Abstract
Purpose
In this single institution retrospective study of patients with stage I medically inoperable non-small cell lung cancer (NSCLC) treated with stereotactic ablative radiotherapy (SABR) we attempt to model overall survival (OS) using initial prognostic variables with specific attention on the Charlson co-morbidity index (CCI).
Methods
Between 2008 and 2013, 335 patients with medically inoperable stage I NSCLC were treated with SABR or hypofractionated radiotherapy (50–60 Gy in at least 5 Gy or 4 Gy fractions respectively) at our institution. Medical comorbidities and Charlson scores were determined by individual chart review. Patients were stratified into 3 groups based on the CCI score (0–1, 2–3, 4–9) and again based on the age-adjusted Charlson Comorbidity score (aCCI). Cumulative survival for each stratum was determined using the Kaplan-Meier method. Non-significant and confounding variables were identified and discounted from survival modeling. 3 sex stratified Cox regression models were tested: (1) aCCI with age and comorbidity combined; (2) age and CCI; (3) age alone, comorbidity removed.
Results
The median survival was 4.4 years and the median follow up 4.7 years. The median CCI and aCCI scores were 2 and 5 respectively. Patients with aCCI 7–12 had an increased hazard of death on univariate analysis HR 2.45 (1.15–5.22 95%CI, p = 0.02) and -excluding age as a competing variable- on multivariate analysis HR 2.25 (1.04–4.84 95%CI, p = 0.04). Patients with CCI 4-9 had an increased hazard of death on univariate analysis HR 1.57(1.30–2.90) but not on multivariate analysis. On formalized testing – with either continuous or categorical variables- all three survival models yielded similar coefficients of effect.
Conclusion
We identify male gender, weight loss greater than 10% and age as independent prognostic factors for patients treated with medically inoperable NSCLC treated with SABR or hypofractionated radiotherapy. Based on our survival models, age alone can be used interchangeably with aCCI or CCI plus age with the same prognostic value. Age is more reliably recorded, less prone to error and therefore a more useful metric than Charlson score in this group of patients.
Keywords: Charlson Comorbidity Index, SABR, Non-small cell lung cancer, Medically inoperable
Introduction
Worldwide, lung cancer is the second most common cancer (13% of all cancers) and the leading cause of cancer deaths (19.4% of cancer deaths) [1]. Stage I (T1-2a, N0) accounts for 18% of lung cancer cases [2]. For patients with stage I non-small cell lung cancer (NSCLC), surgical resection is the standard of care [3]. However, up to 25% of patients with early stage NSCLC are medically inoperable due to co-morbidities such as heart disease, emphysema and advanced age [4]. Stereotactic ablative radiotherapy (SABR) is an excellent alternative to surgery in those patients. The local control rates for SABR approach those of lobectomy (88–92% at 3 years) [5], [6], [7]. On the other hand, the reported long term survival rates vary widely and are generally inferior to surgical outcomes for the same stage patient [5]. There is a spectrum of comorbidity in medically inoperable patients that may account for variations in survival. How can we predict treatment outcomes in a group of patients with widely varying medical constitution?
The Charlson Comorbidity Index (CCI) was designed as a measure of risk of mortality (based on comorbidity) in longitudinal studies [8]. It is a weighted index that takes into account the number and the seriousness of comorbid diseases by assigning points for certain illnesses. The CCI score is the sum of the points for each disease. The goal is to create a categorical variable (CCI) where the score is proportional to the burden of disease. The age adjusted score (aCCI) assigns an additional point for each decade above the fourth: The higher the score the older and “sicker” the patient. It is not always clear whether studies using the “Charlson Comorbidity” score are referencing aCCI or CCI.
The aCCI has been applied to various clinical scenarios, including other domains of lung cancer. It is tempting to use given its simplicity. We attempt to correlate both aCCI and CCI to outcome with retrospective analysis in medically inoperable stage I NSCLC. Our objective is to further define prognostic factors that can be used to select patients who benefit from aggressive management and possibly those who may avoid it altogether.
