Abstract
Introduction
Anemia has long been associated with poor prognosis in patients with cervical cancer. Recently, additional hematologic parameters have emerged as potential indicators of worse outcome in this patient group. In a cohort of cervical cancer patients treated with chemoradiotherapy (CRT) and brachytherapy, we report on the prognostic significance of hematologic parameters including anemia, leukocytosis, neutrophil to lymphocyte ratio (NLR), and thrombocytosis, the effect of combining anemia with other hematologic parameters, and the effect of changes in hemoglobin levels during treatment.
Materials and methods
Two-hundred fifty-seven cervical cancer patients were retrospectively identified from a single cancer institution’s database. Hematologic parameters were categorized as: anemia (hemoglobin ≤115 g/L), leukocytosis (white blood cell count >10 × 109/L), thrombocytosis (platelets >400 × 109/L), and NLR (ratio >5). The association between clinical factors and hematologic parameters on progression-free survival (PFS) and overall survival (OS) were assessed at 5 years.
Results
At 5 years, both pre-treatment anemia (PFS: 60% vs 34%, p < 0.0001; OS: 68% vs 41%, p < 0.0001) and on-treatment anemia (PFS: 62% vs 40%, p < 0.0001; OS: 70% vs 48%, p < 0.0001) were significantly associated with worse survival. This adverse effect on 5-year PFS and OS was increased in patients with both pre-treatment anemia and leukocytosis (PFS: 72% vs 42%, p < 0.0001; OS: 68% vs 37%, p < 0.0001) and pre-treatment anemia and elevated NLR (PFS: 61% vs 30%, p < 0.0001; OS: 68% vs 37%, p < 0.0001). Five-year PFS (50% vs 31%) and OS (60% vs 36%) was better in patients whose pre-treatment anemia improved to normal hemoglobin levels on treatment vs those patients who were anemic both pre- and on-treatment.
Conclusion
Pre-treatment and on-treatment anemia were significant, independent predictors of worse PFS and OS. Anemia and other hematologic parameters remain prognostic markers for cervical cancer patients. Improvement in PFS and OS was seen in patients with normalization of hemoglobin.
Abbreviations: Hgb, hemoglobin; NLR, neutrophil-to-lymphocyte ratio; CRT, chemoradiotherapy; EBRT, external beam radiotherapy; BT, brachytherapy; WBC, white blood cell; Plt, platelet; PFS, progression free survival; OS, overall survival; AOTHgb, average on treatment hemoglobin; LDR, low dose rate; HDR, high dose rate; PA, paraortic; PTHgb, pre-treatment hemoglobin
Keywords: Cervical cancer, Leukocytosis, Anemia, Thrombocytosis, Prognosis
Introduction
Cervical cancer remains a significant worldwide health challenge and many patients die from the disease. Clinically meaningful prognostic markers to inform clinical practice are needed. In addition to advanced tumour stage, anemia has been described as a poor prognostic factor in cervical cancer patients [1], [2], [3], [4], [5], [6]; however, the mechanism is poorly understood and several hypotheses have been explored, including tumour hypoxia, tumour size, and the impact of transfusion [1], [2], [3], [4], [5], [6], [7].
Most reports concur that anemia in cervical cancer patients portends a worse prognosis; however, there is disagreement as to whether pre-treatment or on-treatment hemoglobin (Hgb) is most prognostic [1], [2], [3], [4], [6]. The use of transfusion and other means of improving Hgb levels have been investigated and results are also conflicting [1], [2], [3], [4], [6]. Recently, a review of cervical cancer patients, with various FIGO stages and treatments, has challenged the notion of anemia being a poor prognostic marker [8].
In addition to anemia, other hematologic parameters have been investigated; most notably is tumour related leukocytosis as a poor prognostic marker in several cancers including anal canal [9] and cervical [5], [10], [11], [12], [13], [14], [15]. Others have reported on more general hematologic markers and markers of inflammation such as neutrophilia [15] and elevated neutrophil-to-lymphocyte ratio (NLR) [10], [11], [14].
