Abstract
Neuromyelitis optica spectrum disorders (NMOSD) are mostly relapsing inflammatory disorders of the central nervous system (CNS). Optic neuritis (ON) is the first NMOSD-related clinical event in 55% of the patients, which causes damage to the optic nerve and leads to visual impairment. Retinal optical coherence tomography (OCT) has emerged as a promising method for diagnosis of NMOSD and potential individual monitoring of disease course and severity. OCT not only detects damage to the afferent visual system caused by ON but potentially also NMOSD-specific intraretinal pathology, i.e. astrocytopathy. This article summarizes retinal involvement in NMOSD and reviews OCT methods that could be used now and in the future, for differential diagnosis, for monitoring of disease course, and in clinical trials.
Keywords: Neuromyelitis optica; Tomography, optical coherence; Diagnosis, differential; Optic neuritis; Retina; Disease progression; Vision disorders
Neuromyelitis optica spectrum disorders
Neuromyelitis optica spectrum disorders (NMOSD) are autoimmune inflammatory conditions of the central nervous system (CNS) with a mostly relapsing disease course [1]. Clinical hallmarks of NMOSD are optic neuritis (ON), longitudinally extensive transverse myelitis (LETM) in the spinal cord spanning three or more vertebral segments, and brain stem encephalitis including area postrema syndrome [2–5]. Neuropathic pain [6], fatigue [7], and depression [8] are important secondary symptoms. A serum autoantibody against the astrocytic water channel, aquaporin-4 (AQP4-ab), is detectable in approximately 80% of the patients [9–12]. This antibody was shown to be pathogenic, and its detection together with characteristic clinical, epidemiological, and imaging features allows for the discrimination of NMOSD from multiple sclerosis (MS), the most common autoimmune disorder of the CNS and the most relevant differential disease diagnosis [13–17]. NMOSD has distinct immunopathogenesis from MS, which firmly establishes both of these conditions as separate nosologic entities [18–27]. Consequently, disease-modifying treatment differs fundamentally between NMOSD and MS; as many drugs used in MS have proven ineffective or even harmful in NMOSD [28–34]. Conversely, many patients with NMOSD respond well to B cell targeting therapies with rituximab or immunosuppressive therapies with azathioprine or mycophenolate mofetil [29, 33–37]. Recently, an antibody against myelin oligodendrocyte glycoprotein (MOG-ab) was detected in a subgroup of exclusively AQP4-ab seronegative NMOSD patients [38–44], recurrent idiopathic optic neuritis (RION) patients, and a few MS patients [45, 46], further complicating the disease spectrum. Currently, there is controversy over whether these MOG-ab seropositive patients are part of the NMOSD disease spectrum or if they belong to a separate disease entity (“MOG-ab positive encephalomyelitis” or MOG-EM) [47–49]. This article reviews OCT techniques and discusses associations between structural retinal damage and visual function in NMOSD. It will also describe the potential future relevance of OCT for differential diagnosis, patient profiling, individual monitoring of disease course, and for clinical trials with immunosuppressive or potential causal therapies. This article is an updated and extended English version of a recently published article in German [50].
Optical coherence tomography
In vivo imaging of the retinal anatomy by OCT
OCT is an interferometric technique employing low-coherent light to produce structural cross-sectional images [51]. The light emitted from the device is backscattered and reflected in a manner dependent on the structural composition of the retina; the interference with a reference beam allows anatomical reconstruction with an axial resolution of a few micrometers (currently approximately 5 μm) [52, 53]. Since its introduction in 1991 by Huang et al. [54], the OCT research has been fast paced. Currently, the most widely used OCT setup is composed of a fixed reference mirror and simultaneous analysis of echoes from all retinal layers by Fourier transformation, thus being called Fourier domain OCT (FD-OCT) or spectral domain OCT (SD-OCT). SD-OCT achieves highly reduced motion artifacts, better reproducibility, and 50 to 100 times faster acquisition than previous methods [55–57]. The novel OCT technology involves the use of a short-cavity swept laser for even higher speed and resolution called swept-source OCT (SS-OCT) [58], as well as the incorporation of volumetric angiography images called optical coherence tomography angiography (OCTA) [59].
Next to MS and NMOSD, a retinal examination by OCT is increasingly applied as a non-invasive technique to evaluate key features of various neurological disorders, e.g., in Susac syndrome, anti-NMDA receptor encephalitis, and also in neurodegenerative diseases [60–65]. OCT provides high-resolution 3D images of retinal structures and can be employed to evaluate the first three neurons of the visual pathway and their interneurons, where one key application is the quantification of neuro-axonal retinal damage (Fig. 1) [66–69].
Fig. 1.
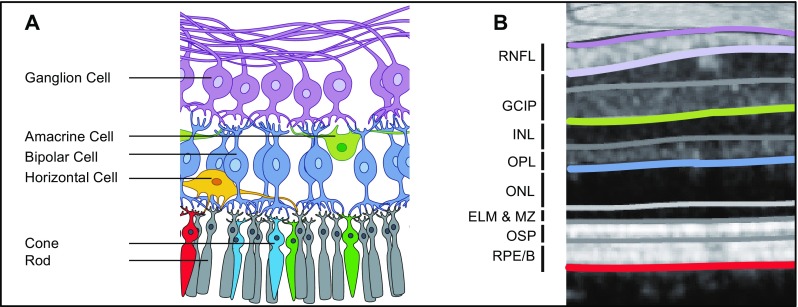
Anatomy of the retina (a) with corresponding layers measured by OCT as suggested by Staurenghi et al. [172] and Cruz-Herranz et al. [97] (b). Parts of the figure are provided by courtesy of www.neurodial.de [173]. OCT optical coherence tomography, RNFL retinal nerve fiber layer, GCIP combined ganglion cell and inner plexiform layer, INL inner nuclear layer, OPL outer plexiform layer, ONL outer nuclear layer, ELM & MZ external limiting membrane and myoid zone, OSP outer segments of photoreceptors (ellipsoid zone), RPE/B retinal pigment epithelium and Bruch’s complex
Retinal layer thinning and its quantification
The peripapillary retinal nerve fiber layer thickness (pRNFL or sometimes just RNFL or RNFLT) has become a reliable OCT marker for diagnostic evaluation in translational research and care [70–77]. The eponymous retinal nerve fibers are unmyelinated axons of retinal ganglion cells, originating in the retina and leaving the eye through the optic nerve head towards the lateral geniculate nuclei thereby forming the optic nerve. Therefore, these nerve fibers are a suitable model to investigate neuro-axonal damage and neuroprotection in diseases presenting with ON, such as NMOSD, where they are representative of anterograde sections of axons directly affected by ON [78–81]. The pRNFL is measured in ring scans of defined circumference (most commonly 12° or 3.5 mm) around the optic nerve head as mean thickness (in μm) (Fig. 1). By using a ring scan circling the optic nerve head virtually, all axons leaving the eye are included in the measurement, thereby allowing representation of the full axonal content of the respective optic nerve.
The ganglion cell and inner plexiform layer thickness or volume (GCIP or sometimes GCIPL) regularly complements the pRNFL as an imaging marker. The main targets of interest are the very ganglion cell bodies associated with axons in the retinal fiber layers described previously. Due to the poor differentiability in OCT imaging, the ganglion cell layer is usually measured in combination with the adjacent inner plexiform layer as GCIP (Fig. 1). The GCIP is mostly measured as the perifoveal volume (in mm3), since the ganglion cells are highly concentrated parafoveally and account for about 34% of the macular volume [78, 82, 83]. Up to 3 months after acute ON when the pRNFL is regularly affected by swelling, the GCIP serves especially well as a stable parameter to quantify retinal neuro-axonal damage [84–87]. Recently, the inner nuclear layer (INL) was suggested to have a swelling specific to an inflammation in autoimmune disorders of the CNS that present with ON [88–91]. The outer retinal layers are currently of lesser interest in neuroinflammatory diseases. Although changes have been described, e.g., after ON or branch retinal artery occlusion in Susac syndrome, high vulnerability to variability from imaging, such as patient positioning and poor reproducibility, makes the interpretation of the outer retinal layer measurements challenging [60, 92, 93].
Retinal measurements between OCT devices from different manufacturers are usually not comparable. Whereas, pRNFL has a reasonably good standardization and is measured similarly across devices, GCIP and INL measurements lack this standardization, thereby impeding comparability [94]. The establishment of standardized criteria for acquisition and assessment of OCT images like the OSCAR-IB criteria for image quality [95, 96] and the APOSTEL reporting guidelines for studies incorporating OCT strive to improve comparability of retinal layer quantification longitudinally, as well as across cohorts [97].
NMOSD and OCT
Characteristics of ON as the most common manifestation of NMOSD
ON is the first clinical feature observed in about 55% of the patients with NMOSD and usually causes severe structural damage to the optic nerve and retina with resulting functional impairment [98]. NMOSD patients often suffer from bilateral and sometimes simultaneous ON (radiological bilateral ON: MS ~ 20%, NMOSD ~ 80%), frequent relapses, and severely reduced visual acuity or even complete vision loss [98]. Unilateral ON often appears as afferent pupillary defect (RAPD), while this can be concealed in bilateral ON [99]. Typically, subacute visual loss progresses in the course of days or weeks, and recovery is possible within 6 months since onset [100, 101]. One year after ON, only 52% of the NMOSD patients recover a high contrast visual acuity of 20/20 to 20/63, and about 25% suffer from visual impairment with acuity of < 20/200 [102–104]. Apart from a high contrast visual acuity, patients are often afflicted with severe loss of low contrast visual acuity and decreased vision-related quality of life [105, 106].
