Graphical abstract
Abbreviations: RT, Radiotherapy; 18F-FDG, 2-deoxy-2-(18F)fluoro-D-glucose; PET, Positron emission tomography; NSCLC, Non-small cell lung cancer; SUV, Standard uptake value; CCL, Chemokine (CC motif) ligand; RILT, Radiation induced lung toxicity; EGFR, Epidermal growth factor receptor; CT, Computed tomography; GTV, Gross tumor volume; HU, Hounsfield Unit; MMP, Matrix metalloproteinase; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; EORTC QLQ-LC13, EORTC QLQ Lung Cancer 13; IL, Interleukin
Keywords: 18F-FDG, Positron emission tomography, Standardized uptake value, Thoracic radiotherapy, Erlotinib, Lung cancer
Abstract
Purpose
To investigate effects of radiotherapy (RT) and erlotinib on pulmonary glucose uptake using 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG) positron emission tomography (PET) during and after treatment of non-small cell lung cancer (NSCLC) and to identify associations between serum cytokine levels and lung glucose uptake.
Material and methods
Twenty-seven patients with advanced NSCLC, receiving RT alone or concomitant RT and erlotinib therapy, were examined by 18F-FDG PET before, during, and after treatment. A total of 57 18F-FDG PET scans were analyzed. Pulmonary 18F-FDG uptake and radiotherapy dose mapping were used to acquire dose-response curves for each patient, where subsequent linear regression gave a glucose uptake level in the un-irradiated parts of the lungs (SUV0) and a response slope (ΔSUV). Serum cytokine levels at corresponding time points were assessed using a multiplex bioassay. Correlations between the most robust cytokines and lung 18F-FDG dose response parameters were further investigated.
Results
From the dose response analysis, SUV0 at post-therapy was significantly higher (P < 0.001) than at mid- and pre-therapy (45% and 58%, respectively) for the group receiving RT + erlotinib. Also, SUV0 at post-therapy was higher for patients receiving RT + erlotinib compared to RT alone (42%; P < 0.001). No differences in ΔSUV were seen with treatments or time. SUV0 was positively associated (r = 0.47, P = 0.01) with serum levels of the chemokine C–C motif ligand 21 (CCL21) for patients receiving RT + erlotinib.
Conclusions
Concomitant RT and erlotinib causes an elevation in pulmonary 18F-FDG uptake post treatment compared to RT alone. Pulmonary glucose uptake is associated with an upregulation of a chemokine (CCL21) involved in inflammatory reactions.
Introduction
Lung cancer is the leading cause of cancer-related death in the world [1], where non-small cell lung cancer (NSCLC) accounts for about 80–85% of the lung cancer cases [2]. Although thoracic radiation therapy (RT) plays an important role in the management of NSCLC, radiation induced lung toxicity (RILT) such as radiation pneumonitis needs to be considered [3], [4], [5], [6]. Radiation pneumonitis is an inflammatory reaction where inflammatory cells recruit in the lung and within the irradiated lung tissue in response to injury, caused by radiation-induced apoptosis and differentiation of immunoregulatory cells [7], [8], [9]. The onset and severity of radiation pneumonitis depend on many factors such as total radiation dose, number of fractions, volume of the irradiated parenchyma, and the use of other therapies concurrently with RT [10], [11], [12].
Targeted drugs such as erlotinib have been developed to improve outcome for patients with e.g. NSCLC [13]. Erlotinib is a low molecular weight agent that reversibly and selectively inhibits tyrosine kinase activity of the epidermal growth factor receptor (EGFR) [14]. Erlotinib has been shown to be effective after failure of previous chemotherapies and as maintenance therapy of patients with NSCLC, in addition to first line treatment for patients with an activating EGFR mutation [15], [16], [17]. Moreover, it is reported that combination of radiotherapy and EGFR inhibitors can improve local tumor control and prognosis [18], [19]. The most frequent side effects of erlotinib are skin rash and diarrhea [20], [21]. In some case studies pulmonary toxicity and interstitial lung disease are reported following erlotinib therapy [22], [23], [24], [25]. Even though such reported toxicities from erlotinib are not highly frequent, it is vital to identify individual patients at risk of such side effects.
It has been shown that 18F-FDG-PET can potentially visualize and assess radiation pneumonitis [6], [26], [27], [28], [29], [30], as higher 18F-FDG uptake in the lung could result from greater inflammatory response [31]. However, the possible additional effect of erlotinib on lung 18F-FDG uptake has not previously been investigated. Also, previous studies have found associations between RT exposure and serum cytokine levels, pointing to altered regulatory cellular processes related to inflammation [32], [33]. In order to understand such subclinical signs and their role in predicting toxicity, it is important to investigate these regulatory mechanisms in conjunction with the altered lung glucose metabolism seen with 18F-FDG. In this study, we evaluated the 18F-FDG uptake in normal lung tissue before, during, and after therapy for NSCLC patients receiving either RT or concurrent RT and erlotinib. We also looked for relations between 18F-FDG uptake in the lung and serum cytokines related to inflammatory responses.
Materials and methods
Study design
Twenty-seven patients with stage III-IV NSCLC, recruited between November 30, 2012 and May 5, 2015, were prospectively included (NCT02714530). The study was approved by the Regional Committees for Medical and Health Research Ethics. Written informed consent was received from all patients. The median (±SD) patient age was 69.5 ± 7.5 years (range, 47 to 81 years) and 21 patients (78%) were male. Nineteen patients had PET scans before therapy while 25 and 13 had PET examinations at mid- and post-therapy, respectively. Patients received either thoracic RT alone or RT concurrently with oral erlotinib (150 mg p.o.) given daily from the day before start of radiotherapy and during the radiotherapy course. Radiotherapy included two opposed 6 MV photon beams with a total dose of 30 Gy and was delivered in 10 fractions, once every weekday, at a linear accelerator. The treatment was planned based on the tumor location and anatomy of the given patient, as reflected in planning computed tomography (CT) images.