In this study, we report on hematologic parameters (anemia, leukocytosis, thrombocytosis and NLR) and their prognostic significance in cervical cancer patients treated with radical chemoradiotherapy (CRT). Additionally, we evaluated the prognostic significance of Hgb levels pre- and on-treatment. This is the first study to comprehensively evaluate these hematologic factors in this patient population.
Materials and methods
Patients and treatment
With ethics board approval, patients treated with curative intent CRT between 1998 and 2012 were identified through a retrospective review of a single institution’s cervical cancer patient clinicopathologic database. All patients had histologically confirmed cervical cancer and were staged according to FIGO criteria. Pre-treatment investigations included physical examination, imaging, and bloodwork. Patients were treated with external beam radiation (EBRT) to the pelvis (45 Gy in 25 daily fractions over 5 weeks) ±extended field to cover part of the paraortic (PA) nodes or the full PA nodes, with concurrent weekly cisplatin-based chemotherapy at 40 mg/m2 for all patients. Brachytherapy (BT) was administered according to institution practice. Low dose rate (LDR) BT at 20 Gy × 2 fractions over 2 weeks was used up to late 2006 and high dose rate (HDR) BT at either 6.5 Gy × 4 fractions or 8 Gy × 3 fractions weekly to present. BT was prescribed to Point A as per ICRU 38 [16]. Patients had weekly bloodwork during CRT, and institution guidelines recommended transfusion to maintain hemoglobin >100 × 109 g/L. No patients were treated with erythopoietin. Patients were followed every 3–4 months for the first 2 years, then every 6 months up to 5 years, then discharged back to their community physicians.
Diagnostic definition of hematologic parameters
Leukocytosis was defined as a white blood cell (WBC) count >10 × 109/L [12]. Patients with a documented infection or pre-existing hematologic disorder were excluded from analysis. Anemia was defined as Hgb ≤115 × 109g/L [5], thrombocytosis was defined as platelet (Plt) count >400 × 109/L, and NLR was calculated as the absolute number of neutrophils/lymphocytes, and a ratio greater than 5 was considered elevated [10]. All pre-treatment hematologic parameters were evaluated from the same blood sample taken at the time of diagnosis, prior to any interventions (including transfusions or CRT). On-treatment Hgb and WBC measurements were recorded and calculated as the mean on-treatment value.
Statistical analysis
Baseline patient, tumour and treatment characteristics were evaluated with descriptive statistics using Wilcoxon’s rank sum, Pearson’s χ2 test and Fisher’s exact tests where appropriate. Progression-free survival (PFS) and overall survival (OS) were assessed at 5-years and were measured from date CRT was completed and date of diagnosis respectively. Overall follow-up was measured from date of diagnosis to last known date alive, as interpreted through retrospective chart review. The log-rank test was utilized for comparisons between groups of interest visualized with Kaplan–Meier graphs. Clinically relevant variables were included in multivariate analysis and backwards approach was used to establish final Cox proportional hazard regression models. Lymph node status was determined as negative or positive, for pelvic and/or PA nodes, based on diagnostic CT imaging. All p-values reported were 2-sided, and p < 0.05 was considered to be statistically significant. Statistical analyses were performed using Stata version 12.0.
Results
Patient and treatment characteristics
Patient, tumour, and treatment characteristics are summarized in Table 1. Two hundred-fifty-seven patients were included. Forty-five percent (n = 116) of tumours were FIGO stage II and 87% (n = 222) were squamous cell carcinomas. Ninety-eight percent (n = 252) of patients received brachytherapy in addition to pelvic EBRT, 5.8% (n = 15) were treated with full pelvic and PA node radiation technique, and 94.6% (n = 243) received 3 or more cycles of concurrent cisplatin-based chemotherapy. Fifty-eight (22.6%) patients had documented transfusions as part of their treatment course.
Table 1.