Neuro-axonal damage of the retina after ON
So far, no published studies have investigated acute ON specifically in NMOSD. Studies investigating isolated or idiopathic acute ON, without the distinction of underlying pathologies, have shown that during clinical onset of acute ON, OCT measurements typically give a highly swollen pRNFL that is not representative of retrograde axonal damage [107]. At this time, GCIP thickness is similar in both the affected and the unaffected fellow eye (Table 1) [86]. After acute ON, the loss of retinal axons and ganglion cells proceeds over a period of 6 months [89, 106]. Since the optic nerve is often affected near the chiasm in the AQP4-ab positive NMOSD, potential carryover affects could radiologically or clinically impact the contralateral optic nerve after the unilateral ON [98]. Recurrent ONs in NMOSD give rise to severely thinned pRNFL and GCIP (Fig. 2) [122]. In the case of severe optic nerve atrophy resulting from multiple ON attacks, with pRNFL values lower than 30 μm, further neuro-axonal loss is hard to detect due to flooring effects [99] and the influence of retinal blood vessels running through the measured layers [123]. While retinal damage after ON in MS exhibits a temporal preponderance, all segments can be affected in NMOSD [106, 124]. Pattern variances between NMOSD subtypes are still under investigation: a recent publication suggests a temporal preponderance of retinal damage in MOG-ab seropositive patients as well [111]. Single ONs seem to have less severe effects in MOG-ab seropositive patients compared to AQP4-ab seropositive patients; although the higher frequency of ONs in MOG-ab seropositive patients may result in similar long-range prognoses and may still be unfavorable with respect to visual outcome [119, 125]. After ON, high-contrast visual acuity and low-contrast visual acuity impairment are highly correlated cross-sectionally with reduced pRNFL and GCIP, suggesting both imaging markers as appropriate structural correlates for visual function loss [100, 106, 113, 114]. Relapsing ONs cause pathological latencies of visual evoked potentials (VEP) and severe visual impairment up to complete vision loss [119, 126].
Table 1.
Most important recent publications on OCT in NMOSD
| Reference | Study patients | Controls | Findings |
|---|---|---|---|
| [108] | N = 30, 66% AQP4-ab-p. | No | ↓ pRNFL only in NMOSD with past ON over 18 months follow-up, independent from relapses |
| [102] | N = 29, 48% AQP4-ab-p., 100% ON, and LETM |
N(HC) = 45 N(LETM only) = 29 N(MS-ON) = 29 N(MS-NON) = 44 |
↓ pRNFL in NMOSD vs. all other groups ↓ pRNFL after ON in NMOSD vs. MS ↓ GCIP in NMOSD vs. HC and LETM ↑ INL in NMOSD vs. HC |
| [109] | N = 25, 100% AQP4-ab-p. | No | Microcystic alterations in INL in 15% of the eyes and 24% of the eyes after ON |
| [110] | N = 21, 90% AQP4-ab-p. | N(HC) = 34 | Time since onset +~ atrophy of gray matter pRNFL +~ pericalcarine cortex thickness |
| [111] |
N(AQP4-ab-p.) = 19 N(MOG-ab-p.) = 13 |
N(HC) = 13 | ↓ pRNFL in MOG-ab-p. vs. AQP4-ab-p. NMOSD temporal atrophy in MOG-ab-p. NMOSD |
| [112] | N = 72, 69% ON | N(HC) = 34 | ↓ fovea thickness in NMOSD with and without ON vs. HC; foveal thickness +~ low contrast VA |
| [113] | N = 15, 100% AQP4-ab-p. |
N(HC) = 23 N(MS) = 15 |
↓ pRNFL, high contrast and low contrast VA in NMOSD vs. MS and HC |
| [114] | N = 33, 100% ON, 52% AQP4-ab-p. |
N(HC) = 41 N(MS) = 60 N(LETM) = 28 |
↓ pRNFL and high contrast VA in NMOSD after ON vs. all other groups ↓ pRNFL in LETM vs. HC |
| [115] | N = 18, 100% ON, 100% AQP4-ab-p. | N(MS) = 14 | ↓ pRNFL in NMOSD vs. MS pRNFL +~ high contrast VA pRNFL −~ number of attacks and −~ time until high-dose corticosteroid treatment |
| [116] | N = 31, 71% ON, 100% AQP4-ab-p. | N(HC) = 34 | ↓ foveal thickness and FA in NMOSD with and without ON vs. HC ↓ pRNFL in NMOSD only after ON vs. HC |
| [50] | N = 40, 92, 5% AQP4-ab-p. | No | Vessel artifacts in pRNFL measurements −~ pRNFL |
| [72] | N = 23, 70% ON, 56% AQP4-ab-p. |
N(HC) = 75 N(MS) = 110 |
= pRNFL in NMOSD and MS after ON ↓ temporal pRNFL without ON in MS vs. NMOSD ↓ pRNFL and GCIP in NMOSD without ON vs. HC |
| [117] | N = 9, 100% ON, 67% AQP4-ab-p. | No | No RNFL or macular thinning observed over 4 years follow-up |
| [118] | N = 22, 77% ON, 100% AQP4-ab-p. | N(MS) = 47 | ↓ pRNFL after ON in NMOSD vs. to MS More severe superior and inferior affection in NMOSD |
| [119] |
N(AQP4-ab p.) = 16 N(MOG-ab-p.) = 16 |
N(HC) = 16 | ↓ pRNFL, GCIP, high contrast VA in AQP4-Ak-p., and MOG-ab-p. NMOSD vs. HC = structural and functional parameters in AQP4-ab-p. vs. MOG-ab-p. NMOSD ↑ ON rate in MOG-ab-p. vs. AQP4-ab-p. NMOSD |
| [120] | N = 26, 100% ON, 60% AQP4-ab-p. |
N(HC) = 77 N(MS) = 378 N(LETM) = 17 |
↓ pRNFL and TMV after ON in NMOSD vs. to MS = pRNFL and TMV in non-ON NMOSD eyes and HC |
| [105] | N = 31, 74% ON, 65% AQP4-ab-p. | N(MS) = 31 | ↓ vision-related quality of life in NMOSD vs. MS vision-related quality of life +~ high contrast and low contrast VA and pRNFL and GCIP |
| [106] | N = 17, 60% ON, 94% AQP4-ab-p. |
N(HC) = 17 N(MS) = 17 |
↓ pRNFL, GCIP and low contrast VA in NMOSD vs. HC ↑ INL and outer retinal layers in NMOSD after ON vs. NMOSD without ON, MS, and HC |
| [89] | N = 39 | N(HC) = 39 | ↓ pRNFL, GCIP, outer retinal layers, and low contrast VA in NMOSD vs. HC microcystic INL alterations in 26% of the NMOSD patients (after ON only) |
| [121] | N(MOG-ab-p) = 6 | N(AQP4-ab-p.) = 10 | ↓ pRNFL and VA after ON in AQP4-ab-p. vs. MOG-ab-p. NMOSD |
| [86] | N = 22, 73% AQP4-ab-p. |
N(HC vs. NMOSD) = 22 N(HC vs. MS) = 50 N(MS) = 98 N(acute ON) = 20 |
↓ pRNFL, GCIP, and TMV after ON vs. without ON in NMOSD and MS ↓ GCIP in non-ON NMOSD vs. HC |
N number, vs. versus, ↓ reduction, ↑ increase, +~ positive correlation, -~ negative correlation, pRNFL peripapillary retinal nerve fiber layer, GCIP combined ganglion cell and inner plexiform layer, INL inner nuclear layer, ON optic neuritis, AQP4-ab-p. aquaporin-4 antibody positive, FA fractional anisotropy, HC healthy controls, LETM longitudinally extensive transverse myelitis, MS multiple sclerosis, MOG-ab-p. myelin oligodendrocyte glycoprotein antibody positive, VA visual acuity
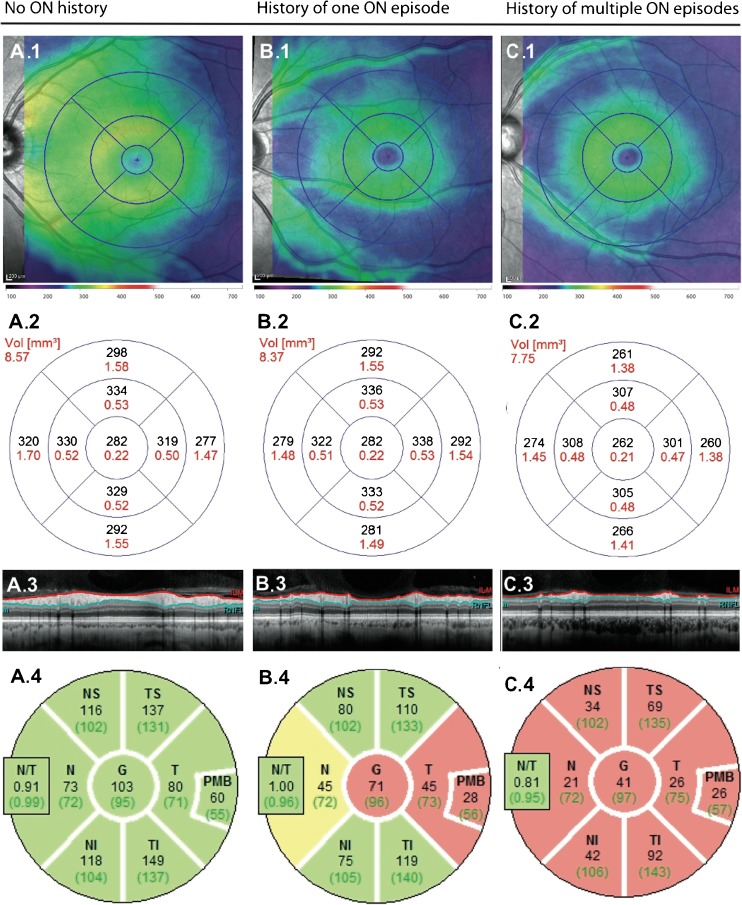
Fig. 2.
