Abstract
The paper is motivated by severe concerns regarding currently applied care of the pregnancy-associated breast cancer (PABC) characterised by particularly poor outcomes of the disease. Psychological and ethical aspects play a crucial role in PABC: the highest priority not to damage the foetus significantly complicates any treatment generally, and it is quite usual that patients disclaim undergoing any breast cancer treatment during pregnancy. Although, due to global demographic trends, PABC is far from appearing rarely now, severe societal and economic consequences of the disease are still neglected by currently applied reactive medical approach. These actualities require creating new strategies which should be better adapted to the needs of the society at large by advancing the PABC care based on predictive diagnostic approaches specifically in premenopausal women, innovative screening programmes focused on young female populations, targeted prevention in high-risk groups, and optimised treatment concepts. The article summarises the facts and provides recommendations to advance the field-related research and medical services specifically dedicated to the PABC care.
Keywords: Predictive preventive personalised medicine, Breast cancer, Pregnancy, Risk assessment, Multi-level diagnostics, Recommendations
Motivation of the paper and inclination of new concepts in PABC care
Severe concerns regarding the pregnancy-associated breast cancer (PABC) are not really a new issue in medical care. Already by the middle of previous century, the field-dedicated physicians have addressed the problem providing clear evidence for particularly poor outcomes of the disease and stets pessimistic prognosis [1]. Since almost seven decades, the demographic profile of current populations underwent sufficient changes, and the problem, which has been rather neglected that time, now appears to provoke severe consequences and, therefore, attracts much more attention by medical, social, and economic fields of the society at large. What are exactly the main issues? Let us summarise the facts.
Early twenty-first century is characterised by the breast cancer epidemic: about two million of new cases and a half of million disease-related deaths are registered annually [2]; this actuality prompts to reconsider currently applied strategies in breast cancer management, generally.
Specifically, PABC represents a particularly aggressive type of breast cancer with poor individual outcomes [3–6].
Psychological and ethical aspects play a crucial role in PABC: the highest priority not to damage the foetus significantly complicates any treatment approach in pregnancy generally. In early pregnancy (first trimester), its termination in case of PABC is usual but not a trivial decision. Furthermore, it happens frequently that patients disclaim undergoing any breast cancer treatment during their pregnancy. Consequent postpartum breast cancer (BC) treatment is more costly but less effective, due to advanced stages of the disease [7–9].
Delayed diagnosis and treatment (if any) in combination with particularly aggressive cancer type result in dramatically decreased overall survival in PABC patient cohort [10].
No any population (screening) programme, no generally accepted risk factors, no standardised diagnostic approach, and no targeted preventive measures dedicated specifically to the PABC are currently established [4], perhaps due to persisting consideration of PABC as a rare disease with minor impacts to the society.
However, PABC is far from appearing rarely now: its incidence is rapidly increasing up to 1:3000 of all pregnancies and more, that by about 250 million pregnancies registered annually [11] may comprise around 100,000 PABC new cases per year worldwide. Moreover, according to the currently available statistics, about a half of all unintended pregnancies ends in abortion. In turn, an induced abortion is a strong causal factor for breast cancer development, therefore, increasing the real portion of PABC by “masked” cases. To this end, in some cohort studies, the PABC portion is considered to represent up to 20% and more of all breast cancer cases [12].
Specifically in Western countries, relatively high and stets increasing age at first gestation, on the one hand, is the characteristic attribute of their current demographic profiles; on the other hand, it represents one of the best acknowledged risk factors of PABC (for women older than 30 years of age) [10, 13].
The above listed facts require creating new strategies which should be adapted to the needs of the society at large by advancing the PABC care based on innovative predictive approaches, dedicated screening programmes, targeted prevention, and optimised treatment concepts. Below provided chapters create a robust platform for that.
The definition of pregnancy-associated breast cancer
PABC was originally understood as carcinoma of the breast in pregnant and breastfeeding patients. It was defined either as the breast cancer (BC) diagnosed during pregnancy or pregnancy occurred during the disease treatment [14]. Due to rapidly increasing knowledge about PABC aetiology and pathomechanisms, the definition of PABC underwent several modifications: from earlier “BC occurring during gestation and/or within one year after childbirth” [15, 16], and, later on, considering a sufficiently prolonged time-frame up to 2 years [17, 18] and longer after the postpartum [19]. Due to completely different treatment approaches applied, the subdivision into “breast cancer in pregnancy” and “postpartum breast cancer” is broadly practiced. Although both terms emphasise the doubtlessly important time point of diagnosis, they address neither the time frame of the disease development (that means unclear risk factors) nor the mechanism of the disease progression which are crucial for the disease prediction, personalised treatment, and prognosis.
Epidemiology of pregnancy-associated breast cancer
PABC incidence is rapidly rising from 1:10,000 registered at the beginning of the millennium to the currently registered 1:3000 of all pregnancies [16, 20–22]. The median patient’s age at diagnosis has been reported by some studies at the level of 33 years [23]. Importantly, the portion of PABC amongst all BC cases appears up to more than 15% in women below 35 years of age [24]. An increasing incidence of PABC affects both developed and developing countries. Specifically in developed countries, a rapidly increasing PABC incidence is currently demonstrated for a number of countries and regions. Hence, a large population study performed in Sweden showed that the number of PABC cases doubled from 16.0 up to 37.4 cases per 100,000 deliveries within the time frame 1963–2002 [22]. As detailed below, the age over 30 years at first gestation is one of the best acknowledged risk factors of PABC [10, 13]. There are several reasons which strongly motivate women to postpone their first delivery such as advanced education and career interests. According to the UN’s annual Human Development Report (2016), percentage of female population with at least some secondary school education was documented as 100% in Canada, 89.1% in Denmark, 87.8% in Sweden, 69.8% in China, and 35.8% in India [25]. Overall, developed countries demonstrate in average 88.4% of their female populations against 51.7% in developing countries who have been educated at the secondary school. Therefore, in average, women are better educated and have higher chances receiving jobs in developed countires that may significantly enhance their age at first childbearing and, consequently, impact the PABC incidence as discussed below.
Pathophysiology of pregnancy-associated breast cancer
Several key players and molecular mechanisms driving PABC development and progression have been already identified. They demonstrate synergetic effects with each other potentiating their individual impacts and including pregnancy-specific hormonal profiles and alterations of the immune systems during pregnancy as well as transformation of the breast tissue during and after both—the pregnancy and breastfeeding [16] as detailed below.
PABC supportive hormonal profiles during pregnancy
There is a meaningful transformation in the entire hormonal profile of the maternal organism during pregnancy and lactation period including elevated levels of circulating oestrogen, progesterone, and a great number of growth factors. These molecular profiles (described in below provided subchapters) are essential for the physiologic foetal development but, on the other hand, they also effectively promote a particularly quick tumour progression from pre-lesions and early stage of malignant transformation into advanced cancer and aggressive metastatic disease [26–30].
Compromised immune tolerance during pregnancy
Significant changes described for the immune reactivity as specific for pregnancy may deliver an important contribution to the PABC development and progression [31]. One of the primary functions of the human foetal placenta is to promote the tolerance of the maternal organism against the semi-allogeneic foetus. Consequent pregnancy-specific modulation of both innate and adaptive immune function ensures that the embryo safely escapes removing from the maternal body [32–34]. However, as the negative side-effect of the provisional pregnancy-associated tolerance, malignant cells more easily overcome a confrontation with the immune system and may maintain for further proliferation [31]. In addition to that, pro/inflammatory processes triggered in the breast tissue during the postpartum and post-lactation period may effectively contribute to the PABC development and progression [15, 35].
Under compromised immune tolerance during pregnancy, further investigations are needed to clarify the possibility of a metastatic spread specifically to the placenta thoroughly investigating the neonate for potential metastases, in case they are detected in the villous placental system.
Breast tissue involution after delivery and discarded breastfeeding
The PABC development is considered to be, possibly associated with the process of breast involution that physiologically occurs after the pregnancy and lactation period [36, 37]. During this process, the fully differentiated functional mammary gland regresses back into the immature, pre-pregnant state. Such tissue transformation is extremely complex at both - molecular and cellular levels including extensive apoptosis of the epithelial cells, rigorous stromal remodelling, adipogenesis, and activation of inflammatory response accompanied by comprehensive alterations in a number of molecular pathways demonstrating great similarity with wound healing mechanisms, which, if being impaired, generate pro-oncogenic microenvironment [35, 38–42].