Planning CT, PET/CT acquisition, and contouring
Before the initiation of the treatment, patients underwent a planning CT scan (GE medical systems, USA). Planning CT images were imported into a radiotherapy planning system (Oncentra® External Beam, Elekta, Sweden) for delineation of regions of interest (ROIs) such as gross tumor volume (GTV), lymph nodes, lungs, heart, thoracic vertebrae, and esophagus. Patients also underwent at most three 18F-FDG PET/CT examinations using a Biograph 16-scanner (Siemens, Germany); prior to radiotherapy, at mid-therapy (after median delivery of 9 Gy (range 6 to 18 Gy), and six weeks after completion of radiotherapy. All patients fasted for at least 6 h prior to intravenous administration of 18F-FDG. The amount of 18F-FDG administered was 377 MBq (range; 263–445 MBq) irrespective of patients’ body weight. Patients included in this study had blood glucose level <11 mmol/L when imaged.
Image registration and voxel-wise quantification
Here, we describe a framework for voxel-wise quantification of radiation- and erlotinib-induced changes in PET image features of the unaffected (non-tumor) lung tissue, and for studying the dose-response sensitivity of these features. A set of tools was developed for registering different image series and scoring PET signal vs RT dose. All procedures described were implemented in IDL (Interactive Data Language, v 8.3, Research Systems, Boulder, CO, USA).
The PET images were transformed into standardized uptake value (SUV) images, using injected activity, time interval between 18F-FDG injection and image acquisition, and patients’ body weight. RT dose images, containing the voxel-wise mapping of planned doses in units of [Gy], were exported from the treatment planning system. PET/CT, planning CT, and RT dose images were interpolated to the same isotropic reference resolution (3 mm). PET/CT images taken at different time points were rigidly registered to the planning CT. This was done by thresholding bone in the respective CT series and subsequently applying a correlation maximization algorithm [34] to derive the optimal translation from the PET/CT series to the CT planning series. The RT dose image series were inherently co-registered to the planning CT. Having all images aligned voxel by voxel; the delineated lung ROI from the planning CT was transferred to the registered PET/CT and RT dose images. To avoid possible PET signal spillover from tissues other than the normal lung, the ROIs for GTV, lymph nodes, heart, esophagus, and thoracic vertebrae including additional margins of 1.2–1.5 cm were removed from the lung ROI. Furthermore, to ensure that only lung parenchyma was included, voxels exclusively having Hounsfield units (HU) between -924 and -224 HU in the CT images were considered in the analysis.
Dose response curves
From co-registered PET and RT dose images; the 18F-FDG uptake (in terms of SUV) could be analyzed against the dose to given lung voxels. First, dose was classified into bins of size 0.5 Gy, and SUVmean was then calculated for each radiotherapy dose, D, category up to the prescription dose for each patient at a given PET session. On a population level, a further average of SUVmean from individual patients was calculated at each dose category. Because only half of the total RT dose was delivered at mid-therapy, the lung dose ranged between 0 and 15 Gy for this PET session. Furthermore, the dose was between 0 and 30 Gy at the post-therapy session. Thus, data are presented in terms of % of delivered dose. Linear regression was used to investigate the relationship between lung 18F-FDG uptake and dose at mid- and post-therapy, both on an individual and a population level. Mathematically, this may be formulated as SUVmean = SUV0 + ΔSUV × D. This provides two response parameters; the 18F-FDG uptake level in the un-irradiated parts of the lung (SUV0) and a response slope (ΔSUV; change in SUV per % dose). The analysis was also carried out for the PET data taken prior to therapy.
Cytokine measurements
As described previously [35], patient blood samples were taken at different time points before, during and after treatment. In the current work, blood samples collected at time points corresponding to the PET sessions pre-, mid-, and post-therapy were employed. Serum levels for a panel of 57 cytokines and matrix metalloproteinases (MMP’s) were assessed using a multiplex bioassay (Bio-Rad, Hercules, CA, USA). Proteins associated with inflammatory responses (annotated with GO:0006954) were identified using gene ontology [36], [37]. This gave a resulting panel of 24 cytokines (see additional file 1). From this panel, the most robust group of cytokines showing the smallest patient-to-patient variation in log-transformed pre-therapy level was identified using Jenks natural breaks classification. This final panel consisted of the 8 cytokines: CXCL1, CXCL2, CXCL10, CCL7, CCL13, CCL21, CCL22, and CCL25.
Statistics
Population-based SUV0 and ΔSUV from the dose response relationships for different imaging sessions and treatments were compared for statistical significance using 95% confidence intervals (CIs) estimated from the linear regression. SUV0 and ΔSUV in individual patients were compared using Mann–Whitney test with a two-sided significance level of 0.05. Pearson’s correlation was employed to evaluate associations between SUVs and serum cytokine levels.