Patient, tumour, and treatment characteristics for the cohort (n = 257).
| Variable | n | % |
|---|---|---|
| Age (years) | ||
| Median | 50 | |
| Range | 21–89 | |
| FIGO Stage | ||
| IB (NOS) | 7 | 2.7 |
| IB1 | 22 | 8.6 |
| IB2 | 26 | 10.1 |
| IIA | 22 | 8.6 |
| IIB | 94 | 36.6 |
| IIIA | 10 | 3.9 |
| IIIB | 69 | 26.9 |
| IVA | 7 | 2.7 |
| Tumour size (cm) | ||
| Median | 5 | |
| Range | 1–10 | |
| ≤5 cm | 132 | 51.4 |
| >5 cm | 102 | 39.7 |
| Unknown | 23 | 9.0 |
| Histology | ||
| Squamous cell | 222 | 86.4 |
| Adeno variation | 31 | 12.1 |
| Other | 4 | 1.6 |
| Nodal status | ||
| Negative | 198 | 77.0 |
| Positive | 55 | 21.4 |
| Unknown | 4 | 1.6 |
| Treatment | ||
| Concurrent CRT | 243 | 94.6 |
| EBRT + BT | 252 | 98.1 |
| RT Fields | ||
| Pelvic alone | 171 | 66.5 |
| Pelvic + partial PA | 58 | 22.6 |
| Pelvic + full PA | 15 | 5.8 |
| Unknown | 13 | 5.1 |
| Transfusion status | ||
| No | 186 | 72.4 |
| Yes | 58 | 22.6 |
| Unknown | 13 | 5.1 |
NOS = not otherwise specified; CRT = chemoradiotherapy; EBRT = external beam radiotherapy; BT = brachytherapy; RT = radiotherapy; PA = paraortic.
For the entire cohort, median follow-up was 40.8 months (range 4.4–167.1), median PFS was 31 months (range 0.5–163) and median OS was 40 months (range 4–167). 5-year PFS and OS was 52% and 60% respectively. On univariate analysis, worse PFS and OS was associated with larger tumour size (>5 cm), [HR 1.73 (95% CI 1.17–2.56), p = 0.006] and [HR 1.70 (95% CI 1.09–2.64), p = 0.019], respectively, and FIGO stage (I-II vs III-IV) [HR 1.9 (95% CI 1.33–2.80), p = 0.001] and [HR 2.00 (95% CI 1.32–3.03), p = 0.001]. Result details for the univariate analysis for all clinical variables can be found in Supp. Table 1.
Hemoglobin level
Anemia was documented in 28.8% (n = 74) of patients. Median pre-treatment Hgb (PTHgb) was 128 g/L (Table 2). On univariate analysis, pre-treatment anemia was associated with worse 5-year PFS and OS: 60% vs 34%, [HR 2.4 (95% CI 1.67–3.56), p < 0.001] and 68% vs 41%, [HR 2.49 (95% CI 1.64–3.81), p < 0.001] respectively. This association remained significant on multivariate analysis: 5-year PFS [HR 2.0 (95% CI 1.38–2.97), p < 0.001] and OS [HR 2.76 (95% CI 1.79–4.25), p < 0.001]. Median average on-treatment hemoglobin (AOTHgb) was 118 g/L (Table 2). AOTHgb levels were also significantly associated with worse 5-year PFS and OS on univariate analysis: 62% vs 40%, [HR 2.24 (95% CI 1.54–3.26), p < 0.001] and 70% vs 48%, [HR 2.44 (95% CI 1.60–3.73), p < 0.001] respectively. On multivariate analysis, AOTHgb was independently associated with outcome: 5-year PFS [HR 2.02 (95% CI 1.38–2.97), p < 0.001] and OS [HR 2.18 (95% CI 1.41–3.36), p < 0.001]. Detailed results for multivariate analysis can be found in Supp. Table 2.
Table 2.
Pre-treatment and average on-treatment hematologic characteristics for the cohort (n = 257).