Neuro-axonal damage after ON in NMOSD for A an eye not affected by ON in an NMOSD patient compared to B an eye after one single ON in an NMOSD patient and C an eye after multiple ONs of an NMOSD patient. (1) TMV around the fovea with (2) corresponding macular volume of represented segments. (3) Peripapillary ring scan around the optic nerve head with marked retinal nerve fiber layer for pRNFL measurements. (4) Color-coded image of the pRNFL thicknesses compared to a healthy cohort from the device’s normative database: green: not reduced compared to a healthy cohort (> fifth percentile), yellow: borderline thinned compared to a healthy cohort (< fifth percentile), red: severely reduced compared to a healthy cohort (< first percentile). ON optic neuritis, NMOSD neuromyelitis optica spectrum disorders, pRNFL peripapillary retinal nerve fiber layer, TMV total macular volume
Primary retinal pathology in NMOSD
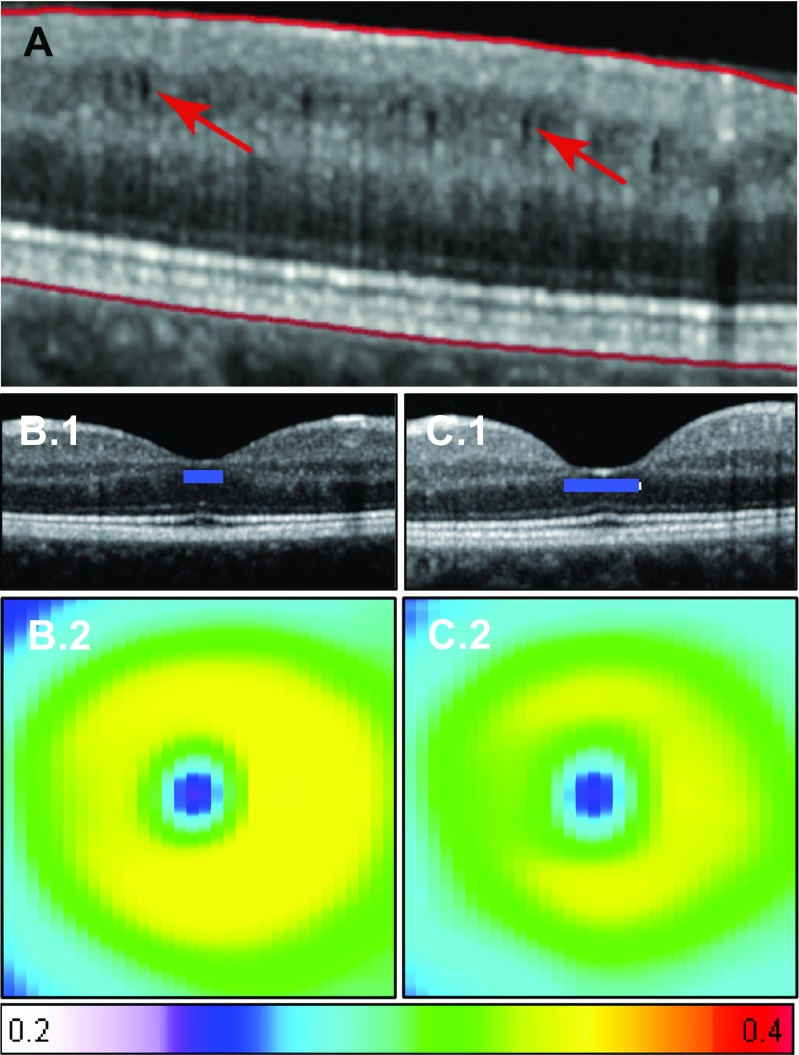
Around 20% of the NMOSD patients have microcystic alterations of the INL after ON (Fig. 3) [89, 90, 109, 127]. This so-called microcystic macular edema (MME) is characteristic for a range of optic neuropathies and is not specific for NMOSD. It has also been reported, although not as frequently, in MS patients with ON and from patients with non-inflammatory optic neuropathies [90, 128, 129]. Its formation in NMOSD seems to be dynamic and caused through intraretinal processes, although vitreous traction might play an additional role in some cases [127, 130]. The detailed pathology of MME is not yet clear; possible explanations include vascular damage with extracellular fluid accumulation, the aforementioned vitreous traction and Mueller cell pathology [128, 127, 131].
Fig. 3.
Primary retinal pathology in NMOSD. A Macular microcysts in the INL of a NMOSD after ON (arrows: microcysts). B (1) OCT and (2) mean shape surface reconstruction with shape variation (color code: thickness in mm + 1 SD) of healthy cohort compared to C (1) OCT and (2) mean shape surface reconstruction with shape variation of broadened fovea surface in a NMOSD cohort. ON optic neuritis, NMOSD neuromyelitis optica spectrum disorders, INL inner nuclear layer, SD standard deviation
Mueller cells are astrocytic cells of the retina residing mainly in the INL and might also play a role in NMOSD beyond MMO and ON-inflicted damage. They have multiple responsibilities, including water homeostasis, energy metabolism, and neurotransmitter recycling. Since they express AQP4 water channel proteins, they might be a direct target of AQP4-ab and a potential cause of a primary retinopathy in NMOSD [132–134]. Animal studies and human autopsy reports support the concept of NMOSD as a primary astrocytopathy. In one study, a retinal damage model in rats showed complement-independent loss of AQP4 in Mueller cells [132]. Autopsies of the afferent visual system demonstrated complement-independent loss of retinal Müller cells also in humans [135]. This is further substantiated by in vivo data from the fovea in NMOSD, where Mueller cells reside in high concentration. In AQP4-ab seropositive NMOSD, the foveal and parafoveal regions are thinned while the pRNFL and the GCIP seem to be unaffected in patients without a history of ON (Fig. 3) [112, 116]. The presumed primary retinopathy could potentially enable a quicker diagnosis and sensible tracking of disease course in the future, but research in this regard is still lacking [136, 137]. While a recent study by Tian et al. [138] found that there is also retinal neuro-axonal damage without ON in NMOSD, longitudinal studies investigating neuro-axonal damage without ON in NMOSD have not been performed extensively. The only two studies published so far have shown conflicting results, where Bouyon et al. [108] showed RNFL thinning over 18 months in patients with a past ON, but Manogaran et al. [117] were not able to show RNFL or macular thinning over a 4-year follow-up. Thus, further longitudinal studies are required, to investigate the development of retinal damage in NMOSD beyond ON and their potential functional relevance.
Association between OCT and magnetic resonance imaging
The magnetic resonance imaging (MRI) of brain and spinal cord is an indispensable tool and a part of the diagnostic criteria for MS and NMOSD [4, 139–144]. In NMOSD, the association between brain tissue alterations and intraretinal or afferent visual system changes is not completely understood. Retrograde and anterograde trans-synaptic degeneration following ON potentially causes subsequent alterations in the retina, optic nerve, and anatomically connected tracts [145–148]. Consequently, a combination of lesion length in the optic nerve measured by MRI and retinal findings by OCT offers the unique possibility of predicting visual outcome after ON [125]. Recently, a study with a mixed AQP4-ab seropositive and seronegative NMOSD cohort that had cortical atrophy showed a correlation between pRNFL and pericalcarine cortex thickness, further supporting the concept of trans-synaptic degeneration being responsible for some detectable brain atrophy [110]. Also, intracerebral changes are accentuated in the optic radiation and can consequently be understood as ON-associated transmitted damage [149]. Nevertheless, a functional MRI study by Finke et al. suggests that not only are there degenerative processes that contribute to impaired vision in NMOSD but maladaptive plasticity after ON may also play a role [150].
While numerous studies exist describing brain tissue alterations in MS (global atrophy, atrophy of grey and white matter, microstructural changes by diffusion-weighted imaging (DWI)), only few studies have investigated MRI characteristics in NMOSD [149, 151–154]. The existence of diffuse tissue alterations with global or regional atrophy in NMOSD is therefore still a matter of debate [155, 156]. Up to 80% of the AQP4-ab seropositive NMOSD patients present with cerebral lesions in AQP4-rich sites like the hypothalamus and periependymal regions; where up to 15% would formally fulfil the diagnostic criteria for MS [157, 158]. In contrast to MS, cortical lesions are rare in NMOSD [159, 160]. Joint analyses of diffusion tensor imaging of the optic radiation and OCT data from AQP4-ab seropositive patients suggest microstructural damage of the afferent visual system also in patients without a history of ON, supporting diffuse brain changes detectable by MRI outside of trans-synaptic degeneration [116, 138]. In line with this, a study from Ventura et al. showed a spinal cord atrophy in patients without LETM and spinal cord lesions, which points towards an attack-independent tissue damage in the spinal cord [161]. Ultimately, the latter three studies included only a few patients and further studies investigating attack-independent tissue alterations in NMOSD with higher sample sizes and in different anatomical regions are highly warranted.
The relevance of OCT for clinical trials in NMOSD
To date, no results from randomized controlled trials (RCTs) of disease-modifying therapies (DMTs) in NMOSD have been published. Current treatment strategies (e.g., rituximab, azathioprine, mycophenolate mofetil, oral prednisolone, recently also tocilizumab) are based on retrospective case series or uncontrolled trials [29, 30, 33–37]. Importantly, DMTs used in MS (e.g., beta-interferon, glatiramer acetate, natalizumab, fingolimod, alemtuzumab) are ineffective in NMOSD patients or can even provoke relapses [31, 32, 162]. Therefore, the development of safe and effective DMTs for NMOSD is highly warranted [163, 164]. Several RCTs in this regard are currently conducted or planned [165–167]. In these and future trials, OCT may serve as a valuable outcome parameter to evaluate the structural sequelae of ON attacks or to track subclinical retinal changes. To date, multiple RCTs in MS and ON have successfully used OCT measures like pRNFL as primary or secondary endpoints [73, 168, 169]. In NMOSD, smaller retrospective studies evaluating the effect of therapies based on OCT parameters were performed that suggest the superiority of the combination of plasmapheresis and corticosteroid therapy compared to corticosteroid therapy alone and confirmed a preserving effect on RNFL of early high-dose methyl prednisolone therapy in acute ON [113, 115]. Future RCTs in NMOSD may incorporate the predictive value of structural OCT parameters for visual function in parallel to common clinical endpoints, such as pRNFL and GCIP as markers of neuro-axonal damage and INL as a marker for inflammation [84, 170].