Clinical picture of PABC
It is important to know the stage of the disease, patient’s decision on completing the pregnancy, age at the gestation as well as integrity of the foetus, in order to adequately consider the treatment options during pregnancy [43]. The main challenge in applying the life-saving therapy for the mother is to avoid any life-threatening harm on the foetus. Therefore, it is essential to know the exact clinical behaviour along with the biology of PABC to apply the most efficient individualised treatment for each patient [44].
Generally, PABC appears more aggressive compared with the age-matched non-PABC. The tumours are often diagnosed in more advanced stages, with larger tumour mass, higher grade, evident lympho-vascular invasion, more frequent nodal involvement, and distant metastases [3–6, 45–47]. Advanced disease stages, in addition to more aggressive tumour phenotype, can be well explained by the substantial time delay in PABC diagnosis [15, 19, 48, 49]. The definitive PABC diagnosis is often delayed by 1 up to 13 months after the appearance of typical symptoms such as lump, pain, nipple discharge, and skin change, amongst others [4]. The early signs of cancer are frequently neglected by explaining their appearance to be physiological transformation of the breast tissue related to pregnancy and lactation. Also the physiological increase of breast density hinders breast cancer diagnostics by a conventional imaging approach. Finally, in contrast to the 40+-year-old female subpopulations, there are no specialised breast screening programmes adapted to the specifics and needs of young women in child-bearing age that automatically exclude this particularly PABC relevant population from standardised and routinely performed BC diagnostics. All the above listed aspects hinder predictive medical services and cost-effective preventive measures which are crucial for an effective combating PABC in the society at large.
Pathophysiological characteristics of PABC
Pathophysiological characteristics of PABC are extensively under investigation. Invasive ductal carcinoma is the most frequently occurring histological type observed in 75–90% of cases, followed by invasive lobular and rarely inflammatory subtypes of breast carcinoma [50]. Current PABC treatment strategies rely on the protein expression status of oestrogen and progesterone receptors and human epidermal growth factor (HER2/neu) [51].
Expression levels of oestrogen and progesterone receptors are often reduced in PABC
The expression of oestrogen (ER) and/or progesterone (PR) receptors is often reduced in PABC against the BC patient cohort in general. Hence, investigations performed in 797 PABC versus 4177 age-matched non-PABC patients revealed significantly bigger portions of ER-negative (39.3 vs. 28%, P < 0.01) and PR-negative tumours (39.7 vs. 28.4%, P < 0.01) in PABC [3]. In consensus, two other groups demonstrated sufficiently higher frequency of ER- (54.4 vs. 37.4%, P = 0.02; 59 vs. 31%, P < 0.001) and PR-negative tumours (54.6 vs. 26.9%, P = 0.0001; 72 vs. 40%, P < 0.001) in PABC [52]. Several other studies have demonstrated similar findings [4, 53, 54].
Expression levels of HER2/neu are unremarkable in PABC
In general, although the pathological activation of oncogenes is involved in tumour development, their physiological activation during human ontogenesis is associated with normal foetal development. Of note, overexpression of HER2/neu is observed in physiologic placenta and foetal epithelial cells. Furthermore, the expression levels of p105 (proteolytic breakdown product corresponding to the extracellular domain of oncoprotein p185) in blood serum show a significant decrease in the first and second trimesters, whereas there is a significant increase during the third trimester in normal pregnancies [55]. A very recent study which has involved 344 PABC and 668 non-PABC patients demonstrated a parity in HER2-positive tumours for both groups (20.1 vs. 19.3% respectively, P = 0.52) [4]. This finding is sustainable by consensus with other groups [45].
Gene expression patterns characteristic for PABC
Multiomic approach has been demonstrated as particularly useful in detecting gene expression patterns specific for PABC compared to non-PABC patients: the approach includes genomics, epigenomics, metabolomics, and miRNA panels, amongst others [16]. The substantial increase in invasiveness and aggressiveness of PABC epithelial cells was observed, as these cells experienced gain in copy numbers of genes associated with morphogenesis, angiogenesis, and metastasis, and loss in copy numbers of genes for tumour suppressors, cell adhesion, and macromolecular complex assembly or intra-cellular trafficking [56]. More recently, highly specific gene expression patterns have been identified in PABC compared to non-PABC patients [57]. Noteworthy, the specific molecular setup detected could persist in breast tissue up to 10 years after postpartum. Authors concluded that the molecular signature identified was best attributable to the triple-negative BC subtype which is highly aggressive and the most frequently linked to the PABC.
Risk factors identified for PABC
There are several major conditions clearly associated with PABC development as summarised in Table 1 including family history, specific syndromes, early age at menarche, higher age at first pregnancy, too short or no breastfeeding, and abnormal body mass index, amongst others [16]. These factors are per evidence linked to an increased risk of PABC as demonstrated or proposed by a number of studies discussed below.
Table 1.
Risk factors of PABC
| Risk factors | Significance | Reference |
|---|---|---|
| Family history of breast cancer | Increased risk of PABC ≤ 2 years postpartum (HR 3.28, P = 0.001) | [12] |
| Genetic predisposition | BRCA1/2 carriers in PABC (25 vs. 11.5%, P = 0.034) | [12, 58] |
| Age at first pregnancy > 30 years old |
5.3% increased risk of PABC per each year postponing pregnancy after 25 years of age | [59] |
| No long-term BC protection from 1st pregnancy after age 35 years | [60] | |
| Early age at menarche | Increased risk of PABC with early age at menarche (< 13 vs. ≥ 13 years) (HR 2.165, P < 0.001) | [4] |
| Little-to-no breastfeeding | 4.3% decreased lifetime risk of BC by each 12 months of breastfeeding (P < 0.0001) | [61] |
| Decreased risk of BC with breastfeeding > 12 months (HR 0.74, P < 0.001) | [62] | |
| Overweight | Overweight women are at higher risk of PABC (HR 1.427, P = 0.011) | [4] |
Family history of breast cancer
Family history of BC is one of the best acknowledged risk factors for the development of the breast malignancies generally [2, 63]. BC family history has been identified as an independent risk factor for the PABC: in the large-scale study including 1715 premenopausal women, BC family history was associated with an increased risk of PABC up to 2 years after delivery (HR 3.28; 95%CI 1.05–10.3, P = 0.001) [12], although the contrary results have to be mentioned [64]. Further, BRCA1 and/or BRCA2 mutation carriers are per evidence at high risk for the familial BC [65, 66]. However, whether the inherited BRCA1/2 mutations do increase the PABC risk remains inconclusive [12, 67].
First pregnancy after 30 years of age
Strong evidence is provided that the first pregnancy after 30 years of age increases the risk of PABC and BC in general [15, 22, 68]. The age at first delivery is an important risk factor amongst parous women aged over 25 years: postponing the childbirth by 1 year each is associated with 5.3% increased risk of BC development [59]. This result is well in consensus with other groups concluding that specifically in Western countries, relatively high and stets increasing age at first gestation is a dangerous trend of the demographic profiles [15, 60, 68]. In contrast, no any link between the BC risk and mother’s age at the second or further births has been found.
Early age at menarche
The long period of time between the first menstrual bleeding (menarche) and the first full-term pregnancy is a well-known risk factor for the BC carcinogenesis [69, 70]. The prolongation of this time period leads to the substantial increase in hormonal stress resulting in the development of pre-cancerous sites in the breast tissue and thus the more pronounced predisposition to BC later in life [2]. The early age at menarche (below 13 years) is clearly associated with an increased risk of PABC development (HR 1.762, 95%CI 1.322–2.348, P < 0.001) [4].
Little-to-no breastfeeding
A number of studies identified important protective effects of breastfeeding and its extended duration against the BC in general and PABC specifically. In contrast, little-to-no breastfeeding was associated with a significantly increased risk specifically of the triple-negative (but not other types) BC amongst women aged ≤ 56 years [71]. In consensus, a large-scale comparative study including 1424 BC patients against 2022 BC-free controls who experienced an increased number of breastfed children and increased number of months of breastfeeding per child has clearly demonstrated that an extended duration of breastfeeding reduces specifically a risk of the basal-like BC (but not other subtypes) [72]. Recent meta-analysis showed that mothers who breastfed for more than 12 months had a 26% decreased risk of BC compared to not breastfeeding women (50 studies; HR 0.74, 95%CI 0.69–0.79, P < 0.001) [62]. Another meta-analysis has included over 50,000 BC patients and almost 100,000 BC-free controls demonstrated the relative lifetime risk of BC to be decreased by 4.3% (P < 0.0001) for every 12 months of breastfeeding [61]. Noteworthy, the women with a total of ≥ 55 months of the lifetime breastfeeding do disease significantly rarely on BC compared to the parous women with no breastfeeding (HR 0.73, P = 0.049). In conclusion, an extended breastfeeding is a natural and very effective protection against BC generally and against PABC specifically.
