Abstract
Objective
Abnormalities in exocrine pancreatic function have been reported in diabetes mellitus (DM). We reviewed published literature to determine the nature of structural and functional alterations in the exocrine pancreas in DM.
Methods
We identified and abstracted data from original studies (n = 50) describing morphological, structural, and functional changes in the exocrine pancreas in types 1 and 2 DM.
Results
Pancreatic weight and volume are markedly lower in type 1 DM (P < 0.005) with insignificant decrease in type 2 DM compared with age-, sex-, and body mass index–matched controls. Pancreatic histopathological changes seen in most subjects with DM at autopsy (n = 7 studies, 1272 autopsies) include mild-to-marked interacinar fibrosis, scant inflammatory infiltrate, no pancreatic ductal changes, and hyalinization of arteries. In subjects with DM, pooled prevalence of decreased fecal elastase 1 (<200 μg/g) is higher, coefficient of fat absorption is near normal (mean, 91%–94%), and pancreatic exocrine dysfunction is nonprogressive over time. Diabetes mellitus is asymptomatic in regard to the exocrine pancreas.
Conclusions
In types 1 and 2 DM, moderate-to-severe subclinical pancreatic fibrosis and modest exocrine dysfunction occurs in the absence of clinical or histopathological evidence of chronic pancreatitis. We call this novel entity “diabetic exocrine pancreatopathy.”
Keywords: chronic pancreatitis, diabetes mellitus
Type 1 diabetes mellitus (DM), previously called juvenile-onset or insulin-dependent DM, is caused by autoimmune destruction of pancreatic beta cells; islet histopathology shows characteristic “insulitis” or islet inflammation.1 Type 2 DM, previously called maturity-onset or non–insulin-dependent DM, is associated with metabolic syndrome and insulin resistance; islet amyloid is the characteristic histopathological finding in islets.2 Diabetes mellitus secondary to chronic pancreatitis (CP) is due to loss of islet mass secondary to fibro-inflammatory destruction of the pancreas, and the exocrine pancreas has characteristic features in CP.3 This review pertains to changes in the exocrine pancreas in types 1 and 2 DM, assuming a rough equivalence between current and older terminologies.
On the basis of the frequent observation that exocrine pancreatic function is abnormal in types 1 and 2 DM, it has been concluded that DM causes pancreatic exocrine insufficiency.4–9 It has been known for more than a century that there are significant changes in exocrine pancreas in patients presenting with types 1 and 2 DM.10 Because of both structural and functional changes in the exocrine pancreas in types 1 and 2 DM, some authors have speculated that these changes represent CP.4,10–12 Because DM is not associated with clinical symptoms of exocrine pancreatic disease, it is postulated that the changes may represent silent CP.4
“Chronic pancreatitis” is the only term currently available to describe fibro-atrophic changes in the exocrine pancreas.3 Chronic pancreatitis encompasses a wide spectrum of diseases associated with progressive fibro-inflammatory damage to the exocrine pancreas, which, if the injury is widespread, leads to failure of exocrine and endocrine pancreatic function requiring treatment. Chronic pancreatitis has a wide range of presentations depending on etiology, the most frequent being clinically acute pancreatitis, imaging evidence of calcification, dilated pancreatic duct, pancreatic atrophy, steatorrhea, diabetes, and jaundice.
To better understand the nature of exocrine pancreatic changes in types 1 and 2 DM, we performed a comprehensive review of the literature to identify studies on morphology, histopathology, and exocrine pancreatic function in DM. On the basis of this review, we conclude that DM is indeed associated with significant alterations in pancreatic exocrine structure and function, more so in type 1 DM, which share some similarities with but also have distinct differences from those described in CP. We suggest the term diabetic exocrine pancreatopathy (DEP) to describe this entity. We discuss the implications of recognizing this new entity for the study of the endocrine and exocrine pancreas and the poorly understood interactions between the two.
METHODS
Literature Review
Search Strategy
Using defined (MESH) terms and key words, MEDLINE, Scopus, EMBASE, and Web of Science were searched for studies published from database inception through January 2015. Search terms used included “diabetes mellitus” in combination with the key words “pancreatic volume,” “pancreatic size,” “pancreatic function,” “insulitis,” “pancreatic fibrosis,” “fatty pancreas,” “pancreatic exocrine insufficiency,” “steatorrhea,” “fecal elastase,” “secretin,” “bicarbonate,” and “cholecystokinin” (see Supplementary Appendix 1, http://links.lww.com/MPA/A487, for detailed search strategy). To complement this search, the references in the manuscripts were manually screened for additional (usually older) studies on the subject.
Study Selection
Search results from the different databases were combined, duplicates were removed electronically, and results were checked manually for accuracy. Abstracts with nonrelevant titles were excluded. Abstracts and full texts were reviewed independently by 2 reviewers (S.M. and S.M.) for inclusion. Any disagreements about inclusion or exclusion of these studies were resolved by consensus, and a third senior reviewer (S.T.C.) was consulted to resolve any remaining disagreements. Studies that seemed to fulfill the following eligibility criteria and those for which information in the abstract was not sufficient for exclusion were read in full.
Inclusion Criteria
Original articles that reported on exocrine pancreatic morphology (volume, gross morphology, and ductal morphology), histopathology, pancreatic exocrine function (tested by direct function tests [pancreatic stimulation by secretin, cholecystokinin/pancreazymin, or secretin-cholecystokinin/pancreazymin] or indirect function tests [fecal elastase 1 (FE1) concentration], coefficient of fat absorption (CFA), and steatorrhea were eligible for this review.