| Variable | Pre-treatment |
Average on-treatment |
||
|---|---|---|---|---|
| n | % | n | % | |
| Hemoglobin (g/L) | ||||
| Median | 128 | 118 | ||
| Range | 46–164 | 88–152 | ||
| ≤115 | 74 | 28.8 | 109 | 42.4 |
| >115 | 181 | 70.4 | 145 | 56.4 |
| Missing | 2 | 0.8 | 3 | 1.2 |
| WBC (109/L) | ||||
| Median | 8.3 | 6.0 | ||
| Range | 2.9–24.7 | 2.1–17.5 | ||
| ≤10 | 175 | 68.1 | 230 | 89.5 |
| >10 | 69 | 26.9 | 14 | 5.5 |
| Missing | 13 | 5.1 | 13 | 5.1 |
| NLR | ||||
| Median | 3.2 | |||
| Range | 1.04–31.8 | |||
| ≤5 | 191 | 74.3 | ||
| >5 | 52 | 20.2 | ||
| Missing | 14 | 5.5 | ||
| Platelets (109/L) | ||||
| Median | 321.5 | |||
| Range | 115–1069 | |||
| ≤400 | 181 | 70.4 | ||
| >400 | 63 | 24.5 | ||
| Missing | 13 | 5.1 | ||
WBC = white blood cell; NLR = neutrophil-to-lymphocyte ratio.
Leukocytosis and NLR
Leukocytosis was documented in 26.9% (n = 69) of patients, and an elevated NLR in 20.2% (n = 52) of patients (Table 2). On univariate analysis, pre-treatment leukocytosis had worse 5-year OS 63% vs 53% [HR 1.56 (95% CI 1.00–2.42), p = 0.050]. Elevated NLR was associated with worse 5-year PFS 58% vs 36% [HR 1.76 (95% CI 1.16–2.68), p = 0.008] and worse OS 64% vs 45% [HR 1.73 (95% CI 1.09–2.74), p = 0.020). On multivariate analysis, these associations did not reach significance (Supp. Table 3). On-treatment leukocytosis was not significantly associated with PFS or OS on either univariate or multivariate analysis (Supp. Table 4).
Platelets
Thrombocytosis occurred in 24.5% (n = 63) patients and median pre-treatment Plt was 321.5 × 109/L (range 115–1069) (Table 2). On univariate analysis, thrombocytosis was associated with worse 5-year PFS 57% vs 43%, [HR 1.72 (95% CI 1.15–2.58), p = 0.008] and OS 66% vs 46%, [HR 2.03 (95% CI 1.31–3.14), p = 0.001]. This association remained significant for 5-year OS on multivariate analysis [HR 1.73 (95% CI 1.09–2.75), p = 0.021] (Supp. Table 5).
Hematologic parameters related to field technique
Treatment with full pelvic and PA RT fields (n = 15) was strongly correlated with lower PTHgb status (p < 0.001) and lower AOTHgb status (p = 0.007). Additionally, full pelvic and PA RT treatment was correlated with NLR status (p = 0.017) but not with pre-treatment Plt or WBC. Worse 5-year PFS and OS was seen for these patients [HR 1.75 (95% CI: 1.25–2.450, p = 0.001] and [HR 1.70 (95% CI: 1.19–2.43), p = 0.004] respectively.
Hemoglobin in combination with other hematologic parameters
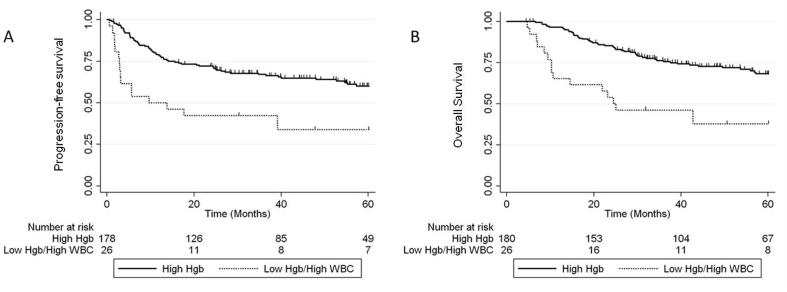
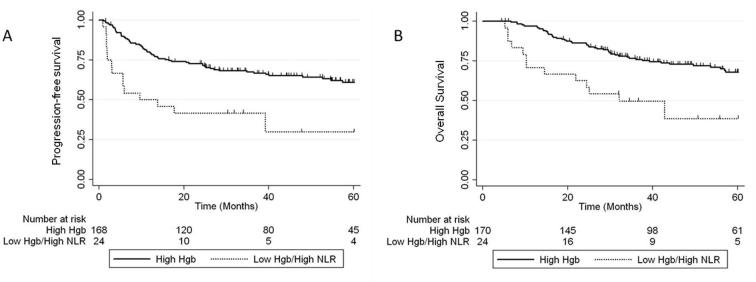
The prognostic impact of combining pre-treatment anemia with other hematologic parameters was examined. On multivariate analysis, there was an additional risk of worse 5-year PFS and OS in patients who had both pre-treatment anemia and leukocytosis: 5-year PFS 72% vs 42%, [HR 3.05 (95% CI 1.75–5.35), p < 0.001] and OS HR 68% vs 37%, [HR 3.91 (95% CI 2.00–6.49), p < 0.001] (Fig. 1). A similar effect was observed for patients who had both pre-treatment anemia and elevated NLR: 5-year PFS 61% vs 30%, [HR 1.9 (95% CI 1.02–3.54), p = 0.042] and OS 68% vs 37%, [HR 3.40 (95% CI 1.82–6.37), p < 0.001] (Fig. 2).
