currently serves as the Medical Director of the Institute. He has been involved with numerous educational and research initiatives centered around the treatment of acute and chronic wounds. Michael L. Sabolinski, MD, has more than 25 years of experience in clinical research and medical products development, including regenerative medicine. He was integrally involved in securing the FDA approval of a living skin substitute, pioneering the path for other cell therapies. Michelle Skornicki, MPH, currently works at Precision Health Economics. In her time at Organogenesis, Inc., she was the Manager of health economics and outcomes research. Nathan B. Parsons, RN, BSN, is Director of Medical Affairs at Organogenesis, Inc.
References
- 1.Phillips TJ, Dover JS. Leg ulcers. J Am Acad Dermatol 1991;25:965–987 [DOI] [PubMed] [Google Scholar]
- 2.Jones KR. Why do chronic venous leg ulcers not heal? J Nurs Care Qual 2009;24:116–124; quiz 125–126 [DOI] [PubMed] [Google Scholar]
- 3.Abbade LPF, Lastória S. Venous ulcer: epidemiology, physiopathology, diagnosis and treatment. Int J Dermatol 2005;44:449–456 [DOI] [PubMed] [Google Scholar]
- 4.Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol 2001;44:401–421 [DOI] [PubMed] [Google Scholar]
- 5.Krasner D. Painful venous ulcers: themes and stories about their impact on quality of life. Ostomy Wound Manage 1998;44:38–49 [PubMed] [Google Scholar]
- 6.Phillips T, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol 1994;31:49–53 [DOI] [PubMed] [Google Scholar]
- 7.Green J, Jester R. Health-related quality of life and chronic venous leg ulceration: part 1. Br J Community Nurs 2009;14:S12, S14, S16–S17 [DOI] [PubMed] [Google Scholar]
- 8.Sen CK, Gordillo GM, Sashwati R, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice JB, Desai U, Cummings AKG, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. J Med Econ 2014;17:347–356 [DOI] [PubMed] [Google Scholar]
- 10.Callam MJ, Harper DR, Dale JJ, Ruckley CV. Chronic ulcer of the leg: clinical history. Br Med J (Clin Res Ed) 1987;294:1389–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Rep Regen 2004;12:163–168 [DOI] [PubMed] [Google Scholar]
- 12.Margolis DJ, Berlin JA, Strom BL. Risk factors associated with the failure of a venous leg ulcer to heal. Arch Dermatol 1999;135:920–926 [DOI] [PubMed] [Google Scholar]
- 13.Kantor J, Margolis DJ. A multicenter study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol 2000;142:960–964 [DOI] [PubMed] [Google Scholar]
- 14.Gelfand JM, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol 2002;119:1420–1425 [DOI] [PubMed] [Google Scholar]
- 15.Falanga V, Margolis D, Alvarez O, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Arch Dermatol 1998;134:293–300 [DOI] [PubMed] [Google Scholar]
- 16.Landsman AS, Cook J, Cook E, et al. A retrospective clinical study of 188 consecutive patients to examine the effectiveness of a biologically active cryopreserved human skin allograft (TheraSkin) on the treatment of diabetic foot ulcers and venous leg ulcers. Foot Ankle Spec 2011;4:29–41 [DOI] [PubMed] [Google Scholar]
- 17.Landsman A, Rosines E, Houck A, et al. Characterization of a cryopreserved split-thickness human skin allograft-TheraSkin. Adv Skin Wound Care 2016;29:399–406 [DOI] [PubMed] [Google Scholar]
- 18.Net Health. http://nethealth.com/products/wound-care/woundexpert-software-wound-care-ehr (last accessed December27, 2016)
- 19.Toh S, Rodriguez LAG, Hernan MA. Analyzing partially missing confounder information in comparative effectiveness and safety research of therapeutics. Pharmacoepidemiol Drug Saf 2012;21:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motheral B, Brooks J, Clark MA, et al. A checklist for retrospective database studies—report of the ISPOR task force on retrospective databases. Value Health 2003;6:90–97 [DOI] [PubMed] [Google Scholar]
- 21.Eaglstein WH, Kirsner RS. Expectations for comparative effectiveness and efficacy research [editorial]. JAMA Dermatol 2013;149:18–19 [DOI] [PubMed] [Google Scholar]
- 22.Berger ML, Mamdani M, Atkins D, Johnson ML. Good research practices for comparative effectiveness research: defining, reporting and interpreting nonrandomized studies of treatment effects using secondary data sources: the ISPOR good research practices for retrospective database analysis task force report—part I. Value Health 2009;12:1044–1052 [DOI] [PubMed] [Google Scholar]
- 23.Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM, editors. Developing a Protocol for Observational Comparative Effectiveness Research: A User's Guide. AHRQ Publication No. 12(13)-EHC099. Rockville, MD: Agency for Healthcare Research and Quality, 2013. www.effectivehealthcare.ahrq.gov/Methods-OCER.cfm (last accessed February24, 2017) [PubMed] [Google Scholar]
- 24.Marston WA, Hanft J, Norwood P, Pollak R, for the Dermagraft Diabetic Foot Ulcer Study group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers. Diabetes Care 2003;26:1701–1705 [DOI] [PubMed] [Google Scholar]