Results
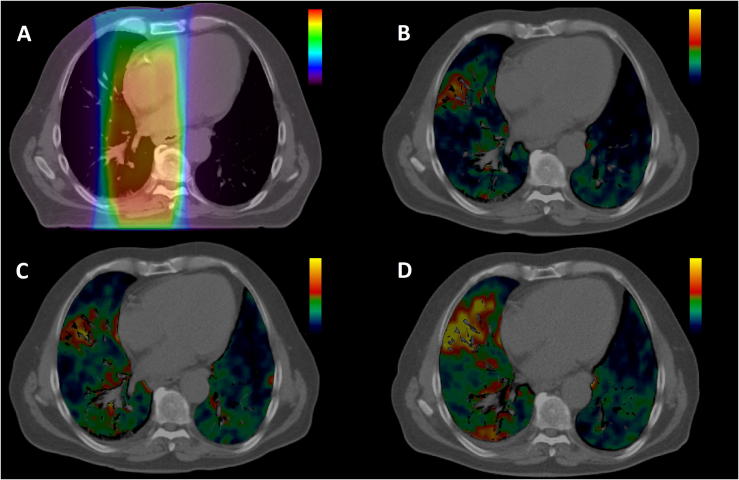
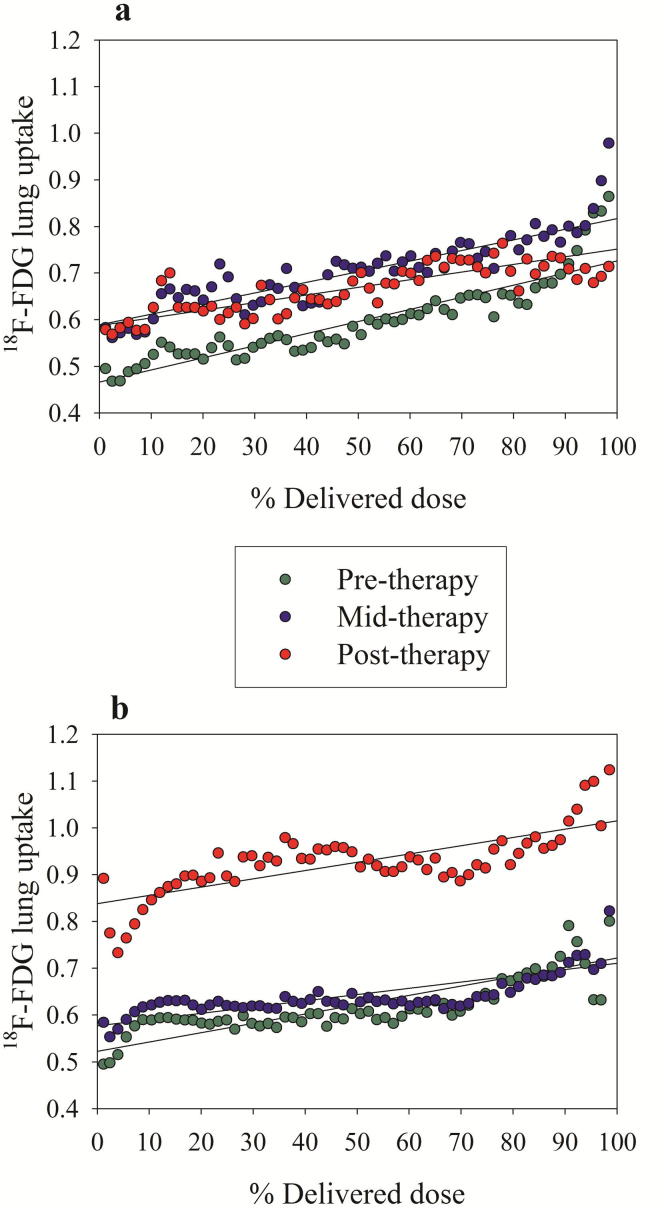
Patients’ characteristics and number of patients eligible for analysis in each treatment group at the three PET sessions are given in Table 1. The radiotherapy dose distribution together with PET/CT images taken at pre-, mid-, and post-radiotherapy and erlotinib for one patient is shown in Fig. 1. As seen, there is a marked increase in 18F-FDG uptake in the lungs after therapy, although the high uptake regions do not seem to be co-localized with high radiation dose. Fig. 2 shows the 18F-FDG uptake in the lungs, averaged over all patients in the respective treatment groups, against percentage delivered dose at pre-, mid-, and post-therapy sessions. First, all dose response relationships appear rather parallel, regardless of treatment group or imaging session. Second, there are small differences in the relationships between pre-, mid-, and post-therapy PET sessions for the RT group. However, a higher 18F-FDG pulmonary uptake is observed in mid- and post-therapy compared to pre-therapy (P < 0.05). Third, pulmonary 18F-FDG uptake at the post-therapy session for the RT + erlotinib group seems to be shifted to a considerably higher level compared to pre- and mid-therapy (P < 0.001). Fourth, at post-therapy PET a significantly higher pulmonary 18F-FDG uptake was observed in the RT + erlotinib group compared to the RT group (P < 0.001). This is further reflected in SUV0s and ΔSUVs from the linear regressions applied on the dose response curves (Table 2), where the only parameter significantly different from the rest was SUV0 post-therapy for the RT + erlotinib group. These findings were confirmed in a sub-group analysis for patients receiving radiotherapy + erlotinib having 3 consecutive PET scans (data not shown).
Table 1.
