To the Editor
We previously reported the results of a nonrandomized, controlled trial that compared survival among patients with Ebola virus (EBOV) disease who were treated with convalescent plasma with survival among historical controls. Although no safety concerns were identified, efficacy was not shown.1 Notably, the levels of total anti-EBOV IgG and neutralizing antibodies in the infused plasma were unknown at the time of administration.1,2 We now report on the association between the amount of total anti-EBOV IgG and neutralizing antibodies received and patient survival and on the changes in the amount of EBOV in their blood 24 hours after transfusion, expressed as the change in the cycle-threshold value in the polymerase-chain-reaction (PCR) analysis. The cycle-threshold value is the number of cycles required for the fluorescence signal to cross the threshold for a positive result on the EBOV PCR assay; lower values indicate higher viral loads.
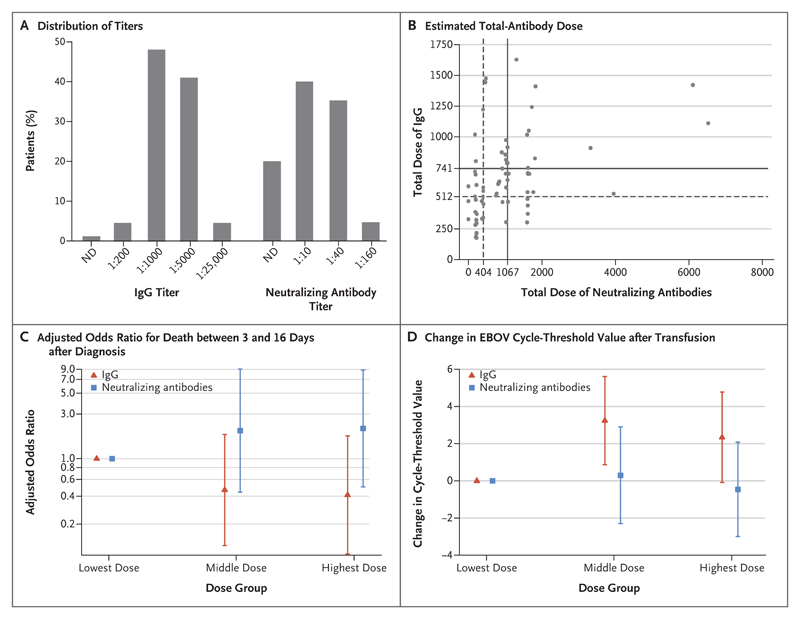
The level of antibodies in the 85 donations was determined by means of an enzyme-linked immunosorbent assay (ELISA) and a plaque-neutralization assay (see the Supplementary Appendix, available with the full text of this letter at NEJM.org). ELISA titers for 94% of the donations were at least 1:1000. In the 50% plaque-neutralizing activity assay, most donations (75%) had a titer of 1:10 or 1:40, and only 4 (5%) had a titer of 1:160 (Fig. 1A). For each patient, a total-antibody dose was calculated by multiplying the volume of convalescent plasma infused by the EBOV antibody titer in the donation. The analysis was restricted to adults, because the dosing of convalescent plasma was done differently in children.
Figure 1. Titers of Total IgG and Neutralizing Antibodies against the Ebola Virus (EBOV) in Plasma from Convalescent Donors, Distribution of Total-Antibody Doses Given to Patients, Odds Ratios for Death between Days 3 and 16, and Changes in Cycle-Threshold Value after Transfusion.