Outlook
The retina is one of the most affected CNS regions in NMOSD. The OCT is an easy-to-use diagnostic tool to assess neuroinflammatory and neurodegenerative processes in the retina and thus the visual system. An early examination of the retina by OCT in NMOSD might provide useful information on the severity of structural damage that may be predictive of functional outcomes, as well as in the long-term disease course [171]. With regard to NMOSD-specific pathology, OCT measurements can also provide key information for differential diagnosis against other disease entities. In the future, OCT might also help to evaluate the success of NMOSD-specific therapies. Adequately powered studies investigating longitudinal changes both after ON in NMOSD but also outside ON are currently lacking and should be a priority of future research.
Acknowledgements
We thank Claudia Chien for her support in the English proofreading.
Abbreviations
- AQP4-ab
aquaporin-4 antibodies
- CNS
central nervous system
- DMT
disease modifying therapy
- DWI
diffusion-weight imaging
- ELM
external limiting membrane
- FA
fractional anisotropy
- FD-OCT
Fourier domain optical coherence tomography
- GCIP
combined ganglion cell and inner plexiform layer
- HC
healthy control
- INL
inner nuclear layer
- LETM
longitudinally extensive transverse myelitis
- MMO
microcystic macular edema
- MOG-ab
myelin-oligodendrocyte glycoprotein antibody
- MOG-EM
MOG-Enzephalomyelitis
- MRI
magnet resonance imaging
- MS
multiple sclerosis
- MZ
myoid zone
- NMOSD
neuromyelitis optica spectrum disorders
- OCT
optical coherence tomography
- OCTA
optical coherence tomography angiography
- ON
optic neuritis
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- OSP
outer segments of photoreceptors
- pRNFL
peripapillary retinal nerve fiber layer
- RAPD
relative afferent pupillary defect
- RCT
randomized controlled trial
- RION
relapsing isolated optic neuritis
- RNFL
retinal nerve fiber layer
- RPE/B
retinal pigment epithelium and Bruch’s membrane complex
- SD
standard deviation
- SD-OCT
spectral-domain optical coherence tomography
- SS-OCT
swept-source optical coherence tomography
- TMV
total macular volume
- VA
visual acuity
- VEP
visual evoked potential
Compliance with ethical standards
Conflict of interest
F. C. Oertel reports no conflicts of interest. H. Zimmermann received speaker honorary from TEVA and Bayer Healthcare, independent from this work. A. U. Brandt received consulting fees unrelated to this study for research from Novartis, Biogen, Motognosis, Teva, and Bayer. FP received research support from the German Ministry for Education and Research (BMBF/KKNMS; Competence Network Multiple Sclerosis), the Deutsche Forschungsgemeinschaft (DFG) (grant exc. 257), and from the Guthy Jackson Charitable Foundation and National Multiple Sclerosis Society as well as research grants and speaker honoraria from Bayer, Teva, Genzyme, Merck, Novartis, MedImmune and is member of the steering committee of the OCTIMS study (Novartis).
Contributor Information
Frederike C. Oertel, Email: frederike-cosima.oertel@charite.de
Hanna Zimmermann, Email: hanna.zimmermann@charite.de.
Friedemann Paul, Email: friedemann.paul@charite.de.
Alexander U. Brandt, Phone: +49-30-450-539757, Email: alexander.brandt@charite.de
References
- 1.Wildemann B, Jarius S, Paul F. Neuromyelitis optica. Nervenarzt. 2013;84(4):436–441. doi: 10.1007/s00115-012-3602-x. [DOI] [PubMed] [Google Scholar]
- 2.Jarius S, Paul F, Franciotta D, Waters P, Zipp F, Hohlfeld R, et al. Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol. 2008;4(4):202–14. 10.1038/ncpneuro0764. [DOI] [PubMed]
- 3.Jarius S, Wildemann B, Paul F. Neuromyelitis optica: clinical features, immunopathogenesis and treatment. Clin Exp Immunol. 2014;176(2):149–164. doi: 10.1111/cei.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–89. 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed]
- 5.Kremer L, Mealy M, Jacob A, Nakashima I, Cabre P, Bigi S, et al. Brainstem manifestations in neuromyelitis optica: a multicenter study of 258 patients. Mult Scler. 2014;20(7):843–7. 10.1177/1352458513507822. [DOI] [PubMed]
- 6.Zhao S, Mutch K, Elsone L, Nurmikko T, Jacob A. Neuropathic pain in neuromyelitis optica affects activities of daily living and quality of life. Mult Scler. 2014;20(12):1658–1661. doi: 10.1177/1352458514522103. [DOI] [PubMed] [Google Scholar]
- 7.Chanson J-B, Zéphir H, Collongues N, Outteryck O, Blanc F, Fleury M, et al. Evaluation of health-related quality of life, fatigue and depression in neuromyelitis optica. Eur J Neurol. 2011;18(6):836–41. 10.1111/j.1468-1331.2010.03252.x. [DOI] [PubMed]
- 8.Chavarro VS, Mealy MA, Simpson A, Lacheta A, Pache F, Ruprecht K, et al. Insufficient treatment of severe depression in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2016;e286:3. doi: 10.1212/NXI.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metz I, Beißbarth T, Ellenberger D, Pache F, Stork L, Ringelstein M, et al. Serum peptide reactivities may distinguish neuromyelitis optica subgroups and multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;e204:3. doi: 10.1212/NXI.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zekeridou A, Lennon VA. Aquaporin-4 autoimmunity. Neurol Neuroimmunol Neuroinflamm. 2015;2(4):e110. doi: 10.1212/NXI.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul F, Jarius S, Aktas O, Bluthner M, Bauer O, Appelhans H, et al. Antibody to aquaporin 4 in the diagnosis of neuromyelitis optica. PLoS Med. 2007;e133:4. doi: 10.1371/journal.pmed.0040133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarius S, Ruprecht K, Wildemann B, Kuempfel T, Ringelstein M, Geis C, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. 2012;9:14. 10.1186/1742-2094-9-14. [DOI] [PMC free article] [PubMed]
- 13.Borisow N, Kleiter I, Gahlen A, Fischer K, Wernecke K-D, Pache F, et al. Influence of female sex and fertile age on neuromyelitis optica spectrum disorders. Mult Scler. 2017;23(8):1092–103. 10.1177/1352458516671203. [DOI] [PubMed]
- 14.Sinnecker T, Schumacher S, Mueller K, Pache F, Dusek P, Harms L, et al. MRI phase changes in multiple sclerosis vs neuromyelitis optica lesions at 7T. Neurol Neuroimmunol Neuroinflamm. 2016;e259:3. doi: 10.1212/NXI.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarius S, Franciotta D, Paul F, Ruprecht K, Bergamaschi R, Rommer PS, et al. Cerebrospinal fluid antibodies to aquaporin-4 in neuromyelitis optica and related disorders: frequency, origin, and diagnostic relevance. J Neuroinflammation. 2010;7(1):52. 10.1186/1742-2094-7-52. [DOI] [PMC free article] [PubMed]
- 16.Hinson SR, Pittock SJ, Lucchinetti CF, Roemer SF, Fryer JP, Kryzer TJ, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69(24):2221–31. 10.1212/01.WNL.0000289761.64862.ce. [DOI] [PubMed]
- 17.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 18.Bennett JL, O’Connor KC, Bar-Or A, Zamvil SS, Hemmer B, Tedder TF, et al. B lymphocytes in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. 2015;e104:2. doi: 10.1212/NXI.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melamed E, Levy M, Waters PJ, Sato DK, Bennett JL, John GR, et al. Update on biomarkers in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. 2015;e134:2. doi: 10.1212/NXI.0000000000000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeshita Y, Obermeier B, Cotleur AC, Spampinato SF, Shimizu F, Yamamoto E, et al. Effects of neuromyelitis optica-IgG at the blood-brain barrier in vitro. Neurol Neuroimmunol Neuroinflamm. 2017;e311:4. doi: 10.1212/NXI.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarius S, Paul F, Fechner K, Ruprecht K, Kleiter I, Franciotta D, et al. Aquaporin-4 antibody testing: direct comparison of M1-AQP4-DNA-transfected cells with leaky scanning versus M23-AQP4-DNA-transfected cells as antigenic substrate. J Neuroinflammation. 2014;11(1):129. doi: 10.1186/1742-2094-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarius S, Paul F, Franciotta D, de Seze J, Münch C, Salvetti M, et al. Neuromyelitis optica spectrum disorders in patients with myasthenia gravis: ten new aquaporin-4 antibody positive cases and a review of the literature. Mult Scler. 2012;18(8):1135–43. 10.1177/1352458511431728. [DOI] [PubMed]
- 23.Bove R, Elsone L, Alvarez E, Borisow N, Cortez MM, Mateen FJ, et al. Female hormonal exposures and neuromyelitis optica symptom onset in a multicenter study. Neurol Neuroimmunol Neuroinflamm. 2017;e339:4. doi: 10.1212/NXI.0000000000000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collongues N, Marignier R, Jacob A, Leite MI, Siva A, Paul F, et al. Characterization of neuromyelitis optica and neuromyelitis optica spectrum disorder patients with a late onset. Mult Scler. 2014;20(8):1086–94. 10.1177/1352458513515085. [DOI] [PubMed]
- 25.Hertwig L, Pache F, Romero-Suarez S, Stürner KH, Borisow N, Behrens J, et al. Distinct functionality of neutrophils in multiple sclerosis and neuromyelitis optica. Mult Scler. 2016;22(2):160–73. 10.1177/1352458515586084. [DOI] [PubMed]
- 26.Jarius S, Paul F, Franciotta D, Ruprecht K, Ringelstein M, Bergamaschi R, et al. Cerebrospinal fluid findings in aquaporin-4 antibody positive neuromyelitis optica: results from 211 lumbar punctures. J Neurol Sci. 2011;306(1-2):82–90. 10.1016/j.jns.2011.03.038. [DOI] [PubMed]
- 27.Pache F, Wildemann B, Paul F, Jarius S. Neuromyelitis optica. Fortschr Neurol Psychiatr. 2017;e1:85. doi: 10.1055/s-0035-1567186. [DOI] [PubMed] [Google Scholar]