Fig. 1.
5-year PFS (A) and OS (B) for patients with Hgb levels >115 g/L (high Hgb) vs patients with both anemia and leukocytosis.
Fig. 2.
5-year PFS (A) and OS (B) for patients with Hgb levels >115 g/L (high Hgb) vs patients with both anemia and elevated NLR.
Hemoglobin status during treatment
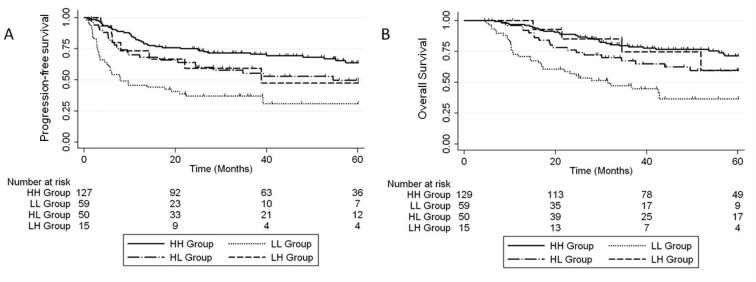
To examine whether a change in a patient’s Hgb status between normal and anemic had an effect on PFS and OS, a comparison of patients with normal Hgb level both pre- and during-treatment (HH group), normal pre-treatment and low during treatment (HL group), low pre-treatment and normal during treatment (LH group), and low both pre- and during-treatment (LL group) was performed. Five-year PFS and OS was highest in the HH group: 64% and 71%; and lowest in the LL group: 31% and 36% (Fig. 3). Patients whose Hgb status changed during treatment had intermediate 5-year PFS and OS, 47% and 60% for the HL group, and 50% and 60% for the LH group. PFS was significantly different between HH vs LL (p < 0.0001), HH vs HL (p = 0.047), HH vs LH (p = 0.023), and LL vs HL (p = 0.011). Five-year OS was significantly different between: HH vs LL (p < 0.0001), LL vs HL (p = 0.01), and LL vs LH (p = 0.039).
Fig. 3.
5-year PFS (A) and OS (B) comparing patient’s Hgb status throughout treatments: Hgb levels >115 g/L both pre- and during-treatment (HH group), normal pre-treatment and low during treatment (HL group), low pre-treatment and normal during treatment (LH group), and low both pre- and during-treatment (LL group).
Discussion
In this uniformly treated cohort, anemia was an independent prognostic marker for 5-year PFS and OS in this patient cohort, consistent with other studies [1], [2], [3], [4], [6], [7], [11], [13], [15], [17], [18]. Additional hematologic parameters identified patient cohorts at increased risk of adverse outcomes.
Patients with low PTHgb levels had worse PFS and OS compared to patients with normal PTHgb levels. AOTHgb was also a significant prognostic marker for PFS and OS; Winter et al. [2] had similar finding with 5-year PFS of 73% for patients with normal AOTHgb levels vs 40% for patients with low AOTHgb, while Grogan et al. [3] reported 5-year OS for patients with normal AOTHgb was 74% vs 45% for patients with low AOTHgb. Escande et al. [15] evaluated Hgb at weekly intervals, and also found worse OS in patients with on-treatment anemia. Although Bishop et al. [8] concluded that anemia does not have a strong role in determining outcome in cervical cancer patients, they found that Hgb at the <100 g/L cut-point was significantly correlated with disease specific survival in both the large cohort and in the subset of patients treated with CRT [8].