Panel A shows the distribution of titers of total anti-EBOV IgG and neutralizing antibodies against EBOV in 85 donations from 58 convalescent donors whose plasma was used in the trial. ND denotes not detected. Panel B shows the distribution of the total dose of antibodies administered to patients with EBOV disease. To calculate the total dose that a patient received, the volume of the plasma unit was multiplied by the corresponding optical-density value from the enzyme-linked immunosorbent assay (for total anti-EBOV IgG) or antibody titer (for neutralizing antibodies); the sum of this measure for all plasma units that a patient received represented the estimated total-antibody dose. The total dose of neutralizing antibodies has been divided by a factor of 10. Infused plasma in which no antibodies were detected were allocated a zero dose in the estimation of the total dose. The dashed lines show the cutoff for the lowest-dose group of estimated antibody dose, and the corresponding solid lines show the cutoff for the middle-dose group (Spearman’s rho = 0.425; P<0.001). Panel C shows the adjusted odds ratio for death between days 3 and 16 after diagnosis among 71 patients 16 years of age or older. The analysis used the lowest-dose group as the reference group, with adjustment for age and pretransfusion cycle-threshold value. I bars indicate 95% confidence intervals. Patients who died before day 3 after the diagnosis of EBOV disease were excluded.1,2 In a test for association assuming a linear trend, after adjustment for age and cycle threshold, P = 0.21 for IgG and P = 0.32 for neutralizing antibodies. Panel D shows the change in EBOV cycle-threshold values from before to after transfusion among 83 patients 16 years of age or older. The analysis used the lowest-dose group as the reference group, with adjustment for age and pretransfusion cycle-threshold value. The cycle-threshold value is the number of cycles required for the fluorescence signal to cross the threshold for a positive result on the EBOV polymerase-chain-reaction assay; lower values indicate higher viral loads. I bars indicate 95% confidence intervals. More patients were included in this analysis than in the mortality analysis (see the Supplementary Appendix). In tests for heterogeneity between the dose groups (with adjustment for age and pretransfusion cycle-threshold value), P = 0.02 for IgG and P = 0.82 for neutralizing antibodies. In a test for a linear trend (with adjustment for age and pretransfusion cycle-threshold values), P = 0.06 for IgG and P = 0.69 for neutralizing antibodies.
Patients were categorized into one of three equally sized groups on the basis of the estimated total-antibody dose (Fig. 1B). By chance, the pretransfusion cycle-threshold values were lowest in the highest-dose group for IgG and in the middle-dose group for neutralizing antibodies, and there was significant imbalance in the neutralizing-antibodies dose groups (see the Supplementary Appendix). Adjusting for age and pretransfusion cycle-threshold value, we observed lower mortality with higher IgG doses and higher mortality with higher doses of neutralizing antibodies, but neither of these associations was significant (Fig. 1C). The change in cycle-threshold values from before to after transfusion differed significantly according to IgG dose group (P = 0.02). However, there was little difference between the two higher-dose groups (Fig. 1D) and only weak evidence of a linear trend overall (P = 0.06). No association was apparent with the dose of neutralizing antibodies.
In conclusion, most patients received plasma with anti-EBOV IgG antibodies, but levels of neutralizing antibodies were low in many donations. The dose of IgG antibodies showed an association with larger increases in cycle-threshold values after transfusion but no significant association with mortality. Neither outcome showed an association with the estimated doses of neutralizing antibodies received. Further studies are needed to assess the effectiveness of an antibody dose higher than the doses used in this study, the antibody measure that best correlates with virologic and clinical outcomes, and the potential mechanism and clinical effect of viral clearance by anti-EBOV IgG antibodies.
Supplementary Material
A complete list of collaborators in the Ebola-Tx Consortium is provided in the Supplementary Appendix, available with the full text of this letter at NEJM.org.
Acknowledgments
Supported by a grant (666094) from the European Union Horizon 2020 research and innovation program and by the Department of Economy, Science and Innovation of the Flemish government. The mobile plasma unit used in this trial was provided to Guinea by the Bill and Melinda Gates Foundation.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Johan van Griensven, Institute of Tropical Medicine, Antwerp, Belgium
Tansy Edwards, London School of Hygiene and Tropical Medicine, London, United Kingdom
Sylvain Baize, Institut Pasteur, Lyon, France
References
- 1.van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374:33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards T, Semple MG, De Weggheleire A, et al. Design and analysis considerations in the Ebola_Tx trial evaluating convalescent plasma in the treatment of Ebola virus disease in Guinea during the 2014-2015 outbreak. Clin Trials. 2016;13:13–21. doi: 10.1177/1740774515621056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.