Patients’ characteristics.
| n = 27 (%) | |||
| Treatment group | |||
| RT: | 13 (48) | ||
| RT + erlotinib: | 14 (52) | ||
| Gender | |||
| Male: | 21 (78) | ||
| Female: | 6 (22) | ||
| Smoking history | |||
| Current: | 7 (26) | ||
| Former: | 20 (74) | ||
| Stage | |||
| III: | 7 (26) | ||
| IV: | 20 (74) | ||
| Histology | |||
| Adenocarcinoma: | 15 (56) | ||
| Squamous cell carcinoma: | 9 (33) | ||
| NOS: | 3 (11) | ||
| COPD | |||
| Positive: | 10 (37) | ||
| Negative: | 17 (63) | ||
| Available PET/CT Scans | |||
| Pre-therapy: | RT | 10 (37) | |
| RT + erlotinib | 9 (33) | ||
| Mid-therapy: | RT | 13 (48) | |
| RT + erlotinib | 12 (44) | ||
| Post-therapy: | RT | 5 (18) | |
| RT + erlotinib | 8 (30) | ||
Abbreviations: RT: Radiotherapy, NOS: Not otherwise specified, COPD: Chronic obstructive pulmonary disease.
Fig. 1.
A) Dose distribution and CT image at treatment planning for a patient receiving RT and erlotinib. Dose range is 0–30 Gy. PET/CT images at: B) pre-, C) mid-, and D) post therapy. SUV range is 0.1–2.5. Only the 18F-FDG uptake within the CT lung window is displayed.
Fig. 2.
Population based 18F-FDG uptake (in terms of SUV) in the lung versus percentage dose at pre-, mid-, and post-therapy across patients separated into a) RT group and b) RT + erlotinib group. The solid lines correspond to first order linear regressions.
Table 2.
Analysis of dose response relationships.
| Treatment group | Session | ΔSUV (95% CI)† | SUV0 (95% CI) |
|---|---|---|---|
| RT | Pre-Therapy | 0.84 (0.57, 1.11) | 0.46 (0.42,0.51) |
| Mid-Therapy | 0.75 (0.49,1.00) | 0.59 (0.54,0.63)‡ | |
| Post-Therapy | 0.51 (0.10, 0.91) | 0.59 (0.52,0.66)‡ | |
| RT + erlotinib | Pre-Therapy | 0.57 (0.34,0.81) | 0.53 (0.49,0.57) |
| Mid-Therapy | 0.38 (0.17,0.59) | 0.58 (0.54,0.62) | |
| Post-Therapy | 0.56 (0.30,0.82) | 0.84 (0.79,0.87)‡ | |
Population-based dose-response analysis for RT and RT+erlotinib groups at different PET sessions. Numbers given are the ΔSUV (change in SUV per percentage dose increase) and SUV0 (SUV in un-irradiated lung), with corresponding 95% confidence intervals (CIs). †Multiplied by a factor 100. ‡Significantly different from finding at pre-therapy.
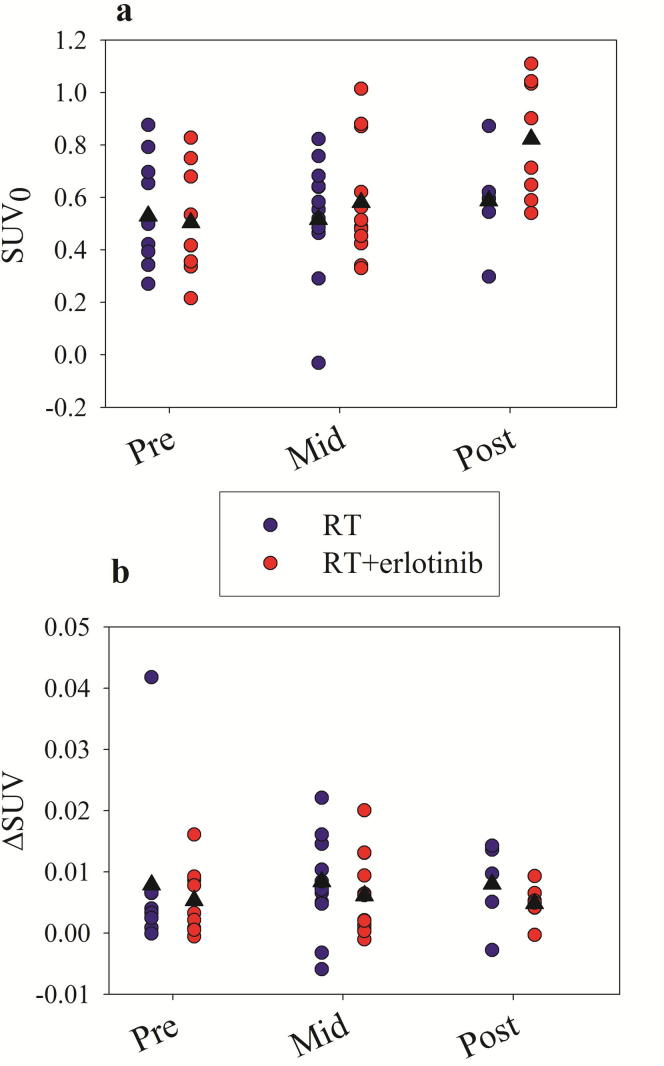
Results from first order linear regressions of the dose response relationships for individual patients are given in Fig. 3. Here, considerable inter-patient scatter is seen in the SUV0s and ΔSUVs. In the RT + erlotinib group, a significantly higher SUV0 at post-therapy compared to pre-therapy (P = 0.02) and mid-therapy (P = 0.01) was found. Although RT + erlotinib on average gave a higher SUV0 at post-therapy compared to RT only, this difference was not significant on an individual patient basis (P = 0.12).
Fig. 3.