Overweight
Abnormally increased body mass index (BMI) is observed more frequently in PABC compared to non-PABC patients. To this end, it is important to note that BMI standard ranges may vary between populations depending on the genetic predisposition, traditional nutrition, and climate, amongst others. Hence, the recent study performed in South Korea has indicated BMI ≥ 23.0 kg/m2 as overweight for their female population with consequently increased risk of PABC (HR 1.427, 95%CI 1.086–1.876, P = 0.011) [4]. Abnormally high portion of the fat tissue provokes BC relevant hormonal dysregulation, increased oestrogen production, and increased blood levels of growth factors such as the insulin-like growth factors (IGF1, IGF2, and IGF3) and pro-inflammatory processes that synergistically promote pro-oncogenic alterations in maternal organism during pregnancy leading to PABC development and progression [29, 73].
Modifiable breast cancer risk factors potentially relevant to PABC
Since cancerous pre-lesions may appear in teenager age [1], the above described pregnancy-associated pro-oncogenic conditions generate highly “fertile” environment for the transformation of preexisting lesions into aggressive breast cancer and quickly progressing metastatic disease. Contextually, all known risk factors specific for the youngest female populations are highly relevant to PABC development and progression. BC risk factors have been comprehensively overviewed [2, 74]; however, the majority of them have not been investigated in the context of PABC yet. Here we analyse their relevance for PABC as summarised in Table 2.
Table 2.
Breast cancer risk factors potentially relevant to PABC
| Risk factors | Significance | Reference |
|---|---|---|
| Smoking cigarettes | 21% increased risk of BC by smoking more than 40 cigarettes per day | [75–77] |
| 57% increased risk of BC by smoking longer than 40 years | ||
| Strong association with BC, if smoking for 5 years (or longer) before the first full-term pregnancy | ||
| Alcohol consumption | Regular alcohol consumption associated with 16.4% of all breast cancers | [75] |
| Most critical in the period of time between menarche and the first full-term pregnancy | [78] | |
| Physical inactivity and sedentary lifestyle | Associated with 3.9% of all breast cancers | [75] |
| 20% lower risk of BC amongst physically active women | [79] | |
| Rotating shift and night work | Increased risk of BC, particularly amongst shift worker during adolescence | [80–84] |
| Occupation as a flight attendant | Increased risk of BC (in addition to an increased risk of melanoma) | [85–87] |
| Abnormally low BMI | Increased risk of metastatic BC with poor outcomes compared to the standard (20–25 kg/m2) range BMI | [88] |
| Diabetes mellitus | Acknowledged risk factor of BC and poorer outcomes | [89, 90] |
| Increased risk of BC by 2 and more pregnancies with gestational diabetes | [91] | |
| Flammer syndrome (FS) | FS-affected females are at higher risk specifically for aggressive subtypes of BC and metastatic disease | [92–94] |
Synergic effects by patho/physiological factors and detrimental societal trends
Cumulative risks by early menarche, long time period between menarche and the first full-term pregnancy, and low number of full-term pregnancies
Early age at menarche (e.g. due to increased protein and fat percentage in the dietary composition) and older age at first delivery (altered occupational exposure such as professional career) both are clearly associated with the development of BC generally and PABC specifically as described above. This condition is new, being typical for the early twenty-first century compared to the past [22, 95]. Further, low number of full-term pregnancies (due to ubiquitous application of contraceptives and about 50% abortion rates in unintended pregnancies—both strong risks of BC) is currently the detrimental trend. Longer period of time between early menarche and first full-time pregnancy together with low number of full-term pregnancies result in dramatically increased lifelong total number of menstrual cycles and ovulations in affected women. Cumulatively, this leads to highly increased hormonal stress, consequent early development of cancerous lesions in breast tissue getting, further, promoted into aggressive PABC by pregnancy, and follow-up processes as described above.
Physical inactivity and sedentary lifestyle
Physical inactivity and sedentary lifestyle is highly prevalent in childhood and adolescence with clear predominance in Western countries. This condition has adverse health effects and significantly increases BC risks in a multi-factorial way [75, 79, 96]. Various mechanisms with synergic effects have been proposed such as premature ageing, obesity-related hormonal dysregulation, altered insulin sensitivity, inflammation, and increased cytokine and oestrogen production, amongst others. Contextually, it was found that regular physical activity reduces exposure to sex hormones and activates specific tumour suppressor genes [97, 98]. These protective effects are strongly beneficial against PABC development.
Risky prolactin initiation linked to abortion and short intervals between pregnancies
In contrast to the protective effects of breastfeeding, elevated levels of the milk-producing hormone prolactin are associated with a significant increase in BC risk [99]. Beginning with the 8th weeks onwards pregnancy, prolactin levels do rapidly increase. The joint effects of high prolactin and progesterone levels may initiate abnormal cellular changes with a strong potential for BC development that is particularly relevant to the pregnancy-associated malignant breast tissue transformation in the case of abortion, when the pregnancy is not followed by the consequent breastfeeding. Further, too short time intervals between two pregnancies, especially first and second ones, result in joint stimulatory effects by oestrogen, progestogen, and prolactin leading to the malignant transformation of epithelial breast cells [99]. A prospective cohort study on 377 BC patients demonstrated that high concentration of plasma prolactin might increase BC risk regardless the menopausal and hormone receptor status [100].
External risk factors
Environmental risk factors
The higher BC incidence amongst affected populations was observed in various studies [1] evaluating environmental risk factors such as an industrial air pollution [101], toxic environmental contamination with heavy metals and polychlorinated biphenyl [102], drinking water and food contamination [103], ionising radiation exposure [104–106], tobacco smoke [76, 77], or nutritional risk factors (such as excessive alcohol consumption) [107–109]. These factors could be highly relevant to the PABC development, due to rapidly increasing number of children and young adults, who are frequently or even permanently exposed to the toxic environment.
Smoking cigarettes before the first full-term pregnancy
Tobacco smoke is the well-acknowledged risk factor of BC incidence and mortality [75–77]. Recent cohort study conducted on 89,835 women by Canadian National Breast Screening programme showed that the total duration of smoking as well as the number of cigarettes per day used is significantly associated with an increased risk of BC [76]. Women smoking more than 40 cigarettes per day demonstrate 21% increased risk and women smoked more than 40 years are at 57% increased risk of BC versus non-smokers. Particularly relevant to the PABC development might be the smoking exposure in early (teenager) age and 5 years or longer before the first full-term pregnancy [1].
Alcohol consumption
Recently performed large-scale epidemiological study including more than 1.5 million cancer patients has clearly demonstrated that a regular alcohol consumption results in development of 6.4% of all female malignancies and 16.4% of all BCs [75]. Thereby, several mechanisms are related to BC risks such as an oxidative stress and toxic effects by hormonal dysregulation and triggered carcinogenesis [108]. Particularly relevant to PABC, the alcohol intake is most critical for breast cancer development in the time period between menarche and first full-term pregnancy [1]. Alcohol consumption in this period, adjusted for drinking after first pregnancy, was associated with significantly higher risk of BC (HR 1.11 per 10 g/day intake, 95%CI 1.00 to 1.23, P = 0.01) [78]. This risk might be, further, potentiated by a longer period of time between menarche and the first full-term pregnancy that is an acknowledged risk factor of PABC as discussed above.
Professional occupation-related risk factors
Rotating shift and night work
Rotating shift and night work is associated with a number of adverse health effects, syndromes, and disorders such as fatigue, anxiety, depression, sleep disorder, digestive and metabolic disorders, and cardiovascular diseases as well as cancer [110–112]. Several mechanisms have been suggested as elevating BC risks in rotating shift and night workers including the suppression of melatonin and vitamin D synthesis, disruption of circadian rhythm, suppressed immune system, and sleep deprivation [80–83]. Recent large-scale analysis including more than 190,000 women with long-term follow-up medical records clearly showed that the rotating night shift work is associated with an increased BC risk, particularly significant for persons performing shift work in adolescence [84] that might be of particular relevance for the PABC development.
Flight attendants and elevated BC risk
Women working as a flight attendant are at higher BC risk (in addition to an increased risk of melanoma) that has been concluded mainly by population-based studies [85–87]. Specific mechanisms are supposed to include an exposure to the cosmic radiation, circadian rhythm and sleep disruption. However, recent studies emphasise the crucial role of coincidence of contributing risk factors such as reproductive aspects (older age at first pregnancy and lower parity), amongst others [113]. Collectively, they may potentiate PABC risks—the connections which have not been investigated so far.