Exclusion Criteria
Case reports, nonsystematic reviews, non-English articles, and animal studies were excluded. Pancreatic size based on abdominal ultrasound and pancreatic volume index based on computerized tomography (CT) scan and magnetic resonance imaging (MRI) were excluded as the data were not comparable with other imaging studies. Also excluded from this review were studies of pancreatic function in patients with symptomatic pancreatobiliary disease and indirect function tests other than FE1. Histopathological studies of the pancreas in DM that focused on islet pathology and did not provide detailed description of the exocrine pancreas were also excluded.
Data Extraction, Quality Assessment, and Statistical Analysis
One reviewer extracted relevant data into a standardized form. These data were verified by a second reviewer, and discrepancies were resolved by consensus. For included studies, we abstracted data on study populations, interventions, outcomes, quality, and applicability.
Two investigators independently rated risk of bias using the Newcastle Ottawa scale for nonrandomized studies. Disagreements were adjudicated by consensus among 2 independent investigators or by obtaining a third reviewer’s opinion when consensus could not be reached. On the basis of the extracted results, textual summaries and tables were created by the reviewers. Similarities and differences across the textual summaries were further inspected to avoid contradiction. Figure 1 demonstrates the details of data extraction process.
FIGURE 1.
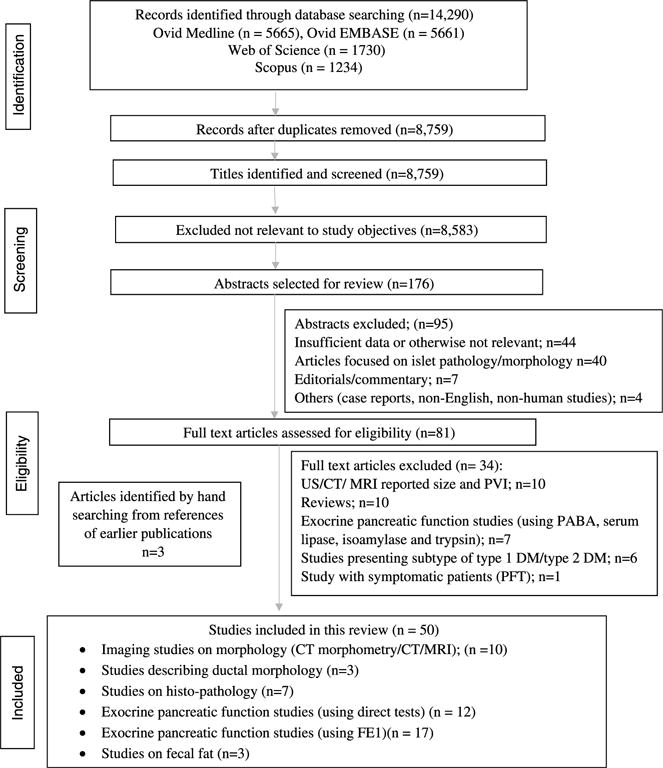
Study flow diagram.
Statistical pooled analysis was only applied to FE1 results measured by monoclonal antibody (Schebo); χ2 test was used to compare percentage of subjects with decreased FE1 in type 1 DM and type 2 DM versus controls. Tables for morphological, structural, and fecal fat (FF) test abnormalities were also created for comparison, and heterogeneous data in disaggregated form were presented where appropriate in the text. As noted in the study flow diagram (Fig. 1), 8759 records were identified after duplicates were removed, and 176 abstracts were identified and screened to assess the eligibility. Of these, 50 met the final inclusion criteria for this review; some studies reported on more than 1 aspect of interest.9,13–16
RESULTS
Pancreatic Parenchymal Weight, Volume, and Size in DM
Type 1 DM has been consistently associated with marked (20%–50%) decrease in pancreatic weight, volume, and size compared with nondiabetic controls measured directly17 or by computerized morphometry18 at autopsy and CT imaging19–21 and MRI22–25 in living subjects (Table 1). This has been shown to occur early in the course of type 1 DM,17 although its progression over time has not been tracked. There are conflicting reports of changes in pancreatic volume in type 2 DM; whereas some studies reported decrease in volume compared with nondiabetic controls,20,25 others found no difference.19 Pancreatic volume is affected by age, sex, and body mass index (BMI).20 In 1 study20 that controlled for these factors, there was a small (~7%) but significant decrease in total pancreas volume in 165 subjects with type 2 DM compared with age-, sex-, and BMI-matched non-DM controls. Some studies have reported no correlation between pancreatic volume with duration of DM.19,24 A recent study21 described decrease in pancreatic volume in DM, which correlated with low FE1 concentration or low chymotrypsin activity, suggesting a correlation between pancreatic atrophy and exocrine deficiency. However, the same study found no significant difference in pancreatic volume in type 1 versus type 2 DM.21
TABLE 1.