- 28.Gelfand JM, Cotter J, Klingman J, Huang EJ, Cree BAC. Massive CNS monocytic infiltration at autopsy in an alemtuzumab-treated patient with NMO. Neurol Neuroimmunol Neuroinflamm. 2014;1(3):e34. doi: 10.1212/NXI.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stellmann J-P, Krumbholz M, Friede T, Gahlen A, Borisow N, Fischer K, et al. Immunotherapies in neuromyelitis optica spectrum disorder: efficacy and predictors of response. J Neurol Neurosurg Psychiatry. 2017;88(8):639–47. 10.1136/jnnp-2017-315603. [DOI] [PMC free article] [PubMed]
- 30.Trebst C, Jarius S, Berthele A, Paul F, Schippling S, Wildemann B, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS) J Neurol. 2014;261(1):1–16. doi: 10.1007/s00415-013-7169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayzenberg I, Schöllhammer J, Hoepner R, Hellwig K, Ringelstein M, Aktas O, et al. Efficacy of glatiramer acetate in neuromyelitis optica spectrum disorder: a multicenter retrospective study. J Neurol. 2016;263(3):575–582. doi: 10.1007/s00415-015-7991-1. [DOI] [PubMed] [Google Scholar]
- 32.Kleiter I, Hellwig K, Berthele A, Kümpfel T, Linker RA, Hartung H-P, et al. Failure of natalizumab to prevent relapses in neuromyelitis optica. Arch Neurol. 2012;69(2):239–45. 10.1001/archneurol.2011.216. [DOI] [PubMed]
- 33.Nosadini M, Alper G, Riney CJ, Benson LA, Mohammad SS, Ramanathan S, et al. Rituximab monitoring and redosing in pediatric neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2016;e188:3. doi: 10.1212/NXI.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valentino P, Marnetto F, Granieri L, Capobianco M, Bertolotto A. Aquaporin-4 antibody titration in NMO patients treated with rituximab: a retrospective study. Neurol Neuroimmunol Neuroinflamm. 2017;4(2):e317. doi: 10.1212/NXI.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mealy MA, Wingerchuk DM, Palace J, Greenberg BM, Levy M. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: multicenter study of treatment efficacy. JAMA Neurol. 2014;71(3):324–330. doi: 10.1001/jamaneurol.2013.5699. [DOI] [PubMed] [Google Scholar]
- 36.Mealy MA, Kim S-H, Schmidt F, López R, Jimenez Arango JA, Paul F, et al. Aquaporin-4 serostatus does not predict response to immunotherapy in neuromyelitis optica spectrum disorders. Mult Scler. 2017;1352458517730131. 10.1177/1352458517730131. [DOI] [PMC free article] [PubMed]
- 37.Rommer PS, Dörner T, Freivogel K, Haas J, Kieseier BC, Kümpfel T, et al. Safety and clinical outcomes of rituximab treatment in patients with multiple sclerosis and neuromyelitis optica: experience from a national online registry (GRAID) J Neuroimmune Pharmacol. 2016;11(1):1–8. doi: 10.1007/s11481-015-9646-5. [DOI] [PubMed] [Google Scholar]
- 38.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation. 2016;13(1):279. doi: 10.1186/s12974-016-0717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13(1):280. [DOI] [PMC free article] [PubMed]
- 40.Sato DK, Callegaro D, Lana-Peixoto MA, Waters PJ, de Jorge FM, H, Takahashi T, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82(6):474–81. 10.1212/WNL.0000000000000101. [DOI] [PMC free article] [PubMed]
- 41.Waters P, Woodhall M, O’Connor KC, Reindl M, Lang B, Sato DK, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm. 2015;e89:2. doi: 10.1212/NXI.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamid SHM, Whittam D, Mutch K, Linaker S, Solomon T, Das K, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J Neurol. 2017;264(10):2088–94. 10.1007/s00415-017-8596-7. [DOI] [PMC free article] [PubMed]
- 43.Kim S-M, Woodhall MR, Kim J-S, Kim S-J, Park KS, Vincent A, et al. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflamm. 2015;e163:2. doi: 10.1212/NXI.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chalmoukou K, Alexopoulos H, Akrivou S, Stathopoulos P, Reindl M, Dalakas MC. Anti-MOG antibodies are frequently associated with steroid-sensitive recurrent optic neuritis. Neurol Neuroimmunol Neuroinflamm. 2015;2(4):e131. doi: 10.1212/NXI.0000000000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spadaro M, Gerdes LA, Krumbholz M, Ertl-Wagner B, Thaler FS, Schuh E, et al. Autoantibodies to MOG in a distinct subgroup of adult multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;e257:3. doi: 10.1212/NXI.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Körtvélyessy P, Breu M, Pawlitzki M, Metz I, Heinze H-J, Matzke M, et al. ADEM-like presentation, anti-MOG antibodies, and MS pathology: TWO case reports. Neurol Neuroimmunol Neuroinflamm. 2017;e335:4. doi: 10.1212/NXI.0000000000000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reindl M, Rostasy K. MOG antibody-associated diseases. Neurol Neuroimmunol Neuroinflamm. 2015;2(1):e60. doi: 10.1212/NXI.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm. 2015;2(1):e62. doi: 10.1212/NXI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sepúlveda M, Armangué T, Sola-Valls N, Arrambide G, Meca-Lallana JE, Oreja-Guevara C, et al. Neuromyelitis optica spectrum disorders: comparison according to the phenotype and serostatus. Neurol Neuroimmunol Neuroinflamm. 2016;e225:3. doi: 10.1212/NXI.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oertel FC, Zimmermann H, Brandt AU, Paul F. Optische Kohärenztomographie bei Neuromyelitis optica-Spektrum-Erkrankungen. Nervenarzt. 2017; 10.1007/s00115-017-0444-6. [DOI] [PubMed]
- 51.Zimmermann H, Oberwahrenbrock T, Brandt AU, Paul F, Dörr J-M. Optical coherence tomography for retinal imaging in multiple sclerosis. Degener Neurol Neuromuscul Dis. 2014:153–62. [DOI] [PMC free article] [PubMed]
- 52.Frohman AR, Schnurman Z, Conger A, Conger D, Beh S, Greenberg B, et al. Multifocal visual evoked potentials are influenced by variable contrast stimulation in MS. Neurology. 2012;79(8):797–801. 10.1212/WNL.0b013e3182661edc. [DOI] [PMC free article] [PubMed]
- 53.Jindahra P, Hedges TR, Mendoza-Santiesteban CE, Plant GT. Optical coherence tomography of the retina: applications in neurology. Curr Opin Neurol. 2010;23(1):16–23. doi: 10.1097/WCO.0b013e328334e99b. [DOI] [PubMed] [Google Scholar]
- 54.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bock M, Brandt AU, Dörr J, Pfueller CF, Ohlraun S, Zipp F, et al. Time domain and spectral domain optical coherence tomography in multiple sclerosis: a comparative cross-sectional study. Mult Scler. 2010;16(7):893–6. 10.1177/1352458510365156. [DOI] [PubMed]
- 56.Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT. Evaluation of time domain and spectral domain optical coherence tomography in the measurement of diabetic macular edema. Invest Ophthalmol Vis Sci. 2008;49(10):4290–4296. doi: 10.1167/iovs.08-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yaqoob Z, Wu J, Yang C. Spectral domain optical coherence tomography: a better OCT imaging strategy. Biotechniques. 2005;39(6):S6–13. doi: 10.2144/000112090. [DOI] [PubMed] [Google Scholar]
- 58.Lavinsky F, Lavinsky D. Novel perspectives on swept-source optical coherence tomography. Int J Retina Vitr. 2016;2(1):25. doi: 10.1186/s40942-016-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kashani AH, Chen C-L, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, et al. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017;60:66–100. 10.1016/j.preteyeres.2017.07.002. [DOI] [PMC free article] [PubMed]
- 60.Brandt AU, Oberwahrenbrock T, Costello F, Fielden M, Gertz K, Kleffner I, et al. Retinal lesion evolution in Susac syndrome. Retina. 2016;36:366–374. doi: 10.1097/IAE.0000000000000700. [DOI] [PubMed] [Google Scholar]
- 61.Roth NM, Saidha S, Zimmermann H, Brandt AU, Oberwahrenbrock T, Maragakis NJ, et al. Optical coherence tomography does not support optic nerve involvement in amyotrophic lateral sclerosis. Eur J Neurol. 2013;20:1170–1176. doi: 10.1111/ene.12146. [DOI] [PubMed] [Google Scholar]
- 62.Roth NM, Saidha S, Zimmermann H, Brandt AU, Isensee J, Benkhellouf-Rutkowska A, et al. Photoreceptor layer thinning in idiopathic Parkinson’s disease. Mov Disord. 2014;29(9):1163–70. 10.1002/mds.25896. [DOI] [PubMed]
- 63.Brandt AU, Oberwahrenbrock T, Mikolajczak J, Zimmermann H, Prüss H, Paul F, et al. Visual dysfunction, but not retinal thinning, following anti-NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm. 2016;e198:3. doi: 10.1212/NXI.0000000000000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stricker S, Oberwahrenbrock T, Zimmermann H, Schroeter J, Endres M, Brandt AU, et al. Temporal retinal nerve fiber loss in patients with spinocerebellar ataxia type 1. PLoS One. 2011;6(7):e23024. 10.1371/journal.pone.0023024. [DOI] [PMC free article] [PubMed]
- 65.Ascaso FJ, Cruz N, Modrego PJ, Lopez-Anton R, Santabárbara J, Pascual LF, et al. Retinal alterations in mild cognitive impairment and Alzheimer’s disease: an optical coherence tomography study. J Neurol. 2014;261(8):1522–30. 10.1007/s00415-014-7374-z. [DOI] [PubMed]
- 66.Fercher AF, Hitzenberger CK, Drexler W, Kamp G, Sattmann H. In vivo optical coherence tomography. Am J Ophthalmol. 1993;116(1):113–114. doi: 10.1016/S0002-9394(14)71762-3. [DOI] [PubMed] [Google Scholar]
- 67.Swanson EA, Izatt JA, Hee MR, Huang D, Lin CP, Schuman JS, et al. In vivo retinal imaging by optical coherence tomography. Opt Lett. 1993;18(21):1864–1866. doi: 10.1364/OL.18.001864. [DOI] [PubMed] [Google Scholar]
- 68.Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia A-S, McNamara JO, et al. Central projections of retinal ganglion cells. 2001 [cited 2016Jan 22]; Available from:http://www.ncbi.nlm.nih.gov/books/NBK11145/
- 69.Zimmermann H, Brandt AU, Paul F. Optische Kohärenztomographie in der Neurologie—Methodik und Anwendung in Forschung und Klinik. Klin Neurophysiol. in press.