Collateral syndromes and disorders potentiating PABC risks
Metabolic syndrome
Diabetic history is a well-acknowledged risk factor of BC development as well as poorer individual outcomes compared to the general population and non-diabetic BC patients, respectively [89, 90]. Metabolic syndrome is characterised by increased levels of growth factors and inflammatory processes associated with BC development, progression and aggressive metastatic disease [89, 114]. Although association of gestational diabetes with BC risk remains controversially discussed [115], recent studies report two and more pregnancies complicated by gestational diabetes as clearly associated with an increased BC risk (HR 1.68, 95%CI 1.15–2.44) [91]. Due to the rapidly increasing rates of diabetes mellitus type 2 in teenager populations and youth [116], its relevance for PABC as the collateral pathology potentiating the risks has to be considered.
Abnormal BMI: high versus low values
Abnormally high BMI has been demonstrated to increase PABC risks by several pathomechanisms presented in the above chapters [4]. Abnormally low BMI is less investigated in the context of BM. However, the large-scale meta-analysis performed basing on 82 studies with altogether over 200,000 BC patients demonstrated that underweight women (BMI < 20) are at higher BC risk and related mortality compared to the standard BMI (20–25) range [88]. Since the so-called eating disorders such as anorexia nervosa are relatively common especially in teenagers and young females [117, 118], their relevance for PABC risks should get thoroughly investigated in follow-up studies.
Flammer syndrome and PABC: facts and hypotheses
Strong evidence provided recently demonstrates that systemic hypoxia is the generator and promotor of the cancer-friendly microenvironment [119]. On the other hand, Flammer syndrome (FS), a well-described suboptimal health condition [120], is known to predispose affected individuals to the systemic hypoxia with its clinical onset early in life (teenager age) [121]. Symptoms and molecular patterns specific for the systemic hypoxia can be objectively monitored and measured in the FS-affected individuals utilising nailfold capillary microscopy, imaging tool, and multiomic approach utilising blood samples [122]. Systemic hypoxia has been identified as one of the key drivers of the aggressive BC subtypes and metastatic disease [74, 119, 123]. Consequently, young women affected by FS are at the meaningful risk to develop quickly progressing BC [123], that has been confirmed by a series of the topic dedicated studies [92–94] concluding the FS relevance for particularly aggressive BC subtypes such as the triple-negative breast cancer which is typical for PABC patient cohort. Currently, there is no any evidence provided for the FS incidence in the PABC patient cohort. However, according to the above summarised facts, we hypothesised that FS phenotyping in young female populations might be extremely useful for innovative screening programmes specifically dedicated to selection of persons at high risk for the PABC development.
Conclusions and expert recommendations
Early twenty-first century is characterised by the breast cancer epidemic. Specifically, PABC represents a particularly aggressive type of breast cancer with poor individual outcomes. Delayed diagnosis and treatment of PABC dramatically decrease overall survival of the affected women. It happens quite frequently that patients disclaim undergoing any cancer treatment during their pregnancy. Consequent postpartum BC treatment is more costly but less effective, due to advanced stages of the disease. PABC is far from appearing rarely now: its incidence is rapidly increasing up to 1:3000 of all pregnancies and even more; moreover, a big portion of the PABC cases are “masked” by abortions that further increase the PABC contribution to the breast cancer aetiology. Specifically in Western countries, relatively high and stets increasing age at first gestation is the characteristic attribute of the demographic profile being also one of the best acknowledged risk factors of PABC (women over 30 years of age).
All these facts and actualities are known. However, our society is currently not skilled to effectively combat the PABC with all ethical, social, and economic consequences negatively impacting healthcare and the society at large in a long-term manner. No any population (screening) programme, no generally accepted risk factors, no standardised diagnostic approaches, and no targeted preventive measures specifically focused on combating the PABC are currently established. The appropriate measures can happen only if new concepts advancing medical devices in the overall PABC management will be created—see the below provided recommendations.
Recommendations
The dimension of currently underestimated PABC prevalence should be reconsidered, since the PABC causal contribution to the overall breast cancer aetiology in the reality appears to be much higher than it is traditionally assumed.
Innovative screening programmes should be adapted to the needs of young populations (teenagers, youth) relevant to PABC. To this end, imaging modalities (ultra-sound and MRI diagnostics) are effective for early breast cancer diagnosis and treatment prior to a planned pregnancy.
Phenotyping of predisposed individuals such as FS-affected individuals might be of great importance to start with, e.g. applied to teenagers by introducing dedicated questionnaires at the level of primary care (general practitioners).
Multilevel diagnostics including family history, questionnaires, imaging tools, and multiomic approach analysing predisposition and disease-specific molecular patterns (saliva and blood samples) are essential for predictive medical approaches applied to the persons at high risk for premenopausal breast cancer. This approach is strongly recommended specifically for planned pregnancies, in order to avoid a clinical manifestation of the PABC by targeted treatments. Hence, an innovative diagnostic approach has been most recently proposed for the premenopausal triple-negative breast cancer relevant to the PABC manifestation [124].
Preventive measures tailored to the patient, if based on the individualised diagnostic approach, will save lives and improve the cost-effectiveness of medical services in the area.
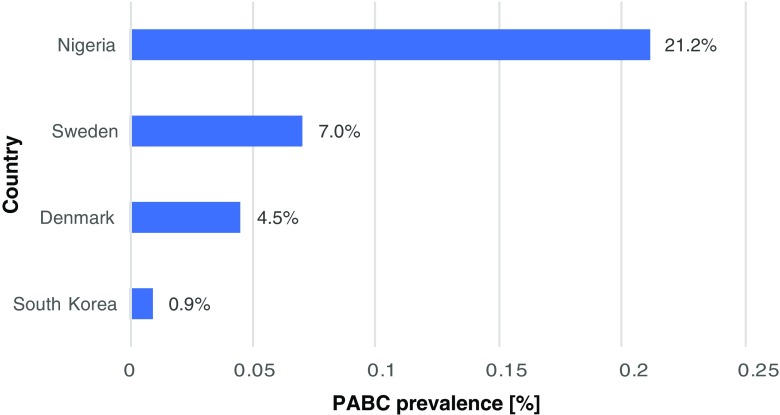
In conclusion, Fig. 1 demonstrates huge deviations in PABC prevalence recorded worldwide. Certainly, the difference is primarily attributed to particularities of individual populations as discussed above. However, an adequate PABC definition has to be consolidated as stated in this chapter. Due to the emergency impacting the society at large, the authors emphasise the necessity and urgency of the measures listed above.
Fig. 1.
The diagram demonstrates a big range in PABC prevalence recorded worldwide. The earliest study (in years 1963–2002) performed in Sweden based on The Swedish Cancer Register and Swedish Multi-Generation Register considering premenopausal women aged between 15 and 44 years old demonstrated the 7% prevalence of PABC defined as the breast cancer diagnosed during pregnancy and up to 2 years of postpartum [22]. The population-based study (in years 1977–2006) performed in Denmark based on Danish Cancer Registry considering premenopausal women aged between 15 and 44 years old demonstrated 4.5% prevalence of PABC defined as the breast cancer diagnosed during pregnancy and up to 1 year of postpartum [125]. The large-scaled study (in years 1999–2013) performed in South Korea based on Korean Breast Cancer Registry (102 hospitals) considering premenopausal women aged between 20 and 45 years old demonstrated 0.9% PABC prevalence defined as breast cancer (invasive ductal carcinoma, invasive lobular carcinoma, ductal carcinoma in situ) diagnosis during pregnancy up to 1 year postpartum [4]. The large-scaled study (in years 1998–2011) performed in Nigeria based on the database of the University College Hospital in Ibadan considering premenopausal women aged between 21 and 50 years old demonstrated 21.2% PABC prevalence defined as breast cancer diagnosis during pregnancy up to 2 years postpartum; this study specifically fixed the following inclusion criteria for matching the PABC versus non-PABC patients for comparison: age at menarche, parity, age at the first live birth, duration of breastfeeding, and abortion [12]
Authors’ contribution
OG is the project coordinator who has created the main scientific concepts presented in the manuscript. JP Jr. has performed the literature search, analysed the data, and drafted the manuscript. IA has contributed to the literature search, data collection and analysis and concepts’ development. OG and JP Jr. have designed the final version of the manuscript.
Funding information
This work was supported by the Charles University Research Fund (Progres Q39), by the MH CZ - DRO (Faculty Hospital Plzen - FNPl, 00669806), and by the National Sustainability Program I (NPU I) Nr. LO1503 provided by the Ministry of Education Youth and Sports of the Czech Republic. The authors thank the European Association for Predictive, Preventive and Personalised Medicine (EPMA, Brussels) for professional and financial support of the project.