Pancreatic Volume by MR or CT in DM
| No. Subjects With DM
|
Pancreatic Volume, mL
|
Volume/Size* (Percentage Decrease vs Controls) |
P, Controls vs Type 1 DM/Type 2 DM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Author (Year) | Type 1 | Type 2 | Controls | Imaging Test | Type 1 | Type 2 | Controls | ||
| Bilgin et al26 (2009) | 28 | — | 21 | MRI/MRCP | — | — | — | 4* | <0.0001/— |
| Williams et al24 (2007) | 12 | — | 12 | MRI | 52.4 ± 17.1 | — | 101 ± 19.5 | 48 | <0.001/— |
| Sequeiros et al23 (2010) | 12 | — | 12 | MRI | 52.5 | — | 104.8 | — | <0.0001/— |
| Williams et al22 (2012) | 20 | — | 24 | MRI | 91.9 ± 6.4 | — | 121.3 ± 6.5 | 26 | 0.003/— |
| Burute et al25 (2014) | — | 32 | 50 | MRI | — | 72.7 ± 20.7 | 89.6 ± 22.7 | — | —/<0.001 |
| Goda et al19 (2001) | 26 | 29 | 22 | CT | 45.2 ± 19.5 | 68.7 ± 18.8 | 71.5 ± 18.7 | 20 | <0.001/NS |
| Saisho et al20 (2007) | — | 165 | 1721 | CT | — | 70.0 ± 26.5 | 74.9 ± 27.0 | 7 | —/<0.05 |
| Philippe et al21 (2011) | 24 | 28 | — | CT | 42 | — | — | — | |
Histopathological Changes in the Exocrine Pancreas in DM
In general, histopathology studies of the pancreas in DM have tended to focus on islet pathology. We identified 7 studies10,11,27–31 encompassing 1272 subjects with DM who commented on the changes in exocrine pancreas (see Supplementary Table 1, http://links.lww.com/MPA/A487, for details on individual studies and summary of all studies in Supplementary Table 2, http://links.lww.com/MPA/A487). Four studies included controls27–30; however, only in 2 studies were findings in the exocrine pancreas compared between DM versus non-DM controls28,29 (Table 2). Collectively in all 7 studies,10,11,27–31 255 (59.4%) of 429 patients had pancreatic fibrosis, with all studies reporting greater than 50% prevalence of fibrosis in those with long-standing DM; Gepts30 reported 18% prevalence of fibrosis in those with juvenile-onset DM dying less than 2 years of onset of disease. Two case-control studies provide more detailed grading of fibrosis28,29 (Table 2). Although any amount of fibrosis (graded 1+ to 3+) without inflammation was seen equally in cases and controls, moderate-to-severe fibrosis was twice as frequent in DM as in non-DM controls.28,29 In contrast to the high prevalence of fibrosis, inflammatory infiltrates are scant and infrequent in DM (Table 2).28,29
TABLE 2.
Histopathological Findings in DM Versus Controls
| Kim28 (1977)
|
Lazarus and Volk29 (1961)
|
|||||
|---|---|---|---|---|---|---|
| Histologic Features,* N (%) | DM (n = 95) | Controls (n = 95) | P | Maturity-Onset DM (n = 50) | Controls (n = 50) | P |
| Interacinar fibrosis | 36 (38) | 16 (17) | 0.004 | 15 (30) | 8 (16) | 0.09 |
| Interlobular fibrosis | 24 (36) | 14 (15) | 0.004 | 19 (38) | 4 (8) | 0.0003 |
| Periductular fibrosis | 33 (35) | 1 (1) | 0.004 | — | — | |
| Acinar atrophy | 21 (22) | 7 (7) | 0.004 | 24 (48) | 12 (24) | 0.001 |
| Inflammation | 13 (14) | 10 (11) | NS | † | † | |
| Lipomatosis | 13 (14) | 12 (13) | NS | 25 (50) | 20 (40) | NS |
| Arteriosclerosis | 37 (39) | 14 (15) | 0.004 | 11 (22) | 4 (8) | 0.05 |
Age range (years) for cases/controls: Kim et al,28 12–85/not stated; Lazarus and Volk,29 40–85/matched for age and sex.
Both studies graded features as mild, moderate, and severe (1+ to 3+): data shown are prevalence of moderate or higher.
Reported as found in “some,” equally in diabetic and nondiabetic pancreases.
NS indicates Not Significant.
Gepts30 described a focal or diffuse lesion of acute pancreatitis in the patients with acute juvenile DM compared with less frequent involvement by the infiltrates in chronic juvenile DM. In a study from Scotland, Foulis et al32 reported lymphocytic infiltrates in the exocrine pancreas in only 9 (9.4%) of 95 cases of type 1 DM. Lymphocytic infiltration of the exocrine pancreas was seen in 22 (46.8%) of the 47 Japanese patients with type 1 DM suggesting immune-mediated destruction of both exocrine and endocrine pancreas in the Japanese population with DM.27 In a Danish autopsy study of 394 consecutive patients,33 higher prevalence of DM (19% vs 7%) was reported in subjects with mild chronic inflammation compared with those without chronic inflammation.
Pancreatic Ductal Morphology in DM
One of the striking features of descriptions of pancreatic exocrine histology in DM is the complete absence of ductal changes typically seen in CP, including ductal distortion (strictures and dilatation), intraductal protein plugs, and calcification (Fig. 2). This is corroborated by the only ERP study in subjects with primary DM (25 type 1 and 15 type 2)34 from Sudan, which showed normal pancreatogram in type 1 DM and minimal changes in 2 of 15 type 2 DM. Analysis of other ERP and magnetic resonance cholangiopancreatography (MRCP) studies in people with diabetes is confounded by the fact that they have included variable proportion of symptomatic patients with suspected pancreatobiliary disease12,26 or did not provide any clinical history.35 For example, Hardt et al12 retrospectively analyzed pancreatograms of 156 symptomatic patients with DM with suspected pancreaticobiliary disease and found characteristic changes of CP in a large proportion (76.7%) of patients. Similarly, Bilgin et al26 reported MRCP abnormalities of CP in 32% patients with pancreatobiliary disease consistent with symptoms of CP.
FIGURE 2.
