Abstract
Background
Liver fibrosis (LF) is a chronic disease, associated with many collateral diseases including reproductive dysfunction. Although the normal liver has a large regenerative capacity the complications of LF could be severe and irreversible. Hormone and sex-related issues of LF development and interactions with male reproductive have not been finally studied. The aim was to study the reproductive function of male rats in experimental CCl4-induced liver fibrosis rat model, and the capability for restoration of both the liver and male reproduction system.
Materials
Studies were conducted on 20 3-month old Wistar male rats. The experimental animals were injected with freshly prepared 50% olive oil solution of carbohydrate tetrachloride (CCl4). On the 8th week after injection we noted the manifestations of liver fibrosis. The rats were left to self-healing of the liver for 8 weeks. All male rats underwent ultrasound and biopsy of the liver and testes on the 8th and 16th weeks. The male rats were mated with healthy females before CCl4 injection, after modeling LF on the 8th week, and after self-healing of the liver. Pregnancy was monitored on ultrasound.
Results
On the 8th week of experiment we observed ultrasound manifestation of advanced liver fibrosis, including hepatosplenomegaly, portal hypertension. Ultrasound exam of the rat testes showed testicular degeneration, hydrocele, fibrosis, scarring, petrifications, size reduction, and restriction of testicular descent; testes size decreased from 1.24 ± 0.62 ml to 0.61 ± 0.13, p < 0.01. Liver histology showed granular dystrophy of hepatocytes, necrotic areas, lipid inclusions in parenchyma. Rats with liver fibrosis demonstrated severe injury of the reproductive system and altering of fertility: the offspring of male rats with advanced LF was 4.71 ± 0.53 born alive vs 9.55 ± 0.47 born from mating with healthy males, p < 0.001. Eight weeks after last CCl4 injection, we revealed signs of liver regeneration, significant recovery of its structure. The ALT and AST levels significantly decreased and reached background measurements. As a result of the second interbreeding after liver self-healing no significant difference was found vs previous mating.
Conclusion
Carbohydrate tetrachloride induces injury of liver parenchyma evoking fast and severe liver fibrosis, and is associated with irreversible structural and functional changes in testes, reducing fertility, decreasing potential pregnancy rate, and affecting its development. Liver showed high potential to regenerate, however the self-restoring after liver fibrosis was not accompanied with recovery of the reproductive system.
Keywords: Predictive preventive personalized medicine, Liver fibrosis, Liver regeneration, Male reproductive system, Fertility, Animal model, Wistar rats, Ultrasound, Translation, Men health
Overview
Liver fibrosis and male fertility associations within the concept of predictive, preventive, and personalized medicine
Nonalcoholic fatty liver disease (NAFLD) is a global health problem, represents a hepatic metabolic syndrome and includes fatty liver (simple steatosis), steatohepatitis, liver fibrosis (LF), and cirrhosis [1–3]. In 1980, the term nonalcoholic steatohepatitis (NASH) was suggested which is now considered to be one of the manifestations of the broader NAFLD spectrum, characterized by fatty and inflammatory changes, Mallory bodies, fibrosis and cirrhosis. The disease was more common in women, the obese, those with diabetes mellitus, gallstones, and thyroid disease [4].
The “two-hit” hypothesis for the progression of NASH has been suggested, which claims the pathophysiology start with steatosis (the primary hit), which primes the liver to oxidative stress (a secondary hit) [5, 6]. Obesity is a risk factors for NAFLD [2]. Many other risk factors can also serve as the secondary hit [5] such as gut-derived endotoxins, pro-inflammatory cytokines, endoplasmic reticulum (ER) stress, and insulin resistance (IR), inflammation [7], mitochondrial dysfunction [8], oxidative stress, the role of Cytochrome P450 3A4 [6], etc.
Liver fibrosis (LF) is a chronic disease of the liver, is a frequent form of metabolic syndrome (MetS), often connected to obesity, diabetes, insulin resistance, and associated with the male reproductive tract function—the processes of functional sperm production [9–12]. Biosynthesis of estrogens and androgens plays a role in the development of liver disease [13, 14], and gender differences in the relative risk of developing metabolic complications were demonstrated [15].
Although the normal liver has a large regenerative capacity [16], the complications of LF on the reproductive system could be severe and irreversible.
Dramatic falling birth rates and fertility rates of modern societies in recent decades is closely associated with the increased incidence of metabolic syndrome [17, 18]. The tasks of predictive, preventive, and personalized medicine (PPPM) are to develop a well-balanced family life through all life spans in aging society and promote sustainable reproduction health and new healthy generations [19–21]. Women’s health has been widely assessed within the large scope of factors affecting fertility, providing clear recommendations for gender-related pathology. In men’s health, we still observe a lack of such clear concept, focused attention in research and health care [22]. Recently, we studied antioxidative effects of nanoceria on male infertility and suggested an extensive multiparameter diagnostic assessment panel for men’s health and fertility [22].
However, so far many aspects of liver regeneration, hormone and sex-related issues of LF development, and interactions with the reproductive system in males, its impact on fertility, and potential pregnancy development have not been finally elucidated.
The rat models are reliable and have been widely used to study liver fibrosis and related conditions when the regenerative capacity of liver is compromised. This approach can be effected either by partial hepatectomy or using hepatotoxins like carbon tetrachloride (CCl4) [23–27]. CCl4 has been used as a model toxicant and has been the focus of many in vitro and in vivo toxicological studies. It has toxicity on organs and tissues, and most pronounced liver-specific activity with strong hepatotoxic cirrhotic and carcinogenetic effects due to the metabolism of CCl4 via cytochrome P450 (CYP). On the other hand, it is supposed to have generalized toxic effects upon other organs including the reproduction system through inhibition of CYP system [24].
We have chosen the carbon tetrachloride-induced liver fibrosis model [28–31] on 3-month old male Wistar rats using the longitudinal ultrasound survey to study the male reproductive system in liver fibrosis.
The aim is to study the male reproductive function of rats in the CCl4-induced liver fibrosis model, and the regenerative capacity of both the liver and of male reproduction system; and to overview the literature to update the evidence regarding liver fibrosis and reproductive dysfunction.
Methods
Research was conducted in compliance with the standards of the Convention on Bioethics of the Council of Europe’s ‘Europe Convention for the Protection of Vertebrate Animals’ used for experimental and other scientific purposes’ (1997), the general ethical principles of animal experiments, approved by the First National Congress on Bioethics Ukraine (September 2001) in compliance with the Law of Ukraine of 21.02.2006 № 3447-IV “On protection of animals from abuse”, and with other international agreements and national legislation in this field. Animals were kept in a vivarium that was accredited in accordance with the ‘standard rules on ordering, equipment, and maintenance of experimental biological clinics (vivarium)’. Instruments to be used for research are subject to metrological control. No human subjects have been involved to the study.
Preclinical in vivo ultrasound was used during the model that allowed gathering more relevant parameters for keeping animals alive.
Animals and housing conditions
The experiment included 20 3-month old Wistar male rats and 20 healthy females for mating and to study fertility. The animals of each experimental group were individually housed in polypropylene cages in an environmentally controlled clean air room, with a temperature of 22 ± 3 °C, a 12 h light/12 h dark cycle, and a relative humidity of 60 ± 5%.
Experiments
Studies were conducted on 20 3-month old Wistar male rats. The experimental animals were injected with freshly prepared 50% olive oil solution of carbon tetrachloride (CCl4) in a dose of 200 μL/100 g body weight during 2 weeks with a periodicity twice a week (Monday and Thursday) [26, 27]. The subsequent 2 weeks, the animals were injected intraperitoneally with freshly prepared 50% olive oil solution of CCl4 in a dose of 100 μL/100 g of body weight with a periodicity twice a week (Monday and Thursday). During the residual 4 weeks of experiment the animals were injected intraperitoneally with freshly prepared 50% olive oil solution of CCl4 in a dose of 50 μL/100 g body weight with a periodicity of 2 times a week (Monday and Thursday).
Before injection of CCl4 the male rats were mated with females.
On the 8th week of the experiment we noted the signs of liver fibrosis and transaminases increasing as in [26, 27].
Thereafter, we started the second step of the experiment.
The male rats were mated with 20 healthy females and then left untreated during 8 weeks to evoke regeneration (‘self-healing’) of the liver.
After 8 weeks all the male rats underwent ultrasound again and a biopsy of the liver and testes.
Then the male rats were mated again with healthy females.
Thus, mating was performed at the beginning, on the 8th, and 16th week of experiment.
One month later animals were sacrificed according to guidelines on bioethics.
Post-mortem study
A diagnostic algorithm based on the basis of visual assessment of color, size, shape, and consistency of organ edges.
Histological preparations
The pieces of liver and testes were fixed in 10% neutral formalin, embedded in paraffin blocks, sections were prepared with thickness about 5 μm. Tissue sections were stained with hematoxylin-eosin by Romanovsky.
Biochemical blood analysis
We determined levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the sera of rats in the control and experimental groups were determined using photoelectrocolorimeters KFK-2, we used test system manufacturer Audit Diagnostics (Ireland).
Ultrasound (US) of the internal organs and rat testes at pregnancy
All animals underwent ultrasound study of internal organs and the reproductive system at the initial point and on the 8th and on the 16th weeks of the experiment, as well as the biopsy of the liver and testes under US guidance. We performed ultrasonography (US) of testes in rats using linear 5–12 MHz frequency probes of ultrasound scanner Ultrasound Philips/ATL HDI 5000 (Netherlands) according to [32].
An important criterion in assessing the development of liver disease during ultrasound were parameters of changes in the size of the liver, spleen, portal vein diameter, and Doppler of portal blood flow. We obtained transversal and longitudinal measurements of testes and calculated volume. We used the most common formula to calculate a testicular volume for an ellipsoid structure: length (L) × width (W) × height (H) × 0.52.
The pregnancy in rats was monitored on ultrasound.
The overall scheme of the experiment is presented in Fig. 1.