Benefits of correcting low Hgb by transfusion in cervical cancer patients remains controversial as causality has not been definitively shown [1], [4], [6], [7], [11], [13], [17], [18]. The GOG 191 phase III trial was designed, in part, to assess this by randomizing patients to standard treatment (transfusion if Hgb below 100 g/L) and erythopoietin stimulation to maintain Hgb levels >130 g/L [19]. This study was closed prematurely and was not able to show a survival advantage to aggressively increasing Hgb levels [19]. In our cohort, however, OS was better for patients whose PTHgb improved during treatment compared to patients who remained anemic throughout treatment. We also showed that patients who became anemic during treatment had worse OS than patients whose Hgb remained normal throughout treatment. These findings re-inforce the association between anemia and poor prognosis. Although the improvement in Hgb cannot be solely attributed to correction through transfusion, the data highlights the importance of maintaining a normal Hgb level through treatment, which may require transfusion.
Plts and NLR were significantly associated with PFS and OS on univariate analysis and Plts remained significant on multivariate analysis. Neutrophilia, as a component of leukocytosis, has been described by others [10], [12], [15]. Escande et al. [15] found an association between neutrophilia and risk of in-field relapse but was not associated with worse OS. Cho et al. [14] whose study describes a higher locoregional failure risk and worse OS with elevated NLR (but defined this as >2.5 as opposed to >5 in the current study). This difference in cut-point may explain why our study failed to reach significance on multivariable analysis. A cut point >5 was used in this study as has previously been described by Tavares-Murta et al. [10]. Assessing optimal cut point for NLR was beyond the scope of this study, and evaluation using a larger cohort would be important to determine an optimal cut point for NLR. Other recent publications support worse prognosis and response to treatment in both cervical cancer patients and non-cervical cancer patients [20], [21], [22]. It is hypothesized that the elevated NLR is indicative of a widespread inflammatory response which may interfere with the effectiveness of therapy [21].
Additionally, low PTHgb and low AOTHgb and elevated NLR were associated with patients receiving pelvic + full PA node RT. Low AOTHgb may be partly explained by a larger volume of bone marrow being irradiated and a myelosuppressive effect. This group of patients, however, had evidence of abnormal hematologic parameters prior to starting treatment. In their report Escande et al. reported higher infield and out-of-field (in PA region) relapse in patients with leukocytosis and neutrophilia and suggests that this group of patients may benefit from dose escalation [15].
Although this study did not show leukocytosis to be an independent prognostic marker as seen in other reports [9], [10], [12], [14], [15], the decreased PFS and OS associated with the combination of anemia and other hematologic parameters (leukocytosis and elevated NLR) suggests that having more than one abnormal hematologic parameter heralds a worse prognosis compared to anemia alone. Cho et al. reported worse locoregional failure-free survival in patients with both tumour-related leukocytosis and high NLR [14], supporting this concept and may explain some of the conflicting results previously reported when only anemia was considered.
A limitation of this study was its retrospective nature; certain confounding variables could not be controlled, and some data were not complete (Table 1, Table 2). Standard practice was to offer transfusion to patients with Hgb < 100 g/L; however, it was not possible to collect complete information regarding transfusions. Despite these limitations, the outcomes achieved by our patients match favourably to those reported in other trials [8], [11]. The current study is unique, in that it is the first to comprehensively evaluate multiple hematologic markers in cervical cancer patients treated with radical CRT.
Conclusion
Pre-treatment and average on-treatment anemia were significant, independent predictors of worse 5-year PFS and OS. Additionally, patients with both pre-treatment anemia and pre-treatment leukocytosis, pre-treatment anemia and pre-treatment elevated NLR, or pre-treatment thrombocytosis, had an increased risk of worse PFS and OS. A survival advantage was seen in patients with pre-treatment anemia in whom Hgb increased to normal range on-treatment.