- 70.Brandt AU, Zimmermann H, Kaufhold F, Promesberger J, Schippling S, Finis D, et al. Patterns of retinal damage facilitate differential diagnosis between Susac syndrome and MS. PLoS One. 2012;7(6):e38741. 10.1371/journal.pone.0038741. [DOI] [PMC free article] [PubMed]
- 71.Diem R, Molnar F, Beisse F, Gross N, Drüschler K, Heinrich SP, et al. Treatment of optic neuritis with erythropoietin (TONE): a randomised, double-blind, placebo-controlled trial-study protocol. BMJ Open. 2016;6(3):e010956. 10.1136/bmjopen-2015-010956. [DOI] [PMC free article] [PubMed]
- 72.Outteryck O, Majed B, Defoort-Dhellemmes S, Vermersch P, Zéphir H. A comparative optical coherence tomography study in neuromyelitis optica spectrum disorder and multiple sclerosis. Mult Scler J. 2015;21(14):1781–1793. doi: 10.1177/1352458515578888. [DOI] [PubMed] [Google Scholar]
- 73.Raftopoulos R, Hickman SJ, Toosy A, Sharrack B, Mallik S, Paling D, et al. Phenytoin for neuroprotection in patients with acute optic neuritis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(3):259–69. 10.1016/S1474-4422(16)00004-1. [DOI] [PubMed]
- 74.Oberwahrenbrock T, Schippling S, Ringelstein M, Kaufhold F, Zimmermann H, Keser N, et al. Retinal damage in multiple sclerosis disease subtypes measured by high-resolution optical coherence tomography. Mult Scler Int. 2012;2012:530305. [DOI] [PMC free article] [PubMed]
- 75.Martinez-Lapiscina EH, Arnow S, Wilson JA, Saidha S, Preiningerova JL, Oberwahrenbrock T, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15(6):574–84. 10.1016/S1474-4422(16)00068-5. [DOI] [PubMed]
- 76.Brandt AU, Oberwahrenbrock T, Ringelstein M, Young KL, Tiede M, Hartung HP, et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain J Neurol. 2011;134:e193. doi: 10.1093/brain/awr095. [DOI] [PubMed] [Google Scholar]
- 77.Bock M, Brandt AU, Kuchenbecker J, Dörr J, Pfueller CF, Weinges-Evers N, et al. Impairment of contrast visual acuity as a functional correlate of retinal nerve fibre layer thinning and total macular volume reduction in multiple sclerosis. Br J Ophthalmol. 2012;96(1):62–7. 10.1136/bjo.2010.193581. [DOI] [PubMed]
- 78.Galetta KM, Graves J, Talman LS, Lile DJ, Frohman EM, Calabresi PA, et al. Visual pathway axonal loss in benign multiple sclerosis: a longitudinal study. J Neuroophthalmol. 2012;32(2):116–23. 10.1097/WNO.0b013e318240204d. [DOI] [PMC free article] [PubMed]
- 79.Galetta SL, Villoslada P, Levin N, Shindler K, Ishikawa H, Parr E, et al. Acute optic neuritis: unmet clinical needs and model for new therapies. Neurol Neuroimmunol Neuroinflamm. 2015;e135:2. doi: 10.1212/NXI.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petzold A, Wattjes MP, Costello F, Flores-Rivera J, Fraser CL, Fujihara K, et al. The investigation of acute optic neuritis: a review and proposed protocol. Nat Rev Neurol. 2014;10(8):447–58. 10.1038/nrneurol.2014.108. [DOI] [PubMed]
- 81.Petzold A, Balcer LJ, Calabresi PA, Costello F, Frohman TC, Frohman EM, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017;16(10):797–812. 10.1016/S1474-4422(17)30278-8. [DOI] [PubMed]
- 82.Burkholder BM, Osborne B, Loguidice MJ, Bisker E, Frohman TC, Conger A, et al. Macular volume determined by optical coherence tomography as a measure of neuronal loss in multiple sclerosis. Arch Neurol. 2009;66(11):1366–72. 10.1001/archneurol.2009.230. [DOI] [PubMed]
- 83.Costello F. Evaluating the use of optical coherence tomography in optic neuritis. Mult Scler Int. 2011;2011:148394. [DOI] [PMC free article] [PubMed]
- 84.Britze J, Pihl-Jensen G, Frederiksen JL. Retinal ganglion cell analysis in multiple sclerosis and optic neuritis: a systematic review and meta-analysis. J Neurol. 2017;264(9):1837–1853. doi: 10.1007/s00415-017-8531-y. [DOI] [PubMed] [Google Scholar]
- 85.Oberwahrenbrock T, Ringelstein M, Jentschke S, Deuschle K, Klumbies K, Bellmann-Strobl J, et al. Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult Scler. 2013;19(14):1887–95. 10.1177/1352458513489757. [DOI] [PubMed]
- 86.Syc SB, Saidha S, Newsome SD, Ratchford JN, Levy M, Ford E ‘t, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain J Neurol. 2012;135(2):521–533. doi: 10.1093/brain/awr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walter SD, Ishikawa H, Galetta KM, Sakai RE, Feller DJ, Henderson SB, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012;119(6):1250–7. 10.1016/j.ophtha.2011.11.032. [DOI] [PMC free article] [PubMed]
- 88.Saidha S, Sotirchos ES, Ibrahim MA, Crainiceanu CM, Gelfand JM, Sepah YJ, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 2012;11(11):963–72. 10.1016/S1474-4422(12)70213-2. [DOI] [PMC free article] [PubMed]
- 89.Sotirchos ES, Saidha S, Byraiah G, Mealy MA, Ibrahim MA, Sepah YJ, et al. In vivo identification of morphologic retinal abnormalities in neuromyelitis optica. Neurology. 2013;80(15):1406–14. 10.1212/WNL.0b013e31828c2f7a. [DOI] [PMC free article] [PubMed]
- 90.Kaufhold F, Zimmermann H, Schneider E, Ruprecht K, Paul F, Oberwahrenbrock T, et al. Optic neuritis is associated with inner nuclear layer thickening and microcystic macular edema independently of multiple sclerosis. PLoS One. 2013;8(8):e71145. [DOI] [PMC free article] [PubMed]
- 91.Knier B, Berthele A, Buck D, Schmidt P, Zimmer C, Mühlau M, et al. Optical coherence tomography indicates disease activity prior to clinical onset of central nervous system demyelination. Mult Scler. 2016;22(7):893–900. 10.1177/1352458515604496. [DOI] [PubMed]
- 92.Al-Louzi OA, Bhargava P, Newsome SD, Balcer LJ, Frohman EM, Crainiceanu C, et al. Outer retinal changes following acute optic neuritis. Mult Scler. 2016;22(3):362–72. 10.1177/1352458515590646. [DOI] [PMC free article] [PubMed]
- 93.Ouyang Y, Walsh AC, Keane PA, Heussen FM, Pappuru RKR, Sadda SR. Different phenotypes of the appearance of the outer plexiform layer on optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2013;251(10):2311–2317. doi: 10.1007/s00417-013-2308-5. [DOI] [PubMed] [Google Scholar]
- 94.Oberwahrenbrock T, Weinhold M, Mikolajczak J, Zimmermann H, Paul F, Beckers I, et al. Reliability of intra-retinal layer thickness estimates. PLoS One. 2015;10(9):e0137316. [DOI] [PMC free article] [PubMed]
- 95.Schippling S, Balk LJ, Costello F, Albrecht P, Balcer L, Calabresi PA, et al. Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler. 2015;21(2):163–70. 10.1177/1352458514538110. [DOI] [PubMed]
- 96.Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7(4):e34823. 10.1371/journal.pone.0034823. [DOI] [PMC free article] [PubMed]
- 97.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, Saidha S, Martinez-Lapiscina EH, Lagreze WA, et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86(24):2303–9. 10.1212/WNL.0000000000002774. [DOI] [PMC free article] [PubMed]
- 98.Ramanathan S, Prelog K, Barnes EH, Tantsis EM, Reddel SW, Henderson AP, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. 2016;22(4):470–482. doi: 10.1177/1352458515593406. [DOI] [PubMed] [Google Scholar]
- 99.Costello F. Optical coherence tomography in neuro-ophthalmology. Neurol Clin. 2017;35(1):153–163. doi: 10.1016/j.ncl.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 100.Sanchez-Dalmau B, Martinez-Lapiscina EH, Torres-Torres R, Ortiz-Perez S, Zubizarreta I, Pulido-Valdeolivas IV, et al. Early retinal atrophy predicts long-term visual impairment after acute optic neuritis. Mult Scler. 2017;1352458517718628. 10.1177/1352458517718628. [DOI] [PubMed]
- 101.Balcer LJ. Clinical practice. Optic neuritis. N Engl J Med. 2006;354(12):1273–1280. doi: 10.1056/NEJMcp053247. [DOI] [PubMed] [Google Scholar]
- 102.Fernandes DB, Raza AS, Nogueira R, Wang D, Callegaro D, Hood DC, et al. Evaluation of inner retinal layers in patients with multiple sclerosis or neuromyelitis optica using optical coherence tomography. Ophthalmology. 2013;120(2):387–394. doi: 10.1016/j.ophtha.2012.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kitley J, Leite MI, Nakashima I, Waters P, McNeillis B, Brown R, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain J Neurol. 2012;135(6):1834–49. 10.1093/brain/aws109. [DOI] [PubMed]