Fig. 1.
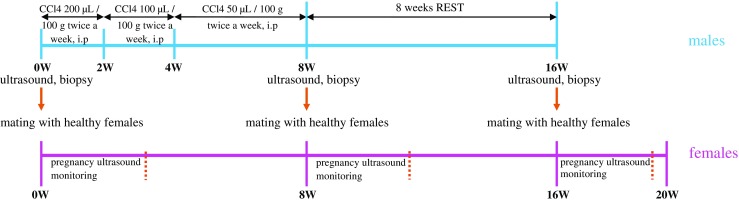
General scheme of the experiment
Statistical analysis
Statistical processing of the results, which included an analysis of the significance of differences in the average values by Student’s test was performed using the MS Exel. All experimental data in this study were expressed as means ± standard deviation (M ± SD). The difference between groups was defined to be statistically significant when a P-value was lower than 0.05.
The number of animals was calculated considering high levels of mortlity due to toxicity of carbon tetrachloride.
Results
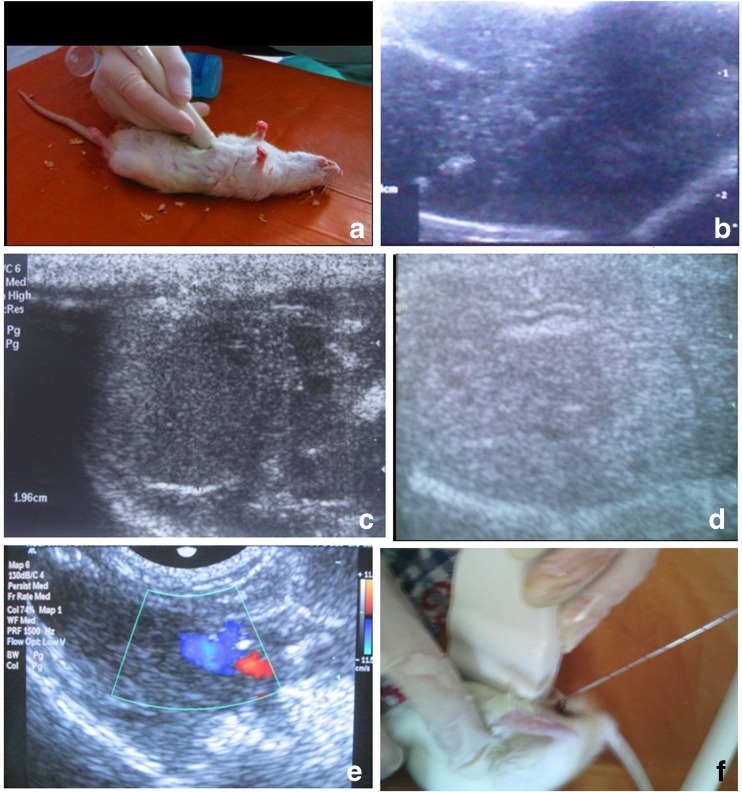
On the 8th week we observed changes typical to advanced fibrosis (cirrhosis) on ultrasound including signs of portal hypertension with the dilatation of the portal and splenic veins, blood flow changes, and signs of nephropathy (hepatorenal syndrome) in all animals (Fig. 2).
Fig. 2.
Ultrasound of liver in rats. a – general view, b, c, d – liver structure; b – liver fibrosis, nodules, petrifications; c – liver measurement; d – changes of liver structure, deformation of tubular structures, cholestasis; e – portal hypertension: portal vein expanded (blue), hepatic artery (red); f – core biopsy under ultrasound guidance
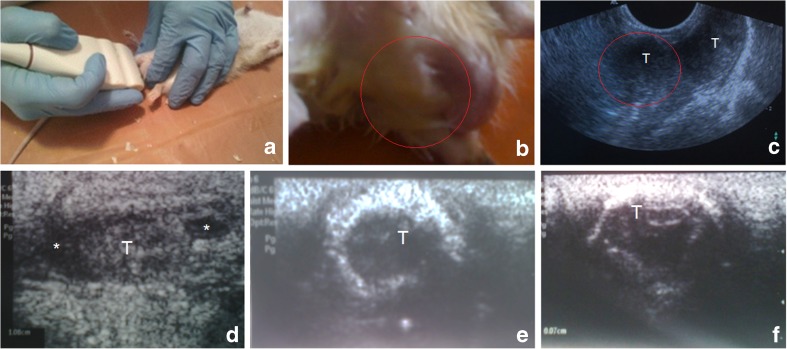
Ultrasound exam of the rat testes since the 8th week showed the presence of testicular degeneration, often the presence of hydrocele testis (fluid in the scrotum) testicular fibrosis, scarring, petrification, size reduction and restriction of testicular descent (Fig. 3). Testicular volumes on ultrasound decreased from 1.24 ± 0.62 ml (at the beginning) to 0.61 ± 0.13, p < 0.01.
Fig. 3.
Assessment of the rat testes: a – view of ultrasound technique to obtain transversal scans of testes in rat; b, c – acquired cryptorchidism under LF model – general view (b) and corresponding US image (c), whereas red circles indicate the right testicle (T) not descended to the scrotum; d, e, f – longitudinal US scan of testes: decreasing size, fibrosis, deformation, petrification, stiff and thick (fibrotic) capsule, irregular contour; * – fluid around a testicle (hydrocele)
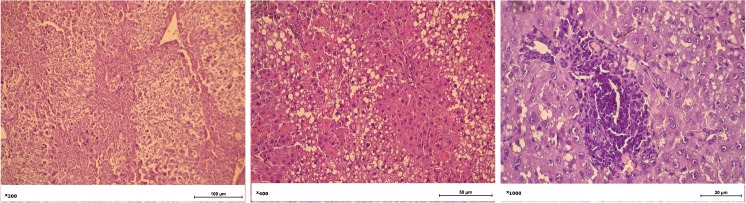
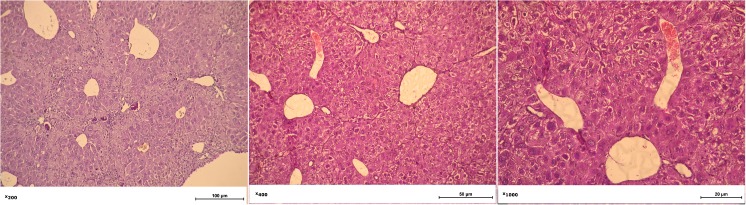
Histology of the liver of rats on the 8th week after CCl4 injection revealed granular dystrophy of hepatocytes, necrotic areas in parenchyma, identified areas with lipid inclusions, which is typical for the development of cirrhosis in humans and lack of typical girder structure of the liver (Fig. 4).
Fig. 4.
Histology of liver of the rats at the 8th week after injection of CCl4. Granular dystrophy of hepatocytes, necrotic areas, areas with lipid inclusions, and lack of typical girder structure of the liver
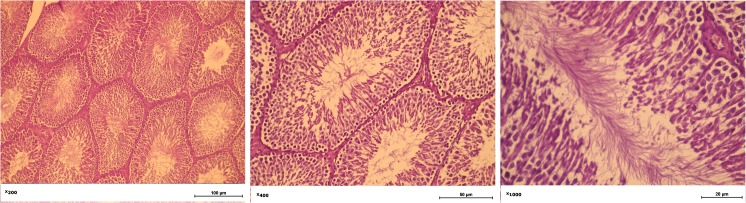
Histology of the testes at stages of the current model of intact rat testis, during liver fibrosis model, and 8 weeks after liver self-healing are presented in Figs. 5, 6, and 7 respectively.
Fig. 5.
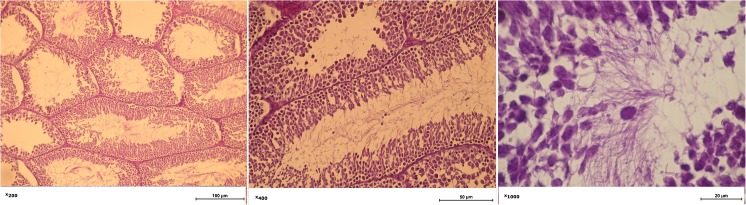
Intact rat testis. The Leydig cells and Sertoli cells preserve their structure
Fig. 6.
The morphology of the testicles of rats with liver fibrosis. The tubular lumen diameter is largest among the study groups; a minor amount of sperm in the lumen; partial necrosis of Leydig cells
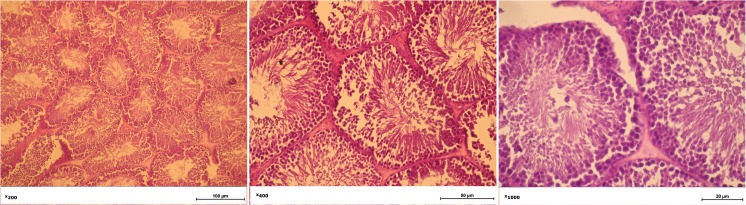
Fig. 7.
The morphology of the testicles of on the 8th week of liver regeneration. Leydig cells not detected; necrosis of Leydig cells; a serous exudate in interfollicular stroma. Seminiferous tubules filled with spermatids and spermatozoa. Sertoli cells preserve their structure
Liver fibrosis causes severe changes in the metabolic processes of an organism and as we hypothesized can be associated with severe disease of the reproductive system. We are aware of potentially biased results by possible direct toxicity of carbon tetrachloride on the reproductive system and not only indirect ones, mediated by liver fibrosis.
The clear proof of the reproductive system injury was demonstrated by the offspring of the male rats under the model who were mated with healthy females. These females had already mated with healthy males and gave birth to an average of 9.55 ± 0.47 rat babies. As a result, at the first birth four female rats died, three female rats did not become pregnant, and the rest gave birth, 4.71 ± 0.53 per one female rat, p < 0.05 (Table 1).
Table 1.