Compliance with ethical standards
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of informed consent
Patients have not been involved in the study.
Statement of human and animal rights
No experiments have been performed including patients and/or animals.
References
- 1.White TT. Carcinoma of the breast and pregnancy; analysis of 920 cases collected from the literature and 22 new cases. Ann Surg. 1954;139(1):9–18. doi: 10.1097/00000658-195401000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golubnitschaja O, Debald M, Yeghiazaryan K, Kuhn W, Pešta M, Costigliola V, Grech G. Breast cancer epidemic in the early twenty-first century: evaluation of risk factors, cumulative questionnaires and recommendations for preventive measures. Tumour Biol. 2016;37(10):12941–12957. doi: 10.1007/s13277-016-5168-x. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez AO, Chew H, Cress R, Xing G, McElvy S, Danielsen B, Smith L. Evidence of poorer survival in pregnancy-associated breast cancer. Obstet Gynecol. 2008;112(1):71–78. doi: 10.1097/AOG.0b013e31817c4ebc. [DOI] [PubMed] [Google Scholar]
- 4.Kim YG, Jeon YW, Ko BK, Sohn G, Kim E-K, Moon B-I, Youn HJ, Kim HA, Korean Breast Cancer Society Clinicopathologic characteristics of pregnancy-associated breast cancer: results of analysis of a nationwide breast cancer registry database. J Breast Cancer. 2017;20(3):264–269. doi: 10.4048/jbc.2017.20.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langer A, Mohallem M, Stevens D, Rouzier R, Lerebours F, Chérel P. A single-institution study of 117 pregnancy-associated breast cancers (PABC): presentation, imaging, clinicopathological data and outcome. Diagn Interv Imaging. 2014;95(4):435–441. doi: 10.1016/j.diii.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez C, Acevedo F, Medina L, Ibáñez C, Razmilic D, Elena Navarro M, et al. Breast cancer and pregnancy: a comparative analysis of a Chilean cohort. Ecancermedicalscience. 2014;8:434. doi: 10.3332/ecancer.2014.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shachar SS, Gallagher K, McGuire K, Zagar TM, Faso A, Muss HB, Sweeting R, Anders CK. Multidisciplinary management of breast cancer during pregnancy. Oncologist. 2017;22(3):324–334. doi: 10.1634/theoncologist.2016-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zagouri F, Psaltopoulou T, Dimitrakakis C, Bartsch R, Dimopoulos M-A. Challenges in managing breast cancer during pregnancy. J Thorac Dis. 2013;5(Suppl 1):S62–S67. doi: 10.3978/j.issn.2072-1439.2013.05.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loibl S, Han SN, von Minckwitz G, Bontenbal M, Ring A, Giermek J, Fehm T, van Calsteren K, Linn SC, Schlehe B, Gziri MM, Westenend PJ, Müller V, Heyns L, Rack B, van Calster B, Harbeck N, Lenhard M, Halaska MJ, Kaufmann M, Nekljudova V, Amant F. Treatment of breast cancer during pregnancy: an observational study. Lancet Oncol. 2012;13(9):887–896. doi: 10.1016/S1470-2045(12)70261-9. [DOI] [PubMed] [Google Scholar]
- 10.Moreira WB, Brandão EC, Soares AN, de Lucena CEM, Antunes CMF. Prognosis for patients diagnosed with pregnancy-associated breast cancer: a paired case-control study. Sao Paulo Med J Rev Paul Med. 2010;128(3):119–124. doi: 10.1590/S1516-31802010000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of Pediatrics - December 2013 Information for the adolescent woman and her parents: abortion and the risk of breast cancer. Issues Law Med. 2017;32:99–104. [PubMed] [Google Scholar]
- 12.Hou N, Ogundiran T, Ojengbede O, Morhason-Bello I, Zheng Y, Fackenthal J, Adebamowo C, Anetor I, Akinleye S, Olopade OI, Huo D. Risk factors for pregnancy-associated breast cancer: a report from the Nigerian Breast Cancer Study. Ann Epidemiol. 2013;23(9):551–557. doi: 10.1016/j.annepidem.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molckovsky A, Madarnas Y. Breast cancer in pregnancy: a literature review. Breast Cancer Res Treat. 2008;108(3):333–338. doi: 10.1007/s10549-007-9616-6. [DOI] [PubMed] [Google Scholar]
- 14.White TT. Carcinoma of the breast in the pregnant and the nursing patient; review of 1,375 cases. Am J Obstet Gynecol. 1955;69(6):1277–1286. doi: 10.1016/S0002-9378(16)38162-5. [DOI] [PubMed] [Google Scholar]
- 15.Amant F, Loibl S, Neven P, Van Calsteren K. Breast cancer in pregnancy. Lancet. 2012;379(9815):570–579. doi: 10.1016/S0140-6736(11)61092-1. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz R, Herrero C, Strasser-Weippl K, Touya D, St Louis J, Bukowski A, et al. Epidemiology and pathophysiology of pregnancy-associated breast cancer: a review. Breast. 2017;35:136–141. doi: 10.1016/j.breast.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Johansson ALV, Andersson TM-L, Hsieh C-C, Jirström K, Dickman P, Cnattingius S, Lambe M. Stage at diagnosis and mortality in women with pregnancy-associated breast cancer (PABC) Breast Cancer Res Treat. 2013;139(1):183–192. doi: 10.1007/s10549-013-2522-1. [DOI] [PubMed] [Google Scholar]
- 18.Callihan EB, Gao D, Jindal S, Lyons TR, Manthey E, Edgerton S, Urquhart A, Schedin P, Borges VF. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat. 2013;138(2):549–559. doi: 10.1007/s10549-013-2437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6(4):281–291. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]
- 20.Pentheroudakis G, Orecchia R, Hoekstra HJ, Pavlidis N, ESMO Guidelines Working Group Cancer, fertility and pregnancy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v266–v273. doi: 10.1093/annonc/mdq198. [DOI] [PubMed] [Google Scholar]
- 21.Kakoulidis I, Skagias L, Politi E. Pregnancy associated breast cancer (PABC): aspects in diagnosis. Breast Dis. 2015;35(3):157–166. doi: 10.3233/BD-150408. [DOI] [PubMed] [Google Scholar]
- 22.Andersson TM-L, Johansson ALV, Hsieh C-C, Cnattingius S, Lambe M. Increasing incidence of pregnancy-associated breast cancer in Sweden. Obstet Gynecol. 2009;114(3):568–572. doi: 10.1097/AOG.0b013e3181b19154. [DOI] [PubMed] [Google Scholar]
- 23.Keyser EA, Staat BC, Fausett MB, Shields AD. Pregnancy-associated breast cancer. Rev Obstet Gynecol. 2012;5(2):94–99. [PMC free article] [PubMed] [Google Scholar]
- 24.Beadle BM, Woodward WA, Middleton LP, Tereffe W, Strom EA, Litton JK, Meric-Bernstam F, Theriault RL, Buchholz TA, Perkins GH. The impact of pregnancy on breast cancer outcomes in women<or=35 years. Cancer. 2009;115(6):1174–1184. doi: 10.1002/cncr.24165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.| Human Development Reports [Internet]. [cited 2018 Jan 2]. Available from: http://hdr.undp.org/en/2016-report.