Methods
Study population
A single institution ethics approved database for medically inoperable early stage 1 NSCLC treated with radiotherapy at our institution, records outcomes for patients treated between November 1994 and December 2013. The database was created for the purpose of observing patterns of failure post radiotherapy. Patients from the database were deemed eligible for the present analysis if they met the following criteria: (1) Pathologic confirmation of NSCLC; (2) clinical stage I lung cancer according to the American Joint Committee in Cancer (AJCC) 7th edition staging manual; (3) treatment with curative intent and with SABR or hypofractionated radiotherapy alone (we define SABR as 50 to 60 Gy delivered in at least 5 Gy fractions and hypofractionated radiotherapy as 50 – 60 Gy in at least 4 Gy fractions). Patients who had received previous thoracic radiation, had a synchronous malignancy more than stage I, or metastatic disease were excluded. 335 patients satisfied these criteria (210 were excluded). After completing their treatment, the patients were followed as per our institution’s usual protocol with chest imaging (CT or X-ray) every 3 months for the first year and then every 6 months until the end of 5 years. Overall survival (OS) is defined as the time between biopsy and death of any cause.
Statistical analysis
The aCCI was calculated for each patient based on the age and medical conditions recorded in the health record at the time of diagnosis. The patients were stratified into three groups based on the aCCI score (1–4, 5–6 and 7–12). In an effort to further separate the impact of comorbidity from age, the CCI was similarly calculated for all patients based on the conditions recorded in the health record. Likewise they were stratified into three groups based on the CCI score (0–1, 2–3, 4–9). Cumulative survival for each stratum was determined using the Kaplan–Meier method. The log-rank test was used to compare OS between aCCI groups and between CCI groups.
With special attention to the Charlson comorbidity score, the variables listed in Table 1 were used to generate a series of statistical models to predict OS in this patient group. The selection of variables begins with a univariate analysis followed by evaluation of the proportional hazards assumption and a purposeful selection of variables in the model [17]. Variables with p-values greater than 0.2 on univariate analysis were not included in subsequent multivariate analyses. In the variable selection process we used the Wald test from logistic regression and a p-value cut-off point of 0.1 to remove the non-significant and confounding variables. After this step, the model is left with variables significant at the 0.05 level and not confounders.
Table 1.
Patient characteristics.
| Variable | % (n = 335) | |
|---|---|---|
| Age | 74.7 | |
| Sex | ||
| Female | 201(60) | |
| Male | 134(40) | |
| Smoking Status | ||
| Never Smoker | 13(4) | |
| Ex-Smoker | 130(39) | |
| Current Smoker | 190(57) | |
| Unknown | 2(1) | |
| Weight Loss | ||
| <5% | 262(78) | |
| 5–10% | 27(8.1) | |
| >10% | 34(10) | |
| Unknown | 12(4) | |
| aCCI | ||
| 1–4 | 135(40) | |
| 5–6 | 136(41) | |
| 7–12 | 64(19) | |
| CCI | ||
| 0–1 | 127(38) | |
| 2–3 | 148(44) | |
| 4–9 | 60(18) | |
| Histology | ||
| Adenocarcinoma | 163(49) | |
| Squamous | 82(24) | |
| Other | 90(27) | |
| Performance Status | ||
| ECOG 0 | 139(41) | |
| ECOG 1 | 112(33) | |
| ECOG ≥ 2 | 84(25) | |
| Radiation Dose | ||
| EQD210 ≤ 80 Gy | 16(5) | |
| 80 Gy < EQD210<100 Gy | 108(32) | |
| 100 Gy ≤ EQD210≤110 Gy | 143(43) | |
| EQD210 > 110 | 68(20) | |
| T - Stage | ||
| T1a | 153(46) | |
| T1 b | 112(33) | |
| T2a | 70(21) | |
3 sex stratified Cox regression models were tested: (1) aCCI with age and comorbidity combined; (2) Age and CCI; (3) Age alone, comorbidity removed. Models were created using age, CCI and aCCI as continuous or categorized (age dichotomized at median [<75 vs.>75], CCI and aCCI as tercile and quartile). The models were compared using Harrell’s C concordance index, Akaike information criterion (AIC) and Bayesian information criterion (BIC). We used Stata version 13.1.