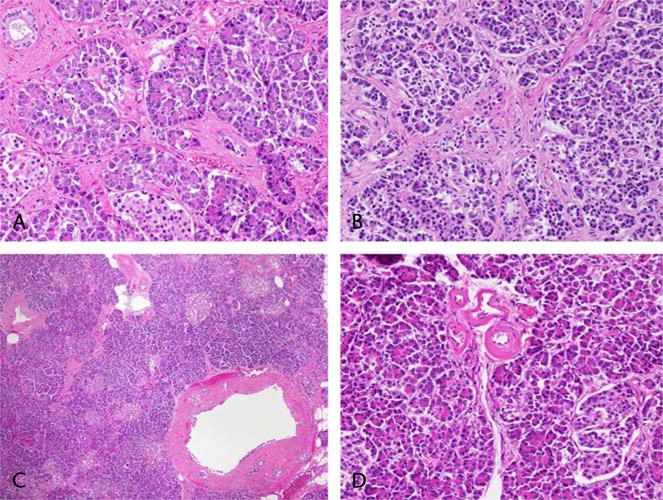
A and B, Interacinar fibrosis without inflammation (200×) in type 2 DM. A small normal duct is present in the upper left corner of A. C, Large duct at lower right has denuded epithelium (likely artifact), but where intact, the epithelium and pancreatic duct glands are normal (40×). D, Vasculopathy: small artery branches with wall thickened by amorphous pink material (200×). Editor’s note: A color image accompanies the online version of this article.
Pancreatic Exocrine Function in DM
Multiple studies in DM have reported high prevalence of abnormal exocrine pancreatic function both on direct16,36–46 (Supplementary Table 3, http://links.lww.com/MPA/A487) and indirect pancreatic function test (FE1) (Table 3). The most common abnormalities observed by direct pancreatic function tests were decreased amylase and bicarbonate output and decreased maximum bicarbonate concentration. A few studies have reported mild-to-moderate reduction in lipase output.16,39 We identified 17 studies5–8,13–16,47–55 (Supplementary Table 4, http://links.lww.com/MPA/A487) where prevalence of decreased FE1 levels (cutoff of <200 μg/g and/or <100 μg/g) measured by monoclonal antibody (Schebo Biotech, Giessen, Germany) was reported. Collectively, these studies have included 940 non-DM controls and 3662 subjects with DM, of which 1724 had type 1 DM. A pooled analysis of these 17 studies (Table 3) shows that decreased FE1 is more prevalent in DM compared with controls (P < 0.00001). Furthermore, low FE1 levels are more commonly seen in type 1 DM versus type 2 DM, with a cutoff of both less than 200 μg/g (38.62% vs 28.12%, P < 0.00001) and less than 100 μg/g (20.11% vs 14.1%, P < 0.00001).
TABLE 3.
Pooled Analysis of Studies of FE1 in DM
| Type of Subjects (No. Studies) | Total No. Subjects Evaluated for Decreased FE1 (<200/<100 μg/g) |
Percentage of Subjects With Decreased FE1 (<200/<100 μg/g) |
P,* DM vs Control |
|---|---|---|---|
| Type 1 DM (14) | 1178/1566 | 39%/20% | <0.001 |
| Type 2 DM (7) | 1938/1928 | 28%/14% | <0.001 |
| Controls (6) | 940/940 | 13%/3% | — |
Total excludes studies by Nunes et al7 (no DM subtypes), Ewald et al5 (percentage for <200 μg/g not available), Hahn et al,16 Laass et al,6 and Vesterhus et al14 (percentage for <100 μg/g unavailable).
P values for types 1 and 2 DM versus controls are <0.00001 and <0.00001 for <200 and <100 μg/g, respectively.
Changes in exocrine function are nonprogressive in DM. In a German study of 20 subjects with type 1 DM, a follow-up secretin-pancreazymin test 11 years after a previously abnormal test found no significant correlation between the duration of DM and the test results for both time points of investigation.36 In fact, only mild abnormalities in pancreatic function were observed after a mean of 22 (±10.9) years of disease. There have been conflicting results regarding FE1 levels and duration of DM; a few studies have reported that increase in the duration of DM is associated with decreased FE1 level,50,52 but others did not find any correlation with duration of DM.8,47,49,53 Many studies49–51 have reported that poor glycemic control in DM was associated with greater reduction in FE1. Other reported associations with reduced FE1 in DM include BMI greater than 257,54 and presence of vascular disease.54
Steatorrhea in DM
In DM, fat balance studies to determine CFA have been performed and correlated with pancreatic function tests (Table 4). A study of 101 German subjects with type 1 DM9 with severe reduction in FE1 (<100 μg/g) showed that 40% had normal CFA (<7 g of fat/day on a 100-g/d fat diet); the mean FF excretion (FFE) (100-CFA in grams per day) in this cohort was only 9.2 ± 5.4 g/d, and only 12% had FFE of greater than 15 g/d. Overall, 1% of 1020 subjects with diabetes had FFE of greater than 15 g/d. To understand the mechanism of steatorrhea in DM, Hahn et al16 measured lipase output, FE1, and FFE in 33 subjects with type 1 DM. Similar to Hardt et al,9 they found that 45.5% of subjects with type 1 DM had low FE1 (<200 μg/g) and 67% had an abnormal FFE (>7 g/d). However, in none of the subjects with abnormal FE1 or increased FFE was lipase output severely (<10% of normal) reduced. In fact, the mean reduction in lipase output was only 18%, which was not sufficient to explain the mild increase in FFE in type 1 DM. They concluded that DM is associated, at best, with only mild-moderate reduction in lipase output that is insufficient to explain mild fat malabsorption seen in DM and speculated that this may be due to small bowel bacterial overgrowth.16 In keeping with these findings, a randomized double-blind control trial5 of pancreatic enzyme replacement therapy (PERT) in 80 patients with low FE1 and DM showed no significant difference in clinical symptoms (stool consistency, flatulence, abdominal pain) between the PERT and placebo groups. However, there was a reduction in the frequency of hypoglycemia in patients on PERT.5
TABLE 4.