Reproductive performance of male rats
| Result | The offspring of healthy male rats | The offspring of the male rats with LF | The offspring of the male rats after 8 weeks of liver regeneration |
|---|---|---|---|
| Female failed to get pregnant | - | 2 | 3 |
| Female failed to give birth, died | - | 3 | 6 |
| Healthy offspring | 20 | 15 | 8 |
| The average number of offspring per birth | 9.55 ± 0.47 | 4.71 ± 0.53* | 4.25 ± 0.45* |
*p < 0.001 compared with healthy rats
After mating we repeated ultrasound studies. We measured sizes of the liver, spleen size, portal vein diameter, and size of the testes (Fig. 8). We found a strong correlation (r = 0.82; p < 0.01) between the size of the liver and portal vein diameter, and moderate correlation with ALT and AST levels (r = 0.63).
Fig. 8.
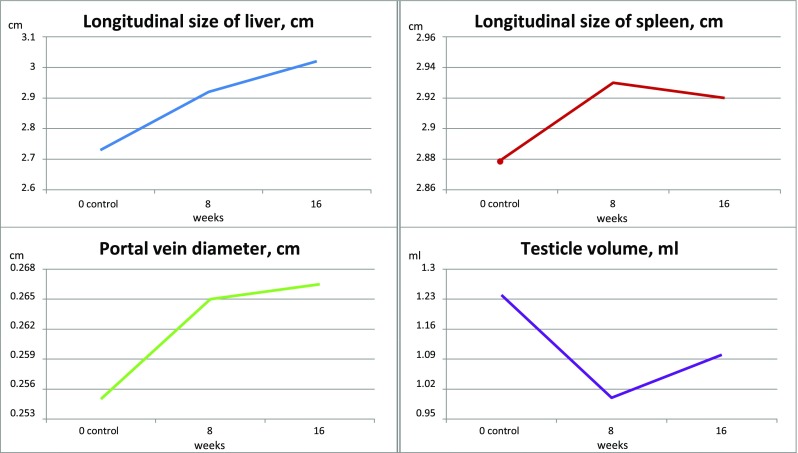
The changes of ultrasound parameters during the study
The manifestations of portal hypertension can vary and modify differences and association between liver and spleen size. Thus, the spleen size is an indirect manifestation of portal hypertension and can be considered as the most relevant parameter of liver fibrosis severity and the tendency to chronic disease progression or improvement.
On 8 week after the last injection we revealed manifestations of the liver regeneration, significant recovery of parenchyma, partial restoring of its lobular-girder structure, reducing the amount of lymphocytic infiltration and lipid inclusions in the hepatocytes. We found a large number of dual-nuclear and tri-nuclear hepatocytes that favor the self-restoring of parenchyma (Fig. 9).
Fig. 9.
Liver morphology of male rat on the 8th week of liver regeneration. Manifestations of the liver regeneration, partial restoring of its lobular-girder structure, reducing the amount of lymphocytic infiltration and lipid inclusions in the hepatocytes; a large number of dual-nuclear and tri-nuclear hepatocytes (a sign of regeneration)
The levels of ALT and AST significantly decreased from 1.95-fold increasing to the level of 1.46-fold increasing normal measurements respectively and reached initial levels.
On the US study we marked an improvement in the structure of the internal organs, while structural injuries of the testes were still identified.
Testicular histology after 8 weeks of liver regeneration (so called ‘self-healing’) did not show significant changes and testicular volume remained at levels as low as 0.65 ± 0.11 ml (changes non-significant).
The same males were mated with females again. As a result of the second interbreeding we noted: six females died during childbirth, three females were not pregnant, and the rest by an average of one female gave birth to 4.25 ± 0.45 rats (Table 1).
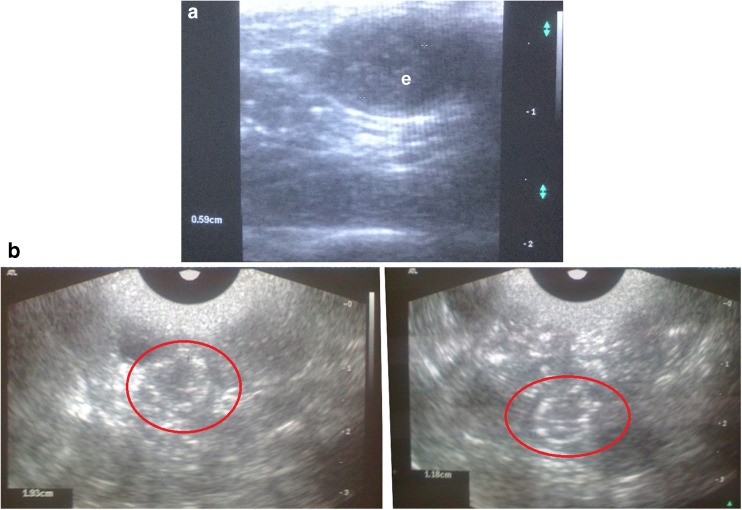
The pregnancy was successfully monitored on ultrasound (Fig. 10).
Fig. 10.
Ultrasound scans of pregnancy in rats. a – detecting embryo in gestational sac at the early term (e); b – several fetuses are visualized in uterus corns in the last trimester; red circles indicate the fetus skull bones (hyperechoic)
We found no significant difference between them, indicating that the improvement in the reproduction function of male rats that remained 2 months under the liver self-healing, was not observed.
Discussion
Mechanism and hypotheses of interplay between liver fibrosis and male fertility: role of inflammation, oxidative stress and direct toxicity
To our best knowledge, this is one of the initial studies of male reproductive function under the liver fibrosis model in the context of reversibility. To clarify background mechanics that are behind the reciprocity between liver disease and male fertility, the roles of several pathways need to be discussed.
The liver fibrosis development mechanism is complex and multifactorial [3, 33–35], and generally progresses from inflammation to fibrosis and finally tumorogenesis [35]. Its pathogenesis has been deeply investigated, especially regarding excessive triacylglycerols (TAGs’) accumulation in liver parenchyma [34].
Inflammation plays a crucial role in different human and experimental liver diseases [36], since the liver is a central immunological organ with a high exposure to circulating antigens and endotoxins from the gut microbiota, particularly enriched for innate immune cells (macrophages, innate lymphoid cells, mucosal-associated invariant T (MAIT) cells). In homeostasis, many mechanisms ensure suppression of immune responses, resulting in tolerance. Conserved mechanisms such as damage-associated molecular patterns (DAMPs, alarmins), Toll-like receptor signaling or inflammasome activation initiate inflammatory responses in the liver. The inflammatory activation of hepatic stellate and Kupffer cells results in the chemokine-mediated infiltration of neutrophils, monocytes, natural killer (NK), and natural killer T (NKT) cells [7].
A growing body of evidence has shown that liver autophagy contributes to basic hepatic functions [37].
Cytochrome P450–2E1 (CYP2E1) metabolizes a variety of small molecule substrates including long-chain fatty acids. Superoxide, the oxidative radical produced from CYP2E1-mediated metabolisms [24], can serve as part of the second hit to advance the severity of NAFLD. CYP2E1 expression in the liver was increased in humans and animal models of NAFLD. An important role of CYP2E1 in the development of NASH using high-fat diet (HFD) was reported [6, 38, 39]. CYP2E1 was suggested to be critically important in NASH development by promoting oxidative/nitrosative stress, protein modifications, inflammation, and insulin resistance [39]. The recent studies conclusively demonstrate that CYP2E1 is the major factor involved in the CCl4-induced hepatotoxicity, CYP2E1 was degraded during the process of CCl4-induced hepatotoxicity [29], while factors such as hyperammonemia and portosystemic shunting observed in cirrhotic animals also play important roles.
Recently the cellular localization of the constitutive expression of steroidogenic and non-steroidogenic cytochrome P450 (CYP) in rat testis was defined [40].
Thus, the toxicity of carbon tetrachloride (CCl 4 ) could also have direct effects on the reproductive system but also indirect ones, mediated by liver fibrosis.
However, the quantity/concentration of CYP in testis is not comparable with those in the liver parenchyma exceeding several times to be supposed as a target of CCl4-induced toxicity. Hence, the mechanics of of CCl4–induced toxicity is very complex, it seems very doubtful that direct toxic effect is prevailing on the reproductive system.
Recently the efficacy of plant-based drugs was studied on so called ‘carbon tetrachloride-induced testicular damage’ [41, 42]. The authors speculated that carbon tetrachloride can directly induce damages on the male reproductive system based on the assumption that CYP genes in the male reproductive organs are targets for CCl4, which causes usual oxidative damage to the lipids and proteins of the reproductive tissues [43]. However, significant increasing of protein carbonyl content was observed in the liver by 138% and only by 21% in testis, and by 51% in lungs. Similar mechanisms are probably responsible for the toxicity of CCl4 in humans [24].
Other molecular targets of CCl 4 in testes are unclear or probably insignificant and do not explain mechanics, reasonable focus is rather on the pituitary-gonadal axis.
However, although the researchers agree that liver is a main target of CCl4 they did not study liver function and did not consider this mechanism via endotoxicity related to LF, which on our opinion should be considered as largely significant. While evaluating efficacy of plant products on so called ‘carbon tetrachloride-induced sperm damages and testicular apoptosis’ [44], liver function has not been studied; however, studied antioxidants could reduce the toxic effects exerted by CCl4 upon testes likely through inhibition of CYP system, mostly represented in the liver tissue.
Few researchers consider liver-mediated mechanism in the study of impairment of reproductive function in a male rat [45].
The human studies demonstrated strong crosslinks between male reproduction and liver disease [46]. Thus, the problems with the male reproductive system are observed in many liver fibrosis models, and are reproducible in, e.g., on NSAIDs-induced LF (male rats are more susceptible to toxic injury according to preliminary data).
Use of adult (sexually mature) rats for modeling, time of development of testicular degeneration under conditions of advanced cirrhotic liver lesion and the dose used can indirectly support the ‘liver-mediated’ consequence hypothesis.