Results
Out of 545 patients treated with SABR or hypofractionated RT between January 2008 and December 2013, 335 were included for analysis. 210 patients were excluded from the study: 74 patients with NSCLC > Stage 1; 25 patients with no pathologic confirmation of malignancy; 14 patients with pathology other than NSCLC; 35 patients with other synchronous malignancy; the remaining 64 patients were treated surgically, palliatively, for oligometastatic disease, received up front chemo, were treated at another centre or received no treatment at all.
The baseline patient characteristics are listed in Table 1. At the time of treatment, the median age was 74.7 years. The median OS was 4.41 years and the median follow up time was 4.7 years (95% CI: 3.9–4.9 years). At the time of manuscript preparation, 53% of the study population was deceased. The median Charlson comorbidity score was 2, while the median age adjusted Charlson score was 5.
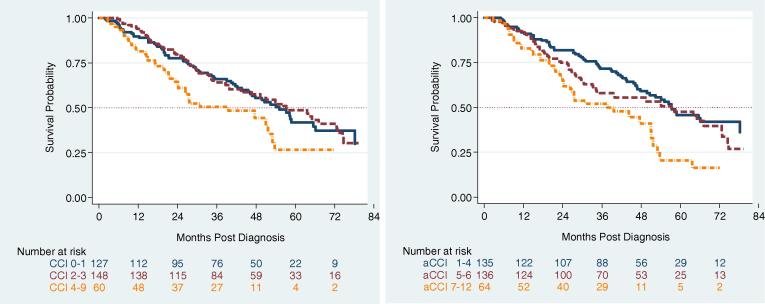
Kaplan–Meier analyses were performed based on the aCCI tercile (1–4, 5–6, and 7–12) and the CCI tercile (0–1, 2–3, and 4–9) the resulting curves are shown in Fig. 1. On univariate analysis (Table 2), weight loss > 10%, sex, and ECOG performance status ≥ 2 were found to be significant predictors of OS. The assumption of proportional hazards was found not valid for sex. However, only patients with the highest comorbidity scores (aCCI 7–12 or CCI 4–9) were found to have a significantly increased hazard of death on univariate analysis. Smoking status, T-stage, histology, and EQD210 were not found to be significant OS predictors on univariate analysis and were dropped from subsequent survival modeling. Similarly, performance status failed to meet the statistical threshold on multivariate refinement and was dropped from the final OS modeling. We fit a stratified Cox model for which the baseline hazard can be different according to sex.
Fig. 1.
(Left) Kaplan-Meier survival curves for Charlson Comorbidity score (CCI) and (Right) for the age adjusted score (aCCI).
Table 2.
Univariate analysis.
| Variable | Hazard Ratio (95% CI) | p-Value | |
|---|---|---|---|
| Age | |||
| <75 | *1.03 (1.01–1.05) | 0.008 | |
| ≥75 | 1.54 (1.14–2.09) | 0.005 | |
| CCI | |||
| 0–1 | *1.13(1.03–1.23) | 0.005 | |
| 2–3 | 0.95 (0.68–1.32) | 0.75 | |
| 4–9 | 1.57 (1.03–2.37)) | 0.03 | |
| aCCI | |||
| 1–4 | *1.07 (0.97–1.18) | 0.169 | |
| 5–6 | 1.22 (0.87–1.71) | 0.241 | |
| 7–12 | 1.94 (1.30–2.90) | 0.001 | |
| Sex | |||
| Female | |||
| Male | 1.47 (1.1–1.99) | 0.01 | |
| Weight Loss 0–5% | |||
| 5–10% | 0.72 (0.33–1.58) | 0.415 | |
| >10% | 2.66 (1.56–4.51) | 0.0001 | |
| Histology Adenocarcinoma | |||
| Squamous | 1.10 (0.76–1.59) | 0.60 | |
| Other | 0.96 (0.68–1.37) | 0.83 | |
| Smoking Status Never Smoker | |||
| Ex Smoker | 1.08 (0.34–3.46) | 0.90 | |
| Current Smoker | 1.25 (0.39–4.04) | 0.71 | |
| Performance Status ECOG 0 | |||
| ECOG 1 | 1.22 (0.86–1.73) | 0.27 | |
| ECOG ≥ 2 | 1.44 (1.00–2.08) | 0.05 | |
| T-stage T1a | |||
| T1b | 1.12 (0.80–1.56) | 0.52 | |
| T2a | 1.24 (0.84–1.83) | 0.28 | |
*Continuous Variable Hazard italicized in first row.