- 26.Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: when they collide. J Mammary Gland Biol Neoplasia. 2009;14(2):87–98. doi: 10.1007/s10911-009-9119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 28.Germain D. Estrogen carcinogenesis in breast cancer. Endocrinol Metab Clin N Am. 2011;40(3):473–484. doi: 10.1016/j.ecl.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Lanzino M, Morelli C, Garofalo C, Panno ML, Mauro L, Andò S, et al. Interaction between estrogen receptor alpha and insulin/IGF signaling in breast cancer. Curr Cancer Drug Targets. 2008;8(7):597–610. doi: 10.2174/156800908786241104. [DOI] [PubMed] [Google Scholar]
- 30.Gupta PB, Proia D, Cingoz O, Weremowicz J, Naber SP, Weinberg RA, Kuperwasser C. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res. 2007;67(5):2062–2071. doi: 10.1158/0008-5472.CAN-06-3895. [DOI] [PubMed] [Google Scholar]
- 31.Shakhar K, Valdimarsdottir HB, Bovbjerg DH. Heightened risk of breast cancer following pregnancy: could lasting systemic immune alterations contribute? Cancer Epidemiol Biomark Prev. 2007;16(6):1082–1086. doi: 10.1158/1055-9965.EPI-07-0014. [DOI] [PubMed] [Google Scholar]
- 32.Graham C, Chooniedass R, Stefura WP, Becker AB, Sears MR, Turvey SE, Mandhane PJ, Subbarao P, CHILD Study Investigators. HayGlass KT. In vivo immune signatures of healthy human pregnancy: inherently inflammatory or anti-inflammatory? PLoS One. 2017;12(6):e0177813. doi: 10.1371/journal.pone.0177813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aagaard-Tillery KM, Silver R, Dalton J. Immunology of normal pregnancy. Semin Fetal Neonatal Med. 2006;11(5):279–295. doi: 10.1016/j.siny.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Rapacz-Leonard A, Dąbrowska M, Janowski T. Major histocompatibility complex I mediates immunological tolerance of the trophoblast during pregnancy and may mediate rejection during parturition. Mediat Inflamm. 2014;2014:579279. doi: 10.1155/2014/579279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, et al. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol. 2010;176(3):1241–1255. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Dev Camb Engl. 1992;115:49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- 37.Strange R, Metcalfe T, Thackray L, Dang M. Apoptosis in normal and neoplastic mammary gland development. Microsc Res Tech. 2001;52(2):171–181. doi: 10.1002/1097-0029(20010115)52:2<171::AID-JEMT1003>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 38.Watson CJ, Kreuzaler PA. Remodeling mechanisms of the mammary gland during involution. Int J Dev Biol. 2011;55(7-8-9):757–762. doi: 10.1387/ijdb.113414cw. [DOI] [PubMed] [Google Scholar]
- 39.Martinson HA, Jindal S, Durand-Rougely C, Borges VF, Schedin P. Wound healing-like immune program facilitates postpartum mammary gland involution and tumor progression. Int J Cancer. 2015;136(8):1803–1813. doi: 10.1002/ijc.29181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo Q, Minnier J, Burchard J, Chiotti K, Spellman P, Schedin P. Physiologically activated mammary fibroblasts promote postpartum mammary cancer. JCI Insight. 2017;2:e89206. doi: 10.1172/jci.insight.89206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avishai E, Yeghiazaryan K, Golubnitschaja O. Impaired wound healing: facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J. 2017;8(1):23–33. doi: 10.1007/s13167-017-0081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stolzenburg-Veeser L, Golubnitschaja O. Mini-encyclopaedia of the wound healing—opportunities for integrating multi-omic approaches into medical practice. J Proteomics. 2018. 10.1016/j.jprot.2017.07.017. [DOI] [PubMed]
- 43.Lenhard MS, Bauerfeind I, Untch M. Breast cancer and pregnancy: challenges of chemotherapy. Crit Rev Oncol Hematol. 2008;67(3):196–203. doi: 10.1016/j.critrevonc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Rovera F, Chiappa C, Coglitore A, Baratelli GM, Fachinetti A, Marelli M, Frattini F, Lavazza M, Bascialla L, Rausei S, Boni L, Corben AD, Dionigi G, Dionigi R. Management of breast cancer during pregnancy. Int J Surg. 2013;11(Suppl 1):S64–S68. doi: 10.1016/S1743-9191(13)60020-5. [DOI] [PubMed] [Google Scholar]
- 45.Murphy CG, Mallam D, Stein S, Patil S, Howard J, Sklarin N, Hudis CA, Gemignani ML, Seidman AD. Current or recent pregnancy is associated with adverse pathologic features but not impaired survival in early breast cancer. Cancer. 2012;118(13):3254–3259. doi: 10.1002/cncr.26654. [DOI] [PubMed] [Google Scholar]
- 46.Stensheim H, Møller B, van Dijk T, Fosså SD. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J Clin Oncol. 2009;27(1):45–51. doi: 10.1200/JCO.2008.17.4110. [DOI] [PubMed] [Google Scholar]
- 47.Basaran D, Turgal M, Beksac K, Ozyuncu O, Aran O, Beksac MS. Pregnancy-associated breast cancer: clinicopathological characteristics of 20 cases with a focus on identifiable causes of diagnostic delay. Breast Care. 2014;9:355–359. doi: 10.1159/000366436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faguy K. Breast disorders in pregnant and lactating women. Radiol Technol. 2015;86:419M–438M. [PubMed] [Google Scholar]
- 49.Taylor D, Lazberger J, Ives A, Wylie E, Saunders C. Reducing delay in the diagnosis of pregnancy-associated breast cancer: how imaging can help us. J Med Imaging Radiat Oncol. 2011;55(1):33–42. doi: 10.1111/j.1754-9485.2010.02227.x. [DOI] [PubMed] [Google Scholar]
- 50.Aziz S, Pervez S, Khan S, Siddiqui T, Kayani N, Israr M, Rahbar M. Case control study of novel prognostic markers and disease outcome in pregnancy/lactation-associated breast carcinoma. Pathol Res Pract. 2003;199(1):15–21. doi: 10.1078/0344-0338-00347. [DOI] [PubMed] [Google Scholar]
- 51.Dolle JM, Daling JR, White E, Brinton LA, Doody DR, Porter PL, et al. Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol Biomarkers Prev. 2009;18:1157–1166. doi: 10.1158/1055-9965.EPI-08-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonnier P, Romain S, Dilhuydy JM, Bonichon F, Julien JP, Charpin C, Lejeune C, Martin PM, Piana L, Société Franĉaise de Sénologie et de Pathologie Mammaire Study Group Influence of pregnancy on the outcome of breast cancer: a case-control study. Societe Francaise de Senologie et de Pathologie Mammaire Study Group. Int J Cancer. 1997;72(5):720–727. doi: 10.1002/(SICI)1097-0215(19970904)72:5<720::AID-IJC3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 53.Ishida T, Yokoe T, Kasumi F, Sakamoto G, Makita M, Tominaga T, Simozuma K, Enomoto K, Fujiwara K, Nanasawa T, Fukutomi T, Hirota T, Fukuda M, Miura S, Koyama H, Inaji H, Sonoo H. Clinicopathologic characteristics and prognosis of breast cancer patients associated with pregnancy and lactation: analysis of case-control study in Japan. Jpn J Cancer Res Gann. 1992;83(11):1143–1149. doi: 10.1111/j.1349-7006.1992.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strasser-Weippl K, Ramchandani R, Fan L, Li J, Hurlbert M, Finkelstein D, Shao ZM, Goss PE. Pregnancy-associated breast cancer in women from Shanghai: risk and prognosis. Breast Cancer Res Treat. 2015;149(1):255–261. doi: 10.1007/s10549-014-3219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mielke S, Meden H, Kuhn W. Expression of the c-erbB-2-encoded oncoprotein p185 (HER-2/neu) in pregnancy as a model for oncogene-induced carcinogenesis. Med Hypotheses. 1998;50(5):359–362. doi: 10.1016/S0306-9877(98)90205-5. [DOI] [PubMed] [Google Scholar]
- 56.Hsiao Y-H, Su YA, Tsai H-D, Mason JT, Chou M-C, Man Y. Increased invasiveness and aggressiveness in breast epithelia with cytoplasmic p63 expression. Int J Biol Sci. 2010;6(5):428–442. doi: 10.7150/ijbs.6.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asztalos S, Pham TN, Gann PH, Hayes MK, Deaton R, Wiley EL, Emmadi R, Kajdacsy-Balla A, Banerji N, McDonald W, Khan SA, Tonetti DA. High incidence of triple negative breast cancers following pregnancy and an associated gene expression signature. Springerplus. 2015;4(1):710. doi: 10.1186/s40064-015-1512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johannsson O, Loman N, Borg A, Olsson H. Pregnancy-associated breast cancer in BRCA1 and BRCA2 germline mutation carriers. Lancet. 1998;352(9137):1359–1360. doi: 10.1016/S0140-6736(05)60750-7. [DOI] [PubMed] [Google Scholar]