FFE in DM
| No. Subjects With DM Studied
|
No. (%) Subjects With DM With Normal and Abnormal FF Estimation
|
% With Abnormal FFE in FE1 Groups
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Author (Year) | Type 1 | Type2 | Mean FFE, g/d | Normal, <7 g/d | Mild, >7–10 g/d | Moderate, 10–15 g/d | Severe, >15 g/d | <200 μg/g | 100 μg/g |
| Hardt et al9 (2003) | 30 | 71 | 9.19 ± 5.39 | 41 (41.3) | 20 (19.8) | 28 (27.7) | 12 (11.9) | — | 60/101 (59.4) |
| Cavalot et al15 (2006) | 66 | — | 6 ± 3.2 | 47 (71.2) | 19 (28.8) | 8/17 (47.0) | 5/7 (71.4) | ||
| Hahn et al16 (2008) | 33 | — | — | 11 (33.3) | 7 (21.2) | 11 (33.3) | 4 (12.1) | 9/15 (60.0) | — |
| Total | 200 | 99/200 (49.5) | 101/200 (50.5) | 17/32 (53.1) | 65/107 (60.7) | ||||
DISCUSSION
A comprehensive review of the published literature on the morphological, histopathological, and functional changes in the exocrine pancreas in types 1 and 2 DM reveals that a significant proportion of subjects have exocrine changes that we term DEP. Diabetic exocrine pancreatopathy is asymptomatic but is associated with (1) a marked decrease in pancreatic weight, size, and volume in type 1 DM, but mild to no decrease in type 2 DM; (2) increased interacinar fibrosis and acinar atrophy with minimal inflammation and no pancreatic ductal changes; (3) a modest reduction in pancreatic enzyme output and FE1 concentrations, more so in type 1 DM, with (4) normal to minimal decrease in CFA; and (5) lack of progression of exocrine dysfunction over time.
Diabetic exocrine pancreatopathy differs significantly from CP in the conspicuous absence of symptoms of exocrine disease (acute pancreatitis and pain), lack of ductal changes including strictures, protein plugs and calculi, lack of significant inflammation, and lack of progression to a calcific state even in a subset of patients. Although a formal case-control study to confirm and validate the histopathological changes noted here is clearly needed, the consistency of findings across studies and the large number of autopsies included (n = 1272)10,11,27–31 strongly support the veracity of the histopathological observations. Similarly, a large study of more than 1000 patients confirmed the abnormalities in FE1 concentrations noted in earlier studies.8
The fundamental significance of this study is in the conclusion that pancreatic fibrosis and exocrine dysfunction frequently occur in the absence of clinical or histopathological evidence of CP; that is, DEP is distinct from CP. This novel observation has significant implications for the study of both the exocrine and endocrine pancreas and the poorly studied interaction between the two. Similar findings have been reported in smokers56 and alcoholics57 without clinical pancreatic disease. The existence of EP radically challenges our perspective on the definition, pathogenesis, and diagnosis of CP. In addition, it questions the specificity of pancreatic function testing and endoscopic ultrasound for the diagnosis CP in asymptomatic subjects with risk factors for EP. A new field of research into EP will be needed to elucidate the relationship between DM and DEP and whether EP worsens DM by reducing islet cell mass. The reasons why DEP rarely progresses to clinically apparent CP also warrant study. New tests will be needed to distinguish EP from CP. In this context, the role of inflammatory markers in pancreatic juice to distinguish CP from EP will be a highly relevant field of study.
What are the possible mechanisms for the alterations in pancreatic structure and function in DM? In type 1 DM, acinar cell atrophy has been attributed to lack of trophic action of insulin. Lohr and Kloppel18 found that exocrine atrophy is due to reduction in size rather than number of acinar cells. However, they could not find a clear relationship between the extent of exocrine atrophy and the residual insulin positivity, the duration of DM, or microangiopathy related to DM. In a study of 11 patients with recent-onset type 1 DM, Foulis and Stewart58 demonstrated that severe pancreatic acinar cell atrophy was present surrounding the insulin-deficient islets whereas acinar cells around the insulin-containing islets were normal suggesting that the exocrine changes could be related to islet-acinar vascular connections and the loss of trophic effects of various islet hormones on pancreatic acini. Fibrosis with minimal inflammation has been attributed to diabetic vasculopathy.28,29
In both forms of DM, parenchymal atrophy and exocrine fibrosis is often accompanied by lesions in the smaller blood vessels and fatty atrophy of the pancreas. Lazarus and Volk29 reported distinct arteriolosclerosis in 66% of the diabetic patients compared with 34% of non-DM controls suggesting that microangiopathy could be the primary factor causing pancreatic fibrosis and exocrine atrophy. However, Lohr and Kloppel18 could not find any significant correlation between the extent of exocrine atrophy and microangiopathy in chronic type 1 DM. In addition, pancreatic function (ductal and acinar) has been shown to decline, secondary to hyperglycemia and hyperinsulinemia.59 There are conflicting reports on fatty atrophy of the pancreas with some suggesting increased fatty change in DM27,28 but others20 describing increase in pancreatic fat associated with increase in BMI rather than presence of DM when compared with non-DM controls.