The final results of the model with categorical variables are shown in Table 3, Table 4, Table 5. On formalized testing –with either continuous or categorical variables- all three survival models yielded very similar coefficients of effect: (1) Age and Charlson comorbidity grouped as a single metric (aCCI); (2) Age and Charlson comorbidity as separate metrics (CCI); (3) Age only. The final prediction tool is shown in Table 6. We could not detect a significant interaction between age and CCI. The strongest independent predictor of poor survival based on univariate and multivariate analysis was greater than 10% weight loss. Age either as a continuous or dichotomous variable, and male sex were found to be associated with increased hazard for death. Smoking status, T-stage, histology, performance status, and EQD210 were not found to be significant independent predictors of survival in this analysis.
Table 3.
Multivariate Analysis with Age Adjusted Charlson Score (aCCI) stratified by sex (MODEL 1).
| Variable | Hazard Ratio (95% CI) | p-Value | |
|---|---|---|---|
| aCCI | |||
| aCCI 1–4 | 1 | ||
| aCCI 5–6 | 1.25 (0.89–1.76) | 0.20 | |
| aCCI 7–12 | 1.80 (1.20–2.69) | 0.004 | |
| Weight Loss | |||
| 0–5% | 1 | ||
| 5–10% | 0.75 (0.40–1.39) | 0.36 | |
| >10% | 2.12 (1.37–3.28) | 0.001 | |
| Unknown | 2.07 (0.95–4.52) | 0.07 | |
c-index = 0.59; AIC = 1585; BIC = 1604.
Table 4.
Multivariate Analysis with Age and CCI separated stratified by sex (MODEL 2).
| Variable | Hazard Ratio (95% CI) | p-Value | |
|---|---|---|---|
| Age | |||
| <75 | 1 | ||
| ≥75 | 1.39 (1.02–1.90) | 0.04 | |
| CCI | |||
| CCI 0–1 | 1 | ||
| CCI 2–3 | 0.95 (0.68–1.33) | 0.78 | |
| CCI 4–9 | 1.36 (0.90–2.07) | 0.148 | |
| Weight Loss | |||
| (0–5%) | 1 | ||
| 5–10% | 0.65 (0.29–1.45) | 0.30 | |
| >10% | 2.52 (1.46–4.36) | 0.001 | |
| Unknown | 1.89 (0.87–4.11) | 0.11 | |
c-index = 0.60; AIC = 1586; BIC = 1609.
Table 5.
Multivariate Analysis using age alone (no Charlson comorbidity) stratified by sex (MODEL 3).
| Variable | Hazard Ratio (95% CI) | p-Value | |
|---|---|---|---|
| Age | |||
| < 75 | 1 | ||
| ≥75 | 1.44 (1.06–1.95) | 0.02 | |
| Weight Loss | |||
| 0–5% | 1 | ||
| 5–10% | 0.73 (0.39–1.35) | 0.31 | |
| >10% | 2.11 (1.37–3.26) | 0.001 | |
| Unknown | 1.95 (0.90–4.23) | 0.09 | |
c-index = 0.59; AIC = 1585; BIC = 1600.
Table 6.
Overall survival hazard ratios based on aCCI as a categorical variable.