Individual a) SUV0s and b) ΔSUVs resulting from linear regressions on the lung 18F-FDG uptake dose response curves at different sessions. The population-based mean is given by a black triangle.
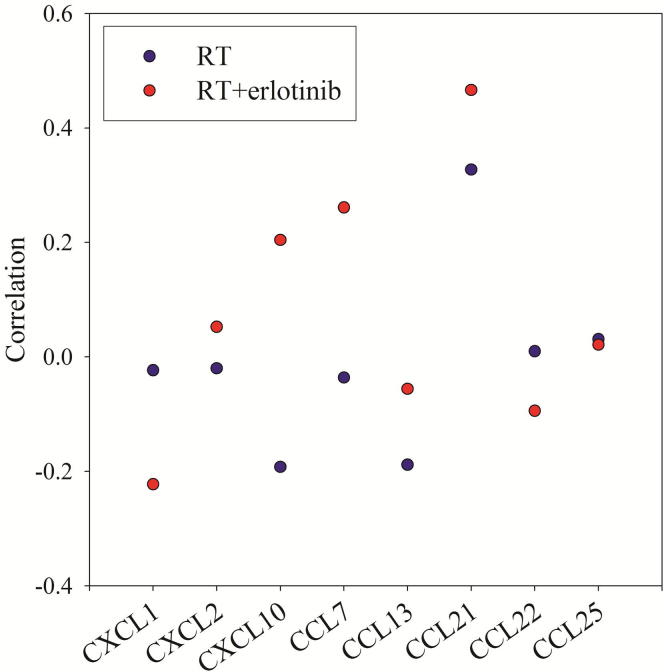
The eight cytokines selected from the natural breaks classification had pre-therapy inter-patient variation ranging from 22% (CCL21) to 67% (CXCL10). In comparison, pre-therapy SUV0 varied by 38%. In a correlation analysis, cytokine levels were systematically tested against SUV0. As ΔSUV did not show any change during therapy or between treatment groups, it was not meaningful to take this parameter into further analysis here. Fig. 4 shows the correlation between SUV0 and the serum level of the eight cytokines taken over all time points for the two treatment groups. Varying associations between lung glucose uptake and serum cytokine levels were found for the RT and RT + erlotinib groups. The strongest positive correlation was found between SUV0 and CCL21 for the patients receiving RT + erlotinib (r = 0.47, P = 0.01). Although CCL21 levels also was positively correlated with SUV0 for patients receiving RT only, this was not significant (r = 0.31, P = 0.09).
Fig. 4.
Correlations between baseline 18F-FDG uptake (SUV0) and serum level for the most robust panel of 8 cytokines associated with inflammatory response.
Discussion
The relationship between the local dose and pulmonary 18F-FDG uptake may contribute to a better understanding of RT-induced lung injury. Guerrero et al. [29] performed restaging examinations based on 18F-FDG PET/CT between 4 and 12 weeks after radiotherapy of 36 esophageal cancer patients. A linear relationship was found between 18F-FDG uptake in the lung and local radiation dose, and the dose response slope (similar to our ΔSUV) varied across patients. Moreover, McCurdy et al. [30] quantified post radiotherapy 18F-FDG pulmonary dose response in lung cancer patients with PET performed approximately 6 weeks after completion of chemo-radiotherapy. In 92% of these patients, a positive linear relationship was found between the local radiation dose and the voxel-averaged post-treatment lung 18F-FDG uptake. Furthermore, the SUV in the irradiated lung was normalized to the levels in the un-irradiated lung, and the slope of the regression line was regarded as the “pulmonary metabolic radiation response”. In the present study, we found a positive linear relationship between the local radiation dose and 18F-FDG uptake in the lung both at mid- and post-therapy in most patients. However, an apparent ‘dose response’ was also found for the pre-therapy PET session, and the magnitude of the apparent response was in most cases comparable to that seen at mid- and post-therapy, with the exception of the RT + erlotinib group at post-therapy. These similarities imply that factors other than radiation have caused the apparent lung 18F-FDG uptake patterns. Such factors may be pre-existing inflammation and/or fibrosis in the lung, since inflammatory and fibrotic cells have been reported to accumulate more 18F-FDG than normal cells [38], [39]. As the pre-therapy lung uptake of 18F-FDG was substantial in the high dose region, this indicates that these other factors coincided with radiation dose. As margins were applied around the other ROIs overlapping with the lungs, it is not likely that the apparent dose response is due to spillover effects. However, it may be that glycolytically active subclinical disease and/or inflammatory cells are enriched in the wider periphery of the tumors. In these regions the radiation dose is high, resulting in the apparent dose response. Furthermore, it cannot be ruled out that the relationships in the reported studies by Guerrero [29] and McCurdy [30] do not reflect a true dose response as these studies did not analyze pre-therapy PET images.
The patient cohort investigated in the current study received palliative radiotherapy with two opposed beams to a total dose of 30 Gy. Thus, the pulmonary response pattern may differ from patients receiving curative treatment with stereotactic radiotherapy or intensity modulated radiotherapy with prescribed doses typically up to 70 Gy. Although clear associations with radiotherapy dose levels were not found in the current work, we saw a pronounced increase in the population-based SUV0 from pre- and mid- to post-therapy for patients receiving RT + erlotinib, and a significantly higher SUV0 in the RT + erlotinib group compared to the RT group at post-therapy. This implies that erlotinib increased the general glucose uptake level in the normal lung compared to radiation alone 6 weeks after completion of treatment, which could point to increased risk of treatment-induced pneumonitis for patients receiving the concomitant treatment. Although we have used the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) and EORTC QLQ Lung Cancer 13 (LC13) to assess clinical changes in the lung function for individual patients, these findings were not correlated to the treatment- or PET-related factors explored in the current work (data not shown). This may be due to that the current population has extensive disease with comorbidities, obscuring any underlying treatment-related side effects.