- 104.Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome) Neurology. 1999;53(5):1107–1114. doi: 10.1212/WNL.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 105.Schmidt F, Zimmermann H, Mikolajczak J, Oertel FC, Pache F, Weinhold M, et al. Severe structural and functional visual system damage leads to profound loss of vision-related quality of life in patients with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2017;11:45–50. 10.1016/j.msard.2016.11.008. [DOI] [PubMed]
- 106.Schneider E, Zimmermann H, Oberwahrenbrock T, Kaufhold F, Kadas EM, Petzold A, et al. Optical coherence tomography reveals distinct patterns of retinal damage in neuromyelitis optica and multiple sclerosis. PLoS One. 2013;8(6):e66151. [DOI] [PMC free article] [PubMed]
- 107.Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59(6):963–9. 10.1002/ana.20851. [DOI] [PubMed]
- 108.Bouyon M, Collongues N, Zéphir H, Ballonzoli L, Jeanjean L, Lebrun C, et al. Longitudinal follow-up of vision in a neuromyelitis optica cohort. Mult Scler. 2013;19(10):1320–2. 10.1177/1352458513476562. [DOI] [PubMed]
- 109.Gelfand JM, Cree BA, Nolan R, Arnow S, Green AJ. Microcystic inner nuclear layer abnormalities and neuromyelitis optica. JAMA Neurol. 2013;70(5):629–633. doi: 10.1001/jamaneurol.2013.1832. [DOI] [PubMed] [Google Scholar]
- 110.von Glehn F, Jarius S, Cavalcanti Lira RP, Alves Ferreira MC, von Glehn FHR, Costa E Castro SM, et al. Structural brain abnormalities are related to retinal nerve fiber layer thinning and disease duration in neuromyelitis optica spectrum disorders. Mult Scler. 2014;20(9):1189–1197. doi: 10.1177/1352458513519838. [DOI] [PubMed] [Google Scholar]
- 111.Havla J, Kümpfel T, Schinner R, Spadaro M, Schuh E, Meinl E, et al. Myelin-oligodendrocyte-glycoprotein (MOG) autoantibodies as potential markers of severe optic neuritis and subclinical retinal axonal degeneration. J Neurol. 2017;264(1):139–51. 10.1007/s00415-016-8333-7. [DOI] [PubMed]
- 112.Jeong IH, Kim HJ, Kim N-H, Jeong KS, Park CY. Subclinical primary retinal pathology in neuromyelitis optica spectrum disorder. J Neurol. 2016;263(7):1343–1348. doi: 10.1007/s00415-016-8138-8. [DOI] [PubMed] [Google Scholar]
- 113.Merle H, Olindo S, Jeannin S, Valentino R, Mehdaoui H, Cabot F, et al. Treatment of optic neuritis by plasma exchange (add-on) in neuromyelitis optica. Arch Ophthalmol Chic. Ill 1960. 2012;130:858–862. doi: 10.1001/archophthalmol.2012.1126. [DOI] [PubMed] [Google Scholar]
- 114.Monteiro MLR, Fernandes DB, Apóstolos-Pereira SL, Callegaro D. Quantification of retinal neural loss in patients with neuromyelitis optica and multiple sclerosis with or without optic neuritis using Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(7):3959–3966. doi: 10.1167/iovs.11-9324. [DOI] [PubMed] [Google Scholar]
- 115.Nakamura M, Nakazawa T, Doi H, Hariya T, Omodaka K, Misu T, et al. Early high-dose intravenous methylprednisolone is effective in preserving retinal nerve fiber layer thickness in patients with neuromyelitis optica. Graefes Arch Clin Exp Ophthalmol. 2010;248(12):1777–1785. doi: 10.1007/s00417-010-1344-7. [DOI] [PubMed] [Google Scholar]
- 116.Oertel FC, Kuchling J, Zimmermann H, Chien C, Schmidt F, Knier B, et al. Microstructural visual system changes in AQP4-antibody–seropositive NMOSD. Neurol Neuroimmunol Neuroinflamm. 2017;e334:4. doi: 10.1212/NXI.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Manogaran P, Traboulsee AL, Lange AP. Longitudinal study of retinal nerve fiber layer thickness and macular volume in patients with neuromyelitis optica spectrum disorder. J Neuroophthalmol. 2016;36:363–368. doi: 10.1097/WNO.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 118.Naismith RT, Tutlam NT, Xu J, Klawiter EC, Shepherd J, Trinkaus K, et al. Optical coherence tomography differs in neuromyelitis optica compared with multiple sclerosis. Neurology. 2009;72(12):1077–82. [DOI] [PMC free article] [PubMed]
- 119.Pache F, Zimmermann H, Mikolajczak J, Schumacher S, Lacheta A, Oertel FC, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflammation. 2016;13(1):282. doi: 10.1186/s12974-016-0720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ratchford JN, Quigg ME, Conger C, Frohman T, Frohman E, Balcer LJ, et al. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology. 2009;73(4):302–8. [DOI] [PMC free article] [PubMed]
- 121.Stiebel-Kalish H, Lotan I, Brody J, Chodick G, Bialer O, Marignier R, et al. Retinal nerve fiber layer may be better preserved in MOG-IgG versus AQP4-IgG optic neuritis: a cohort study. PLoS One. 2017;12(1):e0170847. [DOI] [PMC free article] [PubMed]
- 122.Bennett JL, de Seze J, Lana-Peixoto M, Palace J, Waldman A, Schippling S, et al. Neuromyelitis optica and multiple sclerosis: seeing differences through optical coherence tomography. Mult Scler. 2015;21(6):678–88. 10.1177/1352458514567216. [DOI] [PMC free article] [PubMed]
- 123.Oertel FC, Zimmermann H, Mikolajczak J, Weinhold M, Kadas EM, Oberwahrenbrock T, et al. Contribution of blood vessels to retinal nerve fiber layer thickness in NMOSD. Neurol Neuroimmunol Neuroinflamm. 2017;e338:4. doi: 10.1212/NXI.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bock M, Brandt AU, Dörr J, Kraft H, Weinges-Evers N, Gaede G, et al. Patterns of retinal nerve fiber layer loss in multiple sclerosis patients with or without optic neuritis and glaucoma patients. Clin Neurol Neurosurg. 2010;112(8):647–52. 10.1016/j.clineuro.2010.04.014. [DOI] [PubMed]
- 125.Akaishi T, Sato DK, Nakashima I, Takeshita T, Takahashi T, Doi H, et al. MRI and retinal abnormalities in isolated optic neuritis with myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies: a comparative study. J Neurol Neurosurg Psychiatry. 2016;87(4):446–448. doi: 10.1136/jnnp-2014-310206. [DOI] [PubMed] [Google Scholar]
- 126.Ringelstein M, Kleiter I, Ayzenberg I, Borisow N, Paul F, Ruprecht K, et al. Visual evoked potentials in neuromyelitis optica and its spectrum disorders. Mult Scler. 2014;20(5):617–20. 10.1177/1352458513503053. [DOI] [PubMed]
- 127.Brandt AU, Oberwahrenbrock T, Kadas EM, Lagrèze WA, Paul F. Dynamic formation of macular microcysts independent of vitreous traction changes. Neurology. 2014;83(1):73–77. doi: 10.1212/WNL.0000000000000545. [DOI] [PubMed] [Google Scholar]
- 128.Balk LJ, Killestein J, Polman CH, Uitdehaag BMJ, Petzold A. Microcystic macular oedema confirmed, but not specific for multiple sclerosis. Brain J. Neurol. 2012;135:e226. [DOI] [PMC free article] [PubMed]
- 129.Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain J Neurol. 2012;135(6):1786–93. 10.1093/brain/aws098. [DOI] [PMC free article] [PubMed]
- 130.Barboni P, Carelli V, Savini G, Carbonelli M, La Morgia C, Sadun AA. Microcystic macular degeneration from optic neuropathy: not inflammatory, not trans-synaptic degeneration. Brain J Neurol. 2013;136(7):e239. 10.1093/brain/awt014. [DOI] [PubMed]
- 131.Bringmann A, Reichenbach A, Wiedemann P. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004;36(5):241–249. doi: 10.1159/000081203. [DOI] [PubMed] [Google Scholar]
- 132.Felix CM, Levin MH, Verkman AS. Complement-independent retinal pathology produced by intravitreal injection of neuromyelitis optica immunoglobulin G. J Neuroinflammation. 2016;13(1):275. doi: 10.1186/s12974-016-0746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reichenbach A, Bringmann A. Müller cells in the healthy and diseased retina. 1st ed. New York: Springer New York; 2010. [DOI] [PubMed]
- 134.Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013;61(5):651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- 135.Hokari M, Yokoseki A, Arakawa M, Saji E, Yanagawa K, Yanagimura F, et al. Clinicopathological features in anterior visual pathway in neuromyelitis optica. Ann Neurol. 2016;79(4):605–24. 10.1002/ana.24608. [DOI] [PubMed]
- 136.Yamamura T, Nakashima I. Foveal thinning in neuromyelitis optica: a sign of retinal astrocytopathy? Neurol Neuroimmunol Neuroinflamm. 2017;4(3):e347. doi: 10.1212/NXI.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dalmau J. Precision in neuroimmunology. Neurol Neuroimmunol Neuroinflamm. 2017;4(3):e345. doi: 10.1212/NXI.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tian D-C, Su L, Fan M, Yang J, Zhang R, Wen P, et al. Bidirectional degeneration in the visual pathway in neuromyelitis optica spectrum disorder (NMOSD). Mult Scler. 2017;1352458517727604. 10.1177/1352458517727604. [DOI] [PubMed]