Thus, considering all the above, it is clear that we should stay at the hypothesis point, nevertheless some parameters allow us to be convinced that LF is among the major factors contributing to reproductive dysfunction.
In the study of CCl 4 -induced hepatotoxicity in pregnant and lactating rats the CYP2E1 level in the non-treated lactating rats tended to increase but remained at lower levels until PPD13 compared with that in non-pregnant rats [47]. Thus, the degree of CCl4-induced hepatotoxicity did not correspond to the CYP2E1 levels during lactation. The Authors suggested that during lactation, there may be certain factors other than CYP2E1 expression responsible for the degree of CCl4-induced hepatotoxicity [47].
MetS, LF and male infertility
Certainly, progress in the field of reproduction has been realized in the twenty-first century with advances in the understanding of the regulation of fertility [48], however, a large proportion of infertile males are still diagnosed as idiopathic, reflecting poor understanding of the basic mechanisms regulating spermatogenesis and sperm function [49, 50]. The current clinical evaluation of infertile couples is relatively simple and superficial [48]. Support for innovative reproductive medical care and preventive educational activity for couple health assessment for smart planning social life and reproduction is a large challenge [51, 52].
Currently, there is rigorous evidence to suggest a MetS–male infertility concept. It is known that obesity/overweight may result in hypogonadism, increased scrotal temperatures, impaired spermatogenesis, decreased sperm concentration and motility, and increased sperm DNA damage [53, 54]. Similarly, diabetes mellitus type 2/insulin resistance and dyslipidemia may further decrease fertility contributing to increasing oxidative stress in the testicular microenvironment [53–55].
Oxidative stress as a unified mechanism can trigger changes at an organism level that might serve as a strong link LF with male reproduction system injury [47], our recent data supported effectiveness of nanoceria as an antioxidative treatment of infertility [22]. Oxidative damage to proteins occurs in acute as well as chronic exposure of rats to CCl4 and it may contribute to the pathogenesis of liver injury, while lipid peroxidation plays a major role [29].
Diabetes is associated with increased sperm nuclear and mtDNA damage that may impair the reproductive capability of men [55–60].
MetS impact on fertility might be orchestrated with many pathways, e.g., monosodium glutamate is used for modeling obesity [61–63], and it was reported that MSG may cause partial infertility in male, therefore, the consumption of high dose MSG should be restricted in groups under risk [57].
Sperm apoptosis is related to male age, body mass index (BMI), testicular volume, and FSH. Among the apoptotic markers, only DNA denaturation has been found to predict natural pregnancy better than conventional sperm parameters [63].
Hepatocyte aquaporins (AQPs) are proteinaceous channels that allow facilitated permeation of water and uncharged through cellular membranes. AQPs are widespread in nature and play a number of important roles, e.g., in regulating hepatic TAG synthesis in NAFLD [64] and in subjects with obesity, insulin-resistance, and NAFLD [63]. AQP9 down-regulation and reduction in hepatic glycerol permeability in insulin-resistant conditions were interpreted in a way whereby the hepatocytes counteract further fat accumulation within its parenchyma and diminish hepatic gluconeogenesis during NAFLD. This patho-physiological gender-related pattern is important for both animal and human organism; thus, the extent of AQP9 protein and liver import of glycerol had a distinct profile of control in n3-PUFA (ω3 polyunsaturated fatty acids)-depleted female rats [9, 15, 18, 65].
One among the most important factors associated with decreasing spermatogenesis and steroidogenesis, is hypothalamus–pituitary axis dysregulation leading to reduction of Leydig cell count, which produced more than 60% of androgens, lowering density of LH receptors in the cells, and decreasing the synthesis of testosterone. The apoptosis has been shown to rise with age producing oxidative stress resulting in accelerated germ cell loss in the tissues [66]. Serum levels of testosterone, dihydrotestosterone were reported to become significantly lower in patients with cirrhosis; serum total and unbound E2, serum luteinizing hormone concentration increase in the cirrhotic patients; and an increase of E2 to testosterone ratio (calculated from serum concentration of total or unbound) in cirrhosis [67–69].
Liver transplantation can improve the reproductive function. The data of comparative study prior to and 6 months after liver transplantation demonstrated hypogonadism and feminization of male patients with advanced liver disease, irrespective of the etiology; these abnormalities rapidly improve after successful liver transplantation [70, 71]. After liver transplantation some alterations were reported to persist in some patients, both because of pre-existing gonadal alterations (toxic-metabolic damage) and immunosuppressive pharmacological side effects. Further studies will explain the relationship between hypogonadism and liver transplantation outcome in the early months and in the long term [71], and the role of androgen therapy.
Liver fibrosis/cirrhosis is usually accompanied by portal hypertension, that may lead to ascites, peripheral edema [72]. Congestive changes can affect internal organs and the endocrine system with manifestation in the decrease of libido and potency, testicular atrophy, gynecomastia, and increasing levels of 17 estrodiol β (E2), luteinizing hormone, and FSG. The changes in the balance between the circulating estrogen and androgen plays an important role in the pathogenesis of hepatic cirrhosis gynecomastia [71]. Liver cirrhosis can alter levels of prostate-specific antigen (PSA) [12]. The portal hypertension might also evoke congestive effects in the distant organs of various systems in pulmonary [73–75].
The effects of alcohol consumption on sperm parameters and male infertility have been investigated over the years [76–80]. Alcohol consumption is associated with a deterioration of sperm parameters which may be partially reversible [79, 80]. Testicular biopsy in male rats with alcohol-induced liver fibrosis showed significant histological changes: a decrease in the diameter of the seminiferous tubules and the number of germ cells. Nevertheless, alcohol impact on the male reproductive function is still controversial, its consumption does not seem to have much effect on fertility either in in vitro fertilization programs or population-based studies [80].
Alcohol-induced liver fibrosis was not considered as a core mechanism of impact on male infertility in the studies and still remains unclear.
The liver regeneration and reproductive system
The ancient Greeks were the first who articulated an idea of liver regeneration in the myth of Prometheus, who stolen the secret of fire from the gods of Olympus, and was punished—the eagle preyed on Prometheus’ liver in Caucasus mountains, which was renewed as fast as it was devoured [81]. This ancient myth was not far from reality and is close to a modern evidence-based knowledge, however mythical, on liver regeneration potential.
The normal liver has a remarkable regenerative capacity, acute injury or resection of liver can overload its regenerative ability in the setting two scenarios: development severe acute or chronic liver injury with aberrant liver architecture and fibrosis [16, 82].
The role of the hepatic stellate cell (HSC) as the key fibrogenic driver of hepatic fibrosis in response to chronic liver injury [82, 83], and are also now known to participate in adipokine, angiogenic, and neuroendocrine signaling, interact with other resident cell types, and be regulated by epigenetic and transcriptional mediators. HSCs are emphasized in the emerging mechanisms of the disease and their therapeutic implications. Recently the concept in HSC activation into proliferative, fibrogenic myofibroblasts was updated with novel mediators [83]. Extracellular signals from resident and inflammatory cells including macrophages, hepatocytes, liver sinusoidal endothelial cells, natural killer cells, natural killer T cells, platelets, and B cells further modulate HSC activation. Finally, pathways of HSC clearance have been greatly clarified, and include apoptosis, senescence, and reversion to an inactivated state. Extracellular signals converging upon HSCs to promote their activation include those originating from the extracellular matrix and stimuli from resident and infiltrating inflammatory cells; external stimuli, like high-cholesterol diet can increase liver fibrosis and activation of HSCs [83].
Hypoxia is a common environmental stress factor and is also associated with various physiological and pathological conditions such as fibrogenesis. HSCs activate through TGF-beta signaling pathway [84]. This can provide interesting insights for pre-metastatic niches according to seed and soil theory of cancer metastatic disease development [85].
Cytokine-dependent routine factors [16] indicated that liver regeneration had two routes: cytokine-dependent and non-cytokine-dependent routes, which involved a large amount of associated proteins. The completion of cytokine-dependent routine mainly depends on the involvement of TNF-α, IL-6, and other cytokines (like IL-1, etc.) [86]. IL-6 combines with its receptor IL-6R, whereas IL-6R combines with two sub-units of glycoprotein (GP), which can activate the activity of tyrosine kinase (JAK).
Both innate and adaptive immunity are involved to develop NAFLD [7, 87], the accumulation of pro-fibrogenic myofibroblasts is a central feature of tissue fibrosis; such cytokines as tumor necrosis factor and interleukin-6 induce LF exacerbation, whereas, IL-10 and adiponectin and others are protective [87]. Hedgehog pathway activation leads to hepatic enrichment with natural killer T cells contributing to fibrosis progression, and Kupffer cell depletion prevents the development of diet-induced steatosis and insulin resistance [87].
Autologous bone marrow-derived mesenchymal stem cell transplantation is a promising therapeutic tool promoting liver regeneration after portal vein embolization in cirrhotic rats [88]. Efforts have to be focused on the hepatic stellate cell, as these cells can undergo ‘activation’ into proliferative and fibrogenic myofibroblast-like cells during liver injury [89, 90].
The normal liver is known to attempt to retain an appropriate size relative to the whole body [89, 90].
After an injury or resection the rest of the liver undergoes a sequence of agreed changes to restore its initial volume and structure. This capability is necessary to preserve the previous size to the body weight ratio after hepatomegaly has been induced by such growth factors as triiodothyronine, when the liver decreases to the previous volume [16, 23, 45, 91]. Regeneration of the liver was observed on rat models [69], also on those induced by CCl4 [26–30], e.g., on liver fibrosis rat models in the studies by Weber et al. [28], Singh et al. [91] caused by intraperitoneal injection of CCl 4 (1 ml/kg body weight) twice a week during 2 weeks; a group of animals that were not given any tested treatment demonstrated liver self-repair (Singh, 2015) [91].
Thus, the results of many studies, testing beneficial treatments of liver might be biased due to regenerative potential of liver parenchyma.