This retrospective review, although exhaustive, has limitations and biases. Over time, the terminology of DM (now called types 1 and 2) has changed many times, and the assumption of equivalence, for example, of terms juvenile-onset DM, insulin-dependent DM, and type 1 DM may not be wholly accurate. If there are distinct subtypes of DEP in types 1 and 2 DM, we could not identify their unique histopathological characteristics other than differences in islet pathology. Although findings from more than 1200 autopsies and more than 4600 FE1 measurements have been reported here, many of the studies did not have controls or had limited description of controls. However, findings in uncontrolled studies parallel those seen in case-control studies. Despite these limitations, the observation that more than half of the patients studied had pancreatic fibrosis, often moderate to severe, and nearly 30% to 40% had pancreatic exocrine dysfunction cannot be ignored. The observations need further study to understand its nature and mechanism.
In summary, both types 1 and 2 DM are associated with changes in the exocrine pancreas and decrease in acinar and ductal function. Although an exocrine pancreatopathy does occur in DM, its mechanism and clinical significance remain to be explored. Further studies focusing on the exocrine pancreas are clearly needed to better define DEP and distinguish it from CP.
Supplementary Material
Acknowledgments
Dr Chari was supported by grants from the National Institutes of Health (R01 CA 100685) and the Mayo Clinic Pancreas Cancer SPORE (P50 CA 102701) and the Sandler Kenner Foundation.
Footnotes
The authors declare no conflict of interest.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pancreasjournal.com).
References
- 1.Rowe PA, Campbell-Thompson ML, Schatz DA, et al. The pancreas in human type 1 diabetes. Semin Immunopathol. 2011;33:29–43. doi: 10.1007/s00281-010-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonner-Weir S, O’Brien TD. Islets in type 2 diabetes: in honor of Dr. Robert C. Turner. Diabetes. 2008;57:2899–2904. doi: 10.2337/db07-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klöppel G. Chronic pancreatitis, pseudotumors and other tumor-like lesions. Mod Pathol. 2007;20(suppl 1):S113–S131. doi: 10.1038/modpathol.3800690. [DOI] [PubMed] [Google Scholar]
- 4.Hardt PD, Ewald N. Exocrine pancreatic insufficiency in diabetes mellitus: a complication of diabetic neuropathy or a different type of diabetes? Exp Diabetes Res. 2011;2011:761950. doi: 10.1155/2011/761950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ewald N, Bretzel RG, Fantus IG, et al. Pancreatin therapy in patients with insulin-treated diabetes mellitus and exocrine pancreatic insufficiency according to low fecal elastase 1 concentrations. Results of a prospective multi-centre trial. Diabetes Metab Res Rev. 2007;23:386–391. doi: 10.1002/dmrr.708. [DOI] [PubMed] [Google Scholar]
- 6.Laass MW, Henker J, Thamm K, et al. Exocrine pancreatic insufficiency and its consequences on physical development and metabolism in children and adolescents with type 1 diabetes mellitus. Eur J Pediatr. 2004;163:681–682. doi: 10.1007/s00431-004-1501-2. [DOI] [PubMed] [Google Scholar]
- 7.Nunes AC, Pontes JM, Rosa A, et al. Screening for pancreatic exocrine insufficiency in patients with diabetes mellitus. Am J Gastroenterol. 2003;98:2672–2675. doi: 10.1111/j.1572-0241.2003.08730.x. [DOI] [PubMed] [Google Scholar]
- 8.Hardt PD, Hauenschild A, Nalop J, et al. High prevalence of exocrine pancreatic insufficiency in diabetes mellitus. A multicenter study screening fecal elastase 1 concentrations in 1,021 diabetic patients. Pancreatology. 2003;3:395–402. doi: 10.1159/000073655. [DOI] [PubMed] [Google Scholar]
- 9.Hardt PD, Hauenschild A, Jaeger C, et al. High prevalence of steatorrhea in 101 diabetic patients likely to suffer from exocrine pancreatic insufficiency according to low fecal elastase 1 concentrations: a prospective multicenter study. Dig Dis Sci. 2003;48:1688–1692. doi: 10.1023/a:1025422423435. [DOI] [PubMed] [Google Scholar]
- 10.Cecil RL. A study of the pathological anatomy of the pancreas in ninety cases of diabetes mellitus. J Exp Med. 1909;11:266–290. doi: 10.1084/jem.11.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibb WF, Logan VW. Diabetes mellitus: a study of one hundred and forty seven autopsies. Arch Intern Med (Chic) 1929;43:376–383. [Google Scholar]
- 12.Hardt PD, Killinger A, Nalop J, et al. Chronic pancreatitis and diabetes mellitus. A retrospective analysis of 156 ERCP investigations in patients with insulin-dependent and non-insulin-dependent diabetes mellitus. Pancreatology. 2002;2:30–33. doi: 10.1159/000049445. [DOI] [PubMed] [Google Scholar]
- 13.Vesterhus M, Raeder H, Johansson S, et al. Pancreatic exocrine dysfunction in maturity-onset diabetes of the young type 3. Diabetes Care. 2008;31:306–310. doi: 10.2337/dc07-1002. [DOI] [PubMed] [Google Scholar]