- 59.Robertson C, Primic-Zakelj M, Boyle P, Hsieh CC. Effect of parity and age at delivery on breast cancer risk in Slovenian women aged 25-54 years. Int J Cancer. 1997;73(1):1–9. doi: 10.1002/(SICI)1097-0215(19970926)73:1<1::AID-IJC1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 60.Trichopoulos D, Hsieh CC, MacMahon B, Lin TM, Lowe CR, Mirra AP, et al. Age at any birth and breast cancer risk. Int J Cancer. 1983;31(6):701–704. doi: 10.1002/ijc.2910310604. [DOI] [PubMed] [Google Scholar]
- 61.Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360:187–195. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 62.Chowdhury R, Sinha B, Sankar MJ, Taneja S, Bhandari N, Rollins N, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:96–113. doi: 10.1111/apa.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hadjisavvas A, Loizidou MA, Middleton N, Michael T, Papachristoforou R, Kakouri E, Daniel M, Papadopoulos P, Malas S, Marcou Y, Kyriacou K. An investigation of breast cancer risk factors in Cyprus: a case control study. BMC Cancer. 2010;10(1):447. doi: 10.1186/1471-2407-10-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johansson ALV, Andersson TM-L, Hsieh C-C, Cnattingius S, Dickman PW, Lambe M. Family history and risk of pregnancy-associated breast cancer (PABC) Breast Cancer Res Treat. 2015;151(1):209–217. doi: 10.1007/s10549-015-3369-4. [DOI] [PubMed] [Google Scholar]
- 65.Pollán M. Epidemiology of breast cancer in young women. Breast Cancer Res Treat. 2010;123(Suppl 1):3–6. doi: 10.1007/s10549-010-1098-2. [DOI] [PubMed] [Google Scholar]
- 66.Rousset-Jablonski C, Gompel A. Screening for familial cancer risk: focus on breast cancer. Maturitas. 2017;105:69–77. doi: 10.1016/j.maturitas.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 67.Yu JH, Kim MJ, Cho H, Liu HJ, Han S-J, Ahn T-G. Breast diseases during pregnancy and lactation. Obstet Gynecol Sci. 2013;56(3):143–159. doi: 10.5468/ogs.2013.56.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Albrektsen G, Heuch I, Hansen S, Kvåle G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;92(1):167–175. doi: 10.1038/sj.bjc.6602302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colditz GA, Frazier AL. Models of breast cancer show that risk is set by events of early life: prevention efforts must shift focus. Cancer Epidemiol Biomarkers Prev. 1995;4:567–571. [PubMed] [Google Scholar]
- 70.Warren Andersen S, Trentham-Dietz A, Gangnon RE, Hampton JM, Figueroa JD, Skinner HG, Engelman CD, Klein BE, Titus LJ, Egan KM, Newcomb PA. Reproductive windows, genetic loci, and breast cancer risk. Ann Epidemiol. 2014;24(5):376–382. doi: 10.1016/j.annepidem.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaudet MM, Press MF, Haile RW, Lynch CF, Glaser SL, Schildkraut J, Gammon MD, Douglas Thompson W, Bernstein JL. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. 2011;130(2):587–597. doi: 10.1007/s10549-011-1616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Millikan RC, Newman B, Tse C-K, Moorman PG, Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, Nyante S, Livasy C, Carey L, Earp HS, Perou CM. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simmen FA, Simmen RCM. The maternal womb: a novel target for cancer prevention in the era of the obesity pandemic? Eur J Cancer Prev. 2011;20(6):539–548. doi: 10.1097/CEJ.0b013e328348fc21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Polivka J, Kralickova M, Polivka J, Kaiser C, Kuhn W, Golubnitschaja O. Mystery of the brain metastatic disease in breast cancer patients: improved patient stratification, disease prediction and targeted prevention on the horizon? EPMA J. 2017;8(2):119–127. doi: 10.1007/s13167-017-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, McCullough M, Patel AV, Ma J, Soerjomataram I, Flanders WD, Brawley OW, Gapstur SM, Jemal A. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2017;68(1):31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 76.Catsburg C, Miller AB, Rohan TE. Active cigarette smoking and risk of breast cancer. Int J Cancer. 2015;136(9):2204–2209. doi: 10.1002/ijc.29266. [DOI] [PubMed] [Google Scholar]
- 77.White AJ, Bradshaw PT, Herring AH, Teitelbaum SL, Beyea J, Stellman SD, et al. Exposure to multiple sources of polycyclic aromatic hydrocarbons and breast cancer incidence. Environ Int. 2016;89–90:185–192. doi: 10.1016/j.envint.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y, Colditz GA, Rosner B, Berkey CS, Collins LC, Schnitt SJ, Connolly JL, Chen WY, Willett WC, Tamimi RM. Alcohol intake between menarche and first pregnancy: a prospective study of breast cancer risk. J Natl Cancer Inst. 2013;105(20):1571–1578. doi: 10.1093/jnci/djt213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schottenfeld D, Jr JFF, editors. Cancer epidemiology and prevention. Third edition. Oxford: Oxford University Press; 2006. [Google Scholar]
- 80.Richter K, Acker J, Kamcev N, Bajraktarov S, Piehl A, Niklewski G. Recommendations for the prevention of breast cancer in shift workers. EPMA J. 2011;2(4):351–356. doi: 10.1007/s13167-011-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blakeman V, Williams JL, Meng Q-J, Streuli CH. Circadian clocks and breast cancer. Breast Cancer Res. 2016;18(1):89. doi: 10.1186/s13058-016-0743-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fritschi L, Glass DC, Heyworth JS, Aronson K, Girschik J, Boyle T, Grundy A, Erren TC. Hypotheses for mechanisms linking shiftwork and cancer. Med Hypotheses. 2011;77(3):430–436. doi: 10.1016/j.mehy.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 83.Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer. 2005;41:2023–2032. doi: 10.1016/j.ejca.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 84.Wegrzyn LR, Tamimi RM, Rosner BA, Brown SB, Stevens RG, Eliassen AH, Laden F, Willett WC, Hankinson SE, Schernhammer ES. Rotating night-shift work and the risk of breast cancer in the nurses’ health studies. Am J Epidemiol. 2017;186(5):532–540. doi: 10.1093/aje/kwx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rafnsson V, Tulinius H, Jónasson JG, Hrafnkelsson J. Risk of breast cancer in female flight attendants: a population-based study (Iceland) Cancer Causes Control. 2001;12(2):95–101. doi: 10.1023/A:1008983416836. [DOI] [PubMed] [Google Scholar]
- 86.Linnersjö A, Hammar N, Dammström B-G, Johansson M, Eliasch H. Cancer incidence in airline cabin crew: experience from Sweden. Occup Environ Med. 2003;60(11):810–814. doi: 10.1136/oem.60.11.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pukkala E, Auvinen A, Wahlberg G. Incidence of cancer among Finnish airline cabin attendants, 1967-92. BMJ. 1995;311(7006):649–652. doi: 10.1136/bmj.311.7006.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hardefeldt PJ, Edirimanne S, Eslick GD. Diabetes increases the risk of breast cancer: a meta-analysis. Endocr Relat Cancer. 2012;19(6):793–803. doi: 10.1530/ERC-12-0242. [DOI] [PubMed] [Google Scholar]
- 90.Boyle P, Boniol M, Koechlin A, Robertson C, Valentini F, Coppens K, Fairley LL, Boniol M, Zheng T, Zhang Y, Pasterk M, Smans M, Curado MP, Mullie P, Gandini S, Bota M, Bolli GB, Rosenstock J, Autier P. Diabetes and breast cancer risk: a meta-analysis. Br J Cancer. 2012;107(9):1608–1617. doi: 10.1038/bjc.2012.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park Y-MM, O’Brien KM, Zhao S, Weinberg CR, Baird DD, Sandler DP. Gestational diabetes mellitus may be associated with increased risk of breast cancer. Br J Cancer. 2017;116(7):960–963. doi: 10.1038/bjc.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zubor P, Gondova A, Polivka J, Kasajova P, Konieczka K, Danko J, Golubnitschaja O. Breast cancer and Flammer syndrome: any symptoms in common for prediction, prevention and personalised medical approach? EPMA J. 2017;8(2):129–140. doi: 10.1007/s13167-017-0089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bubnov R, Polivka J, Zubor P, Konieczka K, Golubnitschaja O. “Pre-metastatic niches” in breast cancer: are they created by or prior to the tumour onset? “Flammer syndrome” relevance to address the question. EPMA J. 2017;8(2):141–157. doi: 10.1007/s13167-017-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smokovski I, Risteski M, Polivka J, Zubor P, Konieczka K, Costigliola V, Golubnitschaja O. Postmenopausal breast cancer: European challenge and innovative concepts. EPMA J. 2017;8(2):159–169. doi: 10.1007/s13167-017-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee YY, Roberts CL, Dobbins T, Stavrou E, Black K, Morris J, Young J. Incidence and outcomes of pregnancy-associated cancer in Australia, 1994-2008: a population-based linkage study. BJOG. 2012;119(13):1572–1582. doi: 10.1111/j.1471-0528.2012.03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ballard-Barbash R, Hunsberger S, Alciati MH, Blair SN, Goodwin PJ, McTiernan A, Wing R, Schatzkin A. Physical activity, weight control, and breast cancer risk and survival: clinical trial rationale and design considerations. J Natl Cancer Inst. 2009;101(9):630–643. doi: 10.1093/jnci/djp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bernstein L. Exercise and breast cancer prevention. Curr Oncol Rep. 2009;11(6):490–496. doi: 10.1007/s11912-009-0066-7. [DOI] [PubMed] [Google Scholar]