For the cytokines in our original panel, we extracted the ones directly associated with inflammatory response having the least inter-patient variability at pre-therapy. The latter was done to identify the most robust factors for further testing against the lung SUVs. Therefore, cytokines such as Interleukin 6 (IL-6) and IL-8 were not used in the further analysis, although these factors have previously been associated with RT-induced inflammatory responses [32]. When these cytokines were tested against SUV0 at the different time points, no significant associations were seen (data not shown). For the final panel of eight cytokines, we saw significant associations between CCL21 and SUV0 for patients receiving RT + erlotinib. CCL21 has not previously been associated with therapy response in the normal lung, neither following RT nor erlotinib therapy. Patients with hypersensitivity pneumonitis showed an increase in CCL21 levels and bronchoalveolar lavage fluid compared to healthy volunteers [40]. Moreover, studies have shown that CCL21 is increased in patients with inflammatory skin disease [41], [42], and erlotinib therapy is associated with skin rash and release of inflammatory cytokines [43]. Thus, CCL21 may be a promising candidate for understanding the mechanisms of lung inflammation and subsequent radiation pneumonitis.
Different SUV-based metrics can potentially be used for assessing inflammatory responses. As the slopes (ΔSUVs) from the dose response relationships did not show any significant changes during therapy, it may be simpler to omit the dose-response analysis and extract single parameters from the lung. Metrics such as the 75th percentile of SUV in the normal lung were tested in the current work, and these were highly correlated with SUV0 for patients receiving RT + erlotinib (r = 0.88, P < 0.001). Furthermore, the associations between the 75th percentile SUV and CCL21 showed even stronger correlations (r = 0.53, P = 0.004) compared to those using SUV0. De Ruysscher et al. [6] looked at the maximum lung 18F-FDG uptake (SUVmax), and found that high SUVmax 7 and 14 days into RT was associated with increased risk of RILT. We have tested higher percentiles towards SUVmax, but associations with CCL21 (assumedly linked to RILT) then became slightly weaker compared to the 75th percentile. Thus, it is likely that the high-uptake part of the lungs may be evaluated to assess the response, although an optimal PET metric cannot be defined from the current work.
Further studies on the effect of erlotinib on CCL21 levels should be encouraged as pulmonary glucose uptake and CCL21 level may carry similar information regarding the effect of erlotinib. Although erlotinib is shown to be successful in improving clinical outcomes in a subset of NSCLC patients, potential toxicities in the lung such as inflammation needs to be considered. Strategies to minimize these effects are important to increase patient’s quality of life and overall treatment outcome. Such strategies may well include assessment by 18F-FDG PET and/or CCL21 serum level. Finally, elucidating inflammatory responses in the patients’ normal cells may shed light on aspects such as the use of check-point inhibitors in combination with other immunogenic cancer treatments [44].
Conclusions
In the present study, an increase in the lung uptake of 18F-FDG is observed six weeks post treatment for non-small cell lung cancer patients receiving RT and erlotinib compared to patients receiving RT alone. The lung 18F-FDG uptake is further correlated with chemokine CCL21, pointing to an inflammatory response following concomitant RT and targeted therapy.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
Discussion with Dr. Nina Jeppesen Edin, University of Oslo, Norway, on gene ontology is greatly appreciated. This work was supported by the Norwegian Cancer Society and the regional health authorities in South East Norway.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ctro.2017.04.002.
Contributor Information
Azadeh Abravan, Email: azadeh.abravan@fys.uio.no.
Hanne Astrid Eide, Email: Hanne.Astrid.Eide@rr-research.no.
Ingerid Skjei Knudtsen, Email: i.s.knudtsen@fys.uio.no.
Ayca Muftuler Løndalen, Email: aycloe@ous-hf.no.
Åslaug Helland, Email: aslaug.helland@gmail.com.
Eirik Malinen, Email: eirik.malinen@fys.uio.no.