- 14.Vesterhus M, Haldorsen IS, Raeder H, et al. Reduced pancreatic volume in hepatocyte nuclear factor 1A-maturity-onset diabetes of the young. J Clin Endocrinol Metab. 2008;93:3505–3509. doi: 10.1210/jc.2008-0340. [DOI] [PubMed] [Google Scholar]
- 15.Cavalot F, Bonomo K, Fiora E, et al. Does pancreatic elastase-1 in stools predict steatorrhea in type 1 diabetes? Diabetes Care. 2006;29:719–721. doi: 10.2337/diacare.29.03.06.dc05-1389. [DOI] [PubMed] [Google Scholar]
- 16.Hahn JU, Kerner W, Maisonneuve P, et al. Low fecal elastase 1 levels do not indicate exocrine pancreatic insufficiency in type-1 diabetes mellitus. Pancreas. 2008;36:274–278. doi: 10.1097/MPA.0b013e3181656f8. [DOI] [PubMed] [Google Scholar]
- 17.Campbell-Thompson M, Wasserfall C, Montgomery EL, et al. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA. 2012;308:2337–2339. doi: 10.1001/jama.2012.15008. [DOI] [PubMed] [Google Scholar]
- 18.Lohr M, Kloppel G. Residual insulin positivity and pancreatic atrophy in relation to duration of chronic type 1 (insulin-dependent) diabetes mellitus and microangiopathy. Diabetologia. 1987;30:757–762. doi: 10.1007/BF00275740. [DOI] [PubMed] [Google Scholar]
- 19.Goda K, Sasaki E, Nagata K, et al. Pancreatic volume in type 1 and type 2 diabetes mellitus. Acta Diabetol. 2001;38:145–149. doi: 10.1007/s005920170012. [DOI] [PubMed] [Google Scholar]
- 20.Saisho Y, Butler AE, Meier JJ, et al. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat. 2007;20:933–942. doi: 10.1002/ca.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philippe MF, Benabadji S, Barbot-Trystram L, et al. Pancreatic volume and endocrine and exocrine functions in patients with diabetes. Pancreas. 2011;40:359–363. doi: 10.1097/MPA.0b013e3182072032. [DOI] [PubMed] [Google Scholar]
- 22.Williams AJ, Thrower SL, Sequeiros IM, et al. Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab. 2012;97:E2109–E2113. doi: 10.1210/jc.2012-1815. [DOI] [PubMed] [Google Scholar]
- 23.Sequeiros IM, Hester K, Callaway M, et al. MRI appearance of the pancreas in patients with cystic fibrosis: a comparison of pancreas volume in diabetic and non-diabetic patients. Br J Radiol. 2010;83:921–926. doi: 10.1259/bjr/24009651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams AJ, Chau W, Callaway MP, et al. Magnetic resonance imaging: a reliable method for measuring pancreatic volume in type 1 diabetes. Diabet Med. 2007;24:35–40. doi: 10.1111/j.1464-5491.2007.02027.x. [DOI] [PubMed] [Google Scholar]
- 25.Burute N, Nisenbaum R, Jenkins DJ, et al. Pancreas volume measurement in patients with type 2 diabetes using magnetic resonance imaging-based planimetry. Pancreatology. 2014;14:268–274. doi: 10.1016/j.pan.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 26.Bilgin M, Balci NC, Momtahen AJ, et al. MRI and MRCP findings of the pancreas in patients with diabetes mellitus: compared analysis with pancreatic exocrine function determined by fecal elastase 1. J Clin Gastroenterol. 2009;43:165–170. doi: 10.1097/MCG.0b013e3181587912. [DOI] [PubMed] [Google Scholar]
- 27.Waguri M, Hanafusa T, Itoh N, et al. Histopathologic study of the pancreas shows a characteristic lymphocytic infiltration in Japanese patients with IDDM. Endocr J. 1997;44:23–33. doi: 10.1507/endocrj.44.23. [DOI] [PubMed] [Google Scholar]
- 28.Kim CI. Clinicopathological study of pancreatic fibrosis in diabetes mellitus. Med J Osaka Univ. 1977;28:23–31. [PubMed] [Google Scholar]
- 29.Lazarus SS, Volk BW. Pancreas in maturity-onset diabetes. Pathogenetic considerations. Arch Pathol. 1961;71:44–59. [PubMed] [Google Scholar]
- 30.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- 31.Warren S, LeCompte PM. The Pathology of Diabetes Mellitus. 3rd. Philadelphia, PA: Lea & Febiger; 1952. [Google Scholar]
- 32.Foulis AK, Liddle CN, Farquharson MA, et al. The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia. 1986;29:267–274. doi: 10.1007/BF00452061. [DOI] [PubMed] [Google Scholar]
- 33.Olsen TS. The incidence and clinical relevance of chronic inflammation in the pancreas in autopsy material. Acta Pathol Microbiol Scand A. 1978;86A:361–365. doi: 10.1111/j.1699-0463.1978.tb02057.x. [DOI] [PubMed] [Google Scholar]
- 34.Fedail SS, el Mahadi EM, el Lidir AR, et al. Endoscopic retrograde cholangiopancreatography in Sudanese diabetics. Digestion. 1986;34:226–228. doi: 10.1159/000199333. [DOI] [PubMed] [Google Scholar]
- 35.Nakanishi K, Kobayashi T, Miyashita H, et al. Exocrine pancreatic ductograms in insulin-dependent diabetes mellitus. Am J Gastroenterol. 1994;89:762–766. [PubMed] [Google Scholar]
- 36.Creutzfeldt W, Gleichmann D, Otto J, et al. Follow-up of exocrine pancreatic function in type-1 diabetes mellitus. Digestion. 2005;72:71–75. doi: 10.1159/000087660. [DOI] [PubMed] [Google Scholar]