- 98.Zeng H, Irwin ML, Lu L, Risch H, Mayne S, Mu L, Deng Q, Scarampi L, Mitidieri M, Katsaros D, Yu H. Physical activity and breast cancer survival: an epigenetic link through reduced methylation of a tumor suppressor gene L3MBTL1. Breast Cancer Res Treat. 2012;133(1):127–135. doi: 10.1007/s10549-011-1716-7. [DOI] [PubMed] [Google Scholar]
- 99.Kauppila A, Kyyrönen P, Hinkula M, Pukkala E. Birth intervals and breast cancer risk. Br J Cancer. 2009;101(7):1213–1217. doi: 10.1038/sj.bjc.6605300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tworoger SS, Eliassen AH, Sluss P, Hankinson SE. A prospective study of plasma prolactin concentrations and risk of premenopausal and postmenopausal breast cancer. J Clin Oncol. 2007;25(12):1482–1488. doi: 10.1200/JCO.2006.07.6356. [DOI] [PubMed] [Google Scholar]
- 101.Fazzo L, Carere M, Tisano F, Bruno C, Cernigliaro A, Cicero MR, et al. Cancer incidence in Priolo, Sicily: a spatial approach for estimation of industrial air pollution impact. Geospat Health. 2016;11:320. doi: 10.4081/gh.2016.320. [DOI] [PubMed] [Google Scholar]
- 102.Zimeri AM, Robb SW, Hassan SM, Hire RR, Davis MB. Assessing heavy metal and PCB exposure from tap water by measuring levels in plasma from sporadic breast cancer patients, a pilot study. Int J Environ Res Public Health. 2015;12(12):15683–15691. doi: 10.3390/ijerph121215013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alatise OI, Schrauzer GN. Lead exposure: a contributing cause of the current breast cancer epidemic in Nigerian women. Biol Trace Elem Res. 2010;136(2):127–139. doi: 10.1007/s12011-010-8608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Little MP, McElvenny DM. Male breast cancer incidence and mortality risk in the Japanese atomic bomb survivors—differences in excess relative and absolute risk from female breast cancer. Environ Health Perspect. 2017;125(2):223–229. doi: 10.1289/EHP151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grant EJ, Brenner A, Sugiyama H, Sakata R, Sadakane A, Utada M, Cahoon EK, Milder CM, Soda M, Cullings HM, Preston DL, Mabuchi K, Ozasa K. Solid cancer incidence among the life span study of atomic bomb survivors: 1958-2009. Radiat Res. 2017;187(5):513–537. doi: 10.1667/RR14492.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Cancer. 2013;108(11):2205–2240. doi: 10.1038/bjc.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kotepui M. Diet and risk of breast cancer. Contemp Oncol Poznan Pol. 2016;20:13–19. doi: 10.5114/wo.2014.40560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seitz HK, Pelucchi C, Bagnardi V, La Vecchia C. Epidemiology and pathophysiology of alcohol and breast cancer: update 2012. Alcohol Alcohol. 2012;47(3):204–212. doi: 10.1093/alcalc/ags011. [DOI] [PubMed] [Google Scholar]
- 109.Kim HJ, Jung S, Eliassen AH, Chen WY, Willett WC, Cho E. Alcohol consumption and breast cancer risk in younger women according to family history of breast cancer and folate intake. Am J Epidemiol. 2017;186(5):524–531. doi: 10.1093/aje/kwx137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Härmä M, Kecklund G. Shift work and health—how to proceed? Scand J Work Environ Health. 2010;36(2):81–84. doi: 10.5271/sjweh.2902. [DOI] [PubMed] [Google Scholar]
- 111.Charrier A, Olliac B, Roubertoux P, Tordjman S. Clock genes and altered sleep-wake rhythms: their role in the development of psychiatric disorders. Int J Mol Sci. 2017;18(5):E938. doi: 10.3390/ijms18050938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang X-S, Armstrong MEG, Cairns BJ, Key TJ, Travis RC. Shift work and chronic disease: the epidemiological evidence. Occup Med. 2011;61(2):78–89. doi: 10.1093/occmed/kqr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schubauer-Berigan MK, Anderson JL, Hein MJ, Little MP, Sigurdson AJ, Pinkerton LE. Breast cancer incidence in a cohort of U.S. flight attendants. Am J Ind Med. 2015;58(3):252–266. doi: 10.1002/ajim.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu AH, Kurian AW, Kwan ML, John EM, Lu Y, Keegan THM, Gomez SL, Cheng I, Shariff-Marco S, Caan BJ, Lee VS, Sullivan-Halley J, Tseng CC, Bernstein L, Sposto R, Vigen C. Diabetes and other comorbidities in breast cancer survival by race/ethnicity: the California Breast Cancer Survivorship Consortium (CBCSC) Cancer Epidemiol Biomarkers Prev. 2015;24(2):361–368. doi: 10.1158/1055-9965.EPI-14-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xie C, Wang W, Li X, Shao N, Li W. Gestational diabetes mellitus and maternal breast cancer risk: a meta-analysis of the literature. J Matern-Fetal Neonatal Med. 2017;7:1–11. doi: 10.1080/14767058.2017.1397117. [DOI] [PubMed] [Google Scholar]
- 116.Yeghiazaryan K, Cebioglu M, Golubnitschaja O. Global figures argue in favour of preventive measures and personalised treatment to optimise inadequate diabetes care. New Strateg. Adv. PreDiabetes Care Integr. Approach PPPM [Internet]. Dordrecht : Springer; 2013 [cited 2018 Jan 23]. p. 1–13. Available from: https://link.springer.com/chapter/10.1007/978-94-007-5971-8_1.
- 117.Gaetani S, Romano A, Provensi G, Ricca V, Lutz T, Passani MB. Eating disorders: from bench to bedside and back. J Neurochem. 2016;139(5):691–699. doi: 10.1111/jnc.13848. [DOI] [PubMed] [Google Scholar]
- 118.Jagielska G, Kacperska I. Outcome, comorbidity and prognosis in anorexia nervosa. Psychiatr Pol. 2017;51(2):205–218. doi: 10.12740/PP/64580. [DOI] [PubMed] [Google Scholar]
- 119.Cox TR, Rumney RMH, Schoof EM, Perryman L, Høye AM, Agrawal A, Bird D, Latif NA, Forrest H, Evans HR, Huggins ID, Lang G, Linding R, Gartland A, Erler JT. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522(7554):106–110. doi: 10.1038/nature14492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 120.Konieczka K, Ritch R, Traverso CE, Kim DM, Kook MS, Gallino A, Golubnitschaja O, Erb C, Reitsamer HA, Kida T, Kurysheva N, Yao K. Flammer syndrome. EPMA J. 2014;5(1):11. doi: 10.1186/1878-5085-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Golubnitschaja O, Debald M, Kuhn W, Yeghiazaryan K, Bubnov RV, Goncharenko VM, et al. Flammer syndrome and potential formation of pre-metastatic niches: a multi-centred study on phenotyping, patient stratification, prediction and potential prevention of aggressive breast cancer and metastatic disease. EPMA J. 2016;7:A25. doi: 10.1186/s13167-016-0072-4. [DOI] [Google Scholar]
- 122.Yeghiazaryan K, Flammer J, Golubnitschaja O. Predictive molecular profiling in blood of healthy vasospastic individuals: clue to targeted prevention as personalised medicine to effective costs. EPMA J. 2010;1(2):263–272. doi: 10.1007/s13167-010-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Golubnitschaja O. Feeling cold and other underestimated symptoms in breast cancer: anecdotes or individual profiles for advanced patient stratification? EPMA J. 2017;8(1):17–22. doi: 10.1007/s13167-017-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Golubnitschaja O, Filep N, Yeghiazaryan K, Blom HJ, Hofmann-Apitius M, Kuhn W. Multi-omic approach decodes paradoxes of the triple-negative breast cancer: lessons for predictive, preventive and personalised medicine. Amino Acids. 2017; 10.1007/s00726-017-2524-0. [DOI] [PubMed]
- 125.Eibye S, Kjær SK, Mellemkjær L. Incidence of pregnancy-associated cancer in Denmark, 1977-2006. Obstet Gynecol. 2013;122(3):608–617. doi: 10.1097/AOG.0b013e3182a057a2. [DOI] [PubMed] [Google Scholar]