Appendix A. Supplementary data
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Traynor A.M., Schiller J.H. Systemic treatment of advanced non-small cell lung cancer. Drugs Today (Barc) 2004;40(8):697–710. doi: 10.1358/dot.2004.40.8.850472. [DOI] [PubMed] [Google Scholar]
- 3.Hernando M.L., Marks L.B., Bentel G.C., Zhou S.-M., Hollis D., Das S.K. Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):650–659. doi: 10.1016/s0360-3016(01)01685-6. [DOI] [PubMed] [Google Scholar]
- 4.Rancati T., Ceresoli G.L., Gagliardi G., Schipani S., Cattaneo G.M. Factors predicting radiation pneumonitis in lung cancer patients: a retrospective study. Radiother Oncol. 2003;67(3):275–283. doi: 10.1016/s0167-8140(03)00119-1. [DOI] [PubMed] [Google Scholar]
- 5.Bradley J., Graham M.V., Winter K., Purdy J.A., Komaki R., Roa W.H. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non–small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61(2):318–328. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 6.De Ruysscher D., Houben A., Aerts H.J.W.L., Dehing C., Wanders R., Öllers M. Increased 18F-deoxyglucose uptake in the lung during the first weeks of radiotherapy is correlated with subsequent Radiation-Induced Lung Toxicity (RILT): a prospective pilot study. Radiother Oncol. 2009;91(3):415–420. doi: 10.1016/j.radonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Johnston C.J., Williams J.P., Elder A., Hernady E., Finkelstein J.N. Inflammatory cell recruitment following thoracic irradiation. Exp Lung Res. 2004;30(5):369–382. doi: 10.1080/01902140490438915. [DOI] [PubMed] [Google Scholar]
- 8.Roberts C.M., Foulcher E., Zaunders J.J., Bryant D.H., Freund J., Cairns D. Radiation pneumonitis: a possible lymphocyte-mediated hypersensitivity reaction. Ann Intern Med. 1993;118(9):696–700. doi: 10.7326/0003-4819-118-9-199305010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Ghafoori P., Marks L.B., Vujaskovic Z., Kelsey C.R. Radiation-induced lung injury. Assessment, management, and prevention. Oncology (Williston Park) 2008;22(1):37–47. [PubMed] [Google Scholar]
- 10.Roach M.I., Gandara D.R., Yuo H.-S., Swift P.S., Kroll S., Shrieve D.C. Radiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factors. J Clin Oncol. 1995;13(10):2606–2612. doi: 10.1200/JCO.1995.13.10.2606. [DOI] [PubMed] [Google Scholar]
- 11.Taghian A.G., Assaad S.I., Niemierko A., Kuter I., Younger J., Schoenthaler R. Risk of pneumonitis in breast cancer patients treated with radiation therapy and combination chemotherapy with paclitaxel. J Natl Cancer Inst. 2001;93(23):1806–1811. doi: 10.1093/jnci/93.23.1806. [DOI] [PubMed] [Google Scholar]
- 12.Kimsey F.C., Mendenhall N.P., Ewald L.M., Coons T.S., Layon A.J. Is radiation treatment volume a predictor for acute or late effect on pulmonary function? A prospective study of patients treated with breast-conserving surgery and postoperative irradiation. Cancer. 1994;73(10):2549–2555. doi: 10.1002/1097-0142(19940515)73:10<2549::aid-cncr2820731016>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 13.Lynch T.J., Bell D.W., Sordella R., Gurubhagavatula S., Okimoto R.A., Brannigan B.W. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to Gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 14.Hidalgo M., Siu L.L., Nemunaitis J., Rizzo J., Hammond L.A., Takimoto C. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19(13):3267–3279. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd F.A., Rodrigues Pereira J., Ciuleanu T., Tan E.H., Hirsh V., Thongprasert S. Erlotinib in previously treated non–small-cell lung cancer. N Engl J Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 16.Cappuzzo F., Ciuleanu T., Stelmakh L., Cicenas S., Szczésna A., Juhász E. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11(6):521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 17.Bezjak A., Tu D., Seymour L., Clark G., Trajkovic A., Zukin M. Symptom Improvement in lung cancer patients treated with erlotinib: quality of life analysis of the national cancer institute of canada clinical trials group study BR.21. J Clin Oncol. 2006;24(24):3831–3837. doi: 10.1200/JCO.2006.05.8073. [DOI] [PubMed] [Google Scholar]
- 18.Schneider C.-P., Heigener D., Schott-von-Römer K., Gütz S., Laack E., Digel W. Epidermal growth factor receptor-related tumor markers and clinical outcomes with erlotinib in non-small cell lung cancer: an analysis of patients from german centers in the trust study. J Thorac Oncol. 2008;3(12):1446–1453. doi: 10.1097/JTO.0b013e31818ddcaa. [DOI] [PubMed] [Google Scholar]
- 19.Gurtner K., Deuse Y., Bütof R., Schaal K., Eicheler W., Oertel R. Diverse effects of combined radiotherapy and EGFR inhibition with antibodies or TK inhibitors on local tumour control and correlation with EGFR gene expression. Radiother Oncol. 2011;99(3):323–330. doi: 10.1016/j.radonc.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 20.Pérez-Soler R., Delord J.P., Halpern A., Kelly K., Krueger J., Sureda B.M. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. Oncologist. 2005;10(5):345–356. doi: 10.1634/theoncologist.10-5-345. [DOI] [PubMed] [Google Scholar]
- 21.Hirsh V. Managing treatment-related adverse events associated with egfr tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Curr Oncol. 2011;18(3):126–138. doi: 10.3747/co.v18i3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vahid B., Esmaili A. Erlotinib-associated acute pneumonitis: report of two cases. Can Respir J. 2007;14(3):167–170. doi: 10.1155/2007/832605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peerzada M.M., Spiro T.P., Daw H.A. Pulmonary toxicities of biologics: a review. Anticancer Drugs. 2010;21(2):131–139. doi: 10.1097/CAD.0b013e328333d662. [DOI] [PubMed] [Google Scholar]