| Weight loss |
Weight loss |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–5% | 5–10% | >10% | Age | 0–5% | 5–10% | >10% | |||
| F | aCCI 1–4 | 1 | 0.98 [0.45–2.14] | 1.78 [0.94–3.38] | F | <75 | 1 | 0.89 [0.41–1.93] | 1.78 [0.94–3.38] |
| aCCI 5–6 | 1.08 [0.69–1.68] | 1.05 [0.42–2.66] | 1.92 [0.88–4.17] | ≥75 | 1.56 [1.04–2.34] | 1.38 [0.59–3.27] | 2.78 [1.33–5.82] | ||
| aCCI 7–12 | 1.67 [0.94–2.96] | 1.63 [0.59–4.51] | 2.97 [1.30–6.82] | ||||||
| M | aCCI 1–4 | 1 | 0.52 [0.19–1.43] | 2.58 [1.41–4.71] | M | <75 | 1 | 0.54 [0.20–1.50] | 2.45 [1.35–4.43] |
| aCCI 5–6 | 1.63 [0.94–2.82] | 0.84 [0.27–2.63] | 4.21 [1.77–9.98] | ≥75 | 1.29 [0.80–2.08] | 0.70 [0.22–2.23] | 3.17 [1.50–6.68] | ||
| aCCI 7–12 | 2.14 [1.19–3.85] | 1.10 [0.34–3.57] | 5.52 [2.37–12.85] | ||||||
Discussion
We observed a statistically significant association between worse survival and aCCI of 7 or higher. This result is consistent with previous studies. For example, in a retrospective review of 88 medically inoperable patients with early stage NSCLC treated with SABR, Kopek et al. [10] found that the aCCI is a significant predictor of overall survival. Mokhles et al. likewise found aCCI to predict survival outcomes in patients with early stage disease managed with SABR or surgery [11]. The relationship between aCCI and overall survival has similarly been described in clinical stage I NSCLC treated with protons [12], conventional radiotherapy [13], and surgery [14].
Curiously we did not observe an association between survival and unadjusted Charlson Comorbidity score (CCI). It is only after adding extra points for age that we found a significant association between Charlson score and survival in patients with Stage 1 medically inoperable lung cancer. In other words we did not observe an association between comorbid illnesses and survival unless age itself was included as a comorbidity. Furthermore, on formalized comparisons, our predictive models are not significantly different whether comorbidity is included as aCCI, CCI or not at all. Previous reports of aCCI score predicting mortality may be primarily due to patient age and not comorbid illnesses. In this group of medically inoperable stage I NSCLC patients treated with radiotherapy, age alone is as good a prognostic indicator as Charlson score. Age is also more reliably recorded and less prone to error and therefore a more useful metric than Charlson score in this group of patients.
Our retrospective study has several limitations which could account for our inability to associate CCI with survival. Firstly, there may be error in the retrospective determination of the Charlson score. For each patient, the score was calculated after careful review of the medical records. It is possible that those records do not accurately reflect the true burden of comorbid disease in these patients. 35 patients who had other uncontrolled cancers were excluded from the analysis. Similarly, 25 patients were excluded because they were treated without a biopsy (possibly because they were too unwell to receive a biopsy). These patients may have had higher aCCI and CCI scores than the patients who were already included, and could possibly have altered the OS models.
This is not the first study that failed to correlate CCI with patient survival in the setting of lung cancer. A retrospective analysis of 617 patients with stage 1–4 NSCLC used multivariate analysis to examine aCCI and CCI independently and found no correlation between CCI and risk of death [15]. Similarly, a prospective observational study of 83 patients over age 70 with untreated NSCLC showed no correlation between CCI and survival [15]. Both studies postulated that CCI was developed for longitudinal studies and may not be useful in predicting short survivals. A Danish study of 22,556 patients (the largest study to date) with lung cancer, found that CCI has a limited effect on survival and only for patients treated with chemotherapy [9]. In another retrospective study of 4072 NSCLC patients treated in the Netherlands, comorbid illnesses were examined independently without using CCI - no independent prognostic effect was found [16].
In a multi-institutional retrospective study of 779 patients, Klement et al. attempted to identify a group of medically inoperable stage I peripheral NSCLC who did not benefit from SBRT. They identified ECOG performance status and operability as the most important predictors of early death (<6 months). Their study similarly identified Charlson comorbidity index (aCCI) as an OS predictor. Neither analysis identified any group of patients who failed to derive any benefit from SBRT [18].
Conclusions
We identify male sex, weight loss greater than 10% and age as independent prognostic factors for patients treated with SABR. While aCCI ≥ 7 was associated with increased hazard for death the effect was due to age and not comorbid illness. Based on our overall survival models, age alone can be used interchangeably with aCCI or CCI plus age with the same prognostic value in this group of medically inoperable stage I NSCLC patients. Charlson comorbidity may play a more important role in a younger group of patients, for example medically operable stage I NSCLC treated with SABR or lobectomy.