- 139.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. 10.1002/ana.22366. [DOI] [PMC free article] [PubMed]
- 140.Dörr J, Wernecke KD, Bock M, Gaede G, Wuerfel JT, Pfueller CF, et al. Association of retinal and macular damage with brain atrophy in multiple sclerosis. PLoS One. 2011;6(4):e18132. 10.1371/journal.pone.0018132. [DOI] [PMC free article] [PubMed]
- 141.Azevedo CJ, Overton E, Khadka S, Buckley J, Liu S, Sampat M, et al. Early CNS neurodegeneration in radiologically isolated syndrome. Neurol Neuroimmunol Neuroinflamm. 2015;e102:2. doi: 10.1212/NXI.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Solomon AJ, Watts R, Dewey BE, Reich DS. MRI evaluation of thalamic volume differentiates MS from common mimics. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e387. doi: 10.1212/NXI.0000000000000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bakshi R, Yeste A, Patel B, Tauhid S, Tummala S, Rahbari R, et al. Serum lipid antibodies are associated with cerebral tissue damage in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;e200:3. doi: 10.1212/NXI.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tao Y, Zhang X, Zivadinov R, Dwyer MG, Kennedy C, Bergsland N, et al. Immunologic and MRI markers of the therapeutic effect of IFN-β-1a in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm.2015;2:e176. [DOI] [PMC free article] [PubMed]
- 145.Gabilondo I, Martínez-Lapiscina EH, Martínez-Heras E, Fraga-Pumar E, Llufriu S, Ortiz S, et al. Trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann Neurol. 2014;75(1):98–107. 10.1002/ana.24030. [DOI] [PubMed]
- 146.Raz N, Bick AS, Ben-Hur T, Levin N. Focal demyelinative damage and neighboring white matter integrity: an optic neuritis study. Mult Scler. 2015;21(5):562–571. doi: 10.1177/1352458514551452. [DOI] [PubMed] [Google Scholar]
- 147.Sinnecker T, Oberwahrenbrock T, Metz I, Zimmermann H, Pfueller CF, Harms L, et al. Optic radiation damage in multiple sclerosis is associated with visual dysfunction and retinal thinning—an ultrahigh-field MR pilot study. Eur Radiol. 2015;25(1):123–31. 10.1007/s00330-014-3358-8. [DOI] [PubMed]
- 148.Kuchling J, Brandt AU, Paul F, Scheel M. Diffusion tensor imaging for multilevel assessment of the visual pathway: possibilities for personalized outcome prediction in autoimmune disorders of the central nervous system. EPMA J. 2017;8(3):279–294. doi: 10.1007/s13167-017-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pache F, Zimmermann H, Finke C, Lacheta A, Papazoglou S, Kuchling J, et al. Brain parenchymal damage in neuromyelitis optica spectrum disorder—a multimodal MRI study. Eur Radiol. 2016;26(12):4413–22. 10.1007/s00330-016-4282-x. [DOI] [PubMed]
- 150.Finke C, Zimmermann H, Pache F, Oertel FC, Kramarenko L, Bellmann-Strobl J, et al. Visual network reorganization in neuromyelitis optica. JAMA Neurol. in press [DOI] [PMC free article] [PubMed]
- 151.Pfueller CF, Paul F, Pfueller CF, Paul F. Imaging the visual pathway in neuromyelitis optica, imaging the visual pathway in neuromyelitis optica. Mult Scler Int. 2011;e869814. [DOI] [PMC free article] [PubMed]
- 152.Saidha S, Al-Louzi O, Ratchford JN, Bhargava P, Oh J, Newsome SD, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol. 2015;78(5):801–813. doi: 10.1002/ana.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Saidha S, Sotirchos ES, Oh J, Syc SB, Seigo MA, Shiee N, et al. Relationships between retinal axonal and neuronal measures and global central nervous system pathology in multiple sclerosis. JAMA Neurol. 2013;70(1):34–43. doi: 10.1001/jamaneurol.2013.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zimmermann H, Freing A, Kaufhold F, Gaede G, Bohn E, Bock M, et al. Optic neuritis interferes with optical coherence tomography and magnetic resonance imaging correlations. Mult Scler. 2013;19(4):443–50. 10.1177/1352458512457844. [DOI] [PubMed]
- 155.Finke C, Heine J, Pache F, Lacheta A, Borisow N, Kuchling J, et al. Normal volumes and microstructural integrity of deep gray matter structures in AQP4+ NMOSD. Neurol Neuroimmunol Neuroinflamm. 2016;e229:3. [DOI] [PMC free article] [PubMed]
- 156.Kremer S, Renard F, Achard S, Lana-Peixoto MA, Palace J, Asgari N, et al. Use of advanced magnetic resonance imaging techniques in neuromyelitis optica spectrum disorder. JAMA Neurol. 2015;72(7):815–22. 10.1001/jamaneurol.2015.0248. [DOI] [PMC free article] [PubMed]
- 157.Kim HJ, Paul F, Lana-Peixoto MA, Tenembaum S, Asgari N, Palace J, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology. 2015;84(11):1165–73. 10.1212/WNL.0000000000001367. [DOI] [PMC free article] [PubMed]
- 158.Filippi M, Rocca MA, Ciccarelli O, De Stefano N, Evangelou N, Kappos L, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016;15(3):292–303. 10.1016/S1474-4422(15)00393-2. [DOI] [PMC free article] [PubMed]
- 159.Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi: 10.1155/2013/398259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sinnecker T, Dörr J, Pfueller CF, Harms L, Ruprecht K, Jarius S, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79(7):708–714. doi: 10.1212/WNL.0b013e3182648bc8. [DOI] [PubMed] [Google Scholar]
- 161.Ventura RE, Kister I, Chung S, Babb JS, Shepherd TM. Cervical spinal cord atrophy in NMOSD without a history of myelitis or MRI-visible lesions. Neurol Neuroimmunol Neuroinflamm. 2016;3(3):e224. doi: 10.1212/NXI.0000000000000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kornberg MD, Newsome SD. Unmasking and provoking severe disease activity in a patient with NMO spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2015;2(2):e66. doi: 10.1212/NXI.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Steinman L, Bar-Or A, Behne JM, Benitez-Ribas D, Chin PS, Clare-Salzler M, et al. Restoring immune tolerance in neuromyelitis optica: part I. Neurol Neuroimmunol Neuroinflamm. 2016;e276:3. doi: 10.1212/NXI.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bar-Or A, Steinman L, Behne JM, Benitez-Ribas D, Chin PS, Clare-Salzler M, et al. Restoring immune tolerance in neuromyelitis optica: part II. Neurol Neuroimmunol Neuroinflamm. 2016;e277:3. doi: 10.1212/NXI.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cree BA, Bennett JL, Sheehan M, Cohen J, Hartung H-P, Aktas O, et al. Placebo-controlled study in neuromyelitis optica—ethical and design considerations. Mult Scler. 2016;22(7):862–872. doi: 10.1177/1352458515620934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Paul F. Hope for a rare disease: eculizumab in neuromyelitis optica. Lancet Neurol. 2013;12(6):529–531. doi: 10.1016/S1474-4422(13)70089-9. [DOI] [PubMed] [Google Scholar]
- 167.Weinshenker BG, Barron G, Behne JM, Bennett JL, Chin PS, Cree BAC, et al. Challenges and opportunities in designing clinical trials for neuromyelitis optica. Neurology. 2015;84(17):1805–15. 10.1212/WNL.0000000000001520. [DOI] [PMC free article] [PubMed]
- 168.Cadavid D, Balcer L, Galetta S, Aktas O, Ziemssen T, Vanopdenbosch L, et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16(3):189–99. 10.1016/S1474-4422(16)30377-5. [DOI] [PubMed]
- 169.Sühs K-W, Hein K, Sättler MB, Görlitz A, Ciupka C, Scholz K, et al. A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann Neurol. 2012;72(2):199–210. 10.1002/ana.23573. [DOI] [PubMed]
- 170.Knier B, Schmidt P, Aly L, Buck D, Berthele A, Mühlau M, et al. Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain J Neurol. 2016;139(11):2855–63. 10.1093/brain/aww219. [DOI] [PubMed]
- 171.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation—EPMA position paper 2016. EPMA J. 2016;7(1):23. 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed]
- 172.Staurenghi G, Sadda S, Chakravarthy U, Spaide RF. International Nomenclature for Optical Coherence Tomography (IN•OCT) Panel. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN•OCT consensus. Ophthalmology. 2014;123(8):1572–1578. doi: 10.1016/j.ophtha.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 173.Schematic Figure –Retina (Creative Commons License) – Neurodiagnostics Laboratory [Internet]. [cited 2017Oct 6]. Available from:http://neurodial.de/2017/08/25/schematic-figure-retina-creative-commons-license/