Funding
This work was supported by Alberta Innovates Health Solutions (AIHS) Collaborative Research & Innovation Opportunities (CRIO) Cancer Care; Alberta Cancer Foundation; Terry Fox Research Institute.
Disclosure of potential conflict of interest
All authors declare that they have no conflicts to declare in relation to the information presented in this report. Dr. TA Koulis is currently doing a clinical research fellowship funded by a CARO Fellowship grant, not related to this current work. Dr. CM Doll received funding in the form of grants in relation to this work from: AIHS CRIO Cancer Care, Alberta Cancer Foundation, and Terry Fox Research Institute. Dr. AM Magliocco reports personal fees from Genoptix Laboratories, personal fees from Ventana Medical Systems, personal fees from Illumina, outside submitted work.
Acknowledgments
We would like to thank AIHS CRIO Cancer Care, Alberta Cancer Foundation, and Terry Fox Research Institute for supporting our research through grants.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ctro.2017.05.001.
Appendix A. Supplementary data
References
- 1.Girinski T., Pejovic-Lenfant M.H., Bourhis J., Campana F., Cosset J.M., Petit C. Prognostic value of hemoglobin concentrations and blood transfusions in advanced carcinoma of the cervix treated by radiation therapy: results of a retrospective study of 386 patients. Int J Radiat Oncol Biol Phys. 1989;16(1):37–42. doi: 10.1016/0360-3016(89)90007-2. [DOI] [PubMed] [Google Scholar]
- 2.Winter W.E., 3rd, Maxwell G.L., Tian C., Sobel E., Rose G.S., Thomas G. Association of hemoglobin level with survival in cervical carcinoma patients treated with concurrent cisplatin and radiotherapy: a Gynecologic Oncology Group Study. Gynecol Oncol. 2004;94(2):495–501. doi: 10.1016/j.ygyno.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Grogan M., Thomas G.M., Melamed I., Wong F.L., Pearcey R.G., Joseph P.K. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer. 1999;86(8):1528–1536. doi: 10.1002/(sici)1097-0142(19991015)86:8<1528::aid-cncr20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Thomas G. The effect of hemoglobin level on radiotherapy outcomes: the Canadian experience. Semin Oncol. 2001;28(2 Suppl. 8):60–65. doi: 10.1016/s0093-7754(01)90215-5. [DOI] [PubMed] [Google Scholar]
- 5.Doll C.M., Aquino-Parsons C., Pintilie M., Klimowicz A.C., Petrillo S.K., Milosevic M. The significance of tumoral ERCC1 status in patients with locally advanced cervical cancer treated with chemoradiation therapy: a multicenter clinicopathologic analysis. Int J Radiat Oncol Biol Phys. 2013;85(3):721–727. doi: 10.1016/j.ijrobp.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Barkati M., Fortin I., Mileshkin L., Bernshaw D., Carrier J.F., Narayan K. Hemoglobin level in cervical cancer: a surrogate for an infiltrative phenotype. Int J Gynecol Cancer. 2013;23(4):724–729. doi: 10.1097/IGC.0b013e31828a0623. [DOI] [PubMed] [Google Scholar]
- 7.Fyles A.W., Milosevic M., Pintilie M., Syed A., Hill R.P. Anemia, hypoxia and transfusion in patients with cervix cancer: a review. Radiother Oncol. 2000;57(1):13–19. doi: 10.1016/s0167-8140(00)00245-0. [DOI] [PubMed] [Google Scholar]
- 8.Bishop A.J., Allen P.K., Klopp A.H., Meyer L.A., Eifel P.J. Relationship between low hemoglobin levels and outcomes after treatment with radiation or chemoradiation in patients with cervical cancer: has the impact of anemia been overstated? Int J Radiat Oncol Biol Phys. 2015;91(1):196–205. doi: 10.1016/j.ijrobp.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee R., Roxin G., Eliasziw M., Joseph K., Maclean A., Buie W.D. The prognostic significance of pretreatment leukocytosis in patients with anal cancer treated with radical chemoradiotherapy or radiotherapy. Dis Colon Rectum. 2013;56(9):1036–1042. doi: 10.1097/DCR.0b013e31829ab0d4. [DOI] [PubMed] [Google Scholar]