Unanswered is the question of whether the liver fibrosis reverses when the toxin completely stops acting [92, 93]. A loong time could be needed for a significant regression, depending on the etiology, duration, and severity of fibrosis and molecular like catecholamine activity [92], fibrillar collagen type I, III, matrix metalloproteinases (MMP-1, −8, −9, and 13), etc. [92, 93].
While performing our rat model, we observed organ regeneration over time as observed in advanced fibrosis; after 12 weeks of model and self-restoring of the liver after the cirrhosis stage was formed. Importantly, note that the longer the liver fibrosis, the less significant the regenerative processes detected.
There are significant sex-related differences in liver fibrosis development under hepatotoxic drugs, including NSAIDs consumption. We recently hypothesized, that gender differences are possible on its effects on liver due to autoimmune hepatitis (type 1) vs drug-induced liver injury (DILI) paths [94], depending on the gender.
Thus, the achievement in understanding the treatment of liver disease should be based on high-quality non-invasive predictive diagnosis. Liver biopsy still remains the gold standard for assessing both diseases-specific pathologies and fibrosis stage, but newer approaches provide complementary information [95]. In general, chronic liver diseases consist of three steps: inflammation, fibrosis, and hepatocarcinogenesis [96]. Prediction risks of transfer to next step is a crucial task for hepatologists. A growing number of studies have demonstrated the accuracy of non-invasive methods to predict significant/advanced fibrosis and cirrhosis and to identify the presence/absence of fibrosis [97–99] and quantify the staging of hepatic fibrosis [100].
Consolidation of the PPPM concept
Thus, current research addresses a few novel findings, answers several questions and raises a few new in this large puzzle:
carbohydrate tetrachloride induces fast and severe liver fibrosis and is associated with irreversible micro-, macrostructural, and functional changes of male reproductive system;
LF hypothetically is a primary cause to induce changes in the male reproductive system, decreasing level of fertility and deteriorating potential pregnancy development if successful;
LF is reversible (on current model);
reproductive system deterioration was not reversible.
Hence, many of the issues of LF were studied in depth, several questions remain open and have been arisen during research as the following:
the toxicity of carbon tetrachloride could also have direct effects on the reproductive system and also indirect ones, mediated by liver fibrosis;
comprehensive mechanisms of liver fibrosis and its stratification;
mechanism of injury to the reproductive system in LF;
how functionally effective and comprehensive is the regenerative potential of the liver?
Is the reproductive dysfunction reversible? What is the regeneration potential of the male reproduction system, what is the molecular mechanism of this phenomena, what are the regulatory pathways beyond hormonal?
If such conditions are related to testosteron levels, can testosterone treatment potentially be used considering risks of inducing hepatocellular carcinoma (HCC) development risks?
How to predict the capacity and limits of liver regeneration using individual profiles?
The mechanics of self-healing body of liver when damaging agent action stops, and the following balance between regeneration and fibrosis under hepatic stellate cells (HSCs) activation.
What is the mechanism of destructive effects on potential pregnancy and health of mated females in this experiment?
Currently, there are limited prospective studies examining the effects of treating metabolic syndrome on male reproduction, and these relationships need to be a focus of further investigation; additional studies are needed to fully elucidate the pathophysiologic link between the components of MetS and male infertility.
Chronic liver disease and MetS including balance between health and disease and an interplay between a genetic component, epigenetic regulations, and environmental factors have been profoundly studied by experts of European Association for Predictive, Preventive and Personalized Medicine (EPMA) [19–21], the effective PPPM solutions were consequently suggested for the PPPM Men Health concept, described in detail in [22].
Predictive medical approach
Translation of the obtained results to the human organism is quite a challenge. Developing the integral LF panel of imaging and molecular biomarkers is an important point to predict individuals who are at risk of developing cirrhosis, HCC, and complications of LF and update national and international guidelines.
The basic message of this study is that a strong potential of liver for regeneration and severe complication in particular in males have been detected. Considering strong crosslinks between hormonal function and sex-dependent effects, perform reliable stratification of patients to pose tailored preventive measures and or personazlied inteventions to decreasing risks of transformation LF to HCC [101]. Hormonal status, a complication on reproductive system might be an additional endpoint to observe in such patients. Progress in defining the cellular and molecular basis of hepatic fibrosis has brought us to a juncture where translation of these discoveries into diagnostic tools and treatments is nearing reality [33]. It is crucial to finalize development and analyze all the existing non-invasive tools for LF in regard to their level of evidence and clinical accessibility with suggesting generalized protocol and update existing algorithms fort liver fibrosis in different kinds of pathology. Genetic markers that predict fibrosis progression risk, combined with new paradigms to stratify the risk of decompensation among patients with cirrhosis, should improve clinical trial design quality and patient selection for antifibrotic therapies [33]. The non-invasive markers besides sonoelastography, like FIB-4, aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio (AAR), AST to platelet count ratio (APRI), and platelet count to spleen diameter (PC/SD) ratio), etc. are definitely underestimated in the clinical set.
Smart reproduction planning and the planning of ones own social life is the earliest and most important time point for effective predictive medicine for future generations in a healthy society. Men Health programs based upon a comprehensive profiling including data of hypothalamic–pituitary–testis axis, microbiome, stress, psyche, emotions, pain, physical activity [102], microbiome and gut–brain axis (GBA), Flammer phenotype [85], and molecular and cellular mechanisms is an important point.
Preventive medical approach
Translation of the obtained data proceeding the animal model to a human organism would be relatively easy, considering the unified biological paradigm put in hypothesis. The strong message for prevention activity in men on liver fibrosis shoud be: No nocere! Do not harm liver with unnecessary medication that might be avoided because it might be successfully self-healed.
Regardless, advanced liver fibrosis is much less reversible in humans than in rodents [87], human liver has a huge potential to regenerate, only a few treatment interventions might be needed in particular groups of patients under risk to prevent development of cirrhosis, HCC, and infertility.
Screening, diagnosis, therapy, and prevention of male genital pathologies, such as varicocele, cryptorchidism, hernia, maldescent testes, testicular and prostate tumors, genitourinary infections, etc.; study infertility and erectile dysfunction in the population involved in sports activities, identify adverse environmental and occupational risk factors, and correct underlying nutritional imbalances. These findings support a cheap and effective preventive paradigm with highest value potentially provided [103, 104].
Personalized medical approach
Finding better noninvasive markers of fibrogenic activity and disease progression is a high priority in order to accelerate progress in developing novel treatments like antifibrotic drugs [33], and antagonists of HCC development [105].
Regenerative medicine successful development provides novel insights to hepatology. Thus, using adult autologous stem cell therapy and autologous biological materials, which recently proved to demonstrate a high level of efficacy in the large study for orthopedic conditions [106, 107], are potentially applicable and have promising perspectives for treatment of liver fibrosis and related diseases. Self-repair/regenerative capacity of using stem cell-based therapies could improve the outcomes in patients with liver fibrosis/cirrhosis [16]. Autologous bone marrow-derived mesenchymal stem cell transplantation is a promising therapeutic tool promoting liver regeneration after portal vein embolization in cirrhotic rats [88]. Novel therapeutic targets continue to be unearthed by new discoveries, including the emergence of the hepatic stellate cell (HSC) as an immunoregulatory cell type [33]. Futuristic technologies of reproductive medicine offer great promise in the near future; however, there are the moral and ethical issues associated with the use of embryonic stem cells. Adult spermatogonial stem cells offer the possibility of a unique source of pluripotent cells with the potential to restore spermatogenesis through autologous transplantation in cases of secondary infertility. These stem cells may also offer a renewable source of cells to be used to correct a variety of diseases of aging, to develop new cell-based therapies for a wide range of diseases and to advance germline gene therapy to ameliorate genetic diseases in offspring [48].
Nanomedicine demonstrates the perspectives to be an effective infertility treatment via reduction of oxidative stress in male reproductive organs, in particular in aging [26, 108].
Beneficial microbe-based drugs and gut-modifiing treatment have huge potential for correcting MetS, LF [109–115]. The growing levels of scientific and clinical evidence show how microbes influence the physiology in many body sites [113]. Well-designed unbiased multicenter studies on evaluation of the gut-microbiome-liver metabolic network and the intervention of these relationships using probiotics and potential prebiotic [116] with personalized nutrition are strongly required in the field.
Development of person-oriented diets is a focus of PPPM, which have a beneficial impact on many aspects of health to prevent liver diasese, and preserving male fertility is one of the pillar factors and an extraordinary task. Both diet and physical activity are neededto improve the many factors associated with the metabolic syndrome. Alcohol is extremely aggressive on liver, however, has a fair negative impact on the reproductive system [76–80]. Popular dietary supplements like monosodium glutamate [60–62] have been reported to evoke a toxic effect on the testicular structure in rats and evoke MetS. The vegetarian diet style that includes soy-based foods including increased levels of phytoestrogens beneficial for MetS and LF might be associated with a higher risk on the male reproductive system [117].
The likely risk factors, such as smoking and drinking habits, and the density and viability of sperm are suggested to be significant predictors of male infertility [118].
Conclusions
Carbohydrate tetrachloride induced injury of liver parenchyma evoking fast and severe liver fibrosis and associated with irreversible structural and functional changes in testes, reducing fertility, decreasing potential pregnancy rate and altered its development. Liver shows high potential for regeneration, however the self-restoring of liver fibrosis was not accompanied with recovery of reproductive system.
Outlooks and recommendations
This study posed several questions and left the hypothesis open regarding causality of a liver-induced mechanism on the reproductive system, since the toxicity of carbon tetrachloride could also have direct effects besides indirect ones, mediated by liver fibrosis.
We suggest to keep open the hypothesis that LF is a major contributing factor on male reproductive dysfunction and to conduct necessary follow up research in the matter.
Thus, comparative study of the male reproductive system on several liver fibrosis models is needed with extensive molecular panel evaluation.
We also recommend the following: to start specific programs on liver fibrosis in men, considering the sex-related nature and severity of complications of LF; to study gender differences in LF development—two different medicine approaches are required; to study the mechanism of acute vs chronic processes in LF and prediction of regeneration, understanding the interplay with function and regulation of the male reproductive systems.