Disclosure
The Authors have nothing to disclose.
References
- 1.Stewart BW,Wild CP World Cancer Report 2014, Lyon France: international Agency for Research on Cancer, issuing body; World Health Organization, issuing body; 2014.
- 2.Chen V.W., Bernardo A.R., Hsieh M.C. Analysis of stage and clinical/prognostic factors for lung cancer from SEER registries: AJCC staging and collaborative stage data collection system. Cancer. 2014;120(23):3793–3806. doi: 10.1002/cncr.29045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howington J., Blum M.G., Chang A.C. Treatment of stage I and II non-small cell lung cancer. Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5):e278s–e313s. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 4.Powell J.W., Dexter E., Scalzetti E.M. Treatment advances for medically inoperable non-small-cell lung cancer: emphasis on prospective trials. Lancet Oncol. 2009;10:885–894. doi: 10.1016/S1470-2045(09)70103-2. [DOI] [PubMed] [Google Scholar]
- 5.Timmerman R., Paulus R., Galvin J. Stereotactic body radiation therapy for inoperable early stage lung cancer. J Amer Med Assoc. 2010:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann P., Nyman J., Hoyer M. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27(20):3290–3296. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann F.B., Geinitz H., Schill S. Stereotactic hypofractionated radiotherapy in stage I (T1–2 N0 M0) non-small-cell lung cancer (NSCLC) Acta Oncol. 2006;45:796–801. doi: 10.1080/02841860600913210. [DOI] [PubMed] [Google Scholar]
- 8.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.Mellemgaard A., Lüchtenborg M., Iachina M. Role of comorbidity on survival after radiotherapy and chemotherapy for nonsurgically treated lung cancer. J Thorac Oncol. 2015;10(2):272–279. doi: 10.1097/JTO.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 10.Kopek N., Paludan M., Petersen J. Co-morbidity index predicts for mortality after stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer. Radiother Oncol. 2009;93:402–407. doi: 10.1016/j.radonc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Mokhles S., Nuyttens J.J., Maat A.P.W.M. Survival and treatment of non-small cell lung cancer stage I-II treated surgically or with stereotactic body radiotherapy: patient and tumor-specific factors affect the prognosis. Ann Surg Oncol. 2014;22(1):316–323. doi: 10.1245/s10434-014-3860-x. [DOI] [PubMed] [Google Scholar]
- 12.Yo S.Y., Bush D.A., Slater J.D. Comorbidity-adjusted survival in early stage lung cancer patients treated with hypofractionated proton therapy. J Oncol. 2010 doi: 10.1155/2010/251208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith S.L., Palma D., Parhar T. Inoperable early stage non-small cell lung cancer: comorbidity, patterns of care and survival. Lung Cancer. 2011;72:39–44. doi: 10.1016/j.lungcan.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Moro-Sibilot D., Aubert A., Diab S. Comorbidities and Charlson score in resected stage I nonsmall cell lung cancer. Eur Resp J. 2005;26:480–486. doi: 10.1183/09031936.05.00146004. [DOI] [PubMed] [Google Scholar]
- 15.Ganti A.K., Siedlik E., Loberiza Marr A.S., FR Predictive ability of Charlson Comorbidity Index on outcomes from lung cancer. Am J Clin Oncol. 2011;34(6):593–596. doi: 10.1097/COC.0b013e3181fe445b. [DOI] [PubMed] [Google Scholar]
- 16.Janssen-Heijnen M.L.G., Smulders S., Lemmens V.E.P.P. Comorbidity on the treatment and prognosis of elderly patients with non-small cell lung cancer. Thorax. 2004;59:602–607. doi: 10.1136/thx.2003.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bursac Z., Gauss C.H., Williams D.K., Hosmer D.W. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klement R.J., Belderbos J., Grills I. Prediction of early death in patients with early-stage NSCLC –Can we select patients without a potential benefit of SBRT as a curative treatment approach? J Thorac Oncol. 2016;11(7):1132–1139. doi: 10.1016/j.jtho.2016.03.016. [DOI] [PubMed] [Google Scholar]