- 24.Makris D., Scherpereel A., Copin M.C., Colin G., Brun L., Lafitte J.J. Fatal interstitial lung disease associated with oral erlotinib therapy for lung cancer. BMC Cancer. 2007;7:150. doi: 10.1186/1471-2407-7-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tammaro K.A., Baldwin P.D., Lundberg A.S. Interstitial lung disease following erlotinib (Tarceva®) in a patient who previously tolerated gefitinib (Iressa®) J Oncol Pharm Pract. 2005;11(3):127–130. doi: 10.1191/1078155205jp158cr. [DOI] [PubMed] [Google Scholar]
- 26.Hicks R.J., Mac Manus M.P., Matthews J.P., Hogg A., Binns D., Rischin D. Early FDG-PET imaging after radical radiotherapy for non–small-cell lung cancer: inflammatory changes in normal tissues correlate with tumor response and do not confound therapeutic response evaluation. Int J Radiat Oncol Biol Phys. 2004;60(2):412–418. doi: 10.1016/j.ijrobp.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 27.Kong F.M.S., Frey K.A., Quint L.E., Haken R.K.T., Hayman J.A., Kessler M. A pilot study of [18F]fluorodeoxyglucose positron emission tomography scans during and after radiation-based therapy in patients with non small-cell lung cancer. J Clin Oncol. 2007;25(21):3116–3123. doi: 10.1200/JCO.2006.10.3747. [DOI] [PubMed] [Google Scholar]
- 28.Hart J.P., McCurdy M.R., Ezhil M., Wei W., Khan M., Luo D. Radiation pneumonitis: correlation of toxicity with pulmonary metabolic radiation response. Int J Radiat Oncol Biol Phys. 2008;71(4):967–971. doi: 10.1016/j.ijrobp.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerrero T., Johnson V., Hart J., Pan T., Khan M., Luo D. Radiation pneumonitis: local dose versus [18F]-fluorodeoxyglucose uptake response in irradiated lung. Int J Radiat Oncol Biol Phys. 2007;68(4):1030–1035. doi: 10.1016/j.ijrobp.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 30.McCurdy M.R., Castillo R., Martinez J., Al Hallack M.N., Lichter J., Zouain N. [18F]-FDG uptake dose–response correlates with radiation pneumonitis in lung cancer patients. Radiother Oncol. 2012;104(1):52–57. doi: 10.1016/j.radonc.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen D.L., Rosenbluth D.B., Mintun M.A., Schuster D.P. FDG-PET imaging of pulmonary inflammation in healthy volunteers after airway instillation of endotoxin. J Appl Physiol. 2006;100(5):1602–1609. doi: 10.1152/japplphysiol.01429.2005. [DOI] [PubMed] [Google Scholar]
- 32.Gridley D.S., Bonnet R.B., Bush D.A., Franke C., Cheek G.A., Slater J.D. Time course of serum cytokines in patients receiving proton or combined photon/proton beam radiation for resectable but medically inoperable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;60(3):759–766. doi: 10.1016/j.ijrobp.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 33.Siva S., MacManus M., Kron T., Best N., Smith J., Lobachevsky P. A pattern of early radiation-induced inflammatory cytokine expression is associated with lung toxicity in patients with non-small cell lung cancer. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0109560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eilertsen K., Skretting A., Tennvassas T.L. Methods for fully automated verification of patient set-up in external beam radiotherapy with polygon shaped fields. Phys Med Biol. 1994;39(6):993–1012. doi: 10.1088/0031-9155/39/6/006. [DOI] [PubMed] [Google Scholar]
- 35.Eide H.A., Halvorsen A.R., Sandhu V., Fåne A., Berg J., Haakensen V.D. Non-small cell lung cancer is characterised by a distinct inflammatory signature in serum compared with chronic obstructive pulmonary disease. Clin Transl Immunol. 2016;5 doi: 10.1038/cti.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill D.P., Smith B., McAndrews-Hill M.S., Blake J.A. Gene Ontology annotations: what they mean and where they come from. BMC Bioinformatics. 2008;9(Suppl 5):S2. doi: 10.1186/1471-2105-9-S5-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gene Ontology Consortium Creating the gene ontology resource: design and implementation. Genome Res. 2001;11(8):1425–1433. doi: 10.1101/gr.180801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basu S., Kumar R., Alavi A. PET and PET-CT imaging in infection and inflammation: Its critical role in assessing complications related to therapeutic interventions in patients with cancer. Indian J Cancer. 2010;47(4):371–379. doi: 10.4103/0019-509X.73562. [DOI] [PubMed] [Google Scholar]
- 39.Groves A.M., Win T., Screaton N.J., Berovic M., Endozo R., Booth H. Idiopathic pulmonary fibrosis and diffuse parenchymal lung disease: implications from initial experience with 18F-FDG PET/CT. J Nucl Med. 2009;50(4):538–545. doi: 10.2967/jnumed.108.057901. [DOI] [PubMed] [Google Scholar]
- 40.Yamashita M., Mouri T., Niisato M., Nitanai H., Kobayashi H., Ogasawara M. Lymphangiogenic factors are associated with the severity of hypersensitivity pneumonitis. BMJ Open Respir Res. 2015;2(1) doi: 10.1136/bmjresp-2015-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eberhard Y., Ortiz S., Ruiz L.A., Kuznitzky R., Serra H.M. Up-regulation of the chemokine CCL21 in the skin of subjects exposed to irritants. BMC Immunol. 2004;26:5–7. doi: 10.1186/1471-2172-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serra H.M., Eberhard Y., Martín A.P., Gallino N., Gagliardi J., Baena-Cagnani C.E. Secondary lymphoid tissue chemokine (CCL21) is upregulated in allergic contact dermatitis. Int Arch Allergy Immunol. 2004;133(1):64–71. doi: 10.1159/000076129. [DOI] [PubMed] [Google Scholar]
- 43.Thatcher N., Nicolson M., Groves R.W., Steele J., Eaby B., Dunlop J. Expert consensus on the management of erlotinib-associated cutaneous toxicity in the u.k. Oncologist. 2009;14(8):840–847. doi: 10.1634/theoncologist.2009-0055. [DOI] [PubMed] [Google Scholar]
- 44.Topalian S.L., Taube J.M., Anders R.A., Pardoll D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.