We recommend to promote educational programs sharing knowledge to avoid toxic drug and liver fibrosis from unspecific and unproved treatments.
Limitations of the study
We are aware of several limitations in our research. First, it was difficult to completely exclude the direct toxic influence of CCl4 upon testes and considering its high toxicity on the whole of the organism as well. Secondly, the study has been done on animals. We considered but did not study at the current stage the issues like extensive metabonomics approach; inflammatory, cytokynes signaling pathways; regenerative potential, stem cells status; apoptosis; plasmin, matrix metalloproteinases, serum galectin-9 biomarker; microbiome and genetics. The use of Masson’s trichome stains might increase informativeness of liver and testes histology via detecting fibrosis. We did not measure obvious markers like testosterone and spermogram, and considered primary endpoint—birthgiving. We did not use sonoelastography to evaluate LF.
Acknowledgements
We acknowledge the EPMA Journal editorial team for the opportunity to publish this work.
Funding
Not applicable.
Compliance with ethical standards
Ethics approval and consent to participate
Not applicable.
Competing interests
Authors declare that they have no competing interests.
Contributor Information
Rostyslav V. Bubnov, Email: rostbubnov@gmail.com
Maria V. Drahulian, Email: parus_major@ukr.net
Polina V. Buchek, Email: evgenijjnuka@rambler.ru
Tamara P. Gulko, Email: ftp_2002@ukr.net
References
- 1.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterology. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–609. [DOI] [PubMed]
- 2.Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol. 2011;17(29):3377–3389. doi: 10.3748/wjg.v17.i29.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig J, Viggiano T, McGill D, Ott B. Nonalcoholic steatohepatitis. Mayo clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 5.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/S0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 6.Abdelmegeed MA, Choi Y, Godlewski G, Ha SK, Banerjee A, Jang S, Song BJ. Cytochrome P450-2E1 promotes fast food-mediated hepatic fibrosis. Sci Rep. 2017;7:39764. doi: 10.1038/srep39764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heymann F, Tacke F. Immunology in the liver--from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13(2):88–110. doi: 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 8.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Rato L, Alves MG, Socorro S, Duarte AI, Cavaco JE. Oliveira PF metabolic regulation is important for spermatogenesis. Nat Rev Urol. 2012;9(6):330–338. doi: 10.1038/nrurol.2012.77. [DOI] [PubMed] [Google Scholar]
- 10.Adekanle O, Ndububa DA, Orji EO, Ijarotimi O. Assessment of the sexual functions of males with chronic liver disease in south West Nigeria. Ann Afr Med. 2014;13(2):81–86. doi: 10.4103/1596-3519.129884. [DOI] [PubMed] [Google Scholar]
- 11.Zifroni A, Schiavi RC, Schaffer F. Sexual function and testosterone levels in men with nonalcoholic liver disease. Hepatology. 1991;14(3):479–482. doi: 10.1002/hep.1840140312. [DOI] [PubMed] [Google Scholar]
- 12.Vicentini FC, Botelho LA, Hisano M, Ebaid GX, Lucon M, Lucon AM, Srougi M. Are total prostate-specific antigen serum levels in cirrhotic men different from those in normal men? Urology. 2009;73(5):1032–1035. doi: 10.1016/j.urology.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Loria P, Carulli L, Bertolotti M, Lonardo A. Endocrine and liver interaction: the role of endocrine pathways in NASH. Nat Rev Gastroenterol Hepatol. 2009;6(4):236–247. doi: 10.1038/nrgastro.2009.33. [DOI] [PubMed] [Google Scholar]
- 14.Lonardo A, Carani C, Carulli N, Loria P. ‘endocrine NAFLD’ a hormonocentric perspective of nonalcoholic fatty liver disease pathogenesis. J Hepatol. 2006;44(6):1196–1207. doi: 10.1016/j.jhep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez A, Marinelli RA, Tesse A, Frühbeck G, Calamita G. Sexual dimorphism of adipose and hepatic Aquaglyceroporins in health and metabolic disorders. Front Endocrinol (Lausanne) 2015;6:171. doi: 10.3389/fendo.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forbes SJ, Newsome PN. Liver regeneration — mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol. 2016;13(8):473–485. doi: 10.1038/nrgastro.2016.97. [DOI] [PubMed] [Google Scholar]
- 17.Lutz W. Fertility rates and future population trends: will Europe's birth rate recover or continue to decline? Int J Androl. 2006;29(1):25–33. doi: 10.1111/j.1365-2605.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 18.Alves MG, Martins AD, Rato L, Moreira PI, Socorro S, Oliveira PF. Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Biochim Biophys Acta. 2013;1832(5):626–635. doi: 10.1016/j.bbadis.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Golubnitschaja O, Costigliola V, EPMA. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, preventive and personalised medicine. EPMA J. 2012;3(1):14. [DOI] [PMC free article] [PubMed]
- 20.Abraham JA, Golubnitschaja O, Akhmetov I, Andrews RJ, et al. EPMA-World Congress 2015 EPMA J. 2016;7(Suppl 1):9. 10.1186/s13167-016-0054-6.
- 21.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, Krapfenbauer K, Mozaffari MS, Costigliola V. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobyliak NM, Falalyeyeva TM, Kuryk OG, Beregova TV, Bodnar PM, Zholobak NM, Shcherbakov OB, Bubnov RV, Spivak MY. Antioxidative effects of cerium dioxide nanoparticles ameliorate age-related male infertility: optimistic results in rats and the review of clinical clues for integrative concept of men health and fertility. EPMA J. 2015;6:12. doi: 10.1186/s13167-015-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cameron GR, Karunaratne WAE. Carbon tetrachloride cirrhosis in relation to liver regeneration. J Pathol. 1936;42(1):1–21. doi: 10.1002/path.1700420104. [DOI] [Google Scholar]
- 24.Abraham P, Wilfred G, Cathrine SP. Oxidative damage to the lipids and proteins of the lungs, testis and kidney of rats during carbon tetrachloride intoxication. Clin Chim Acta. 1999;289:177–179. doi: 10.1016/S0009-8981(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 25.Dragulyan MV, Gulko TP, Kordium VA, Bubnov RV, Deryabina EG. Modelling of toxic liver damage on line ICR mice. Sci Rise. 2014;4/1(4):13–20. 10.15587/2313-8416.2014.29151.
- 26.Gulko TP, Dragulyan MV, Rymar SE, Kordium VA, Levkiv MU, Bubnov RV. Simulation of liver cirrhosis in rats wistar different ages. Faktori eksperimental'noi evolucii organizmiv ISSN 2219–3782. 2013;12:111–114. http://www.utgis.org.ua/journals/index.php/Faktory/article/view/47 Accessed 28.07.2017.
- 27.Gulko TP, Dragulyan MV, Deryabina EG, Kordium VA, Levkiv MU, Bubnov RV. Morphological characteristics of processes injury compensation and devices in pathological changes liver CCl4 Faktori eksperimental'noi evolucii organizmiv. 2014;15:39–44. Accessed 28.02.2017 http://utgis.org.ua/journals/index.php/Faktory/article/view/295/333.
- 28.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33(2):105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 29.Sundari PN, Wilfred G, Ramakrishna B. Does oxidative protein damage play a role in the pathogenesis of carbon tetrachloride-induced liver injury in the rat? Biochim Biophys Acta. 1997;1362(2–3):169–176. doi: 10.1016/S0925-4439(97)00065-3. [DOI] [PubMed] [Google Scholar]
- 30.Manibusan MK, Odin M, Eastmond DA. Postulated carbon tetrachloride mode of action: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25(3):185–209. doi: 10.1080/10590500701569398. [DOI] [PubMed] [Google Scholar]
- 31.Wang M-J, Ling W-W, Wang H, Meng L-W, Cai H, Peng B. Non-invasive evaluation of liver stiffness after splenectomy in rabbits with CCl4-induced liver fibrosis. World J Gastroenterol. 2016;22(46):10166–10179. doi: 10.3748/wjg.v22.i46.10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bubnov RV, Spivak MY, Zholobak NM. Method for experiment to simulate biological processes Patent Ukraine. 2013, UA78082 U -IPC A61B10/00; A61B8/00; A61D99/00, issued 2013.03.11 (Bull. N 5). https://worldwide.espacenet.com/publicationDetails/biblio?FT=D&date=20130311&DB=&locale=en_EP&CC=UA&NR=78082U&KC=U&ND=4 Accessed 28.07.2017.