- 10.Tavares-Murta B.M., Mendonca M.A., Duarte N.L., da Silva J.A., Mutao T.S., Garcia C.B. Systemic leukocyte alterations are associated with invasive uterine cervical cancer. Int J Gynecol Cancer. 2010;20(7):1154–1159. doi: 10.1111/igc.0b013e3181ef8deb. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Arias A., Cetina L., Candelaria M., Robles E., Duenas-Gonzalez A. The prognostic significance of leukocytosis in cervical cancer. Int J Gynecol Cancer. 2007;17(2):465–470. doi: 10.1111/j.1525-1438.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 12.Mabuchi S., Matsumoto Y., Isohashi F., Yoshioka Y., Ohashi H., Morii E. Pretreatment leukocytosis is an indicator of poor prognosis in patients with cervical cancer. Gynecol Oncol. 2011;122(1):25–32. doi: 10.1016/j.ygyno.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Qiu M.Z., Xu R.H., Ruan D.Y., Li Z.H., Luo H.Y., Teng K.Y. Incidence of anemia, leukocytosis, and thrombocytosis in patients with solid tumors in China. Tumour Biol. 2010;31(6):633–641. doi: 10.1007/s13277-010-0079-8. [DOI] [PubMed] [Google Scholar]
- 14.Cho Y., Kim K.H., Yoon H.I., Kim G.E., Kim Y.B. Tumor-related leukocytosis is associated with poor radiation response and clinical outcome in uterine cervical cancer patients. Ann Oncol. 2016;27(11):2067–2074. doi: 10.1093/annonc/mdw308. [DOI] [PubMed] [Google Scholar]
- 15.Escande A., Haie-Meder C., Maroun P., Gouy S., Mazeron R., Leroy T. Neutrophilia in locally advanced cervical cancer: a novel biomarker for image-guided adaptive brachytherapy? Oncotarget. 2016;7(46):74886–74894. doi: 10.18632/oncotarget.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chassagne D, Dutreix A, Almond P, Burgers J, Busch M, Joslin C. ICRU Report No. 38, Dose and volume specification for reporting intracavitary therapy in gynaecology. Bethesda: International commissioning on radiation units and measurements; 1985.
- 17.Demirci S., Ozsaran Z., Ozsaran A., Yavas F., Demircioglu B., Hanhan M. Evaluation of treatment results and prognostic factors in early-stage cervical carcinoma patients treated with postoperative radiotherapy or radiochemotherapy. Eur J Gynaecol Oncol. 2012;33(1):62–67. [PubMed] [Google Scholar]
- 18.Seo Y., Yoo S.Y., Kim M.S., Yang K.M., Yoo H.J., Kim J.H. Nomogram prediction of overall survival after curative irradiation for uterine cervical cancer. Int J Radiat Oncol Biol Phys. 2011;79(3):782–787. doi: 10.1016/j.ijrobp.2009.11.054. [DOI] [PubMed] [Google Scholar]
- 19.Thomas G., Ali S., Hoebers F.J., Darcy K.M., Rodgers W.H., Patel M. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008;108(2):317–325. doi: 10.1016/j.ygyno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y.Y., Choi C.H., Kim H.J., Kim T.J., Lee J.W., Lee J.H. Pretreatment neutrophil:lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Res. 2012;32(4):1555–1561. [PubMed] [Google Scholar]
- 21.Mizunuma M., Yokoyama Y., Futagami M., Aoki M., Takai Y., Mizunuma H. The pretreatment neutrophil-to-lymphocyte ratio predicts therapeutic response to radiation therapy and concurrent chemoradiation therapy in uterine cervical cancer. Int J Clin Oncol. 2015;20(5):989–996. doi: 10.1007/s10147-015-0807-6. [DOI] [PubMed] [Google Scholar]
- 22.Dirican N., Karakaya Y.A., Gunes S., Daloglu F.T., Dirican A. Association of intratumoral tumor infiltrating lymphocytes and neutrophil-to- lymphocyte ratio are an independent prognostic factor in non-small cell lung cancer. Clin Respir J. 2015 doi: 10.1111/crj.12417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.