- 37.Hitanant S, Vannasaeng S, Tan-ngarm-trong D, et al. Exocrine pancreatic function among diabetic patients in Thailand. Am J Gastroenterol. 1986;81:559–561. [PubMed] [Google Scholar]
- 38.Sato M, Yamamoto K, Mayama H, et al. Exocrine pancreatic function in diabetic children. J Pediatr Gastroenterol Nutr. 1984;3:415–420. doi: 10.1097/00005176-198406000-00019. [DOI] [PubMed] [Google Scholar]
- 39.Lankisch PG, Manthey G, Otto J, et al. Exocrine pancreatic function in insulin-dependent diabetes mellitus. Digestion. 1982;25:211–216. doi: 10.1159/000198833. [DOI] [PubMed] [Google Scholar]
- 40.Frier BM, Adrian TE, Saunders JH, et al. Serum trypsin concentration and pancreatic trypsin secretion in insulin-dependent diabetes mellitus. Clin Chim Acta. 1980;105:297–300. doi: 10.1016/0009-8981(80)90472-6. [DOI] [PubMed] [Google Scholar]
- 41.Harano Y, Kim CI, Kang M, et al. External pancreatic dysfunction associated with diabetes mellitus. J Lab Clin Med. 1978;91:780–790. [PubMed] [Google Scholar]
- 42.Frier BM, Saunders JH, Wormsley KG, et al. Exocrine pancreatic function in juvenile-onset diabetes mellitus. Gut. 1976;17:685–691. doi: 10.1136/gut.17.9.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bock OA, Bank S, Marks IN, et al. Exocrine pancreatic function in diabetes mellitus. S Afr Med J. 1967;41:756–758. [PubMed] [Google Scholar]
- 44.Vacca JB, Henke WJ, Knight WA, Jr, et al. The exocrine pancreas in diabetes mellitus. Ann Intern Med. 1964;61:242–247. doi: 10.7326/0003-4819-61-2-242. [DOI] [PubMed] [Google Scholar]
- 45.Chey WY, Shay H, Shuman CR. External pancreatic secretion in diabetes mellitus. Ann Intern Med. 1963;59:812–821. doi: 10.7326/0003-4819-59-6-812. [DOI] [PubMed] [Google Scholar]
- 46.Pollard HM, Miller L, Brewer WA. The external secretion of the pancreas and diabetes mellitus. Am J Dig Dis. 1943;10:20–23. [Google Scholar]
- 47.Hardt PD, Krauss A, Bretz L, et al. Pancreatic exocrine function in patients with type 1 and type 2 diabetes mellitus. Acta Diabetol. 2000;37:105–110. doi: 10.1007/s005920070011. [DOI] [PubMed] [Google Scholar]
- 48.Canaway S, Phillips I, Betts P. Pancreatic exocrine insufficiency and type 1 diabetes mellitus. Br J Nurs. 2000;9:2030–2032. doi: 10.12968/bjon.2000.9.18.12461. [DOI] [PubMed] [Google Scholar]
- 49.Rathmann W, Haastert B, Icks A, et al. Low faecal elastase 1 concentrations in type 2 diabetes mellitus. Scand J Gastroenterol. 2001;36:1056–1061. doi: 10.1080/003655201750422657. [DOI] [PubMed] [Google Scholar]
- 50.Icks A, Haastert B, Giani G, et al. Low fecal elastase-1 in type I diabetes mellitus. Z Gastroenterol. 2001;39:823–830. doi: 10.1055/s-2001-17867. [DOI] [PubMed] [Google Scholar]
- 51.Cavalot F, Bonomo K, Perna P, et al. Pancreatic elastase-1 in stools, a marker of exocrine pancreas function, correlates with both residual beta-cell secretion and metabolic control in type 1 diabetic subjects. Diabetes Care. 2004;27:2052–2054. doi: 10.2337/diacare.27.8.2052. [DOI] [PubMed] [Google Scholar]
- 52.Yilmaztepe A, Ulukaya E, Ersoy C, et al. Investigation of fecal pancreatic elastase-1 levels in type 2 diabetic patients. Turk J Gastroenterol. 2005;16:75–80. [PubMed] [Google Scholar]
- 53.Mueller B, Radko F, Diem P. Pancreatic elastase-1 in stools, a marker of exocrine pancreas function, correlates with both residual beta-cell secretion and metabolic control in type 1 diabetic subjects: response to Cavalot et al. Diabetes Care. 2005;28:2809–2810. doi: 10.2337/diacare.28.11.2809a. [DOI] [PubMed] [Google Scholar]
- 54.Larger E, Philippe MF, Barbot-Trystram L, et al. Pancreatic exocrine function in patients with diabetes. Diabet Med. 2012;29:1047–1054. doi: 10.1111/j.1464-5491.2012.03597.x. [DOI] [PubMed] [Google Scholar]
- 55.Vujasinovic M, Zaletel J, Tepes B, et al. Low prevalence of exocrine pancreatic insufficiency in patients with diabetes mellitus. Pancreatology. 2013;13:343–346. doi: 10.1016/j.pan.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 56.van Geenen EJ, Smits MM, Schreuder TC, et al. Smoking is related to pancreatic fibrosis in humans. Am J Gastroenterol. 2011;106:1161–1166. doi: 10.1038/ajg.2011.43. [DOI] [PubMed] [Google Scholar]
- 57.Pitchumoni CS, Glasser M, Saran RM, et al. Pancreatic fibrosis in chronic alcoholics and nonalcoholics without clinical pancreatitis. Am J Gastroenterol. 1984;79:382–388. [PubMed] [Google Scholar]
- 58.Foulis AK, Stewart JA. The pancreas in recent-onset type 1 (insulin-dependent) diabetes mellitus: insulin content of islets, insulitis and associated changes in the exocrine acinar tissue. Diabetologia. 1984;26:456–461. doi: 10.1007/BF00262221. [DOI] [PubMed] [Google Scholar]
- 59.Lam WF, Gielkens HA, Coenraad M, et al. Effect of insulin and glucose on basal and cholecystokinin-stimulated exocrine pancreatic secretion in humans. Pancreas. 1999;18:252–258. doi: 10.1097/00006676-199904000-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