- 33.Friedman SL. Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1(2):98–105. doi: 10.1038/ncpgasthep0055. [DOI] [PubMed] [Google Scholar]
- 34.Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–171. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 35.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12(7):387–400. doi: 10.1038/nrgastro.2015.94. [DOI] [PubMed] [Google Scholar]
- 37.Ueno T, Komatsu M. Autophagy in the liver: functions in health and disease. Nat Rev Gastroenterol Hepatol. 2017;14(3):170–184. doi: 10.1038/nrgastro.2016.185. [DOI] [PubMed] [Google Scholar]
- 38.Aubert J, Begriche K, Knockaert L, Robin MA, Fromenty B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clin Res Hepatol Gastroenterol. 2011;35(10):630–637. doi: 10.1016/j.clinre.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, Song BJ. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol. 2012;57(4):860–866. doi: 10.1016/j.jhep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilibili RR, Vogl AW, Chang TK, Bandiera SM. Localization of cytochrome P450 and related enzymes in adult rat testis and downregulation by estradiol and bisphenol a. Toxicol Sci. 2014;140(1):26–39. doi: 10.1093/toxsci/kfu070. [DOI] [PubMed] [Google Scholar]
- 41.Yüce A, Türk G, Çeribaşı S, Güvenç M, Çiftçi M, Sönmez M, Özer Kaya Ş, Çay M, Aksakal M. Effectiveness of cinnamon (Cinnamomum Zeylanicum) bark oil in the prevention of carbon tetrachloride-induced damages on the male reproductive system. Andrologia. 2014;46(3):263–272. doi: 10.1111/and.12072. [DOI] [PubMed] [Google Scholar]
- 42.Sönmez M, Türk G, Çeribaşı S, Çiftçi M, Yüce A, Güvenç M, Özer Kaya S, Çay M, Aksakal M. Quercetin attenuates carbon tetrachloride-induced testicular damage in rats. Andrologia. 2014;46(8):848–858. doi: 10.1111/and.12159. [DOI] [PubMed] [Google Scholar]
- 43.Kalla NR, Bansal MP. Effect of carbon tetrachloride on gonadal physiology in male rats. Acta Anat (Basel) 1975;91:380–385. doi: 10.1159/000144399. [DOI] [PubMed] [Google Scholar]
- 44.Türk G, Çeribaşı S, Sönmez M, Çiftçi M, Yüce A, Güvenç M, Kaya ŞÖ, Çay M, Aksakal M. Ameliorating effect of pomegranate juice consumption on carbon tetrachloride-induced sperm damages, lipid peroxidation, and testicular apoptosis. Toxicol Ind Health. 2016;32(1):126–137. doi: 10.1177/0748233713499600. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Liu L, Wang B, Xiong J, Li Q, Wang J, Chen D. Impairment of reproductive function in a male rat model of non-alcoholic fatty liver disease and beneficial effect of N-3 fatty acid supplementation. Toxicol Lett. 2013;222(2):224–232. doi: 10.1016/j.toxlet.2013.05.644. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Liu L, Wang B, Chen D, Wang J. Nonalcoholic fatty liver disease and alteration in semen quality and reproductive hormones. Eur J Gastroenterol Hepatol. 2015;27(9):1069–1073. doi: 10.1097/MEG.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 47.Mochizuki M, Shimizu S, Urasoko Y, Umeshita K, Kamata T, Kitazawa T, Nakamura D, Nishihata Y, Ohishi T, Edamoto H. Carbon tetrachloride-induced hepatotoxicity in pregnant and lactating rats. J Toxicol Sci. 2009;34(2):175–181. doi: 10.2131/jts.34.175. [DOI] [PubMed] [Google Scholar]
- 48.Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14(11):1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferlin A, Raicu F, Gatta V, Zuccarello D, Palka G, Foresta C. Male infertility: role of genetic background. Reprod BioMed Online. 2007;14(6):734–745. doi: 10.1016/S1472-6483(10)60677-3. [DOI] [PubMed] [Google Scholar]
- 50.Vogt PH. Molecular genetic of human male infertility: from genes to new therapeutic perspectives. Curr Pharm Des. 2004;10(5):471–500. doi: 10.2174/1381612043453261. [DOI] [PubMed] [Google Scholar]
- 51.Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G, et al., EAU Working Group on Male Infertility. European Association of Urology guidelines on male infertility: the 2012 update. Eur Urol. 2012;62(2):324–32. [DOI] [PubMed]
- 52.Jungwirth A, Diemer T, Dohle GR, Giwercman A, Kopa Z, Krausz C, Tournaye H. The European Association of Urology (EAU) Guidelines Panel on Male Infertility 2015. https://uroweb.org/wp-content/uploads/17-Male-Infertility_LR1.pdf] Accessed 05 Aug 2017.
- 53.Kasturi SS, Tannir J, Brannigan RE. The metabolic syndrome and male infertility. J Androl. 2008;29(3):251–259. doi: 10.2164/jandrol.107.003731. [DOI] [PubMed] [Google Scholar]
- 54.Morrison CD, Brannigan RE. Metabolic syndrome and infertility in men. Best Pract Res Clin Obstet Gynaecol. 2015;29(4):507–515. doi: 10.1016/j.bpobgyn.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, Mallidis C, et al. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;22(7):1871–1877. doi: 10.1093/humrep/dem077. [DOI] [PubMed] [Google Scholar]
- 56.Sheweita SA, Tilmisany AM, Al-Sawaf H. Mechanisms of male infertility: role of antioxidants. Curr Drug Metab. 2005;6(5):495–501. doi: 10.2174/138920005774330594. [DOI] [PubMed] [Google Scholar]
- 57.La Vignera S, Calogero AE, Condorelli R, Lanzafame F, Giammusso B, Vicari E. Andrological characterization of the patient with diabetes mellitus. Minerva Endocrinol. 2009;34(1):1–9. [PubMed] [Google Scholar]
- 58.Bener A, Al-Ansari AA, Zirie M, Al-Hamaq AO. Is male fertility associated with type 2 diabetes mellitus? Int Urol Nephrol. 2009;41(4):777–784. doi: 10.1007/s11255-009-9565-6. [DOI] [PubMed] [Google Scholar]
- 59.Von Diemen V, Trindade EN, Trindade MR. Experimental model to induce obesity in rats. Acta Cir Bras. 2006;21:425–429. doi: 10.1590/S0102-86502006000600013. [DOI] [PubMed] [Google Scholar]
- 60.Hassan ZA, Arafa MH, Soliman WI, Atteia HH, Al-Saeed HF. The effects of monosodium glutamate on Thymic and Splenic immune functions and role of recovery (biochemical and histological study) J Cytol Histol. 2014;5:283. [Google Scholar]
- 61.Nosseir NS, Ali MM, Ebaid HM. A histological and morphometric study of monosodium glutamate toxic effect on testicular structure and potentiality of recovery in adult albino rat. Res J Biol. 2012;2(2):66–78. http://scientific-journals.co.uk/web_documents/2020210_albino_rats.pdf Accessed 28.07.2017.
- 62.Savcheniuk OA, Virchenko OV, Falalyeyeva TM, Beregova TV, Babenko LP, Lazarenko LM, et al. The efficacy of probiotics for monosodium glutamate-induced obesity: dietology concerns and opportunities for prevention. EPMA J. 2014;5(1):2. doi: 10.1186/1878-5085-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zorn B, Golob B, Ihan A, Kopitar A, Kolbezen M. Apoptotic sperm biomarkers and their correlation with conventional sperm parameters and male fertility potential. J Assist Reprod Genet. 2012;29(4):357–364. doi: 10.1007/s10815-012-9718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gena P, Mastrodonato M, Portincasa P, Fanelli E, Mentino D, Rodríguez A, Marinelli RA, Brenner C, Frühbeck G, Svelto M, et al. Liver glycerol permeability and aquaporin-9 are dysregulated in a murine model of non-alcoholic fatty liver disease. PLoS One. 2013;8:1096. doi: 10.1371/journal.pone.0078139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodríguez A, Gena P, Méndez-Gimenez L, Rosito A, Valentí V, Rotellar F, Sola I, Moncada R, Silva C, Svelto M, et al. Reduced hepatic aquaporin-9 and glycerol permeability are related to insulin resistance in non-alcoholic fatty liver disease. Int J Obes. 2014;38:1213–1220. doi: 10.1038/ijo.2013.234. [DOI] [PubMed] [Google Scholar]
- 66.Levy S, Robaire B. Segment-specific changes with age in the expression of junctional proteins and the permeability of the blood-epididymis barrier in rats. Biol Reprod. 1999;60(6):1392–1401. doi: 10.1095/biolreprod60.6.1392. [DOI] [PubMed] [Google Scholar]
- 67.Lloyd CW, Williams RH. Endocrine changes associated with Laennec's cirrhosis of the liver. Am J Med. 1948;4(3):315–330. doi: 10.1016/0002-9343(48)90248-4. [DOI] [PubMed] [Google Scholar]
- 68.Galvão-Teles A, Anderson DC, Burke CW, Marshall J, Corker CS, Bown RL, Clark ML. Biologically active androgens and œstradiol in men with chronic liver disease. Lancet. 1973;301(7796):173–177. doi: 10.1016/S0140-6736(73)90005-6. [DOI] [PubMed] [Google Scholar]
- 69.Chopra I, Tulchinsky JD, Greenway FL. Estrogen-androgen imbalance in hepatic cirrhosis: studies in 13 male patients. Ann Intern Med. 1973;79(2):198–203. doi: 10.7326/0003-4819-79-2-198. [DOI] [PubMed] [Google Scholar]
- 70.Guéchot J, Chazouillères O, Loria A, Hannoun L, Balladur P, Parc R, Giboudeau J, Poupon R. Effect of liver transplantation on sex-hormone disorders in male patients with alcohol-induced or post-viral hepatitis advanced liver disease. J Hepatol. 1994;20(3):426–430. doi: 10.1016/S0168-8278(94)80020-0. [DOI] [PubMed] [Google Scholar]
- 71.Foresta C, Schipilliti M, Ciarleglio FA, Lenzi A, D'Amico D. Male hypogonadism in cirrhosis and after liver transplantation. Endocrinol Invest. 2008;31(5):470–478. doi: 10.1007/BF03346393. [DOI] [PubMed] [Google Scholar]
- 72.Al-Busafi SA, McNabb-Baltar J, Farag A, Hilzenrat N. Clinical manifestations of portal hypertension. Int J Hepatol. 2012;2012:203794. doi: 10.1155/2012/203794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bozbas SS, Bozbas H. Portopulmonary hypertension in liver transplant candidates. World J Gastroenterol. 2016;22(6):2024–2029. doi: 10.3748/wjg.v22.i6.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Machicao VI, Balakrishnan M, Fallon MB. Pulmonary complications in chronic liver disease. Hepatology. 2014;59(4):1627–1637. doi: 10.1002/hep.26745. [DOI] [PubMed] [Google Scholar]
- 75.Das M, Boerma M, Goree JR, Lavoie EG, Fausther M, Gubrij IB, Pangle AK, Johnson LG. Dranoff JA pathological changes in pulmonary circulation in carbon tetrachloride (CCl4)-induced cirrhotic mice. PLoS One. 2014;9(4):e96043. doi: 10.1371/journal.pone.0096043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Emanuele MA, Emanuele NV. Alcohol's Effects on Male Reproduction. Alcohol Health and Research World 1998;22(3):195. https://pubs.niaaa.nih.gov/publications/arh22-3/195.pdf Accessed 25.07.2017. [PMC free article] [PubMed]
- 77.Cicero TJ, Badger TM. Effects of alcohol on the hypothalamic-pituitary-gonadal axis in the male rat. J Pharmacol Exp Ther. 1977;201(2):427–433. [PubMed] [Google Scholar]
- 78.van Thiel DH, Gavaler JS, Cobb CF, Sherins RJ, Lester R. Alcohol-induced testicular atrophy in the adult male rat. Endocrinology. 1979;105(4):888–895. doi: 10.1210/endo-105-4-888. [DOI] [PubMed] [Google Scholar]
- 79.Condorelli RA, Calogero AE, Vicari E, La Vignera S. Chronic consumption of alcohol and sperm parameters: our experience and the main evidences. Andrologia. 2015;47(4):368–379. doi: 10.1111/and.12284. [DOI] [PubMed] [Google Scholar]
- 80.La Vignera S, Condorelli RA, Balercia G, Vicari E, Calogero AE. Does alcohol have any effect on male reproductive function? A review of literature. Asian J Androl. 2013;15(2):221–5. 10.1038/aja.2012.118. [DOI] [PMC free article] [PubMed]
- 81.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5(10):836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 82.Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7(8):425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- 83.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14(7):397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 84.Shi YF, Fong CC, Zhang Q, Cheung PY, Tzang CH, Wu RS, Yang M. Hypoxia induces the activation of human hepatic stellate cells LX-2 through TGF-beta signaling pathway. FEBS Lett. 2007;581(2):203–210. doi: 10.1016/j.febslet.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 85.Bubnov R, Polivka J, Jr, Zubor P, Koniczka K, Golubnitschaja O. Pre-metastatic niches in breast cancer: are they created by or prior to the tumour onset? “Flammer syndrome” relevance to address the question. EPMA J. 2017;8:141–157. doi: 10.1007/s13167-017-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hart KM, Fabre T, Sciurba JC, Gieseck RL 3rd, Borthwick LA, Vannella KM, Acciani TH, de Queiroz Prado R, Thompson RW, White S, Soucy G, Bilodeau M, Ramalingam TR, Arron JR, Shoukry NH, Wynn TA. Type 2 immunity is protective in metabolic disese but exacerbates NAFLD collaboratively with TGF-β. Sci Transl Med. 2017;9(396). 10.1126/scitranslmed.aal3694. [DOI] [PubMed]
- 87.Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14(3):181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 88.Li T, Zhu J, Ma K, Liu N, Feng K, Li X, Wang S, Bie P. Autologous bone marrow-derived mesenchymal stem cell transplantation promotes liver regeneration after portal vein embolization in cirrhotic rats. J Surg Res. 2013;184(2):1161–1173. doi: 10.1016/j.jss.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 89.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176(1):2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213(2):286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh VK, Kalsan M, Kumar N, Saini A, Chandra R. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3:2. doi: 10.3389/fcell.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arthur MJ. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology. 2002;122:1525–1528. doi: 10.1053/gast.2002.33367. [DOI] [PubMed] [Google Scholar]
- 93.Issa R. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix crosslinking. Gastroenterology. 2004;126:1795–1808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 94.Spivak MY, Bubnov RV, Tymoshok NO. Sposib modelyuvannya medykamentoznoho urazhennya pechinki shchuriv (Method of simulation of drug-induced liver damage in rats). Patent Ukraine. 2015, 98459U -IPC A61B 1/00, issued 27.04.2015 (Bull. N 8). Accessed 28.02.2017 http://uapatents.com/11-98459-sposib-modelyuvannya-medikamentoznogo-urazhennya-pechinki-shhuriv.html Accessed 25.07.2017.
- 95.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 96.Zhang ZY, Dong JH, Chen YW, Wang XQ, Li CH, Wang J, Wang GQ, Li HL, Wang XD. Galectin-9 acts as a prognostic factor with antimetastatic potential in hepatocellular carcinoma. Asian Pac J Cancer Prev. 2012;13(6):2503–2509. doi: 10.7314/APJCP.2012.13.6.2503. [DOI] [PubMed] [Google Scholar]
- 97.Stasi C, Milani S. Evolving strategies for liver fibrosis staging: non-invasive assessment. World J Gastroenterol. 2017;23(2):191–196. doi: 10.3748/wjg.v23.i2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.European Association for Study of Liver Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 99.Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR, Cosgrove D, Dietrich CF, Amy D, Bamber JC, Barr R, Chou YH, Ding H, Farrokh A, Friedrich-Rust M, Hall TJ, Nakashima K, Nightingale KR, Palmeri ML, Schafer F, Shiina T, Suzuki S, Kudo M. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 3: liver. Ultrasound Med Biol. 2015;41(5):1161–1179. doi: 10.1016/j.ultrasmedbio.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 100.Friedrich-Rust M, Poynard T, Castera L. Critical comparison of elastography methods to assess chronic liver disease. Nat Rev Gastroenterol Hepatol. 2016;13(7):402–411. doi: 10.1038/nrgastro.2016.86. [DOI] [PubMed] [Google Scholar]
- 101.Abraham JA, Golubnitschaja O. Time for paradigm change in management of hepatocellular carcinoma: is a personalized approach on the horizon? Pers Med. 2016;13(5):455–67. [DOI] [PubMed]
- 102.NY Dynamic Neuromuscular Rehabilitation & Physical Therapy Clinic. https://nydnrehab.com/ Accessed 05 Aug 2017.
- 103.Akhmetov I, Bubnov RV. Innovative payer engagement strategies: will the convergence lead to better value creation in personalized medicine? EPMA J. 2017;8:1. doi: 10.1007/s13167-017-0078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Akhmetov I, Bubnov RV. Assessing value of innovative molecular diagnostic tests in the concept of predictive, preventive, and personalized medicine. EPMA J. 2015;6:19. doi: 10.1186/s13167-015-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deli A, Kreidl E, Santifaller S, Trotter B, Seir K, Berger W, Schulte-Hermann R, Rodgarkia-Dara C, Grusch M. Activins and activin antagonists in hepatocellular carcinoma. World J Gastroenterol. 2008;14(11):1699–1709. doi: 10.3748/wjg.14.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop. 2016;40:1755–1765. doi: 10.1007/s00264-016-3162-y. [DOI] [PubMed] [Google Scholar]
- 107.Bubnov R, Yevseenko V, Semeniv I. Ultrasound guided injections of platelets rich plasma for muscle injury in professional athletes. Comparative study Med Ultrason. 2013;15(2):101–105. doi: 10.11152/mu.2013.2066.152.rb1vy2. [DOI] [PubMed] [Google Scholar]
- 108.Kobyliak N, Virchenko O, Falalyeyeva T, Kondro M, Beregova T, Bodnar P, Shcherbakov O, Bubnov R, Caprnda M, Delev D, Sabo J, Kruzliak P, Rodrigo L, Opatrilova R, Spivak M. Cerium dioxide nanoparticles possess anti-inflammatory properties in the conditions of the obesity-associated NAFLD in rats. Biomed Pharmacother. 2017;90:608–614. doi: 10.1016/j.biopha.2017.03.099. [DOI] [PubMed] [Google Scholar]
- 109.Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016;65:2035–2044. doi: 10.1136/gutjnl-2016-312729. [DOI] [PubMed] [Google Scholar]
- 110.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Chen Y, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513(7516):59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 111.Minemura M, Shimizu Y. Gut microbiota and liver diseases. World J Gastroenterol. 2015;21(6):1691–1702. doi: 10.3748/wjg.v21.i6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bubnov RV, Spivak MY, Lazarenko LM, Bomba A, Boyko NV. Probiotics and immunity: provisional role for personalized diets and disease prevention. EPMA J. 2015;6(1):14. doi: 10.1186/s13167-015-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reid G, Abrahamsson T, Bailey M, Bindels LB, Bubnov R, Ganguli K, et al. How do probiotics and prebiotics function at distant sites? Benef Microbes. 2017;8(4):521–33. 10.3920/BM2016.0222. [DOI] [PubMed]
- 114.Rincón D, Vaquero J, Hernando A, Galindo E, Ripoll C, Puerto M, Salcedo M, Francés R, Matilla A, Catalina MV, et al. Oral probiotic VSL#3 attenuates the circulatory disturbances of patients with cirrhosis and ascites. Liver Int. 2014;34(10):1504–1512. doi: 10.1111/liv.12539. [DOI] [PubMed] [Google Scholar]
- 115.Marlicz W, Wunsch E, Mydlowska M, Milkiewicz M, Serwin K, Mularczyk M, Milkiewicz P, Raszeja-Wyszomirska J. The effect of short term treatment with probiotic VSL#3 on various clinical and biochemical parameters in patients with liver cirrhosis. J Physiol Pharmacol. 2016;67(6):867–877. [PubMed] [Google Scholar]
- 116.Konopelniuk VV, Goloborodko II, Ishchuk TV, Synelnyk TB, Ostapchenko LI, Spivak MYa, Bubnov RV. Efficacy of Fenugreek-based bionanocomposite on renal dysfunction and endogenous intoxication in high-calorie diet-induced obesity rat model—comparative study. EPMA J. 2017. 10.1007/s13167-017-0098-2. [DOI] [PMC free article] [PubMed]
- 117.Cederroth CR, Zimmermann C, Nef S. Soy, phytoestrogens and their impact on reproductive health. Mol Cell Endocrinol. 2012;355(2):192–200. doi: 10.1016/j.mce.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 118.Chia SE, Lim ST, Tay SK, Lim ST. Factors associated with male infertility: a case-control study of 218 infertile and 240 fertile men. BJOG. 2000;107(1):55–61. doi: 10.1111/j.1471-0528.2000.tb11579.x. [DOI] [PubMed] [Google Scholar]